Abstract
The presence of starch phosphate monoesters in native starch, especially in tuberous storage starch types, has for many years been known to impart unique and valuable functional assets of importance for food and materials applications. The quest of delineating the incorporation of phosphate groups in storage starch of crops, the general misconception over many years that starch phosphorylation is a “metabolic mistake” or a “side reaction”, was disproved when the starch phosphorylator was discovered some 15 years ago. Over the recent years, additional data have evolved to demonstrate that phosphorylation of starch granules in plants is a built-in metabolic feature that is essential for well-functioning starch metabolism. This chapter will embrace the ubiquitous presence of starch phosphorylation in plants and its impact on plant metabolism and starch functionality and very recent studies demonstrating its tremendous impact on crop performance and future prospects for starch bioengineering and polysaccharide innovation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Starch Phosphorylation: Discovery and Early Work
1.1 Discovery of Starch Phosphate Esters
The first indication of the presence of charged substituents in starch was made in 1897 (Coehn 1897) showing that starch displayed an anodic migration behaviour as proven by electrodialysis of different hydrocolloids. Some years after (Fernbach 1904) acid hydrolysis was employed to demonstrate that the anionic nature was due to the presence of phosphate groups in the starch. Using the electrodialysis approach originally applied by Coehn followed by acid hydrolysis, organic phosphate monoesters were shown to be present in the amylopectin fraction(Samec 1914), and later (Takeda and Hizukuri 1982) it was concluded that only trace amounts were present in amylose. Using amylolytic hydrolysis and subsequent chemical analysis of the generated products, some unique resistant maltooligosaccharides could be detected and phosphate found as monoesters bound at the C-6 and the C-3 positions in this polyose material (Posternak 1935). This was later verified using nuclear magnetic resonance, NMR (Bay-Smidt et al. 1994; Blennow et al. 1998a; Hizukuri et al. 1970; Tabata and Hizukuri 1971). Phosphate esterified at the very labile C-2 position of the glucose units has been discussed (Tabata and Hizukuri 1971), but the presence of this phosphoester has not yet been verified. The physical effects, especially paste viscosity, of starch phosphate esters in naturally phosphorylated starch, especially potato starch, were notified somewhat later (Samec and Blinc 1941; Schreiber 1958). These discoveries paved the way for a multitude of potential applications of phosphorylated starch, which will be further detailed in the next sections of this chapter.
1.2 The Chemistry of Starch Phosphate Esters
1.2.1 The Presence of Phosphate Monoesters in the Amylopectin Chains
Amylopectin is a clustered and semi-crystalline polysaccharide having polymodal chain length distribution (Chaps. 1 and 3; Damager et al. 2010; Pérez and Bertoft 2010). The fact that the presence of phosphate esters mainly is in the amylopectin fraction is seemingly dependent on the biosynthesis and presence of these amylopectin structures. The concentration of phosphate esters in the starch is typically very low and depends on plant origin. For example, cereals normally contain ≤1 nmol/mg (Lim et al. 1994), while tubers are highly phosphorylated having up to 33 nmol phosphate/mg starch (Blennow et al. 2000; Hoover 2001; Carciofi et al. 2011; Table 12.1). The highest concentrations are found in tubers, especially those with modified starch having as high as 60 nmol phosphate/mg starch which translates to only 1 out of 200 glucose residues being substituted (Schwall et al. 2000; Blennow et al. 2005b; Wischmann et al. 2005). Yet, the physical and metabolic effects of the phosphate groups are extraordinary. It is now well confirmed that the phosphate groups are monoesterified at both the C-3 and the C-6 positions of the anhydrous pyranosidic glucose residues; C-6 phosphate esters are in majority contributing with approximately 70 % of the total phosphorylation degree (Hizukuri et al. 1970; Tabata and Hizukuri 1971; Bay-Smidt et al. 1994).
1.2.2 The Phosphorylated Chains
Phosphorylated chains in the starch granule have some specific features. As compared to non-phosphorylated amylopectin chains, the phosphorylated chains are typically longer, between degree of polymerisation (DP) 10 and 100 (Blennow et al. 1998a,2000; Tabata and Hizukuri 1971; Takeda and Hizukuri 1982). Hence, starch types having long chains, typically tuber starches, are highly phosphorylated. Since these starch types typically crystallise as the B-type crystalline polymorph, the crystalline polymorph can be readily predicted from the degree of phosphorylation or vice versa (Blennow et al. 2000). Early work clarified that an amylopectin unit chain can contain one or more phosphate groups (Blennow et al. 1998a); no phosphate groups are esterified on the nonreducing end or closer than nine glucosyl units from an α-1,6 branch point (Takeda and Hizukuri 1982). Dephosphorylated chains as short as 10 glucose units were detected in potato starch supporting these data but do not exclude phosphorylation close to the branch point on the main chain. The structure of phospho-oligosaccharides prepared by hydrolytic degradation of potato starch has provided additional valuable information on the relative positioning of the phosphate esters in the starch. Using a combination of neopullulanase and α-amylases (Kamasaka et al. 1997a), the relative clustering of phosphate groups in the starch chain was determined (Kamasaka et al. 1997b). C-6 or C-3 phosphates can be very closely placed and as close as two glucose residues apart. However, adjacent C-3 and C-6 phosphorylated glucose residues were also found in the hydrolysates indicating the presence of very dense local phosphorylation in the starch granule. In the amylopectin molecule, the α-1,6 branch points are clustered to generate highly branched regions termed clusters.
1.2.3 The Phosphorylated Chain Clusters
The so-called cluster model of amylopectin structure in the starch granule suggests that the amorphous lamellae present in the starch granule contain most of the α-1,6-glucosidic linkages and these are constructed of building blocks along the long chains of amylopectin, which collectively form a backbone of the macromolecule (Bertoft and Blennow 2009; Bertoft et al. 2012). The branched clusters are about DP50–DP130 and smaller densely branched building blocks typically being DP5–DP13 but can also be as large as >DP20 (Bertoft et al. 2012). Specifically, the cluster size for a normal potato starch and a transgenic low-phosphate starch with suppressed α-glucan, water dikinase (GWD) activity was DP127 and DP86, respectively (Jensen et al. 2013b). For a high-amylose/high-phosphate potato starch, generated by antisense suppression of starch branching enzyme, the cluster sizes were smaller and contained highly phosphorylated phospho-clusters with low degree of branching as compared to control (Wikman et al. 2011). These data indicate that, even though the effects on cluster size are minor, phosphate monoesters have some effect of the branch clusters in the amylopectin and are depicted in Fig. 12.1.
Schematic representation of phosphorylated amylopectin as organised in the potato starch granule being engineered or selected for low (LPS low-phosphate starch), intermediate (NAMS normal amylopectin starch) and high phosphate (HPS high-phosphate starch) content. Cylinders represent amylopectin double helices, black lines amylopectin chains and grey lines amylose chains. The crystalline lamellae (C) and the amorphous lamellae (A) are enlarged in HPS. C-6-bound phosphate (black circle symbol) is enriched in the amorphous lamellae, whereas C-3 (grey circle symbol)-bound phosphate is more evenly distributed between crystalline and amorphous lamellae. DP degree of polymerisation (Reprinted with permission from Wikman et al. 2013. Copyright (2013) Wiley)
1.2.4 Phosphate Monoester Distribution in the Starch Granule
The starch granule is a complex but highly ordered structural entity with radial, or nearly radial, alternating amorphous and crystalline lamellae repeated with 9 nm spacing packed to form either hexagonal or pseudo-hexagonal crystalline packing of A-type and/or B-type crystalline polymorphs (Chap. 3; Damager et al. 2010; Pérez and Bertoft 2010). A schematic nano-level cross section of the parallel helices is depicted in Fig. 12.1 where the suggested positions of phosphate groups in the C-3 and C-6 position are indicated. From this model it is evident that phosphate groups can be monoesterified in both crystalline and amorphous parts of the granule. The location of phosphoesters in the so-called Naegeli dextrins, representing the crystalline lamella of the starch granule (Blennow et al. 2000; Wikman et al. 2013), confirmed that part of the phosphate groups were indeed located in highly ordered crystalline parts. Such positioning would require a very tight alignment in the crystalline network. As deduced from molecular models (Engelsen et al. 2003), the C-6 phosphate is tightly packed in one of the groves in the double helix. Such tight position was further indicated by its inability to form complex with copper ions as analysed by electron paramagnetic resonance (EPR, Blennow et al. 2006) and the low mobility as demonstrated by NMR (Larsen et al. 2008). As mentioned above, a substantial portion of the phosphate groups (79–84 %) are also found in the amorphous parts of the starch granule (Blennow et al. 2000; Wikman et al. 2013). As deduced from lintnerisation (slow acid hydrolysis of amorphous starch at high acid concentration), the amorphous parts are more enriched in C-6-bound phosphate than C-3-bound phosphate. As indicated, the distribution of phosphate monoesters in the native starch granule is apparently not random at the nm level. Such lack of phosphate distributional data stems from the inherent difficulty to analyse molecular objects and elements in semi-crystalline matrices like those found in the starch granule. However, at the micrometre scale, previous investigations using chemical surface gelatinisation (Jane and Chen 1993) demonstrated that phosphate concentration was lower in the gelatinised (surface) layers of the starch granules than in the remaining central un-gelatinised parts. This was supported by a particle-induced x-ray emission (PIXE) microscopic study, in which phosphate was found concentrated to the centre of a potato starch granule (Blennow et al. 2005a). However, in more recent studies using confocal laser light scanning microscopy (CLSM) with the phosphate-specific fluorescent phosphoprobe Pro-Q® Diamond, phosphate monoesters were rather found closer to the surface of the granules (Fig. 12.2; Glaring et al. 2006). More precisely, phosphate-related fluorescence was often found at a rim close to the surface or spread out through the starch granule (Fig. 12.2; Glaring et al. 2006). However, variations were found depending on genotype, and it cannot be excluded that the accessibility of phosphate esters in the granule can affect such fluorescence data preventing a strict interpretation of phosphate distribution.
