Abstract
PIKfyve is the only kinase that phosphorylates the 5′-position of phosphatidylinositol (PtdIns) 3-phosphate to produce PtdIns (3,5)-bisphosphate, which is indispensable for intracellular trafficking. Thus, this kinase is a potential regulator of various cellular events, such as autophagy, stress-mediated responses, and membrane and ion transport. However, little is known about the physiological function of PIKfyve in innate immune responses. A chemical that has been subjected to clinical tests for autoimmune diseases for the last decade was recently identified as a PIKfyve inhibitor. Additionally, in 2013, a few studies reported some roles of PIKfyve in Toll-like receptor-mediated responses. Although the reported findings are convincing and interesting, the interpretation of the function of PIKfyve based on these reports remains contradictory. In this review, we survey these reports together with data from the clinical tests of the PIKfyve inhibitor and present our perspective on the role of PIKfyve in innate immunity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Phosphoinositides (PIs), the phosphorylated derivatives of phosphatidylinositol (PtdIns), play a central role in cytoskeletal dynamics and membrane trafficking (Lindmo and Stenmark 2006). At present, we know that 19 PI kinases and 28 PI phosphatases are involved in the PI interconversion reactions that generate seven PIs in mice (Sasaki et al. 2009). The details of these reactions and enzymes are described in other reviews (Krauss and Haucke 2007; Mayinger 2012; Sasaki et al. 2009). The various PIs found on the membrane are markers for the recruitment of a specific and distinct set of proteins that determine the membrane identity. One of the most well-documented PIs associated with membrane trafficking is PtdIns(3)P, which is mainly produced by the class III PI3-kinase Vps34 (Krauss and Haucke 2007). PtdIns(3)P and Vps34 are enriched in subcellular compartments, such as the phagosome, autophagosome, and early endosome (Krauss and Haucke 2007; Mayinger 2012). The fate of newly synthesized PtdIns(3)P on the endosome is not well-documented, but it is known that this is hydrolyzed to form PtdIns and also phosphorylated to form PtdIns(3,5)P2 and PtdIns(3,4)P2. Among these, PtdIns(3,5)P2 is enriched in the acidic late endosome or endolysosome (Ho et al. 2012).
Mammalian PIKfyve, so named after its function and domain structure [PI Kinase for five position containing a Fyve finger; also known as type III PtdIns phosphate kinase (PIPKIII)] is the only lipid kinase that phosphorylates the 5′-positions of PtdIns and PtdIns(3)P to yield PtdIns(5)P and PtdIns(3,5)P2, respectively, in vitro (McCartney et al. 2014). However, in intact cells, it remains unclear whether PIKfyve directly generates most of the cellular PtdIns(5)P and whether PtdIns(3,5)P2 serves as a precursor for PtdIns(5)P (Ho et al. 2012; McCartney et al. 2014). PIKfyve is hypothesized to form a large complex with Vac14 and Sac3 (Ho et al. 2012; McCartney et al. 2014), two components that are necessary for its full activation. General information on PIKfyve, such as its structure, functional domains, associated proteins, and regulation of its activity, is described in some excellent previously published reviews (McCartney et al. 2014; Shisheva 2008; Takasuga and Sasaki 2013).
PIKfyve knockout mouse models have not been available because of embryonic lethality (Ikonomov et al. 2011). In the past years, most of the related findings were deduced from the function of the yeast ortholog, Fab1. However, YM201636 was reported as a specific inhibitor of PIKfyve in 2008 (Jefferies et al. 2008). Since then, this inhibitor, together with small-interfering RNA (siRNA), has been used as a good probe for various experimental approaches. Last year, STA-5326/apilimod was identified as another inhibitor of PIKfyve (Cai et al. 2013). Surprisingly, this new inhibitor has been subjected to a phase II clinical test for autoimmune diseases for a decade. There are many clinical data on the effect of the inhibitor on interleukin (IL)-12 production. In this review, we first trace the history of apilimod and then review the current studies on PIKfyve in terms of immune responses. We also present our perspective on this issue.
