Abstract
Vertebrate rod photoreceptors are exceptionally adapted cells that convert light into image-forming signals, which are conveyed to the brain to create vision. The foundation for the optimal function of rod photoreceptors is the functional compartmentalization of the photosensitive membranes containing the light receptor rhodopsin to the uniquely modified primary cilia that form the specialized organelles, the rod outer segments (ROS). The ciliary transport machinery involved in ROS morphogenesis is composed of intersecting networks of macromolecular complexes that functionally link the small GTPases of the Arf and Rab families. This chapter summarizes the role and mechanisms of photoreceptor ciliary transport, with the main emphases on the ciliary targeting of rhodopsin, which is essential for photoreceptor function and viability.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Primary cilia are exquisitely organized membrane projections that emanate from the plasma membrane of almost all eukaryotic cells, where they function as environmental sensors that capture a wide range of extracellular signals (Singla and Reiter 2006). Sensory receptors and their associated signal transduction machineries are highly concentrated in the ciliary membrane, which is contiguous with, but distinctly different from, the surrounding plasma membrane. The compartmentalization of the sensory membranes is achieved by the directed transport of signaling molecules to the base of the cilia, followed by the selective admission through the periciliary diffusion barrier and retention of specific components in the ciliary membranes (Christensen et al. 2007; Emmer et al. 2010; Leroux 2007; Nachury et al. 2010; Rosenbaum and Witman 2002). Defects in primary cilia affect multiple tissues and organs and cause human diseases and disorders broadly classified as ciliopathies, which are manifested by both retinal degeneration and cystic kidneys, often accompanied by obesity, polydactyly, and sensory impairments (Blacque and Leroux 2006; Fliegauf et al. 2007; Gerdes et al. 2009; Sang et al. 2011).
Primary cilia of retinal rod photoreceptors elaborate specialized light sensing organelles known as the rod outer segments (ROS), which are continuously replenished throughout the lifetime of photoreceptor cells (Insinna and Besharse 2008). This membrane renewal depends on the precise ciliary targeting of light-sensing components followed by their confinement in the ROS membranes. Rod cell polarity is unmatched in other cells. Rod photoreceptors are distinctly divided into three different compartments: the cell body called the rod inner segment (RIS) that houses the biosynthetic and metabolic machinery, the ROS which is specialized for light capture, and the synapse that conveys the image-forming signal to the retinal neurons (Fig. 6.1). The light-sensing receptor rhodopsin that is highly concentrated in the ROS is a prototypic G protein-coupled receptor (GPCR). Because the ROS lack the biosynthetic machinery, rhodopsin is synthesized in the RIS. After the passage through the Golgi complex it is incorporated into rhodopsin transport carriers (RTCs) that bud from the trans-Golgi network (TGN) and deliver rhodopsin to the base of the cilia (Deretic 2006). This specialized RTC-mediated ciliary traffic that supports continuous ROS membrane renewal creates an unparalleled unidirectional membrane flow that represents an extreme case of ciliary receptor targeting. The volume of ROS membrane trafficking is much higher in amphibians than in mammals (Besharse 1986); thus, in amphibians the RTCs that carry the bulk of newly synthesized ROS membranes are reproducibly detected in the immediate proximity of the cilia where they presumably fuse with the plasma membrane (Deretic and Papermaster 1991; Papermaster et al. 1985; Papermaster et al. 1986) (Fig. 6.2). The biochemical characterization of isolated RTCs greatly facilitated identification of proteins that associate with newly synthesized rhodopsin during trafficking to the cilia and revealed the identity of important regulators of rhodopsin trafficking, including the small GTPases Rab6, Rab8, and Rab11 (Deretic et al. 1995; Deretic and Papermaster 1993; Deretic et al. 1996).
Functional organization of retinal rod photoreceptors. Photoreceptor cells are divided into three distinct domains: the ROS, the RIS, and the synapse. RIS contain the biosynthetic and sorting machinery, including the Golgi, and the trans-Golgi network (TGN) that are localized in the myoid region (M), and a dense array of mitochondria involved in the energy metabolism that are localized in the ellipsoid region (E). Rhodopsin transport carriers (RTCs) bud from the TGN and traverse the ellipsoid on their way to the cilium, which emanates from the basal body. RIS are delineated by the adherens junctions (AJ). The synaptic terminal is at the opposite end of the cell from the ROS and contains synaptic ribbons that anchor synaptic vesicles involved in neurotransmitter release. The ROS houses approximately 1,000 stacked disk membranes that contain approximately 104–106 molecules of rhodopsin/disk. The phototransduction cascade is initiated in the disk membrane when light-activated rhodopsin (R*) encounters and activates the trimeric G-protein transducin, composed of Gα and Gβγ subunits. The crystal structure of rhodopsin (Palczewski et al. 2000) reveals seven transmembrane helices that shield the chromophore (dark green) and the cytoplasmic helix 8 that lies perpendicular to the membrane, anchored by two palmitates (green). The oligosaccharides are represented in blue (After Palczewski et al. 2000). In the ROS, the cytoplasmic surface of rhodopsin is a site of interaction for transducin and other regulators of phototransduction such as rhodopsin kinase and arrestin. In the RIS, the cytoplasmic surface of rhodopsin interacts with the regulators of ciliary targeting.
RTCs are delivered to the base of the cilium. (a) Rhodopsin transport carriers (RTCs), detected by electron microscopy (EM) immunocytochemistry with anti-rhodopsin mAb 11D5, are clustered at the base of the cilium (C) in the RIS. Rhodopsin is also concentrated in the ROS disks and plasma membrane, but not in the RIS plasma membrane. BB, basal body; m, mitochondria. (Modified from Deretic and Papermaster 1991.) (b) Vesicular membrane structures at the base of the cilium carry newly synthesized rhodopsin, detected by EM autoradiography. Radiolabeled rhodopsin is also seen in the basal ROS disks. (Reproduced from Papermaster et al. 1986)
6.2 Role of Ciliary Transport
In the ROS, rhodopsin is tightly packed within the membranous disks, at concentrations high enough to capture a single photon and signal its absorption (Baylor et al. 1979). The ability of rods to function as perfect cellular machines that convert light absorbed by rhodopsin into changes in neurotransmitter release is based on the extraordinary magnitude of signal amplification through a succession of interactions that has been well documented and recently described in detail (Arshavsky and Burns 2012; Burns and Arshavsky 2005; Palczewski 2012). Briefly, within a millisecond of photon capture, activated rhodopsin (also known as metarhodopsin II or R*) initiates a G protein (transducin)-mediated cascade of interactions that amplify the signal and convert it into electrical response that is relayed across synapses to other neurons in the retina. The first step of the cascade is transducin activation on membranous disks, which proceeds at the rate higher than any other G-protein activation by GPCRs. Further signal amplification is achieved through the subsequent activation of cGMP phosphodiesterase (PDE), an enzyme with an extraordinary catalytic activity that rapidly lowers cGMP concentration. The decrease in cGMP concentration results in closing of the cGMP channels in the ROS plasma membrane, which causes decrease in inward current that is further amplified by the intrinsic properties of the channels. These processes mediating fast signal propagation are perfectly orchestrated because the organelle that houses the components of the phototransduction cascade is built to optimize their performance. Components of the ROS are either a part of the well-integrated light-sensing network or provide the framework that supports it. Proteins and lipids that participate in phototransduction are efficiently sequestered in the ROS, away from those taking part in other cellular functions that would impede the propagation of the visual signal. The ROS architecture reflects the structure–functional organization of the organelle from which it is derived, the primary cilium. The entry into primary cilia is an extremely selective process that is regulated by the lipid and protein networks, which limit the ciliary access by forming the periciliary diffusion barrier, or the ciliary gate. Once the ciliary gate is established during ciliogenesis, only the selected proteins and lipids can gain ciliary access, thus allowing the exquisitely orchestrated interactions such as the phototransduction cascade to proceed at an optimal rate.