Wheat and potato starch granules visualised by transmission light (left) and the fluorescent phosphoprobe Pro-Q® Diamond (right). (a) Wheat granule showing the distinct equatorial groove and very low fluorescence. (b) Normal potato starch granule with intermediate fluorescence. (c) High-amylose potato granule showing internal cracking and high fluorescence. (d) High-amylopectin potato granule showing intermediate fluorescence, like the normal granule (a). Red bars indicate relative size and that phosphate monoesters are found close to the surface of the granules, more precisely at a rim close to the surface or evenly distributed over the starch granule. However, variations are found depending on genotype (Adapted with permission from Glaring et al. 2006. Copyright (2006) American Chemical Society)
1.2.5 Effects of Phosphate in the Starch Granule
An interesting aspect related to physical and rheological features of starch is that many of these effects are considered to be attributed directly to the glucan configurations like α-1,6 branching structures and amylose content. However, it has recently been proven that many physical properties are directly accredited to the presence of phosphate esters in the starch. For example, their presence in the crystalline lamellae was first indicated by a minor suppression of the crystallinity as judged by differential scanning calorimetry (DSC, Muhrbeck and Eliasson 1991). Subsequent studies using an isogenic potato system (Kozlov et al. 2007) confirmed that the phosphate groups reduce the crystal stability by local amorphisation in the starch granule. The phosphate groups had an effect on the enthalpy of melting (ΔΗ) of the starch granules, but there was no effect on the melting temperature (Tm) supporting very local disturbance inducted by the phosphate groups. Further recent support for an amorphisation effect induced by phosphate was provided by differently phosphorylated lintners (Wikman et al. 2013). A strong negative correlation was found between melting enthalpy of the lintnerised starch and the phosphate content of the native starch granules, and a negative correlation was found between melting enthalpy and the phosphate content of the remaining lintnerised starch (Wikman et al. 2013). Phosphate groups also seem to affect interactions between branched and linear glucan chains in gelatinised starch. At high (30–40 %) starch paste concentrations, phosphate groups in the amylopectin repel chains between amylopectin and amylose likely resulting in phase separation between these polysaccharides in the paste (Jensen et al. 2013a).
Direct evidence for a phosphate-induced starch solubilising effect with relevance for plant cell metabolism was provided by studying crystallised phosphorylated maltodextrins in vitro (Hejazi et al. 2008). The main starch phosphorylating enzyme α-glucan, water dikinase (GWD) (see Sect. 12.4) efficiently catalysed phosphorylation of crystalline maltodextrins and induced solubilisation of both the neutral and the phosphorylated glucans in these crystalline aggregates (Hejazi et al. 2008). Partial dephosphorylation of such A- and B-type crystallised phosphoglucans by the specific Arabidopsis thaliana glucan phosphatase AtSEX4 reduced solubilisation of non-phosphorylated chains in these aggregates (Hejazi et al. 2010).
Molecular force field models provided evidence to show that a phosphate ester at the C-6 position can be linked to amylopectin without disturbing its double helical structure. On the other hand, a phosphate ester at the C-3 position imparts molecular strain in the double helical motif thereby preventing optimal crystalline packing (Fig. 12.3; Blennow et al. 2002; Engelsen et al. 2003; Hansen et al. 2008). A general mechanism for phosphate-induced amorphisation and solubilisation based on such models is discussed in Sect. 12.3.
Molecular force field models demonstrating that C-6 phosphorylation does not disturb the double helical structure, while C-3 phosphorylation is more exposed and induces molecular strain in the double helix. This strain can be released by introducing defects in the crystalline structure resulting in local amorphisation of the starch granule (From Blennow and Engelsen 2010. Reprinted with permission from Elsevier Ltd)
Naturally phosphorylated starch has a significant hydration capacity, and such starch types, especially potato starches, produce clear and very viscous pastes (Muhrbeck and Eliasson 1987; Wiesenborn et al. 1994; Viksø-Nielsen et al. 2001; Jobling 2004) affecting processing (e.g. Jensen et al. 2013b). These effects in relation to starch processing are discussed in more detail in Sect. 12.5.
2 Phosphorylation of the Starch Granule: The Enzymes
Two enzymes are seemingly responsible for the phosphorylation of the starch granule, namely, the α-glucan, water dikinase 1 (GWD1) catalysing the formation of starch phosphate monoesters at the C-6 positions of the glucose units and the α-glucan, water dikinase 3/phosphoglucan, water dikinase GWD3/PWD catalysing phosphorylation at the C-3 positions in the amylopectin. This section will describe the catalysis, specificity and regulation of these two main enzymes. A third dikinase named GWD2 (Glaring et al. 2007) is a homologue of GWD1 but is extra-plastidial, and its function has not yet been elucidated. Generally, GWDs are found throughout the plant kingdom in grasses (Blennow et al. 2013; Tanackovic et al. 2014) and in dicotyledonous plants and algae (Baunsgaard et al. 2005; Glaring et al. 2007) and found in various organs like tubers of potato, sweet potato and yam and cereal endosperm of barley and maize, in fruits like banana and in leaves of Arabidopsis and potato (Mikkelsen and Blennow 2005; Ritte et al. 2000a, b) demonstrating their ubiquitous role in plant metabolism.
2.1 Discovery of R1/GWD1
α-Glucan, water dikinases (GWDs), responsible for the phosphorylation of starch, belong to a family comprising two classes of phosphotransferases with different substrate specificities and products. GWD was first discovered as a protein termed R1 in potato (Solanum tuberosum) by Lorberth et al. (1998). In Arabidopsis the enzyme is also known as SEX1 due to the starch excess phenotype found in the leaves (Ritte et al. 2006). The Arabidopsis mutant was well known before the discovery of the R1 protein (Yu et al. 2001) due to its starch excess phenotype. The discovery of the potato R1 protein was based on the loss of phosphate esters in potato tuber starch following antisense suppression of this protein (Lorberthet al. 1998). Four years later the activity of the GWD1 (EC 2.7.9.4) was elucidated (Ritte et al. 2002). GWD1 was proven to phosphorylate glucose residues in starch by transferring the β-phosphate of ATP to a glucosyl residue of amylopectin chains, and the γ-phosphate was transferred to water (Ritte et al. 2002). GWD1 was later confirmed to phosphorylate glucose residues exclusively at the C-6 position (Ritte et al. 2006).