4.2 History of Apilimod
4.2.1 Clinical Tests
Apilimod first appeared in 2004 as a candidate novel drug for the treatment of inflammatory and autoimmune diseases, such as Crohn’s disease, psoriasis, rheumatoid arthritis, and multiple sclerosis (Borchardt 2004). These diseases are characterized by a T helper (Th) 1 cell response and elevated levels of IL-12 (Wada et al. 2007). IL-23, which shares the p40 protein subunit with IL-12, is also increased. IL-23 causes a Th17 response, which plays a significant role in inflammatory responses (Wada et al. 2007). Thus, antibodies against IL-12/IL-23 may provide a significant medical benefit. However, a small-molecule IL-12 inhibitor that can be administrated orally remains highly desirable. Thus, apilimod has been subjected to clinical experiments as an oral inhibitor for the treatment of autoimmune diseases. The first test we identified in the PubMed database was performed with 73 patients with active Crohn’s disease (Burakoff et al. 2006). Clinical activity was observed in most patients (>70 %), with mild-to-moderate side effects. Preclinical studies demonstrated the successful inhibition of IL-12 and IL-23 production by the drug. Thus, apilimod appeared to be a promising treatment for Crohn’s disease (Billich 2007; Burakoff et al. 2006). The next test was performed as a multicenter, phase II, randomized, double-blinded, placebo-controlled study to evaluate the efficacy of apilimod in the treatment of 220 adult patients with Crohn’s disease (Sands et al. 2010). Unexpectedly, apilimod did not demonstrate efficacy over the placebo in patients with active Crohn’s disease (Sands et al. 2010). A similar phase II study of apilimod was performed in 29 patients with active rheumatoid arthritis (Krausz et al. 2012). Although there was a small but significant reduction in the DAS28 (28-joint Disease Activity Score of rheumatoid arthritis), the researchers did not observe the effect of apilimod on IL-12 and IL-23 expression in CD68+ (macrophage-like) synovial cells. As a conclusion, the authors did not confirm the hypothesis that IL-12/IL-23 inhibition by apilimod is able to induce clinical improvement in rheumatoid arthritis (Krausz et al. 2012). In contrast, an open-label clinical study of oral administration in patients with psoriasis reported a positive conclusion (Wada et al. 2012). Substantial improvements in histology were observed in patients receiving apilimod. The expression of IL-23p19 and IL-12p40 in skin lesions was reduced, whereas that of IL-10 was increased in the apilimod-treated group. Although a statistical analysis revealed a significant effect of apilimod, there were non-responders in terms of a histological response (Wada et al. 2012). Interestingly, the epidermal CD11c+ cells in the lesions were almost completely cleared in the responder group but not in the non-responders. Hence, the authors described that the dramatic reduction of Th1/Th17 responses in the apilimod-treated group is the consequence of the clearance of IL-12-/IL-23-producing CD11c+ cells (Wada et al. 2007). The clinical studies that are found in PubMed are summarized in Table 4.1. In most cases, apilimod is more or less effective, but there were differences among individuals even when the final decision is positive (Wada et al. 2007). The effect of apilimod on IL-12 production was also varied: it was decreased in most people, but extremely increased in a few people (Krausz et al. 2012). These reports led us to hypothesize that the effect of apilimod on IL-12 production varies depending on the substantial cell types involved.
The therapeutic effect of apilimod was also tested in a mouse model (experimental autoimmune uveoretinitis) by oral administration. The researchers who studied this mouse model reported a decreased level of serum IL-12p40 and a suppression of inflammation (Keino et al. 2008). Additionally, in vivo studies using a mouse inflammatory bowel disease model demonstrated that the oral administration of apilimod markedly reduced the inflammatory histological changes in the colon (Wada et al. 2007).