The signal amplification within ROS disk membranes is aided by the low viscosity resulting from the exceptionally high content of unsaturated long-chain phospholipids highly enriched in omega-3 docosahexaenoic acid [DHA, 22:6(n-3)]. However, these unsaturated phospholipids are exquisitely susceptible to light and oxidative damage, thus rendering the ROS environment toxic for its constituents (Anderson and Penn 2004; Winkler et al. 1999). To counteract the constant exposure to damaging agents, light-sensitive membranes are continuously produced and renewed, which creates a high-energy burden for photoreceptor cells. Newly synthesized ROS components have to traverse a maze of obstacles created by tightly packed mitochondria in the RIS and carry passwords that provide access into the cilia. Nevertheless, all these processes are essential for survival because disruptions of the regulatory networks at the entrance to the cilia lead to dysfunctions that cause human retinal dystrophies and syndromic diseases that affect multiple organs, including the eyes.
6.3 Mechanism of Ciliary Transport
6.3.1 Structure–Functional Organization of Primary Cilia and the ROS
Primary cilia are elaborated after the cell division in a process regulated by the centrosome, which is composed of two centrioles that dock at the plasma membrane and become the basal body (BB). During ciliogenesis, one (mother) centriole is modified and acquires appendages, accessory structures, and the pericentriolar material (PCM) that are necessary for the formation of the microtubule-organizing center (MTOC), and one (daughter) centriole remains unmodified (Bettencourt-Dias and Glover 2007) (Fig. 6.3). The mother centriole then elaborates the axoneme containing an orderly array of nine microtubule pairs, with two central single microtubules (structure 9C2) in motile cilia, or without the central pair (structure 9C0) in primary (nonmotile) cilia. The microtubule doublets extend into the axoneme and eventually become singlets. At the ciliary base, the basal body is connected to the ciliary membrane via transition fibers (Fig. 6.3), which are densely packed propeller-like sheets that leave only 60-nm openings severely restricting the passage into cilia (Anderson 1972). Immediately adjacent to the basal body is the short and very narrow, approximately 300-nm, transition zone filled with the doublet microtubules and Y-shaped structures that link them to the ciliary membrane and form a unique organization termed the ciliary necklace (Gilula and Satir 1972). The Y-shaped structures are also observed in the so-called connecting cilium of photoreceptor cells (Besharse et al. 1985; Horst et al. 1987) (Fig. 6.3), which corresponds to the transition zone of primary cilia. The ROS is essentially a significantly enlarged cilium filled with a stacked array of membranous disks (Fig. 6.3). Microtubules are also connected to the ciliary membrane through the highly conserved intraflagellar transport complexes (IFTs) and molecular motors that move membrane proteins along the cilia (Insinna and Besharse 2008). The architecture of the mouse photoreceptor cilium was recently examined by cryo-electron tomography, which revealed tethered vesicles at the base of the cilium, which may correspond to RTCs, although they have not been identified as such (Gilliam et al. 2012). Nevertheless, in amphibian photoreceptors abundant RTCs are detected in the vicinity of the periciliary RIS plasma membrane (Deretic and Papermaster 1991; Papermaster et al. 1985; Papermaster et al. 1986), indicating that this is the site of delivery for the ciliary cargo. Thus, the rules of ciliary entry that restrict the access of non-cilia components have to apply to ROS membrane delivery. To gain access to the ROS, resident lipids and proteins have to pass the periciliary diffusion barrier, also called the ciliary gate.
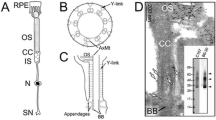
ROS is a modified primary cilium. Parallel organization of the axoneme, transition zone, and the basal body of the primary cilia and the ROS is visualized through the alignment and color-coding that emphasizes the separation of cilia from the rest of the cell. Schematic cross sections on the left depict, from the bottom: the basal body and the transition fibers (green) that extend from the distal end of the basal body to the cell membrane; the transition zone with microtubule doublets and the Y-shaped cross-linkers (red); a circular array of microtubule doublets that fills the axoneme; and the singlets at the distal end of the axoneme. The electron micrographs on the right show the equivalent photoreceptor structures: the basal body and the transition fibers of mouse photoreceptor (reproduced from Sedmak and Wolfrum 2011) and the ciliary necklace in the transition zone (reproduced from Besharse et al. 1985)
6.3.2 The Periciliary Diffusion Barrier
The periciliary diffusion barrier, or the ciliary gate, is formed through specific lipid ordering and the formation of septin rings and multiprotein complexes that are located at the transition zone (Chih et al. 2012; Craige et al. 2010; Garcia-Gonzalo et al. 2011; Garcia-Gonzalo and Reiter 2012; Hu et al. 2010; Nachury et al. 2010; Sang et al. 2011; van Reeuwijk et al. 2011; Vieira et al. 2006; Williams et al. 2011). The periciliary diffusion barrier separates the ciliary membrane from the surrounding plasma membrane through the particularly high lipid ordering caused by the abundance of cholesterol and glycosphingolipids (Godi et al. 2004; Vieira et al. 2006). This increased lipid ordering has also been observed at the base of the photoreceptor cilium where cholesterol-enriched rings were detected by freeze-fracture analysis (Andrews and Cohen 1983). The ROS and the periciliary RIS membranes differ greatly in their composition and fluidity, which presumably limits the mixing of their contents. Septin rings may also participate in the barrier formation at the photoreceptor transition zone, but this has not yet been established.
The ciliary gate is generally formed through NPHP-JBTS-MKS protein complexes located at the centrosomes and the transition zone, which are composed of distinct functional modules linked into networks that build and maintain the primary cilium (Fig. 6.4) (Chih et al. 2012; Garcia-Gonzalo et al. 2011; Garcia-Gonzalo and Reiter 2012; Sang et al. 2011; van Reeuwijk et al. 2011; Williams et al. 2011). These molecular assemblies represent the largest group of the genes linked to photoreceptor degeneration. Mutations in genes encoding for NPHP-JBTS-MKS proteins cause ciliary defects that lead to retinal and neural dysfunction, nephronophthisis, Joubert syndrome, and Meckel syndrome. Nephrocystin-5 (NPHP5) and nephrocystin-6 (NPHP6) form a module at the centrosomes that regulates ciliogenesis (Fig. 6.4). Mutations in NPHP5 cause combined retinitis pigmentosa and nephronophthisis, comprising the renal-retinal Senior-Loken syndrome (Otto et al. 2005). In retinal photoreceptors, NPHP5 functions in conjunction with NPHP2, and the MKS1-related protein B9d2, to support the ciliary transport of rhodopsin (Zhao and Malicki 2011).