Mainly amylopectin, and not amylose, is phosphorylated, which as a consequence explains the apparent substrate recognition requirement of GWD for α-1,6 bonds (Mikkelsen et al. 2004). GWD1 also preferably phosphorylates the long chains (degree of polymerisation, DP30–100) in the amylopectin molecule (Blennow et al. 1998a; Mikkelsen et al. 2004) giving additional evidence for the long phosphorylated chains fond in plants. However, since the catalytic efficiency of the GWD1 is dependent on the chain length of the substrate and that also amylose is being phosphorylated provided that the chains are long enough (Mikkelsen et al. 2004), the requirement of the branch point is possibly an effect of secondary (possibly helical and helical aggregates) structures recognised by the GWD1. The necessity of such folded or aggregated glucan structures was supported by data (Hejazi et al. 2008) revealing that GWD1 readily phosphorylates crystallised maltodextrins of the B-type starch allomorph having longer side chains as compared to A-type crystalline polymorphs. Interestingly, GWD1 exhibited very low activity on identical soluble maltodextrins (Hejazi et al. 2008). However, both A-type and B-type crystalline polymorphs were efficiently catalysed by the GWD1 (and the GWD3/PWD homologue; see below, Hejazi et al. 2009) further pointing at the importance for preferred phosphorylation of granular structures with well-defined physical arrangements, most possibly directed by the α-1,6 bonds in amylopectin.
As mentioned, a third α-glucan, water dikinase named GWD2 (Glaring et al. 2007) is a homologue of GWD1 but is with a function that has not yet been elucidated. Knockout plants for this gene in Arabidopsis show no altered growth or starch and sugar levels, and subcellular localisation studies indicated that this GWD2 is extra-plastidial (starch is mainly synthesised in the plastid) and found expressed in companion cells of the phloem, with expression appearing just before the onset of senescence. Hence, this homologue is seemingly not directly involved in the major routes of transient starch degradation in plants.
2.2 Discovery of PWD/GWD3
Some years after the discovery of the GWD1 activity (Ritte et al. 2002), homologues of this protein in Arabidopsis were found (Baunsgaard et al. 2005; Köttinget al. 2005; Glaring et al. 2007). Some of these homologues were found to have distinctly different substrate specificities and catalytic activities. One type of these homologues was isolated from Arabidopsis and termed GWD3 (EC 2.7.9.5) (Baunsgaard et al. 2005) or phosphoglucan, water dikinase (PWD, Kötting et al. 2005).
The latter name of this enzyme was due to the apparent strict requirement for pre-phosphorylated starch or glucans for its activity (Baunsgaard et al. 2005; Kötting et al. 2005). PWD/GWD3 was demonstrated to strictly phosphorylate pre-phosphorylated starch at the C-3 position of glucose residues in amylopectin chains (Baunsgaard et al. 2005; Kötting et al. 2005; Ritte et al. 2006). The C-3-bound phosphate is a remarkable feature since phosphorylation at this position is rarely seen in nature. The very closely positioned C-3 and C-6 phosphorylated glucose residues found in hydrolysates of potato starch (Kamasaka et al. 1997b) indicate the in vivo action of PWD/GWD3 as well as the requirement for closely situated phosphate groups in the substrate glucan. However, it was later demonstrated that also non-phosphorylated chains could be phosphorylated provided that these were co-crystallised with phosphoglucans (Hejazi et al. 2009). Such differences in substrate specificity between GWD and PWD/GWD3 create metabolic dependence between these two activities in the plant cell that is potentially important for the physiological effect of starch phosphorylation. This is further described in Sect. 12.4.
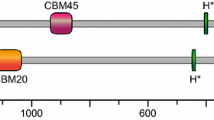
An interesting feature of the GWDs, and many starch-acting enzymes generally, and of potential importance for their catalytic and physiological function, is the presence of starch-binding domains (SBD) of the type carbohydrate-binding module (CBM) from families 20 and 45 (www.CaZy.org). In GWD1 and GWD2 and also in the plastidial α-amylases, CBM45s are placed at the N-terminus, in tandem repeats (Mikkelsen et al. 2006; Glaring et al. 2007). Conserved tryptophan residues act as putative binding sites. Of specific interest is that CBM45 and CBM20 found in plants have lower affinity (mM) towards starch than typical microbial CBMs (μM) (Blennow and Engelsen 2010; Blennow and Svensson 2010; Glaring et al. 2011). In PWD/GWD3 CBMs from family 20 are found. This CBM family is one of the earliest and best characterised families that occur in several starch-active glycosyl hydrolases. CBM20s are 90–130-amino-acid residue long; some of them are conserved. Two tryptophans are suggested to be involved in glucan interaction. Like the CBM45s in the GWDs, the CBM20s show low affinity in PWD/GWD3 (Christiansen et al. 2009). In contrast to the high affinity of the extracellular CBMs, the low affinity found for these intracellular and plastidial CBMs would ensure dynamic and reversible interactions between the enzymes and starch granule in starch phosphorylation at different light regimes and at stress situations (Blennow and Engelsen 2010; Blennow and Svensson 2010; Glaring et al. 2011). Interestingly, starch granule binding of GWD is dependent on the cellular redox potential which varies on a diurnal basis. It remains to be tested of the CBMs that are involved in such diurnal and redox-dependent partitioning of the GWD. GWD in dark-adapted plants is inactive and attached to granules in its oxidised form. Activity can be reversed by reduction using dithiothreitol (DTT) in vitro, and the active form is expected to be soluble during the light period simultaneously with starch biosynthesis (Mikkelsen et al. 2005).
3 Effects on Metabolism
3.1 The Importance of Starch Phosphorylation in Plant Primary Metabolism
The transition from glycogen-based metabolism to photosynthesis-driven starch biosynthesis over evolution has imposed some functions that are essential for long-term carbohydrate storage (Ball et al. 1996, 2011). The evolution of starch phosphorylation in plants and algae is interesting since it is directly linked to the necessity of biosynthesis and long-term deposition dense and efficiently molecularly packed polysaccharides in the form of starch granules in the plant and the directly associated physiological problem to rapidly get metabolic access to the immobilised sugars in the starch granule. Even though it has not been possible to precisely trace the evolutionary origin of GWD and PWD/GWD3 (Ball et al. 2011), their function to permit mobilisation of crystalline glucan (starch) is evident. Starch deposited in virtually all plants is phosphorylated, the only known exception so far being maize storage starch (even though the GWDs are expressed also in this plant, Blennow et al. 2002). I will now describe the current status of the effects of starch phosphorylation on starch degradation and starch biosynthesis with focus on effects related to starch granule structure.
3.2 Effects on Starch Degradation
The evolutionary appearance of starch, being in an insoluble and crystalline state in the cell, and the requirement of starch phosphorylation to permit its physical amorphisation, solubilisation and final amylolytic degradation, forms an important biological strategy for the plant to ensure a robust long-term energy storage capacity combined with regulated mobilisation of carbohydrate energy on metabolic demand (Blennow et al. 2002; Blennow and Engelsen 2010). As mentioned above, the knock-down or knockout of the phosphorylating enzymes, GWDs, in the plant leads to starch excess phenotypes in the leaves strongly suggesting that phosphate is required for efficient and complete degradation of the starch. The preferred location of phosphate monoesters in long-chain, B-type polymorphic starch granules (Takeda and Hizukuri 1982; Tabata and Hizukuri 1971; Blennow et al. 1998a) is an interesting feature as such amylopectin crystalline polymorphs are found in tubers and in green photosynthetic tissue of the plant. This indicates there are specific requirements for organising such starches in the plant. A possible explanation is to provide the ability for these starches to be degraded by specific enzymes in the highly hydrated organs as opposed to dry seeds storing starch having considerably less phosphate. B-type crystalline polymorphs are typically more resistant towards amylolytic hydrolysis in vitro (Wickramasinghea et al. 2009; Jane et al. 2003; Planchot et al. 1997) forming a rational explanation for the phosphorylation-dependent amorphisation of only this type of starch.
The partitioning of GWD between the starch granule surface and the stroma of the plastid is very dynamic and dependent on light and the redox potential in the plastid and also on the surface structure of the starch granule. In potato (Solanum tuberosum) and pea (Pisum sativum) leaf cells, GWD is found bound to starch granule at night where starch degradation takes place (Ritte et al. 2000a, b). Such binding is supposedly dependent both on the enzyme and on the starch granule surface. The importance of the starch granule surface characteristics for GWD interaction was indicated by in vitro binding of GWD showing that GWD was bound more efficiently to dark-adapted granules than to light-treated ones (Ritte et al. 2000a). Moreover, starch extracted from dark-adapted leaves from potato and darkened cells of the unicellular alga Chlamydomonas reinhardtii is more efficiently phosphorylated than light-treated cells (Ritte et al. 2004). However, the binding is more dynamic, and after a time period of phosphorylation and starch degradation, affinity decreases (Ritte et al. 2004). Similar effect can be seen in turion organs of duckweed (Spirodela polyrhiza, Reimann et al. 2002). This effect points at a very intense but transient starch phosphorylation just preceding starch degradation. In turions GWD is potentially autophosphorylated, or at least subject to major conformational changes, prior to starch phosphorylation (Reimann et al. 2004). A transposon insertion in the tomato GWD gene caused male gametophytic lethality associated with a reduction in pollen germination (Nashilevitz et al. 2009). Such data have provided clear relationships between starch phosphorylation and starch degradation and physiologic effect thereof, but do not explain the mechanism behind.