4.2.2 Apilimod, an Interleukin-12 Inhibitor, Is a PIKfyve Inhibitor
In 2007, apilimod was discovered by a biological strategy independent of the clinical tests. Wada et al. discovered this drug among an 80,000-compound library in a cell-based screen aimed at identifying an inhibitor of IL-12 production using interferon (IFN)-γ/lipopolysaccharide (LPS)-stimulated human peripheral blood mononuclear cells (PBMCs) (Wada et al. 2007). The IL-12 production induced by IFN-γ/Staphylococcus aureus Cowan I (SAC) was also susceptible to apilimod in PBMCs (Wada et al. 2007). The cytokine inhibitory spectrum of apilimod that was described by the paper published by Wada et al. is seen in Table 4.2.
When the above-referenced work was published, the inhibitory target of apilimod was not known. The target molecule, PIKfyve, was identified in 2013 through a chemical proteomics approach (Cai et al. 2013). A bioactive analog of apilimod, APA10, was immobilized on gel matrices and incubated with a THP-1 cell extract premixed with either dimethyl sulfoxide or excess apilimod. The specifically bound proteins were eluted and identified by liquid chromatography–tandem mass spectrometry (LC-MS/MS). As a result, only three proteins were identified: PIKfyve, Vac14, and Sac3. As mentioned above, PIKfyve displays its full activation as a large complex with Vac14 and Sac3. These researchers demonstrated that apilimod does not inhibit other lipid kinases or protein kinases (Cai et al. 2013). The specificity is better than YM201636, which inhibits class I PI 3-kinase at higher concentrations (Ikonomov et al. 2009). These researchers mainly used human PBMCs, THP-1 cells and mouse bone marrow-derived cells (BMDCs) to determine the cytokine inhibitory spectrum of apilimod (Table 4.2). As a conclusion, these researchers reported that apilimod is a specific inhibitor of IL-12/IL-23 production induced by Toll-like receptor (TLR) ligation (Cai et al. 2013).
4.3 The Role of PIKfyve in the Intestinal Immune System
PIKfyve knockout mouse embryos die by embryonic day 8.5 (Takasuga et al. 2013). However, intestine-specific knockout mice were recently generated (Takasuga et al. 2013). Interestingly, the mice exhibit diarrhea and bloody stool, and their gut epithelial layers show inflammation and fibrosis, as observed in inflammatory bowel diseases (Takasuga et al. 2013). In the intestinal mucosa of these mice, the mRNA expression levels of inflammatory cytokines, namely tumor necrosis factor (TNF)-α, IL-12p40, C-C chemokine ligand 2 (CCL2), IL-6, C-X-C motif chemokine 5 (CXCL5), IL-1β and transforming growth factor (TGF)-β1, are increased (Takasuga et al. 2013). This effect appears contradictory to the effect of the PIKfyve inhibitor on IL-12 production. However, as mentioned above, the effect of the inhibitor varies depending on the cell type. Most of the works demonstrating the inhibitory effect of apilimod on IL-12 production were performed with human PBMCs or mouse BMDCs. Because intestinal cells are always exposed to resident intestinal bacteria, it is important for them to maintain a balance between protective immunity and tolerance to commensal bacteria. The failure in maintaining this balance results in inflammatory bowel diseases. Regulatory T cells (Tregs) play a substantial role in maintaining immune tolerance (Rouse and Sehrawat 2010). Two different subsets of dendritic cells (DCs), namely CD103+/CD11c+ and CD103+/CD11b+ DCs, are reported to be involved in the differentiation of Tregs in the intestine (Lewis et al. 2011; Persson et al. 2013; Schlitzer et al. 2013). Macrophages also play a role in maintaining immune tolerance through the production of an anti-inflammatory cytokine, namely IL-10 (Murai et al. 2009). It is likely that PIKfyve plays a role in the regulation of the function of these cells to maintain the homeostasis of the intestinal immune system.