The transition zone and the basal body house NPHP-JBTS-MKS and BBS multiprotein complexes that are linked to the small GTPase Rab8. The NPHP-JBTS-MKS network is presented in distinct modules as mapped by Sang et al. 2011, with transition zone proteins involved in cell polarization in blue, basal body network that regulates cilia integrity in gold, and the Hedgehog regulatory network in green. Inversins are shown in gray. BBSome is localized at the basal body and interacts with Arl6 (BBS3), which regulates its function, and with PCM1. BBS6 is located at the daughter centriole. Both the NPHP-JBTS-MKS network and the BBSome interact extensively with the small GTPase Rab8 and its GEF Rabin8, which regulate ciliogenesis and membrane trafficking to the cilia. The activity of Rabin8 is regulated through phosphorylation by the S/T kinase NDR2, which is also involved in ciliopathies
NPHP6, also known as CEP290, is a multifunctional protein mutated in Joubert syndrome (Sayer et al. 2006) and truncated in early-onset retinal degeneration in the rd16 mouse (Chang et al. 2006). In retinal photoreceptors, NPHP6 has been localized to basal bodies and the cilium (Chang et al. 2006; Sayer et al. 2006). It was recently shown that CEP-290/NPHP6 interacts with CEP162, a microtubule-recognition protein that initiates the assembly of the transition zone (Wang et al. 2013). Of high relevance for ciliary membrane trafficking, at the transition zone CEP-290/NPHP6 links NPHP-JBTS-MKS complexes with the small GTPase Rab8 to promote ciliogenesis (Kim et al. 2008; Rachel et al. 2012; Sang et al. 2011; Tsang et al. 2008).
The small GTPase Rab8, which is described in detail later, is a central regulator of the final stages of polarized membrane traffic, carrier fusion, and ciliogenesis (Bryant et al. 2010; Deretic et al. 1995; Moritz et al. 2001; Murga-Zamalloa et al. 2010a; Nachury et al. 2007; Wang et al. 2012; Yoshimura et al. 2007). Rab8 is principally activated by the guanine nucleotide exchange factor (GEF) Rabin8 (Hattula et al. 2002). In rod photoreceptors, Rabin8 is associated with the TGN and the RTCs (Wang et al. 2012). However, in addition to Rabin8 retinal photoreceptors express another Rab8 GEF named retinitis pigmentosa GTPase regulator (RPGR). Mutations in RPGR that reduce its GEF activity cause photoreceptor degeneration in X-linked retinitis pigmentosa (XLRP)(Murga-Zamalloa et al. 2010a). The presence of multiple Rab8 GEFs in rod photoreceptors suggests that the additional or alternative control of Rab8 may be needed for the extraordinary ciliary traffic that supports continuous ROS membrane expansion. Moreover, given the multiple interactors of Rab8 during ciliogenesis and ciliary trafficking it is possible that some of the ciliopathy NPHP-JBTS-MKS proteins with presently unknown function actually perform regulatory roles in the Rab8 activity cycles.
The retina-specific Rab8 GEF RPGR is a key player in ciliary morphogenesis. It is homologous to RCC1, the nucleotide exchange factor for the small GTPase Ran (Meindl et al. 1996). Mutations in the ORF15 isoform of RPGR are linked to photoreceptor degeneration in XLRP (Kirschner et al. 1999). RPGR is anchored to the photoreceptor cilium through RPGRIP1, which is affected in patients with LCA (Dryja et al. 2001; Hong et al. 2000; Hong et al. 2001; Shu et al. 2005; Zhao et al. 2003). RPGR is intimately associated with the NPHP-JBTS-MKS protein network and interacts with NPHP5, NPHP6, and NPHP8, also known as RPGRIP1L (RPGRIP-like), mutations in which cause Joubert syndrome (Arts et al. 2007; Chang et al. 2006; Murga-Zamalloa et al. 2010b; Otto et al. 2005). RPGR is also a risk factor for retinal degeneration in patents with ciliopathies caused by mutations in other genes (Khanna et al. 2009). Given the high susceptibility of the RPGR regulatory network to disease-causing mutations, it is apparent that the activation of Rab8 by RPGR is subject to a tight spatiotemporal regulation at the rod photoreceptor transition zone.
The sheer number of retinopathy-associated ciliary proteins indicates that the malfunction of the ciliary gate has dire consequences for the photoreceptor polarity and health. Different functional networks involved in ciliogenesis and multiple ciliopathy complexes appear to be closely linked to the small GTPase Rab8. In addition to the NPHP-JBTS-MKS network, both Rab8 and Rabin8 also interact directly with the BBSome, a complex required for ciliogenesis that is composed of seven BBS proteins, mutations in which cause the Bardet Biedel syndrome (Jin et al. 2010; Nachury et al. 2007). Because of the BBsome connection to Rab8, some of the defects in Bardet Biedel syndrome may in fact be caused by defects in Rab8-mediated membrane transport to the cilium.
In addition to the universal components of the periciliary diffusion barrier, photoreceptors also have a structure called the periciliary ridge complex (PRC), a specialized domain involved in the fusion of incoming membrane carriers with the RIS plasma membrane in amphibians (Fig. 6.5) (Maerker et al. 2008; Peters et al. 1983). Mammalian photoreceptors have a functionally equivalent region called the periciliary membrane complex (PMC), which houses components of the protein network that is disrupted in Usher syndrome (USH), the most frequent cause of combined deafness and blindness (Jacobson et al. 2008; Maerker et al. 2008; Overlack et al. 2008; Rachel et al. 2012; Williams 2008; Yang et al. 2010). Through its multiprotein interactions, this network appears to support the unique specialization of the periciliary region and the linkage of the ciliary membrane to the surrounding RIS plasma membrane (Maerker et al. 2008).
The periciliary ridge complex (PRC) surrounds the base of the cilium and houses regulators of RTC fusion. (a) RTCs, referred to here as vesicles (v), fuse with the RIS plasma membrane at the periciliary ridge complex (PRC). (Reproduced form Peters et al. 1983.) (b–e) Usher protein whirlin is localized at the PMC in mouse photoreceptors (b, c) and at the PRC in frog photoreceptors (d, e). Longitudinal (b) and transverse (c) views of whirlin that was detected by immuno-EM. (d) Whirlin (green) was localized above the basal bodies marked by γ-tubulin (red) in the longitudinal views of frog photoreceptors. (e) Whirlin appears as circles surrounding the basal bodies in the transverse view. [(b–e) Reproduced from Yang et al. 2010.] (f) EM image detailing RIS structure of a frog photoreceptor. Ellipsoid region (E) is filled with densely packed mitochondria (m). RTCs (arrows) traverse this region and fuse at the base of the cilium (C), at the periciliary ridge complex (PRC, arrow). CP, calycal processes. (g) Anti-whirlin specifically detects the PRC (arrow). (h) SNAREs SNAP-25 (green) and syntaxin 3 (STX3, red) colocalize in the RIS at the RTC fusion sites (yellow, arrows). Asterisk, RIS of green rods. (i) SNAP-25 (green) and whirlin (red) colocalize at the same sites at the base of the cilium (yellow, arrows). (j) Magnified PRC from h shows RTC fusion sites. [(f–j) Reproduced from Mazelova et al. 2009b]
PRC is the site of interaction of the RTC fusion executors syntaxin 3 and SNAP-25, the two plasma membrane SNAREs (soluble N-ethylmaleimide-ensitive factor attachment protein receptors) that control the Rab8-mediated delivery of rhodopsin to the cilium and the ROS (Fig. 6.5) (Mazelova et al. 2009b). The late-acting function of Rab8 in ciliary trafficking indicates that ROS membrane constituents have to enter the Rab8-regulated membrane pathway to gain ciliary access. This is precisely how rhodopsin gets to the cilia, and the way that it achieves that is through interactions with multiple small GTPases and the scaffold proteins that regulate their activation/inactivation cycles and collectively operate upstream of Rab8.