As earlier mentioned, the presence of phosphate groups in the starch granule has some intriguing physical effects, and these can be of importance on how the starch granule is metabolised. Based on molecular models, it was speculated that the phosphate groups could provide molecular signals for starch degradation in the plant (Blennow et al. 2002). It was suggested (Blennow et al. 2002) that the phosphate groups themselves, by restructuring of the starch granule, increase the starch granule degradability. The first support for this hypothesis came some years later where data was provided to support that starch phosphate esters can stimulate hydrolytic enzyme activity in vitro verifying that phosphate esters per se can restructure the starch granule to stimulate its degradation (Edner et al. 2007). In vitro β-amylase activity was stimulated by GWD-catalysed starch phosphorylation. Simultaneous β-amylase- and isoamylase-catalysed starch hydrolysis was higher than for the β-amylase alone. This rate was further enhanced in the presence of GWD and ATP (Edner et al. 2007). Interestingly, modelling suggests that phosphoglucans have a stabilising effect on the β-amylase–glucan ligand complex (Dudkiewicz et al. 2008) supporting that starch phosphate esters can direct amylases to phosphorylated spots on starch granules. From these data it is evident that the presence of starch phosphate esters per se can stimulate starch degradation supporting the previously suggested starch amorphisising hypothesis (Blennow et al. 2002).
From a structural point of view, molecular force field models have provided support for how phosphate esters can stimulate starch degradation. As understood from data collected from nuclear magnetic resonance, x-ray diffraction, differential scanning calorimetry and enzyme kinetics and physiological data as described above, and recently supported by molecular modelling, phosphate exerts a stimulating effect on starch degradation (Blennow and Engelsen 2010). Force field models indicate that phosphate esters localised at the C-6 position of the glucose unit in starch do not necessarily disturb the double helical structure in the granule. However, such C-6 phosphorylation can induce hydration and voids in between the helical arrays to distort helix–helix interactions. Such local disturbance in the starch granule can stimulate endo-active hydrolases. Subsequent C-3 phosphorylation catalysed by the PWD/GWD3 can induce helix break (Fig. 12.3) to permit access for exoenzymes such as β-amylases and debranching enzymes such as isoamylase on these unwounded chains (Blennow et al. 2002; Engelsen et al. 2003; Hansenet al. 2008; Blennow and Engelsen 2010).
This hypothesis has gained support from in vitro studies using B-type crystallised maltodextrins as models for the starch granule (Hejazi et al. 2008). These crystalline aggregates, phosphorylated by GWD1, were readily solubilised. Also chains in these aggregates that were not phosphorylated were efficiently solubilised. Amylolytic enzymes readily attack these solubilised glucans. Further studies demonstrated that both A- and B-type starch crystals were phosphorylated and showed phase transition upon phosphorylation (Hejazi et al. 2010). The hypothesis suggested for these data recommended that phosphorylation by GWD1 at the C-6 position leads to a local structural disturbance to gain access of both non-phosphorylated and phosphorylated glucans to further permit and stimulate C-3 phosphorylation by PWD/GWD3. In conclusion, the two main hypotheses, originally based on molecular models, and followed by physiological and enzymatic data seemingly support each other to form a more robust mechanistic explanation for the stimulation of starch degradation by starch phosphorylation.
Importantly, complete degradation of generated phosphoglucan also requires phosphatases. The phosphatase SEX4 in Arabidopsis and in maize has been demonstrated to efficiently dephosphorylate both C-3 and C-6 phosphate esters (Kötting et al. 2009; Hejazi et al. 2010), while an additional phosphatase studied in Arabidopsis, LSF2 (LIKE SEX FOUR2), is specific for the C-3 phosphate esters (Santelia et al. 2011). The detailed effects and dynamics of starch degradation dependent on these hydrolytic enzymes are described in Chap. 7 of this book.
3.3 Effects on Starch Biosynthesis
As explained in the previous section, there is a clear relationship between starch phosphorylation and starch degradation. Effects on starch biosynthesis are less clear. However, some evidence exists for a role of starch phosphorylation for the formation of a functional and well-structured starch granule. To modulate crystallinity and to prepare the starch granule for dissolution prior to degradation and on physiological demand, most starches are phosphorylated during their biosynthesis (Blennow and Engelsen 2010). Hence, there seems to be a requirement for a biosynthesis-driven pre-phosphorylation. Cooperatively, during starch biosynthesis, branching enzyme, debranching enzyme and GWD act with starch synthases to synthesise proper molecular structures for further growth and crystallisation of the granule permitting efficient molecular packing combined with built-in granule degradability. Starch phosphorylation has not been clearly demonstrated to be an absolutely necessary process in starch biosynthesis. However, some notable effects deserve attention.
Studies on GWD affinity for starch granules and phosphorylating activity have demonstrated that GWD is very dynamic. Its association with starch granules prior to and concurrently with starch degradation seems to be important as described above. However, as deduced from by 32P radiolabelling (Nielsen et al. 1994) and by demonstration of active starch phosphorylation in isolated intact potato amyloplasts fed with sucrose (Wischmann et al. 1999), starch is also phosphorylated during starch biosynthesis, and GWD can have a distinct role also here. GWD activity in leaves has been demonstrated to be dependent on the cellular redox potential, which in turn is directly linked to the dark–light cycle of the plant cell. In potato, it has been found that GWD isolated from dark-adapted plants is inactive and attached to the starch granules in its oxidised form. Binding and GWD activity can be reversed by reducing the enzyme using dithiothreitol (DTT) in vitro (Mikkelsen et al. 2005). These data suggest that, in its active form during light periods, reduced and soluble GWD phosphorylates starch granules simultaneously with starch biosynthesis (Mikkelsen et al. 2005).
In favour of a role of starch phosphorylation in starch biosynthesis, this seems to be affected by starch phosphorylation as indicated by altered starch deposition in plants with suppressed or overexpressed GWD activity. The deposition of starch in a storage organ such as the potato tuber can be regarded a system virtually devoid of starch degradation. Antisense suppression of GWD1 in potato tubers results in starch granules with altered structure (cracks and fissures) and higher amylose content (Viksø-Nielsen et al. 2001; Glaring et al. 2006) supporting the starch phosphorylation has an important role to form a correct granule structure during starch biosynthesis. Data from the cereal system give further support of this view.
Overexpression of the potato GWD1 gene in the barley endosperm results in grain starch with tenfold increased phosphate content (Carciofi et al. 2011). Interestingly, the starch granules had altered topography showing more porous surfaces and irregular shapes as compared to control (Carciofi et al. 2011). However, the yield of starch in the grain was not significantly affected ruling out significant effects of stimulation of starch degradation in this system. This line also degraded grain starch similarly to the control during germination, but protein and β-glucan degradation was stimulated (Shaik et al. 2014). Hence, it is possible that starch phosphorylation, taking place during biosynthesis in grain filling, can dictate starch granule organisation. RNA interference (RNAi)-mediated downregulation of GWD1 in the wheat endosperm resulted in decreased starch-bound phosphate, an increase in grain size and plant biomass but unaltered starch content (Ral et al. 2012). These data are not readily explained as a direct effect of suppressed stimulation of starch degradation in the grain but rather a more complex metabolic change in grain development potentially affecting also starch biosynthesis.
Very recently, interesting effects of GWD1 suppression were demonstrated possibly relating starch phosphorylation to starch biosynthesis. As for the barley grain data, overexpression of GWD1 did not stimulate starch degradation in leaves of Arabidopsis. Moreover, starch degradation was not suppressed until GWD1 was reduced down to 30 % activity (Skeffington et al. 2014). Interestingly, this reduction of GWD1 inhibited starch biosynthesis in the light indicating that starch phosphorylation can stimulate starch biosynthesis as well as starch degradation.
Moreover, starch granules isolated from transgenic Arabidopsis thaliana plants with reduced or no GWD activity had glucan chain structures at the granule surface that was suggested to limit the general accessibility to starch-active enzymes, being degradative or biosynthetic (Mahlow et al. 2014). These data support a very general and biophysic effect of starch phosphorylation as previously postulated (Blennow and Engelsen 2010).