4.4 The Role of PIKfyve in Salmonella Replication
A defect in PIKfyve function induced by siRNA-mediated knockdown or YM201636 treatment inhibits the intracellular replication of Salmonella in epithelial cells (Kerr et al. 2010). PtdIns(3,5)P2 is indispensable for macropinosome–late endosome/lysosome fusion. This PtdIns(3,5)P2-dependent step is required for the proper maturation of the Salmonella-containing vacuole. Although the inhibition of PIKfyve in macrophages inhibits Salmonella replication, it also disrupts the macrophage’s bactericidal response (Kerr et al. 2010).
4.5 The Role of PIKfyve in Pathogen-Associated Molecular Patterns Signals
Innate immune responses are triggered by pathogen-associated molecular patterns (PAMPs), which are recognized by pattern-recognition receptors (PRRs), including TLRs, retinoic acid-inducible gene 1 (RIG-I)-like receptors (RLRs), C-type lectin receptors (CLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), and intracellular sensors for DNA (Kawai and Akira 2011; Sancho and Reis e Sousa 2012; Strowig et al. 2012). CLRs comprise a large family of receptors that bind to carbohydrates (Sancho and Reis e Sousa 2012). Most of the family members are trans-membrane receptors (Sancho and Reis e Sousa 2012). RLRs, NLRs, and the DNA sensors reside in the cytosol and recognize double-stranded RNA (dsRNA), cytoplasmic PAMPs, and double-stranded DNA (dsDNA), respectively (Kawai and Akira 2011; Sancho and Reis e Sousa 2012; Strowig et al. 2012). TLRs can be divided into two groups based on their subcellular localization: TLRs 1, 2, 4, 5, and 6 are cell surface receptors that recognize surface structures of pathogens, whereas TLRs 3, 7, 8, and 9 reside in intracellular compartments and detect microbial nucleic acids (McGettrick and O’Neill 2010). Thus, it is likely that the localization and trafficking of the receptors and their ligands are important factors in the regulation of innate immunity (McGettrick and O’Neill 2010). Hence, PIKfyve may play a role in the appropriate arrangement of these receptors and their ligands.
4.5.1 Toll-Like Receptor (TLR) 9-Mediated Responses
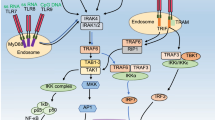
TLR9 is a receptor for oligodeoxynucleotides that contain unmethylated CpG motifs (CpG). Because TLR9 possesses retention signals that maintain it in the endoplasmic reticulum, TLR9 cleavage by proteases is necessary for its recruitment to the endosomes, where it meets its ligand (Latz et al. 2004; Leifer et al. 2006; Park et al. 2008). CpG has been shown to associate with TLR9 after internalization (Fig. 4.1). Thus, the intracellular trafficking of both the receptor and the ligand is essential in TLR9 signaling (Kawai and Akira 2010; Takeshita et al. 2004). Class III PtdIns-3 kinase, which generates endosomal PtdIns(3)P, is involved in TLR9 signaling by regulating CpG uptake (Kuo et al. 2006). We monitored the localization of Rox-CpG and PtdIns(3)P through time-lapse imaging; the latter was visualized with an enhanced green fluorescent protein (EGFP)-fused protein probe. In a normal macrophage, PtdIns(3)P is first co-localized with CpG but rapidly disappears from the CpG-containing endosomes (unpublished data). In contrast, in a macrophage with deficient PIKfyve function, PtdIns(3)P stayed in the CpG-containing endosomes for longer periods (unpublished data). Because TLR9 is waiting for CpG in an acidic late endosome, CpG could not bind to TLR9 in cells with deficient PIKfyve. As a result, the TLR9-mediated signaling events and cytokine production were attenuated in PIKfyve-deficient cells (Hazeki et al. 2013) (Fig. 4.1).