6.3.3 The Rab Family of Small GTPases
In its ciliary transport, rhodopsin interacts with the small GTPases of the Rab and Arf families that play a central role in organizing intracellular membrane trafficking as well as membrane delivery to primary cilia. The hallmark feature of all GTPases, including Rabs and Arfs, is their function as universal molecular switches whose on and off states are triggered by binding and hydrolysis of GTP. This tried-and-true mechanism gives directionality to cellular processes such as signal transduction, cytoskeleton dynamics, and membrane trafficking. The small GTPases undergo activation–inactivation cycles that are regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) that cooperatively control membrane budding and tethering of membranes to other membranes and to cytoskeletal elements (Mizuno-Yamasaki et al. 2012). These regulatory functions are frequently performed by multifunctional proteins that act at the intersection of different cellular pathways and allow crosstalk between different families of small GTPases (Baschieri and Farhan 2012; Deretic 2013). Rabs regulate the delivery of cargo to their particular intracellular destinations through the recruitment of macromolecular complexes that organize membrane microdomains, thus inducing changes in membrane identity during cargo progression through intracellular compartments (Grosshans et al. 2006; Rink et al. 2005; Stenmark 2009; Zerial and McBride 2001). For instance, the succession of Rab6, Rab11, and Rab8 occurs on the photoreceptor biosynthetic membranes during progression of rhodopsin from the Golgi complex toward the cilia (Deretic et al. 1995; Deretic and Papermaster 1993; Deretic et al. 1996). The functional networks of Rab GEF and GAP cascades and positive-feedback loops generated by GEF–effector interactions link the different stages of a particular cellular transport pathway. A prime example of a GEF-effector network is the highly conserved Rab11-Rabin8-Rab8-Sec15 ciliogenesis cascade that is composed of the homologues of the Ypt32p-Sec2p-Sec4p-Sec15p proteins involved in yeast budding (Feng et al. 2012; Knodler et al. 2010; Wang et al. 2012; Westlake et al. 2011).
Rab8 is the linchpin of the Rab11-Rabin8-Rab8-Sec15 ciliogenesis cascade and a central regulator of intracellular membrane trafficking responsible for the final stages of the directed delivery of ciliary membrane components to the ciliary gate (Feng et al. 2012; Knodler et al. 2010; Murga-Zamalloa et al. 2010a; Nachury et al. 2007; Wang et al. 2012; Westlake et al. 2011; Yoshimura et al. 2007). In its active conformation, Rab8 performs multiple functions in ciliogenesis and ciliary targeting through an array of effectors and regulatory proteins. Consistent with its dynamic functions, Rab8 localizes to developing cilia but departs mature cilia (Westlake et al. 2011). The cilia and the ROS of mature photoreceptors are devoid of Rab8, which is present only in the RIS where it is predominantly associated with RTCs (Fig. 6.6) (Deretic et al. 1995; Moritz et al. 2001; Wang et al. 2012). The involvement of Rab8 in rhodopsin trafficking was established early on and subsequently demonstrated in transgenic photoreceptors expressing the dominant-negative GFP-Rab8T22N GTP-binding-deficient mutant that causes accumulation of RTCs below the cilium (Deretic et al. 1995; Moritz et al. 2001). Notably, Rab8 directly interacts with rhodopsin and other ciliary cargo, including polycystin 1 and fibrocystin (Follit et al. 2010; Wang et al. 2012; Ward et al. 2011).
Rab8 regulates RTC fusion at the base of the cilium. (a) Scanning EM of the frog retina reveals the PRC in a cell with the ROS broken off. CP, calycal processes. (b, c) RTCs cluster at the base of the cilium (arrow) where Rab8 (red) and actin detected by phalloidin staining (green) colocalize (yellow). (Reproduced from Deretic et al. 1995.) (d, e) Abundant membranous structures accumulate below the cilium in photoreceptors expressing GFP-Rab8T22N mutant. The accumulated membranes are RTCs as they are labeled with anti-rhodopsin mAb 11D5. (Reproduced from Moritz et al. 2001)
Activation of Rab8 is mediated by Rab11a, through the activation of the Rab8 GEF Rabin8 (Feng et al. 2012; Knodler et al. 2010; Wang et al. 2012; Westlake et al. 2011). In rod photoreceptors, Rabin8 colocalizes with Rab11 at the Golgi/TGN (Wang et al. 2012). Rabin8 also associates with Rab11-containing carriers, including RTCs (Wang et al. 2012; Westlake et al. 2011), and with the basal body (Nachury et al. 2007). Rabin8 is recruited to the membrane through Rab11a, specific phospholipids, and a serine/threonine kinase NDR2 (also called STK38L) (Chiba et al. 2013) (see Fig. 6.4). NDR2 was identified as a canine retinal degeneration gene corresponding to the human ciliopathy Leber congenital amaurosis (LCA)(Berta et al. 2011; Goldstein et al. 2010). The exceptional conservation of the Rab11-Rabin8-Rab8 ciliogenesis cascade that also functions in photoreceptors implies that in its quest to reach the ROS rhodopsin engages the ancient molecular machinery that regulates the polarity and growth of baker’s yeast. However, the road to the cilia is less straightforward than yeast budding and the additional complexity is imparted by Arf4 and its regulator, the Arf GAP ASAP1, which are not expressed in yeast.
6.3.4 The Arf Family of GTPases
The encounter with the small GTPase Arf4 at the Golgi/TGN endows rhodopsin with the initial information on its cellular destination (Deretic et al. 2005; Mazelova et al. 2009a). Arf4 is a member of the Arf family of small GTPases that includes Arf, Arf-like (Arl) and Sar proteins, which collectively regulate lipid metabolism, membrane trafficking, organelle morphology, and cytoskeleton dynamics (Donaldson 2005; Kahn et al. 2006). The mammalian Arfs consist of six isoforms (Arf1–Arf6) that are, with the exception of Arf6, associated with the Golgi. In addition to the distinctive function in the Golgi, Arf4, Arl3, Arl6, and Arl13b are also strongly implicated in specialized membrane transport to primary cilia (Donaldson and Jackson 2011; Mazelova et al. 2009a; Nachury et al. 2007). The precise site of action of Arf GTPases is determined by the intracellular localization of Arf GEFs, because Arf activation is directly coupled with membrane association by the N-terminal “myristoyl switch” that inserts the protein into the membrane. Arfs have very low catalytic activity, and Arf GAPs are absolutely critical for GTP hydrolysis and Arf inactivation providing directionality to membrane transport. Arf GAPs are often incorporated into protein coats that shape the membranes in preparation for budding of distinct transport carriers (Donaldson and Jackson 2011; Kahn et al. 2006). In rhodopsin trafficking, Arf4 and its GAP ASAP1 form the basis of the ciliary targeting complex that regulates its delivery to the cilia. ASAP1 is not only a GAP for Arf4, but is also its effector because a point mutation in Arf4 (I46D) that selectively abolishes ASAP1-mediated GTP hydrolysis disrupts RTC budding, causing rhodopsin mislocalization and rapid retinal degeneration in transgenic frogs (Mazelova et al. 2009a).
The Arf family GTPase Arl6 regulates the function of the BBSome, which is implicated in the delivery of ciliary cargo (Jin et al. 2010; Nachury et al. 2007). Arf GTPases Arl3 and Arl13b are also involved in ciliogenesis, and their impaired function is responsible for retinitis pigmentosa 2 (RP2) and Joubert syndrome, respectively. RP2 protein is a GAP that regulates GTP hydrolysis on Arl3 (Veltel et al. 2008). Arl3 controls the delivery of lipidated proteins to the ROS by specifically releasing lipid-modified ciliary cargo, including the N-acylated transducin α-subunit, from the lipid moiety-binding protein UNC119 at the base of the cilia (Gopalakrishna et al. 2011; Ismail et al. 2012; Schwarz et al. 2012; Veltel et al. 2008; Wright et al. 2011; Zhang et al. 2011). Remarkably, loss of RP2 also reduces rhodopsin content of the ROS, indicating that the transport of lipidated proteins may be functionally linked to the ciliary transport of rhodopsin (Li et al. 2013). Similar to Arl3, Arl13b is a part of a network that regulates ciliary targeting of lipid-modified proteins, which is affected in JBTS and NPHP (Humbert et al. 2012). Remarkably, even the limited number of ubiquitous proteins such as Arfs can regulate very specific cellular processes by being integrated into networks with distinct sets of regulators and effectors. Furthermore, Arfs act at distinct steps of cargo delivery to photoreceptor cilia: whereas Arf4 functions at the Golgi/TGN to direct rhodopsin incorporation into ciliary-targeted RTCs, Arls are specifically localized to the cilia where they directly control the ciliary access of proteins that likely reach the cilia through diverse mechanisms.