In summary, the importance of GWDs in starch degradation is founded on that phosphate groups that decrease the local order within the starch granule and by that facilitate the access of degrading enzymes. However, such effects can also stimulate starch biosynthesis when such transferases are active in the plant. Hence, GWDs play very complex key roles both in the transitory starch metabolism (Ritte et al. 2004; Mikkelsen et al. 2005; Zeeman et al. 2010; Edner et al. 2007) and the cereal endosperm metabolism (Ral et al. 2012; Shaik et al. 2014). Starch phosphorylation affects primary metabolism in the plant as observed both for starch biosynthesis and starch degradation. However, the exact metabolic role of starch phosphorylation remains to be elucidated.
4 Starch Phosphate Bioengineering and Implications for Technology
The main volume of produced starch in the world is chemically or physically modified to achieve proper functionality. Raw starches are not suitable for most applications due to high and unstable viscosity and retrogradation following gelatinisation. Modifications typically include improving processability like reduction of viscosity or melting temperature and improving general performance, such as improving film formation or emulsification properties (Mason 2009). Conventional chemical technology includes esterification, etherification or oxidation as well as acid treatment (Chiu and Solarek 2009). For physical modification extrusion cooking, pregelatinisation by heating in aqueous alcohol or alkaline alcohol and heat–moisture treatments are carried out (Haghayegh and Schoenlechner 2011). Just as for natural phosphorylation, such treatments change the crystalline structure of the starch granule.
4.1 Clean Phosphorylation of Starch Directly in the Crop
Over the recent years a gradual redirection from conventional chemical modification to more clean technologies such as physical modification and bioengineering by, for instance, enzymatic modification, is emerging. For starch phosphorylation, naturally or chemically phosphorylated, this type of modification yields a tremendous hydration capacity producing clear and highly viscous starch pastes (Muhrbeck and Eliasson 1987; Wiesenborn et al. 1994; Viksø-Nielsen et al. 2001; Thygesen et al. 2003; Jobling 2004). If carried out directly in the crop by transgene biotechnology, modern breeding or mutagenesis, starch functionality can be improved with little or no requirement for postharvest processing having tremendous potential economic and environmental advantages. Engineering the expression of GWD1 or GWD3/PWD is the obvious strategy to alter starch phosphate in the crop. However, engineering the expression of other enzymes in starch metabolism can also generate changes in starch phosphorylation. Substantial alterations in the phosphate content are obtained by changing the activities of GWD1 or starch branching enzyme (SBE) in the plant. On the contrary, only minor effects are accomplished by antisense suppression of starch synthases (SSs).
The effects of suppression of the GWD1 in potato (Lorberth et al. 1998) or the SEX1 homologue in Arabidopsis (Yu et al. 2001), being the original discoveries of the origin of starch phosphorylation, result in starch that is almost totally depleted in phosphate. As mentioned, in the Arabidopsis leaf, a starch excess phenotype is observed, and in both Arabidopsis and potato, the amylose content is increased (Viksø-Nielsen et al. 2001). The manipulation of the GWD1 has been described in many patents including the overexpression in wheat (Schewe et al. 2002) and corn (Lanahan and Basu 2005) leading to increased viscosity of the starch paste. Moreover, the use of combinations with the overexpression of both starch synthase II and GWDs in rice and corn has been described (Frohberg 2008). The overexpression in Arabidopsis the GWD3/PWD also results in increased phosphate content as described in a patent (Frohberg et al. 2012). Recently, overexpression of the potato GWD1 in barley was described resulting in increased phosphorylation (Carciofi et al. 2011) and a more “potato-like” starch. The starch functional effects of such engineering must however be further evaluated since many side effects can be noted. For example, in potato tubers with suppressed GWD1, the amylopectin molecular weight is decreased (Viksø-Nielsen et al. 2001) having adverse effects on starch viscosity.
The engineering of other enzymes in starch biosynthesis also often affects starch phosphorylation since the starch substrate for the GWDs is altered, chaining GWD activity. For example, suppression of SBE in most plants results in significantly increased amylose concentrations and often also in increased lengths of the amylopectin unit chains. An interesting side effect is the dramatic approximately threefold increase in the level of starch phosphate (Schwallet al. 2000; Blennow et al. 2005b). The reason for the latter effect is supposedly that the endogenous GWD1 activity is stimulated by long (DP30–DP100) amylopectin chains (Mikkelsen et al. 2004), which are enriched in these plants. Amylose is possibly not phosphorylated in these plants despite of its long chains. Suppression of the granule-bound starch synthase (GBSS) moderately increases the phosphate concentration as an effect of the removal of amylose, accounting for about 20–30 % of the starch, which is not phosphorylated. Suppression of the soluble starch synthases (SSSs), most of which are responsible for the biosynthesis of amylopectin, also affects the phosphate content. However, such engineering is more unpredictable. When the total SSS activity is repressed, the starch phosphate content is somewhat reduced (Lloydet al. 1999), whereas suppression of only the SSIII isoform increases the phosphate content by 70 % (Abel et al. 1996), again possibly due to altered amylopectin structure. Different combinations of SSS suppression in the potato tuber result in very different starch functionalities, for example, freeze–thaw stability, which can be obtained by engineering short starch chains (and low amylose content) combined with a high phosphate content (Jobling et al. 2002).
4.2 Technical Use of Bioengineered Phosphorylated Starch
Phosphorylated starches, either naturally selected or bred, transgenics or mutants, are of tremendous value for technical applications. Potato starch, being highly phosphorylated and very clean, is widely used, especially in paper manufacturing. The optimal performance of potato starch in paper manufacturing is potentially an effect of the phosphate esters providing functional groups and high hydration leading to stickiness of the starch paste. However, in a study of potato starches with different phosphate contents and viscosities, it could be concluded that wet-end paper manufacturing performance did not directly correlate with the phosphate level but with a stickiness parameter, possibly related to the presence of phosphate (Blennow et al. 2003). Starch phosphate esters, being natural plasticisers of the starch, can also be potentially exploited for bioplastic purposes. The function of phosphate is however not readily predictable, and recently a water-nondeformable starch film was generated (Gillgren et al. 2011) using the potato tuber starch engineered for low phosphate content by antisense suppression of GWD1. The phosphate levels of this starch are comparable to the levels obtained in the hyperphosphorylated barley starch overexpressing the potato GWD in the grain (Carciofi et al. 2011) suggesting that starch phosphate can be balanced to obtain optimal levels for bioplastic purposes.
Starch phosphate-induced hydration of starch is also of importance for processing due to the noticeable hydration and swelling of phospho-starch. A striking example is a recent enzyme-assisted modification of starch at high starch concentrations to restrict swelling (Jensen et al. 2013b). Phosphorylated starches behaved very different from typical non-phosphorylated starches like maize starch, and it was demonstrated for branching enzyme modification at these high substrate concentrations that phosphorylated starches reacted more like in solution, while non-phosphorylated starch types could be enzymatically cross-linked due to restricted hydration (Jensen et al. 2013b).
The preparation of phospho-maltooligosaccharides (POs) using enzymatic hydrolysis from potato starch has caught special attention for biomedical and food additives (Kamasaka et al. 2003). For example, POs solubilise calcium and iron salts as a direct effect of phosphate–calcium interactions (Kamasaka et al. 1995, 1997b). POs complexed with calcium (POs–Ca) have been demonstrated to exert high absorption in the intestinal tract (To-o et al. 2003) thereby increasing uptake of calcium in the body. Moreover, POs enhance tooth enamel remineralisation (Tanaka et al. 2012) demonstrating potential for use in dental health.
5 Future Perspectives
5.1 The Future Potential of Starch as a Robust Raw Material
Polysaccharides in general, and starch especially, have tremendous potential for solving future challenges in providing environmentally friendly solutions in the health, technical, food and nonfood sectors. Heading towards more environmentally friendly starch modification, clean, natural starch phosphorylation can potentially partly phase out chemical modification such as phosphorylation as a clean alternative for starch modification. Starch phosphorylation has profound effects on starch biosynthesis and mobilisation in seeds and grain (e.g. Shaik et al. 2014). As an effect, bioengineering phosphorylation in crops also has potential in increasing crop yield as mentioned above (Ral et al. 2012; Weise et al. 2012). Larger volumes and novel, functionalised raw materials are needed to build a robust bioeconomy (Enriques 1998) to take care of expected future major challenges related to climate change and an increasing human population. As such, starch is a multipurpose raw material with tremendous potential to overcome conflicting food, feed and fuel aspects that must be eliminated by increasing crop and starch yield without expanding agricultural area.