In plasmacytoid DCs (pDCs), the CpG in the early endosome causes type I IFN production, whereas CpG in the lysosome causes nuclear factor (NF)-κB activation (Guiducci et al. 2006; Honda et al. 2005; Kerkmann et al. 2005) (Fig. 4.1). It is speculated that PIKfyve disruption may increase type I IFN production and decrease NF-κB activation in pDCs. We are now attempting to demonstrate this hypothesis.
4.5.2 TLR4-Mediated Responses
TLR4 is a well-characterized cell surface TLR that recognizes LPS with the help of some other proteins, such as LPS binding protein (LBP), CD14, and MD2 (Lu et al. 2008). As expected from the effect of apilimod, the siRNA-mediated knockdown of PIKfyve in THP-1 cells results in the inhibition of IL-12p40 production induced by IFN-γ/LPS (Cai et al. 2013). BMDCs from spontaneous mutant mouse ingls, which carries a missense mutation in Vac14, were found to exhibit swollen vacuole formation similar to that observed in apilimod-treated cells (Cai et al. 2013). The TLR7- or TLR4-mediated production of IL-12p40, but not that of CXCL2, was decreased in these mutant cells (Cai et al. 2013). Unexpectedly, however, the PIKfyve inhibitor YM201636 did not inhibit LPS-induced IL-12 production in thioglycollate-induced mouse peritoneal macrophages (Hazeki et al. 2013). Different from apilimod, YM201636 inhibits class I PI 3-kinase and Akt at higher concentrations (Hazeki et al. 2013; Ikonomov et al. 2009). Since class I PI 3-kinase negatively regulates the TLR-mediated IL-12 production (Fukao et al. 2002; Hazeki et al. 2006; Tsukamoto et al. 2008), it is likely that YM201636 increased the IL-12 production by the inhibition of class I PI 3-kinase while decreased it by the inhibition of PIKfyve.
Interestingly, LPS-induced IL-10 production was increased in cells deficient in PIKfyve function (Hazeki et al. 2013). A similar increase in IL-10 production was observed in apilimod-treated human blood stimulated with SAC (TLR2), as described in the Sect. 4.2.1 (Wada et al. 2012).
Because inhibitors of dynamin increase LPS-induce NF-κB activation, the translocation of TLR4/LPS to the endosome was regarded as a negative regulatory mechanism of TLR4 signaling (Husebye et al. 2006). Recently, however, a group of researchers revealed that the inhibition of TLR4 internalization in macrophages results in a loss of LPS-induced IFN regulatory factor 3 (IRF3) phosphorylation without affecting NF-κB activation (Kagan et al. 2008). Thus, although TLR4/LPS translocates to the endosome to be subjected to degradation, TLR4/LPS activates the TIR-domain-containing adapter-inducing IFN-β (TRIF)-dependent pathway to produce type I IFN before its degradation. Thus, it is speculated that the inhibition of PIKfyve may increase NF-κB-mediated cytokine production through the inhibition of receptor degradation, but also attenuates IRF3-dependent type I IFN production (Fig. 4.1).
Gram-negative bacteria-induced IL-1β production is another TRIF-dependent response. The activation of the NLRP3 inflammasome via TRIF ligation is important for the lysosomal degradation of pro-IL-1β (Rathinam et al. 2012). In addition, LPS-induced IL-1β mRNA expression is severely downregulated by either PIKfyve knockdown or YM201636 treatment (Hazeki et al. 2013), which indicates that the maturation of the TLR4/LPS-containing endosome is indispensable for IL-1β mRNA expression. Chloroquine, an inhibitor of endosome acidification, also decreases LPS-induced IL-1β mRNA expression in human PBMCs and macrophages/monocytes (Jang et al. 2006). Hence, it is likely that both the mRNA expression and the processing of pro-IL-1β depend on endosome maturation and acidification in these cells.