6.3.5 The Arf4-Based Ciliary Targeting Complex
The Arf4-based ciliary targeting complex directs rhodopsin into the Rab11-Rabin8-Rab8-Sec15-regulated pathway, thus ensuring its ciliary access. In addition to small GTPases Arf4 and Rab11 and the Arf GAP ASAP1, the complex also contains the Rab11-Arf interacting protein FIP3 (Mazelova et al. 2009a; Wang et al. 2012). ASAP1 is a centerpiece of this complex because of its scaffolding properties that organize components acting sequentially en route to cilia (Fig. 6.7). At the Golgi/TGN, a specific Arf-GEF activates Arf4, which subsequently binds rhodopsin, initiating the assembly of the targeting complex (Deretic et al. 2005; Mazelova et al. 2009a). ASAP1 is recruited to the TGN through distinct lipid and protein interactions and forms a tripartite complex with activated Arf4 and rhodopsin (Wang et al. 2012). Concurrently, ASAP1 binds Rab11a and the Rab11-Arf effector FIP3, completing the initial targeting complex. ASAP1 likely causes membrane deformation through its specialized curvature-inducing BAR domain, which acts as the autoinhibitor of its GAP activity (Jian et al. 2009; Nie et al. 2006). The GTP hydrolysis on Arf4 by ASAP1 that completes this stage is likely assisted by FIP3, which acts as the regulator of ASAP1 GAP activity (Inoue et al. 2008). GTP hydrolysis and inactivation of Arf4 serve as the proofreading mechanism for rhodopsin incorporation into nascent RTCs. However, at this stage RTCs still do not posses the full information on their cellular destination, which is necessary to navigate their way to the cilium. This information is provided during budding of nascent RTCs from the TGN through the ASAP1- and Rab11a-mediated recruitment of Rab8 and its GEF Rabin8 (Wang et al. 2012). Because Rab8 acts at the site of fusion with the periciliary plasma membrane, RTCs marked with Rab8 are endowed with the distinct cellular address. Activation of Rab8 by Rabin8 likely takes place on RTCs, thus rendering them competent for fusion with the periciliary plasma membrane.
Molecular interactions and the sequence of events taking place during rhodopsin trafficking to the cilia. At the TGN, rhodopsin forms a complex with activated Arf4 and ASAP1: this leads to the orderly formation of the Arf4-based ciliary targeting complex, which, in addition to Arf4, contains ASAP1, Rab11, and FIP3. Following GTP hydrolysis on Arf4, ASAP1 and Rab11 recruit Rabin8 and Rab8, which is a critical regulator of fusion with the plasma membrane. On RTCs, ASAP1 serves as a scaffold for activation of Rab8 by Rabin8. Activated Rab8 permits RTC fusion and cargo delivery across the diffusion barrier surrounding the cilium, based in part on its ability to interact with the Sec15 subunit of the exocyst, an octameric membrane tethering complex involved in ciliary targeting. Sec15 directly interacts with Rab8, Rab11, and Rabin8
In the final stages of ciliary targeting, the Rab8a-positive membrane carriers are tethered to the plasma membrane by the conserved octameric complex called the exocyst (Fig. 6.7) (Das and Guo 2011; Heider and Munson 2012; Hsu et al. 2004; Novick et al. 2006). The exocyst, or the Sec6/8 complex, is localized at the base of the cilium in epithelial cells and photoreceptors (Mazelova et al. 2009b; Rogers et al. 2004). The exocyst undergoes dynamic assembly–disassembly cycles that regulate the polarized membrane delivery. The Sec15 subunit of the exocyst interacts directly with Rab8a, Rabin8, and Rab11a and travels to the plasma membrane on transport carriers (Bryant et al. 2010; Feng et al. 2012; Wu et al. 2005; Zhang et al. 2004). Sec15 is a component of the Rab11-Rabin8-Rab8-Sec15 cascade, which in fact constitutes a highly conserved Rab8 GEF-effector interaction network that is essential for yeast budding and membrane trafficking to the cilium (Das and Guo 2011; Feng et al. 2012). In addition to Sec15, the Sec10 component of the exocyst is also involved in cargo delivery. Sec10 interacts directly with IFT20 and the ciliary receptor polycystin 2 and is required for its ciliary localization (Fogelgren et al. 2011).
The establishment of the sequence of events in the ciliary targeting of rhodopsin was recently facilitated through the use of the proximity ligation assay (PLA) for analysis of protein–protein interactions in situ (Wang et al. 2012). The interaction sites of rhodopsin with the Arf4-based ciliary targeting complex and the Rab GTPases are illustrated in Fig. 6.8. Because of the unique geometry of the photoreceptors and the unidirectional progression of newly synthesized rhodopsin toward the cilia, quantification of the fluorescence signals generated by PLA provided invaluable information about the order of protein–protein interactions involved in ciliary targeting. For instance, more than 97 % of rhodopsin-Arf4 interaction sites are contained within the photoreceptor myoid at the Golgi complex, whereas only 3 % are found within the photoreceptor ellipsoid, where RTCs are localized. By contrast, 59 % of rhodopsin-Rab8 interaction sites are at the Golgi and 41 % are associated with RTCs (Fig. 6.8), pointing to the sequential interactions of rhodopsin with these GTPases, with Arf4 almost exclusively operating at the Golgi and upstream of Rab8 that functions on RTCs.
Arf4, ASAP1, and the Rab11a–Rabin8–Rab8 ciliary-targeting complex sequentially interact with rhodopsin transiting from the Golgi/TGN into RTCs. Endogenous protein–protein interactions in situ were detected in a form of fluorescent dots by the proximity ligation assay (PLA). The signal is generated when the fluorescently labeled oligonucleotides are hybridized to the primers covalently linked to secondary antibodies, which recognize in coincidence binding of primary antibodies to two proteins interacting at a range <16 nm. Interaction sites (red dots) were detected by PLA for rhodopsin-Arf4 (a), rhodopsin-ASAP1 (b), rhodopsin-Rab11 (d), Rab11-ASAP1 (e), and rhodopsin-Rab8 (f). c Rhodopsin-Arf4 PLA (red dots) was subsequently stained with antibody to the trans-Golgi marker Rab6 conjugated to Alexa Fluor 488 (green). Arrows indicate rhodopsin–Arf4 interaction sites juxtaposed to the trans-Golgi cisternae (Rab6, green). (Modified from Wang et al. 2012)
6.3.6 The Ciliary Targeting Signals
Clearly, to engage the correct sorting machinery and enter the ciliary pathway rhodopsin and other sensory receptors have to be equipped with adequate ciliary targeting signals. The ciliary targeting sequences that have been identified to date are highly divergent and likely engage an array of binding partners that act at different stages of ciliary membrane traffic (Berbari et al. 2008; Corbit et al. 2005; Fan et al. 2007; Follit et al. 2010; Jin et al. 2010; Kizhatil et al. 2009; Mazelova et al. 2009a; Tao et al. 2009). The extremely conserved rhodopsin ciliary targeting signal (CTS) VxPx directly engages Arf4 to direct rhodopsin to the primary cilia (Deretic et al. 2005). The CTS VxPx is also conserved among other ciliary sensory receptors, including polycystins 1 and 2 and the CNGB1b subunit of the olfactory cyclic nucleotide-gated channel (Fig. 6.9) (Deretic et al. 1998; Deretic et al. 2005; Geng et al. 2006; Jenkins et al. 2006; Ward et al. 2011). Numerous mutations affecting the CTS VxPx cause the most severe forms of autosomal dominant retinitis pigmentosa (ADRP) (Berson et al. 2002), supporting the crucial role of this signal in the directed delivery of rhodopsin to the cilia and ROS. Transgenic expression of rhodopsin carrying mutations in the VxPx motif leads to retinal degeneration in various animal models as a result of rhodopsin mislocalization to multiple cellular compartments, including the synapse that is otherwise devoid of rhodopsin (Concepcion and Chen 2010; Concepcion et al. 2002; Green et al. 2000; Lee and Flannery 2007; Li et al. 1996; Li et al. 1998; Ng et al. 2008; Sommer et al. 2011; Tam et al. 2000). Mislocalized rhodopsin likely exerts a dominant-negative effect on the regulators of functional compartmentalization in photoreceptor cells, even initiating abnormal neurite outgrowth that is observed in animal models and in patients with ADRP (Li et al. 1998).