5.2 The Requirement of Clean, Efficient and Acceptable Production Methods of Modified Starch in Crops
Public concern about transgene (GMO) technology must be taken care of by openly discussing classical and modern breeding procedures including genetic modification, the use of synthetic genes and transgenes or an important upcoming alternatives such as the so-called genome editing (Cermak et al. 2011) which is a collective term for targeting specific genes with mutagenesis or the use of cisgenes (Schouten and Jacobsen 2008; Godwin et al. 2009) which uses genes from the plant itself or genes from sexually compatible and genetically closely related species that can intercross. Classical mutagenesis combined with state-of-the art screening will possibly have an important role in the future (Slade et al. 2012).
5.3 Cisgenesis for Crop Improvement
In favour of a cisgenic strategy, the European Food Safety Authority (EFSA) recently concluded that an equivalent level of hazard can be associated with cisgenesis as for conventional plant breeding. This assessment can open up possibilities for case-by-case exemptions of cisgenic plants from the directives that regulate the use of genetically modified crops (Schouten and Jacobsen 2008; EFSA Panel on Genetically Modified Organisms 2012).
5.4 Genome Editing
A very new and interesting way forward to improve starch quantity and quality like starch phosphorylation directly in the crop can be represented by novel genome editing technologies. Such techniques are based on the specific recognition of gene sequences in the plant and following mutagenesis in that specific gene. If performed using the so-called transient expression of the foreign genes in these systems, i.e. the gene is not permanently integrated in the plant genome, these techniques can be regarded as a non-GMO approach. To date three main systems are available including zinc-finger domains, TALEN factors and CRISPR. Zinc-fingers are built-in functions in transcription factors and zinc-finger nucleases permitting specific recognition of gene segments and their subsequent cleavage and mutagenesis (Desjarlais and Berg 1992). The so-called TALEN (transcription activator-like effector nuclease) system contains DNA-binding domains linked to artificial restriction enzymes generated by fusing a TAL effector DNA-binding domain to a DNA cleavage domain (Boch et al. 2009; Cermak et al. 2011). An even more recent genome editing technology is the so-called CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9/sgRNA system for targeted gene mutagenesis.
Zinc-finger transcription factors, TALENs and CRISPR technologies (Townsend et al. 2009; Boch et al. 2009; Miller et al. 2011; Sanjana et al. 2012) can become powerful tools for targeting genes in starch biosynthesis and phosphorylation. TALENs and zinc-finger nucleases have proved highly efficient in targeting precise genes and disrupting loci in rice and maize (Shukla et al. 2009; Li et al. 2012), and the CRISPR system was tested in Arabidopsis, tobacco, sorghum and rice models for knocking out specific genes (Jiang et al. 2013). Interestingly, problems related to side effects by complete removal of proteins in enzyme complexes by using traditional knockout or knock-down expression are solved by these technologies since they can confer site-directed mutagenesis of catalytically important active amino acid residues without affecting protein biosynthesis.
To this end genome editing has not been documented for starch bioengineering. However, classical mutagenesis strategies are well founded with many examples from the cereal high-amylose system (Blennow et al. 2013). Recently, advanced mutant screening including targeting induced local lesions (TILLING) in genomes of wheat (Slade et al. 2012) and simple sequence repeat (SSR) markers in BEIII in rice (Yang et al. 2012) was accomplished as non-GMO strategies to isolate high-amylose lines. Since BE is involved in the biosynthesis of amylopectin, it should be noted that the so-called high-amylose starch synthesised by BE-deficient plants actually synthesises modified, long-chain amylopectin, even though such starch types very much resemble natural amylose with respect to molecular size (e.g. Carciofi et al. 2012).
Future non-GMO efforts for more advanced starch functionalisation like phosphorylation await development and establishment.
6 Concluding Remarks
Starch phosphorylation plays a fundamental role in plant and crop performance by restructuring the starch granule on metabolic demand. For starch mobilisation, granule physical amorphisation and solubilisation permit starch granule amylolytic degradation. Phosphorylation also unambiguously affects starch biosynthesis, but the effects are less clear, and further research is needed to understand how phosphorylation affects starch deposition in the plant. Starch is a multipurpose raw polysaccharide with invaluable importance for all living organisms and human society. As such, starch has a wide application potential for food, feed and fuel purposes. Most starches must be industrially modified prior to use. Often these processes are expensive and affecting the environment. Phosphorylated starch has unique properties, and upcoming clean starch bioengineering strategies including postharvest enzyme-based and in planta (such as genome editing) modulation of starch phosphorylation can provide society with high-value and environmentally friendly modified raw materials to help realising a true bioeconomy.
References
Abel GJW, Springer F, Willmitzer L, Kossmann J (1996) Cloning and functional analysis of a cDNA encoding a novel 139 kDa starch synthase from potato (Solanum tuberosum L.). Plant J 10:981–991
Ball S, Guan HP, James M et al (1996) From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell 86:349–352
Ball S, Colleoni C, Cenci U et al (2011) The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J Exp Bot 62:1775–1801
Baunsgaard L, Mogensen HL, Mikkelsen R et al (2005) A novel isoform of glucan water dikinase phosphorylates prephosphorylated α-glucans and is involved in starch degradation in Arabidopsis. Plant J 41:595–605
Bay-Smidt AM, Wischmann B, Olsen CE, Nielsen TH (1994) Starch bound phosphate in potato as studied by a simple method for determination of organic phosphate and p-31-nmr. Starch/Stärke 46:167–172
Bertoft E, Blennow A (2009) Chapter 4: Structure of potato starch. In: Singh J, Kaur L (eds) Advances in potato chemistry and technology. Academic Press (imprint of Elsevier), Burlington, pp 83–98
Bertoft E, Koch K, Åman P (2012) Building block organisation of clusters in amylopectin from different structural types. Int J Biol Macromol 50:1212–1223
Blennow A, Engelsen SB (2010) Helix-breaking news: fighting crystalline starch energy deposits in the cell. Trends Plant Sci 15:236–240
Blennow A, Svensson B (2010) Dynamics of starch granule biogenesis – the role of redox regulated enzymes and low affinity CBMs. Biocatal Biotransform 28:3–9
Blennow A, Bay-Smidt AM, Wischmann B et al (1998a) The degree of starch phosphorylation is related to the chain length distribution of the neutral and the phosphorylated chains of amylopectin. Carbohydr Res 307:45–54
Blennow A, Bay-Smidt AM, Olsen CE et al (1998b) Analysis of glucose-3-P in starch using high performance ion exchange chromatography. J Chromatogr 829:385–391
Blennow A, Bay-Smidt AM, Olsen CE et al (2000) The distribution of covalently bound starch-phosphate in native starch granules. Int J Biol Macromol 27:211–218
Blennow A, Engelsen SB, Nielsen TH et al (2002) Starch phosphorylation – a new front line in starch research. Trends Plant Sci 7:445–450
Blennow A, Bay-Smidt A, Leonhardt P et al (2003) Starch paste stickiness is a relevant native starch selection criterion for wet-end paper manufacturing. Starch/Staerke 55:381–389
Blennow A, Sjöland KA, Andersson R et al (2005a) The distribution of elements in the native starch granule as studied by particle-induced X-ray emission and complementary methods. Anal Biochem 347:327–329
Blennow A, Wischmann B, Houborg K et al (2005b) Structure - function relationships of transgenic starches with engineered phosphate substitution and starch branching. Int J Biol Macromol 36:159–168
Blennow A, Houborg K, Andersson R et al (2006) Phosphate positioning and availability in the starch granule matrix as studied by EPR. Biomacromolecules 7:965–974