4.5.3 Viral Infection-Mediated Responses
Another role of PIKfyve in the antiviral response was discovered by an in vitro screening system. During bacterial infection, IRF3 controls the expression of the IFN-β gene. In the quiescent state, IRF3 is located in the cytoplasm. Upon stimulation with viral infection or LPS challenge, it is phosphorylated on serine and threonine residues to form a dimer and moves to the nucleus (Fitzgerald et al. 2003). TANK-binding kinase 1 (TBK1) and inducible IκB-kinase (IKK-i) are responsible for IRF3 phosphorylation (Fitzgerald et al. 2003). When recombinant TBK1 and IRF3 were mixed and subjected to in vitro kinase reaction, the addition of the lipid fraction from human embryonic kidney (HEK) 293 T cells increased the TBK1-mediated IRF3 activation. The screening of the lipids identified PtdIns(5)P as the factor required for the phosphorylation of IRF3 by TBK1 (Kawasaki et al. 2013). In mouse embryonic fibroblast (MEF) cells, the PIKfyve inhibitor YM201636 inhibited the LPS- or poly(I:C) (TLR3)-induced production of IFN-β (Kawasaki et al. 2013). A reporter assay in HEK293 cells revealed that the overexpression of PIKfyve increases the promoter activity of the IFN-stimulated response element (ISRE) induced by LPS or poly(I:C) without influencing NF-κB promoter activity (Kawasaki et al. 2013). In addition, siRNA targeting PIKfyve was found to inhibit the IFN-β mRNA expression induced by LPS or poly(I:C) in MEF cells. Intriguingly, synthetic-C8-PtdIns(5)P promotes the production of IFN-β. As a conclusion, PtdIns(5)P directly binds to IRF3, and this binding is necessary for phosphorylation by TBK1 (Kawasaki et al. 2013) (Fig. 4.1).
4.6 Concluding Remarks
The effect of PIKfyve inhibitors in vivo and in vitro is summarized in Tables 4.1 and 4.2, respectively. The inhibitor apilimod has been regarded exclusively as an IL-12 inhibitor, but a detailed analysis of the data from the clinical tests showed that the inhibitory effect of apilimod on IL-12 production appears to vary among individuals (Wada et al. 2012). Furthermore, it does not inhibit IL-12 production at least in macrophage-like synoviocytes of human origin (Krausz et al. 2012). Additionally, the IL-12p40 production in the mouse intestinal mucosa deficient in PIKfyve is rather increased (Takasuga et al. 2013). It is known that the polarized cells, such as the cells in the intestinal mucosa, and professional phagocytic cells, such as macrophages, have many more different types of endosomes than other cell types. The function of PIKfyve may also vary in these cells. We have generated CD11b- or CD11c-specific PIKfyve knockout mice to unravel the role of PIKfyve in IL-12 production. The results may contribute to the design of new therapeutic strategies.
Both CpG-containing endosomes and Salmonella-containing vacuoles do not mature in the absence of PIKfyve (Hazeki et al. 2013; Kerr et al. 2010). The fact that TLR9-dependent signals are attenuated by PIKfyve inhibition suggests that TLR3- or TLR7/8-mediated events are also susceptible to PIKfyve inhibition. However, because the CpG signal in pDCs emerges from the early endosome and not from the late endosome (Guiducci et al. 2006; Honda et al. 2005; Kerkmann et al. 2005), it is likely that PIKfyve-dependent endosome maturation negatively regulates the TLR9-dependent type I IFN production. Thus, not all of the responses induced by endosomal TLRs may be inhibited by PIKfyve inhibition. Some viruses and bacteria are known to translocate to the late endosome via the autophagosome. Because PtdIns(3,5)P2 controls autophagosome maturation, PIKfyve may also play a role in these autophagosome-mediated events.
TLR4 is a cell surface receptor, but LPS-mediated IRF3 phosphorylation to induce IFN-β gene expression is initiated by TRIF-dependent signals in the endosome. Thus, it is possible that PIKfyve contributes to the LPS-induced activation of IRF3 via endosome maturation. In addition, PIKfyve produces PtdIns(5)P, which accelerates the phosphorylation and dimerization of IRF3. Both mechanisms may be involved in the PIKfyve-mediated regulation of IFN-β production. There is another aspect in the maturation of cell surface receptor containing endosomes. It is generally accepted that cell surface receptors are downregulated by internalization, and PIKfyve-mediated endosome maturation may be involved in this negative regulation. Thus, it is likely that PIKfyve is involved in cell surface receptor signaling either negatively or positively.