The recently identified CTS of rhodopsin, the so-called CTS FR, is composed of amino acids phenylalanine and arginine (AA 313 and 314) localized within its cytoplasmic helix 8 (Wang et al. 2012). In addition to rhodopsin, CTS FR is conserved in ciliary-targeted GPCRs such as Smoothened and SSTR3 (Corbit et al. 2005) (Fig. 6.9). The CTS FR is a recognition motif for the Arf GAP ASAP1 (Wang et al. 2012). This signal is essential for the delivery, to primary cilia of mouse kidney IMCD3 cells, of a fusion protein composed of bovine rhodopsin and eGFP, followed by the C-terminal VxPx motif (Rh-GFP-VxPx). When the CTS FR is replaced with alanines, the fusion protein designated [FR-AA]Rh-GFP-VxPx does not interact with ASAP1, fails to connect with Rab8, and consequently does not localize to cilia (Wang et al. 2012). Thus, CTSs VxPx and FR engage two binding partners, Arf4 and ASAP1, which cooperatively assure the entry into the Rab8-regulated membrane pathway that provides ciliary access. During ciliary trafficking, rhodopsin is not just a passive cargo that is sorted into appropriate carriers but actively recruits the relevant sorting machinery involved in ciliary targeting, which is also shared by other ciliary sensory receptors.
Although the VxPx motif is a key to ciliary localization of rhodopsin, the only other known ROS constituent that has the functional VxPx targeting signal is a lipidated protein retinol dehydrogenase, but the mechanism of its targeting has not been examined (Luo et al. 2004). Other ROS membrane components have different targeting signals and employ different trafficking mechanisms. For example, targeting of peripherin/rds to ROS requires its C terminus, and particularly the valine at position 332 that is downstream of the conserved amphipathic helix, although the protein that recognizes this motif has not been identified (Salinas et al. 2013; Tam et al. 2004). Notably, NPHP and MKS proteins that regulate the ciliary transport of rhodopsin are not involved in the transport of peripherin/rds, supporting their different ciliary routes (Zhao and Malicki 2011). Guanylyl cyclase 1 (GC1) appears to have a diffuse targeting signal and may be cotransported to ROS with other proteins, possibly including rhodopsin (Bhowmick et al. 2009; Karan et al. 2011). Interestingly, in the absence of GC1, trafficking of lipidated ROS proteins is also affected, again suggesting the link between the integral and peripheral components of the ROS light-sensing membranes (Karan et al. 2008). The targeting of cyclic nucleotide-gated (CNG) channels that localize exclusively to the ROS plasma membrane is distinctly different from that of the disk membrane constituents and requires interaction with ankyrin-G (Kizhatil et al. 2009). Other ROS proteins are delivered to the cilia through a multitude of mechanisms that have been recently uncovered and reviewed in depth (Pearring et al. 2013).
6.4 Concluding Remarks
Functional compartmentalization of retinal rod photoreceptors endows these cells with qualities essential for the capture and propagation of visual signals. The underlying structure of the light-sensing organelle, the ROS, is that of a primary cilium, with specializations that include exceptional proliferation of sensory membranes that convert light absorbed by rhodopsin into changes in neurotransmitter release. Thus, the role of ciliary transport is to replenish the light-sensing membranes and deliver rhodopsin and the components of the phototransduction cascade to the exact periciliary location in the photoreceptor cell and nowhere else. The mechanism of ciliary membrane targeting employed by photoreceptors is highly conserved among ciliated cells and involves ordered recruitment and activation of small GTPases of the Rab and Arf families through scaffold proteins and multiprotein complexes, some of which are located at the entrance to the cilia. The disruption of the delicate balance of activities that coordinate the events in light-sensitive membrane renewal ultimately results in the loss of visual function.
Abbreviations
- ADRP:
-
Autosomal dominant retinitis pigmentosa
- CTS:
-
Ciliary targeting signal
- GAP:
-
GTPase activating protein
- GEF:
-
Guanine nucleotide exchange factor
- RIS:
-
Rod inner segment(s)
- ROS:
-
Rod outer segment(s)
- RTC(s):
-
Rhodopsin transport carrier(s)
- TGN:
-
Trans-Golgi network
References
Anderson RG (1972) The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol 54:246–265
Anderson RE, Penn JS (2004) Environmental light and heredity are associated with adaptive changes in retinal DHA levels that affect retinal function. Lipids 39:1121–1124
Andrews LD, Cohen AI (1983) Freeze-fracture studies of photoreceptor membranes: new observations bearing upon the distribution of cholesterol. J Cell Biol 97:749–755
Arshavsky VY, Burns ME (2012) Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287:1620–1626
Arts HH, Doherty D, van Beersum SE, Parisi MA, Letteboer SJ, Gorden NT, Peters TA, Marker T, Voesenek K, Kartono A, Ozyurek H, Farin FM, Kroes HY, Wolfrum U, Brunner HG, Cremers FP, Glass IA, Knoers NV, Roepman R (2007) Mutations in the gene encoding the basal body protein RPGRIP1L, a nephrocystin-4 interactor, cause Joubert syndrome. Nat Genet 39:882–888
Baschieri F, Farhan H (2012) Crosstalk of small GTPases at the Golgi apparatus. Small GTPases 3:80–90
Baylor DA, Lamb TD, Yau KW (1979) Responses of retinal rods to single photons. J Physiol (Lond) 288:613–634
Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K (2008) Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Mol Biol Cell 19:1540–1547
Berson EL, Rosner B, Weigel-DiFranco C, Dryja TP, Sandberg MA (2002) Disease progression in patients with dominant retinitis pigmentosa and rhodopsin mutations. Invest Ophthalmol Vis Sci 43:3027–3036
Berta AI, Boesze-Battaglia K, Genini S, Goldstein O, O’Brien PJ, Szel A, Acland GM, Beltran WA, Aguirre GD (2011) Photoreceptor cell death, proliferation and formation of hybrid rod/S-cone photoreceptors in the degenerating STK38L mutant retina. PLoS One 6:e24074
Besharse JC (1986) Photosensitive membrane turnover: differentiated membrane domains and cell–cell interaction. In: Adler R, Farber D (eds) The retina: a model for cell biological studies. Academic, New York, pp 297–352
Besharse JC, Forestner DM, Defoe DM (1985) Membrane assembly in retinal photoreceptors. III. Distinct membrane domains of the connecting cilium of developing rods. J Neurosci 5:1035–1048
Bettencourt-Dias M, Glover DM (2007) Centrosome biogenesis and function: centrosomics brings new understanding. Nat Rev Mol Cell Biol 8:451–463
Bhowmick R, Li M, Sun J, Baker SA, Insinna C, Besharse JC (2009) Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic 10:648–663
Blacque OE, Leroux MR (2006) Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport. Cell Mol Life Sci 63:2145–2161
Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE (2010) A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12:1035–1045
Burns ME, Arshavsky VY (2005) Beyond counting photons: trials and trends in vertebrate visual transduction. Neuron 48:387–401
Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, Shou C, Hildebrandt F, Williams DS, Heckenlively JR, Swaroop A (2006) In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet 15:1847–1857