Blennow A, Jensen SL, Shaik SS et al (2013) Future cereal starch bioengineering – cereal ancestors encounter gene-tech and designer enzymes. Cereal Chem 90:274–287
Boch J, Scholze H, Schornack S et al (2009) Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326:1509–1512
Carciofi M, Shaik SS, Jensen SL et al (2011) Hyperphosphorylation of cereal starch. J Cereal Sci 54:339–346
Carciofi M, Blennow A, Jensen SL et al (2012) Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol 12(223):1–16
Cermak T, Doyle EL, Christian M et al (2011) Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res 39:e82
Chiu C-W, Solarek D (2009) Modification of starches. In: BeMiller JN, Whistler RL (eds) Starch: chemistry and technology. Academic, New York, pp 629–655
Christiansen C, Abou Hachem M, Glaring MA et al (2009) A CBM20 low affinity starch binding domain from glucan, water dikinase. FEBS Lett 583:1159–1163
Coehn A (1897) Über elektrische Wanderung von Kolloiden. Z Electrochem 4:63–67
Damager I, Engelsen SB, Blennow A et al (2010) First principles insight into starch-like α-glucans: their synthesis, conformation and hydration. Chem Rev 110:2049–2080
Desjarlais JR, Berg JM (1992) Toward rules relating zinc finger protein sequences and DNA binding site preferences. Proc Natl Acad Sci U S A 89:7345–7349
Dudkiewicz M, Siminska J, Pawlowski K et al (2008) Bioinformatics analysis of oligosaccharide phosphorylation effect on the stabilization of the β-amylase ligand complex. J Carbohydr Chem 27:479–495
Edner C, Li J, Albrecht T, Mahlow S et al (2007) Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial β-amylases. Plant Physiol 145:17–28
EFSA Panel on Genetically Modified Organisms (2012) Scientific opinion addressing the safety assessment of plants developed through cisgenesis and intragenesis. EFSA J 10(2):2561
Engelsen SB, Madsen MAO, Blennow A et al (2003) The phosphorylation site in double helical amylopectin as investigated by a combined approach using chemical synthesis, crystallography and molecular modeling. FEBS Lett 541:137–144
Enriques J (1998) Genomics – genomics and the world’s economy. Science 281(5379):925–926
Fernbach A (1904) Quelques observations sur la composition de l’amidon de pommes de terre. C R Acad Sci 138:428–430
Frohberg, C (2008) Genetically modified plants which synthesize a starch having increased swelling power. International patent WO 08/017518
Frohberg C, Kötting O, Ritte G et al (2012) Plants with increased activity of a starch phosphorylating enzyme. US patent application 2012/0017333 A1
Gillgren T, Blennow A, Pettersson AJ, Stading M (2011) Modulating rheo-kinetics of native starch films towards improved wet-strength. Carbohydr Polym 83(2):383–391
Glaring MA, Koch KB, Blennow A (2006) Genotype specific spatial distribution of starch molecules in the starch granule: a combined CLSM and SEM approach. Biomacromolecules 7:2310–2320
Glaring MA, Zygadlo A, Thorneycroft D et al (2007) A cytosolic isoform of α-glucan, water dikinase from Arabidopsis is expressed in the companion cells of the phloem. J Exp Bot 58(14):3949–3960
Glaring MG, Baumann MJ, Hachem MA et al (2011) Characterization of the CBM45 family of low-affinity starch-binding domains involved in plastidial starch metabolism. FEBS J 278:1175–1185
Godwin ID, Williams SB, Pandit PS et al (2009) Multifunctional grains for the future: genetic engineering for enhanced and novel cereal quality. In Vitro Cell Dev Biol Plant 45(4):383–399
Haghayegh G, Schoenlechner R (2011) Physically modified starches: a review. J Food Agric Environ 9:27–29
Hansen PI, Spraul M, Dvortsak P et al (2008) Starch phosphorylation – maltosidic restrains upon 3’- and 6’- phosphorylation investigated by chemical synthesis, molecular dynamics modeling and NMR spectroscopy. Biopolymers 9:179–193
Hejazi M, Fettke J, Haebel S et al (2008) Glucan, water dikinase phosphorylates crystalline maltodextrins and thereby initiates solubilization. Plant J 55:323–334
Hejazi M, Fettke J, Paris O et al (2009) The two plastidial starch-related dikinases sequentially phosphorylate glucosyl residues at the surface of both the A- and B-type allomorphs of crystallized maltodextrins but the mode of action differs. Plant Physiol 150:962–976
Hejazi M, Fettke J, Kötting O et al (2010) The laforin-like dual-specificity SEX4 from Arabidopsis thaliana hydrolyses both C-6 and C-3-phosphate esters introduced by starch-related dikinases and thereby affects phase transition of α-glucans. Plant Physiol 152:711–722
Hizukuri S, Tabata S, Nikuni Z (1970) Studies on starch phosphate. Part 1. Estimation of glucose-6-phosphate residues in starch and the presence of other bound phosphate(s). Starch/Stärke 22:338–343
Hoover R (2001) Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym 45:253–267
Jane J-L, Chen JJ (1993) Internal structure of the potato starch granule revealed by chemical gelatinization. Carbohydr Res 247:279–290
Jane J-L, Ao Z, Duvick SA et al (2003) Structures of amylopectin and starch granules: how are they synthesized? J Appl Glycosci 50:167–172
Jensen SL, Larsen FH, Bandshom O et al (2013a) Stabilization of semi-solid-state starch by branching enzyme-assisted chain-transfer catalysis at extreme substrate concentration. Biochem Eng J 72:1–10
Jensen SJ, Zhu F, Vamadevan V et al (2013b) Structural and physical properties of branching enzyme stabilized starch. Carbohydr Polym 98:1490–1496
Jiang W, Zhou H, Bi H et al (2013) Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice. Nucleic Acids Res 41:e188
Jobling S (2004) Improving starch for food and industrial applications. Curr Opin Plant Biol 7:210–218
Jobling SA, Westcott RJ, Tayal A et al (2002) Production of a freeze-thaw-stable potato starch by antisense inhibition of three starch synthase genes. Nat Biotechnol 20(3):295–929
Kamasaka H, Uchida M, Kusaka K et al (1995) Inhibitory effect of phosphorylated oligosaccharides prepared from potato starch on the formation of calcium phosphate. Biosci Biotechnol Biochem 59:1412–1416
Kamasaka H, To-o K, Kusaka K et al (1997a) Action pattern of neopullulanase on phosphoryl oligosaccharides prepared from potato starch. J Appl Glycosci 44:275–283
Kamasaka H, To-o K, Kusaka K et al (1997b) A way of enhancing the inhibitory effect of phosphoryl oligosaccharides on the formation of a calcium phosphate precipitate using the coupling reaction of cyclomaltodextrin glucanotransferase. J Appl Glycosci 44:285–293
Kamasaka H, Diesuke I, Kentaro M et al (2003) Production and application of phosphoryl oligosaccharides prepared from potato starch. Trends Glycosci Glycotechnol 15:75–89
Kötting O, Pusch K, Tiessen A et al (2005) Identification of a novel enzyme required for starch metabolism in Arabidopsis leaves. The phosphoglucan, water dikinase. Plant Physiol 137:242–252
Kötting O, Santelia D, Edner C et al (2009) STARCH-EXCESS4 is a laforin-like phosphoglucan phosphatase required for starch degradation in Arabidopsis thaliana. Plant Cell 21:334–346
Kozlov SS, Blennow A, Krivandin AV et al (2007) Structural and thermodynamic properties of starches extracted from GBSS- and GWD suppressed potato lines. Int J Biol Macromol 40:449–460
Lanahan MB, Basu SS (2005) Modified starch, uses methods for production thereof. International patent WO 05/002359
Larsen FH, Blennow A, Engelsen SB (2008) Starch granule hydration – A MAS NMR investigation. Food Biophys 3:25–32
Li T, Liu B, Spalding MH et al (2012) High efficiency TALEN-based gene editing produces disease-resistant rice. Nat Biotechnol 30:390–392
Lim S-T, Kasemsuwan T, Jane J-L (1994) Characterization of phosphorus in starch by 31P-nuclear magnetic resonance spectroscopy. Cereal Chem 71(5):488–493
Lloyd JR, Landschütze V, KossmannJ J (1999) Simultaneous antisense inhibition of two starch-synthase isoforms in potato tubers leads to accumulation of grossly modified amylopectin. Biochem J 338:515–521
Lorberth R, Ritte G, Willmitzer L et al (1998) Inhibition of a starch-granule bound protein leads to modified starch and repression of cold sweetening. Nat Biotechnol 16:473–477
Mahlow S, Hejazi M, Kuhnert F et al (2014) Phosphorylation of transitory starch by a-glucan, water dikinase during starch turnover affects the surface properties and morphology of starch granules. New Phytol 203:495–507
Mason WR (2009) Starch use in foods. In: BeMiller JN, Whistler RL (eds) Starch: chemistry and technology. Academic, New York, pp 745–795