The possible role of PIKfyve in innate immunity is summarized in Fig. 4.1. The currently available knowledge is too limited to provide a rational interpretation for the function of PIKfyve. To clarify the tangled effect of PIKfyve inhibition, a cell type-specific analysis will prove informative.
References
Billich A (2007) Drug evaluation: apilimod, an oral IL-12/IL-23 inhibitor for the treatment of autoimmune diseases and common variable immunodeficiency. IDrugs 10:53–59
Borchardt JK (2004) Focus on small molecule inhibitors for treatment of inflammatory and autoimmune diseases. Drug News Perspect 17:607–614
Burakoff R, Barish CF, Riff D et al (2006) A phase 1/2A trial of STA 5326, an oral interleukin-12/23 inhibitor, in patients with active moderate to severe Crohn’s disease. Inflamm Bowel Dis 12:558–565
Cai X, Xu Y, Cheung AK et al (2013) PIKfyve, a class III PI kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in Toll-like receptor signaling. Chem Biol 20:912–921
Fitzgerald KA, McWhirter SM, Faia KL et al (2003) IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat Immunol 4:491–496
Fukao T, Tanabe M, Terauchi Y et al (2002) PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 3:875–881
Guiducci C, Ott G, Chan JH et al (2006) Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J Exp Med 203:1999–2008
Hazeki K, Kinoshita S, Matsumura T et al (2006) Opposite effects of wortmannin and 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride on toll-like receptor-mediated nitric oxide production: negative regulation of nuclear factor-{kappa}B by phosphoinositide 3-kinase. Mol Pharmacol 69:1717–1724
Hazeki K, Uehara M, Nigorikawa K et al (2013) PIKfyve regulates the endosomal localization of CpG oligodeoxynucleotides to elicit TLR9-dependent cellular responses. PLoS One 8:e73894
Ho CY, Alghamdi TA, Botelho RJ (2012) Phosphatidylinositol-3,5-bisphosphate: no longer the poor PIP2. Traffic 13:1–8
Honda K, Ohba Y, Yanai H et al (2005) Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035–1040
Husebye H, Halaas Ø, Stenmark H et al (2006) Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J 25:683–692
Ikonomov OC, Sbrissa D, Shisheva A (2009) YM201636, an inhibitor of retroviral budding and PIKfyve-catalyzed PtdIns(3,5)P2 synthesis, halts glucose entry by insulin in adipocytes. Biochem Biophys Res Commun 382:566–570
Ikonomov OC, Sbrissa D, Delvecchio K et al (2011) The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem 286:13404–13413
Jang CH, Choi JH, Byun MS et al (2006) Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 45:703–710
Jefferies HB, Cooke FT, Jat P et al (2008) A selective PIKfyve inhibitor blocks PtdIns(3,5)P(2) production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9:164–170
Kagan JC, Su T, Horng T et al (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9:361–368
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650
Kawasaki T, Takemura N, Standley DM et al (2013) The second messenger phosphatidylinositol-5-phosphate facilitates antiviral innate immune signaling. Cell Host Microbe 14:148–158
Keino H, Watanabe T, Sato Y et al (2008) Therapeutic effect of the potent IL-12/IL-23 inhibitor STA-5326 on experimental autoimmune uveoretinitis. Arthritis Res Ther 10:R122
Kerkmann M, Costa LT, Richter C et al (2005) Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem 280:8086–8093
Kerr MC, Wang JT, Castro NA et al (2010) Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J 29:1331–1347
Krauss M, Haucke V (2007) Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep 8:241–246
Krausz S, Boumans MJ, Gerlag DM et al (2012) Brief report: a phase IIa, randomized, double-blind, placebo-controlled trial of apilimod mesylate, an interleukin-12/interleukin-23 inhibitor, in patients with rheumatoid arthritis. Arthritis Rheum 64:1750–1755