Chiba S, Amagai Y, Homma Y, Fukuda M, Mizuno K (2013) NDR2-mediated Rabin8 phosphorylation is crucial for ciliogenesis by switching binding specificity from phosphatidylserine to Sec15. EMBO J 32(6):874–885
Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS (2012) A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol 14:61–72
Christensen ST, Pedersen LB, Schneider L, Satir P (2007) Sensory cilia and integration of signal transduction in human health and disease. Traffic 8:97–109
Concepcion F, Chen J (2010) Q344ter mutation causes mislocalization of rhodopsin molecules that are catalytically active: a mouse model of Q344ter-induced retinal degeneration. PLoS One 5:e10904
Concepcion F, Mendez A, Chen J (2002) The carboxyl-terminal domain is essential for rhodopsin transport in rod photoreceptors. Vision Res 42:417–426
Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF (2005) Vertebrate Smoothened functions at the primary cilium. Nature (Lond) 437:1018–1021
Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB (2010) CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol 190:927–940
Das A, Guo W (2011) Rabs and the exocyst in ciliogenesis, tubulogenesis and beyond. Trends Cell Biol 21:383–386
Deretic D (2006) A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vision Res 46:4427–4433
Deretic D (2013) Crosstalk of Arf and Rab GTPases en route to cilia. Small GTPases 4(2):70–77
Deretic D, Papermaster DS (1991) Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol 113:1281–1293
Deretic D, Papermaster DS (1993) Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci 106:803–813
Deretic D, Huber LA, Ransom N, Mancini M, Simons K, Papermaster DS (1995) rab8 in retinal photoreceptors may participate in rhodopsin transport and in rod outer segment disk morphogenesis. J Cell Sci 108:215–224
Deretic D, Puleo Scheppke B, Trippe C (1996) Cytoplasmic domain of rhodopsin is essential for post-Golgi vesicle formation in a retinal cell-free system. J Biol Chem 271:2279–2286
Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH (1998) Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proc Natl Acad Sci USA 95:10620–10625
Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A (2005) Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci USA 102:3301–3306
Donaldson JG (2005) Arfs, phosphoinositides and membrane traffic. Biochem Soc Trans 33:1276–1278
Donaldson JG, Jackson CL (2011) ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol 12:362–375
Dryja TP, Adams SM, Grimsby JL, McGee TL, Hong DH, Li T, Andreasson S, Berson EL (2001) Null RPGRIP1 alleles in patients with Leber congenital amaurosis. Am J Hum Genet 68:1295–1298
Emmer BT, Maric D, Engman DM (2010) Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci 123:529–536
Fan S, Fogg V, Wang Q, Chen XW, Liu CJ, Margolis B (2007) A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. J Cell Biol 178:387–398
Feng S, Knodler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peranen J, Guo W (2012) A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem 287:15602–15609
Fliegauf M, Benzing T, Omran H (2007) When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8:880–893
Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH (2011) The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7:e1001361
Follit JA, Li L, Vucica Y, Pazour GJ (2010) The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J Cell Biol 188:21–28
Garcia-Gonzalo FR, Reiter JF (2012) Scoring a backstage pass: mechanisms of ciliogenesis and ciliary access. J Cell Biol 197:697–709
Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, Garcia-Verdugo JM, Katsanis N, Hildebrandt F, Reiter JF (2011) A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43:776–784
Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119:1383–1395
Gerdes JM, Davis EE, Katsanis N (2009) The vertebrate primary cilium in development, homeostasis, and disease. Cell 137:32–45
Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151:1029–1041
Gilula NB, Satir P (1972) The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53:494–509
Godi A, Di Campli A, Konstantakopoulos A, Di Tullio G, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, De Matteis MA (2004) FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6:393–404
Goldstein O, Kukekova AV, Aguirre GD, Acland GM (2010) Exonic SINE insertion in STK38L causes canine early retinal degeneration (erd). Genomics 96:362–368
Gopalakrishna KN, Doddapuneni K, Boyd KK, Masuho I, Martemyanov KA, Artemyev NO (2011) Interaction of transducin with uncoordinated 119 protein (UNC119): implications for the model of transducin trafficking in rod photoreceptors. J Biol Chem 286:28954–28962
Green ES, Menz MD, LaVail MM, Flannery JG (2000) Characterization of rhodopsin mis-sorting and constitutive activation in a transgenic rat model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 41:1546–1553
Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA 103:11821–11827
Hattula K, Furuhjelm J, Arffman A, Peranen J (2002) A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell 13:3268–3280
Heider MR, Munson M (2012) Exorcising the exocyst complex. Traffic 13:898–907
Hong DH, Pawlyk BS, Shang J, Sandberg MA, Berson EL, Li T (2000) A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc Natl Acad Sci USA 97:3649–3654
Hong DH, Yue G, Adamian M, Li T (2001) Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem 276:12091–12099
Horst CJ, Forestner DM, Besharse JC (1987) Cytoskeletal–membrane interactions: a stable interaction between cell surface glycoconjugates and doublet microtubules of the photoreceptor connecting cilium. J Cell Biol 105:2973–2987
Hsu SC, TerBush D, Abraham M, Guo W (2004) The exocyst complex in polarized exocytosis. Int Rev Cytol 233:243–265
Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329:436–439
Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S (2012) ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc Natl Acad Sci USA 109:19691–19696
Inoue H, Ha VL, Prekeris R, Randazzo PA (2008) Arf GTPase-activating protein ASAP1 interacts with Rab11 effector FIP3 and regulates pericentrosomal localization of transferrin receptor-positive recycling endosome. Mol Biol Cell 19:4224–4237
Insinna C, Besharse JC (2008) Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn 237:1982–1992
Ismail SA, Chen YX, Miertzschke M, Vetter IR, Koerner C, Wittinghofer A (2012) Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J 31:4085–4094
Jacobson SG, Cideciyan AV, Aleman TS, Sumaroka A, Roman AJ, Gardner LM, Prosser HM, Mishra M, Bech-Hansen NT, Herrera W, Schwartz SB, Liu XZ, Kimberling WJ, Steel KP, Williams DS (2008) Usher syndromes due to MYO7A, PCDH15, USH2A or GPR98 mutations share retinal disease mechanism. Hum Mol Genet 17(15):2405–2415
Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR (2006) Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr Biol 16:1211–1216
Jian X, Brown P, Schuck P, Gruschus JM, Balbo A, Hinshaw JE, Randazzo PA (2009) Autoinhibition of Arf GTPase-activating protein activity by the BAR domain in ASAP1. J Biol Chem 284:1652–1663
Jin H, White SR, Shida T, Schultz S, Aguiar M, Nachury MV (2010) The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141:1208–1219
Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A (2006) Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol 172:645–650