Mikkelsen R, Blennow A (2005) Functional domain organization of the potato a-glucan, water dikinase (GWD): evidence for separate site catalysis as revealed by limited proteolysis and deletion mutants. Biochem J 385:355–361
Mikkelsen R, Baunsgaard L, Blennow A (2004) Functional characterization of α-glucan, water dikinase, the starch phosphorylating enzyme. Biochem J 377:525–532
Mikkelsen R, Mutenda K, Mant A et al (2005) α-glucan, water dikinase (GWD): a plastidic enzyme with redox-regulated and coordinated catalytic activity and binding affinity. Proc Natl Acad Sci U S A 102:1785–1790
Mikkelsen R, Suszkiewicz K, Blennow A (2006) A novel type of carbohydrate binding module identified in α-glucan, water dikinases specific for regulated plastidial starch metabolism. Biochemistry 45:4674–4682
Miller JC, Tan SY, Qiao GJ et al (2011) A TALE nuclease architecture for efficient genome editing. Nat Biotechnol 29:143–148
Muhrbeck P, Eliasson A–C (1987) Influence of pH and ionic strength on the viscoelastic properties of starch gels – a comparison of potato and cassava starches. Carbohydr Polym 7:291–300
Muhrbeck P, Eliasson A-C (1991) Influence of the naturally-occurring phosphate-esters on the crystallinity of potato starch. J Sci Food Agric 55:13–18
Nashilevitz S, Melamed-Bessudo C, Aharoni A et al (2009) The legwd mutant uncovers the role of starch phosphorylation in pollen development and germination in tomato. Plant J 57:1–13
Nielsen TH, Wischmann B, Enevoldsen K et al (1994) Starch phosphorylation in potato tubers proceeds concurrently with de novo biosynthesis of starch. Plant Physiol 105:111–117
Perez S, Bertoft E (2010) The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch/Staerke 62:389–420
Planchot V, Colonna P, Buleon A (1997) Enzymatic hydrolysis of a-glucan crystallites. Carbohydr Res 298:319–326
Posternak T (1935) Sur le phosphore des amidons. Helv Chim Acta 18:1351–1369
Ral JP, Bowerman AF, Li Z et al (2012) Downregulation of glucan water-dikinase activity in wheat endosperm increases vegetative biomass and yield. Plant Biotechnol J 10:871–882
Reimann R, Ritte G, Steup M et al (2002) Association of α-amylase and the R1 protein with starch granules precedes the initiation of net starch degradation in turions of Spirodela polyrhiza. Physiol Plant 114:2–12
Reimann R, Hippler M, Machelett B et al (2004) Light induces phosphorylation of glucan water dikinase, which precedes starch degradation in turions of the duckweed Spirodela polyrhiza. Plant Physiol 135:121–128
Ritte G, Lorberth R, Steup M (2000a) Reversible binding of the starch related R1 protein to the surface of transitory starch granules. Plant J 21:387–391
Ritte G, Eckermann N, Haebel S et al (2000b) Compartmentation of the starch-related R1 protein in higher plants. Starch/Staerke 52:179–185
Ritte G, Lloyd JR, Eckermann N et al (2002) The starch-related R1 protein is an alpha-glucan, water dikinase. Proc Natl Acad Sci U S A 99:7166–7171
Ritte G, Scharf A, Eckermann N et al (2004) Phosphorylation of transitory starch is increased during degradation. Plant Physiol 135:1–10
Ritte G, Heydenreich M, Mahlow S et al (2006) Phosphorylation of C6- and C3-positions of glucosyl residues in starch is catalysed by distinct dikinases. FEBS Lett 580:4872–4876
Samec M (1914) Studien über Pflanzenkolloide, IV. Die Verschiebungen des Phosphorgehaltes bei der Zustandsänderungen und dem diastatischen Abbau der Stärke. Kolloidchem Beih 4:2–54
Samec M, Blinc M (1941) Die neuere Entwicklung der Kolloidchemie der Stärke. Velag von Theodor Steinkopff, Dresten
Sanjana NE, Cong L, Zhou Y et al (2012) A transcription activator-like effector toolbox for genome engineering. Nat Protoc 7:171–192
Santelia D, Kötting O, Seung D et al (2011) The phosphoglucan phosphatase LIKE SEX FOUR2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell 23:4096–4111
Schewe G, Knies P, Amati SF et al (2002) Monocotyledon plant cells and plants which synthesise modified starch. International patent WO 02/34923
Schouten H, Jacobsen E (2008) Cisgenesis and intragenesis, sisters in innovative plant breeding. Trends Plant Sci 13(6):260–261
Schreiber K (1958) Chemie und Biochemie unter besonderer Berücksichtigung qualitätbestimmender Faktoren. In: Schick R, Klinkowski M (eds) Die Kartoffel Ein Handbuch. VED Deutscher Lantvirtschaftverlag, Berlin, pp 193–352
Schwall GP, Safford R, Westcott RJ et al (2000) Production of very-high-amylose potato starch by inhibition of SBE A and B. Nat Biotechnol 18:551–554
Shaik SS, Carciofi M, Martens HJ et al (2014) Starch bioengineering affects cereal grain germination and seedling establishment. J Exp Bot 65(9):2257–2270
Shukla VK, Doyon Y, Miller JC et al (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459:437–441
Skeffington AW, Graf A, Duxbury Z et al (2014) Glucan, water dikinase exerts little control over starch degradation in Arabidopsis leaves at night. Plant Physiol 165(2):866–879
Slade AJ, McGurie C, Loeffler D et al (2012) Development of high amylose wheat through TILLING. BMC Plant Biol 12:1–17
Tabata S, Hizukuri S (1971) Studies on starch phosphate. Part 2. Isolation of glucose 3-phosphate and maltose phosphate by acid hydrolysis of potato starch. Starch-Starke 23:267–272
Takeda Y, Hizukuri S (1982) Location of phosphate groups in potato amylopectin. Carbohydr Res 102:321–327
Tanackovic V, Svensson JT, Jensen SL, Buléon A, Blennow A (2014) The deposition and characterization of starch in Brachypodium distachyon. J Exp Bot 65(18):5179–5192
Tanaka T, Kobayashi T, Kuriki T (2012) Optimization of calcium concentration of saliva with phosphoryl oligosaccharides of calcium (POs-Ca) for enamel remineralization in vitro. Arch Oral Biol 58:174–180
Thygesen LG, Blennow A, Engelsen SB (2003) The effects of amylose and starch phosphate on starch gel retrogradation studied by low-field 1H NMR relaxometry. Starch-Starke 55:241–249
To-o K, Kamasaka H, Nakabou Y (2003) Absorbability of calcium from calcium-bound phosphoryl oligosaccharides in comparison with that from various calcium compounds in the rat ligated jejunum loop. Biosci Biotechnol Biochem 67:1713–1718
Townsend JA, Wright DA, Winfrey RJ et al (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459:442–445
Viksø-Nielsen A, Blennow A, Kristensen KH et al (2001) Structural, physicochemical, and pasting properties of starches from potato plants with repressed r1-gene. Biomacromolecules 3:836–841
Weise SE, Aung K, Jarou ZJ et al (2012) Engineering starch accumulation by manipulation of phosphate metabolism of starch. Plant Biotechnol J 10:545–554
Wickramasinghea HAM, Blennow A, Noda T (2009) Physico-chemical and degradative properties of in-planta re-structured potato starch. Carbohydr Polym 77:118–124
Wiesenborn DP, Orr PH, Casper HH et al (1994) Potato starch paste behavior as related to some physical/chemical properties. J Food Sci 59:644–648
Wikman J, Larsen FH, Motawia MS et al (2011) Phosphate esters in amylopectin clusters of potato tuber starch. Int J Biol Macromol 48:639–649
Wikman J, Blennow A, Buléon A et al (2013) Influence of amylopectin structure and degree of phosphorylation on the molecular composition of potato starch lintners. Biopolymers 101:257–271
Wischmann B, Nielsen TH, Møller BL (1999) In vitro biosynthesis of phosphorylated starch in intact potato amyloplasts. Plant Physiol 119:455–462
Wischmann B, Blennow A, Madsen F et al (2005) Functional characterisation of potato starch modified by specific in planta alteration of the amylopectin branching and phosphate substitution. Food Hydrocoll 19:1016–1024
Yang R, Sun C, Bai J et al (2012) A putative gene sbe3-rs for resistant starch mutated from SBE3 for starch branching enzyme in rice (Oryza sativa L.). PLoS ONE 7(8):e43026
Yu T-S, Kofler H, Häusler RE et al (2001) SEX1 is a general regulator of starch degradation in plants and not the chloroplast hexose transporter. Plant Cell 13:1907–1918
Zeeman SC, Kossmann J, Smith AM (2010) Starch: its metabolism, evolution, and biotechnological modification in plants. Annu Rev Plant Biol 61:209–234
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Blennow, A. (2015). Phosphorylation of the Starch Granule. In: Nakamura, Y. (eds) Starch. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55495-0_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-55495-0_12
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55494-3
Online ISBN: 978-4-431-55495-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)