Kuo CC, Lin WT, Liang CM (2006) Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J Immunol 176:5943–5949
Latz E, Schoenemeyer A, Visintin A et al (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5:190–198
Leifer CA, Brooks JC, Hoelzer K et al (2006) Cytoplasmic targeting motifs control localization of toll-like receptor 9. J Biol Chem 281:35585–35592
Lewis KL, Caton ML, Bogunovic M et al (2011) Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity 35:780–791
Lindmo K, Stenmark H (2006) Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119:605–614
Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151
Mayinger P (2012) Phosphoinositides and vesicular membrane traffic. Biochim Biophys Acta 1821:1104–1113
McCartney AJ, Zhang Y, Weisman LS (2014) Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. Bioessays 36:52–64
McGettrick AF, O’Neill LA (2010) Localisation and trafficking of Toll-like receptors: an important mode of regulation. Curr Opin Immunol 22:20–27
Murai M, Turovskaya O, Kim G et al (2009) Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol 10:1178–1184
Park B, Brinkmann MM, Spooner E et al (2008) Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat Immunol 9:1407–1414
Persson EK, Uronen-Hansson H, Semmrich M et al (2013) IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity 38:958–969
Rathinam VA, Vanaja SK, Waggoner L et al (2012) TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619
Rouse BT, Sehrawat S (2010) Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol 10:514–526
Sancho D, Reis e Sousa C (2012) Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu Rev Immunol 30:491–529
Sands BE, Jacobson EW, Sylwestrowicz T et al (2010) Randomized, double-blind, placebo-controlled trial of the oral interleukin-12/23 inhibitor apilimod mesylate for treatment of active Crohn’s disease. Inflamm Bowel Dis 16:1209–1218
Sasaki T, Takasuga S, Sasaki J et al (2009) Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res 48:307–343
Schlitzer A, McGovern N, Teo P et al (2013) IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity 38:970–983
Shisheva A (2008) PIKfyve: partners, significance, debates and paradoxes. Cell Biol Int 32:591–604
Strowig T, Henao-Mejia J, Elinav E et al (2012) Inflammasomes in health and disease. Nature 481:278–286
Takasuga S, Sasaki T (2013) Phosphatidylinositol-3,5-bisphosphate: metabolism and physiological functions. J Biochem 154:211–218
Takasuga S, Horie Y, Sasaki J et al (2013) Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proc Natl Acad Sci U S A 110:1726–1731
Takeshita F, Gursel I, Ishii KJ et al (2004) Signal transduction pathways mediated by the interaction of CpG DNA with Toll-like receptor 9. Semin Immunol 16:17–22
Tsukamoto K, Hazeki K, Hoshi M et al (2008) Critical roles of the p110 beta subtype of phosphoinositide 3-kinase in lipopolysaccharide-induced Akt activation and negative regulation of nitrite production in RAW 264.7 cells. J Immunol 180:2054–2061
Wada Y, Lu R, Zhou D et al (2007) Selective abrogation of Th1 response by STA-5326, a potent IL-12/IL-23 inhibitor. Blood 109:1156–1164
Wada Y, Cardinale I, Khatcherian A et al (2012) Apilimod inhibits the production of IL-12 and IL-23 and reduces dendritic cell infiltration in psoriasis. PLoS One 7:e35069
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Hazeki, K., Nigorikawa, K., Hazeki, O. (2015). The Role of PIKfyve in Toll-Like Receptor-Mediated Responses. In: Seya, T., Matsumoto, M., Udaka, K., Sato, N. (eds) Inflammation and Immunity in Cancer. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55327-4_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-55327-4_4
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55326-7
Online ISBN: 978-4-431-55327-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)