Karan S, Frederick JM, Baehr W (2008) Involvement of guanylate cyclases in transport of photoreceptor peripheral membrane proteins. Adv Exp Med Biol 613:351–359
Karan S, Tam BM, Moritz OL, Baehr W (2011) Targeting of mouse guanylate cyclase 1 (Gucy2e) to Xenopus laevis rod outer segments. Vision Res 51:2304–2311
Khanna H, Davis EE, Murga-Zamalloa CA, Estrada-Cuzcano A, Lopez I, den Hollander AI, Zonneveld MN, Othman MI, Waseem N, Chakarova CF, Maubaret C, Diaz-Font A, Macdonald I, Muzny DM, Wheeler DA, Morgan M, Lewis LR, Logan CV, Tan PL, Beer MA, Inglehearn CF, Lewis RA, Jacobson SG, Bergmann C, Beales PL, Attie-Bitach T, Johnson CA, Otto EA, Bhattacharya SS, Hildebrandt F, Gibbs RA, Koenekoop RK, Swaroop A, Katsanis N (2009) A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet 41:739–745
Kim J, Krishnaswami SR, Gleeson JG (2008) CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Hum Mol Genet 17:3796–3805
Kirschner R, Rosenberg T, Schultz-Heienbrok R, Lenzner S, Feil S, Roepman R, Cremers FPM, Ropers HH, Berger W (1999) RPGR transcription studies in mouse and human tissues reveal a retina-specific isoform that is disrupted in a patient with X-linked retinitis pigmentosa. Hum Mol Genet 8:1571–1578
Kizhatil K, Baker SA, Arshavsky VY, Bennett V (2009) Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 323:1614–1617
Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107(14):6346–6351
Lee ES, Flannery JG (2007) Transport of truncated rhodopsin and its effects on rod function and degeneration. Invest Ophthalmol Vis Sci 48:2868–2876
Leroux MR (2007) Taking vesicular transport to the cilium. Cell 129:1041–1043
Li T, Snyder WK, Olsson JE, Dryja TP (1996) Transgenic mice carrying the dominant rhodopsin mutation P347S: evidence for defective vectorial transport of rhodopsin to the outer segments. Proc Natl Acad Sci USA 93:14176–14181
Li ZY, Wong F, Chang JH, Possin DE, Hao Y, Petters RM, Milam AH (1998) Rhodopsin transgenic pigs as a model for human retinitis pigmentosa. Invest Ophthalmol Vis Sci 39:808–819
Li L, Khan N, Hurd T, Ghosh AK, Cheng C, Molday R, Heckenlively JR, Swaroop A, Khanna H (2013) Ablation of the X-linked rRetinitis pPigmentosa 2 (Rp2) gene in mice results in opsin mislocalization and photoreceptor degeneration. Invest Ophthalmol Vis Sci 54(7):4503–4511
Luo W, Marsh-Armstrong N, Rattner A, Nathans J (2004) An outer segment localization signal at the C terminus of the photoreceptor-specific retinol dehydrogenase. J Neurosci 24:2623–2632
Maerker T, van Wijk E, Overlack N, Kersten FF, McGee J, Goldmann T, Sehn E, Roepman R, Walsh EJ, Kremer H, Wolfrum U (2008) A novel Usher protein network at the periciliary reloading point between molecular transport machineries in vertebrate photoreceptor cells. Hum Mol Genet 17:71–86
Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D (2009a) Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 28:183–192
Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D (2009b) Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci 122:2003–2013
Meindl A, Dry K, Herrmann K, Manson F, Ciccodicola A, Edgar A, Carvalho MR, Achatz H, Hellebrand H, Lennon A, Migliaccio C, Porter K, Zrenner E, Bird A, Jay M, Lorenz B, Wittwer B, D'Urso M, Meitinger T, Wright A (1996) A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat Genet 13:35–42
Mizuno-Yamasaki E, Rivera-Molina F, Novick P (2012) GTPase networks in membrane traffic. Annu Rev Biochem 81:637–659
Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS (2001) Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell 12:2341–2351
Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H (2010a) Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Hum Mol Genet 19:3591–3598
Murga-Zamalloa CA, Desai NJ, Hildebrandt F, Khanna H (2010b) Interaction of ciliary disease protein retinitis pigmentosa GTPase regulator with nephronophthisis-associated proteins in mammalian retinas. Mol Vis 16:1373–1381
Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129:1201–1213
Nachury MV, Seeley ES, Jin H (2010) Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol 26:59–87
Ng YF, Chan HH, Chu PH, To CH, Gilger BC, Petters RM, Wong F (2008) Multifocal electroretinogram in rhodopsin P347L transgenic pigs. Invest Ophthalmol Vis Sci 49:2208–2215
Nie Z, Hirsch DS, Luo R, Jian X, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA (2006) A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Curr Biol 16:130–139
Novick P, Medkova M, Dong G, Hutagalung A, Reinisch K, Grosshans B (2006) Interactions between Rabs, tethers, SNAREs and their regulators in exocytosis. Biochem Soc Trans 34:683–686
Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, De Paepe A, Reinhardt R, Klages S, Tsuda M, Kawakami I, Kusakabe T, Omran H, Imm A, Tippens M, Raymond PA, Hill J, Beales P, He S, Kispert A, Margolis B, Williams DS, Swaroop A, Hildebrandt F (2005) Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 37:282–288
Overlack N, Maerker T, Latz M, Nagel-Wolfrum K, Wolfrum U (2008) SANS (USH1G) expression in developing and mature mammalian retina. Vision Res 48:400–412
Palczewski K (2012) Chemistry and biology of vision. J Biol Chem 287:1612–1619
Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745
Papermaster DS, Schneider BG, Besharse JC (1985) Vesicular transport of newly synthesized opsin from the Golgi apparatus toward the rod outer segment. Ultrastructural immunocytochemical and autoradiographic evidence in Xenopus retinas. Invest Ophthalmol Vis Sci 26:1386–1404
Papermaster DS, Schneider BG, Defoe D, Besharse JC (1986) Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem 34:5–16
Pearring JN, Salinas RY, Baker SA, Arshavsky VY (2013) Protein sorting, targeting and trafficking in photoreceptor cells. Prog Retin Eye Res 36:24–51
Peters KR, Palade GE, Schneider BG, Papermaster DS (1983) Fine structure of a periciliary ridge complex of frog retinal rod cells revealed by ultrahigh resolution scanning electron microscopy. J Cell Biol 96:265–276
Rachel RA, Li T, Swaroop A (2012) Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia 1:22
Rink J, Ghigo E, Kalaidzidis Y, Zerial M (2005) Rab conversion as a mechanism of progression from early to late endosomes. Cell 122:735–749
Rogers KK, Wilson PD, Snyder RW, Zhang X, Guo W, Burrow CR, Lipschutz JH (2004) The exocyst localizes to the primary cilium in MDCK cells. Biochem Biophys Res Commun 319:138–143
Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3:813–825
Salinas RY, Baker SA, Gospe SM 3rd, Arshavsky VY (2013) A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS One 8:e54292
Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, Huntzicker EG, Sfakianos MK, Sandoval W, Bazan JF, Kulkarni P, Garcia-Gonzalo FR, Seol AD, O’Toole JF, Held S, Reutter HM, Lane WS, Rafiq MA, Noor A, Ansar M, Devi AR, Sheffield VC, Slusarski DC, Vincent JB, Doherty DA, Hildebrandt F, Reiter JF, Jackson PK (2011) Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell 145:513–528
Sayer JA, Otto EA, O’Toole JF, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hildebrandt F (2006) The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet 38:674–681
Schwarz N, Novoselova TV, Wait R, Hardcastle AJ, Cheetham ME (2012) The X-linked retinitis pigmentosa protein RP2 facilitates G protein traffic. Hum Mol Genet 21:863–873
Sedmak T, Wolfrum U (2011) Intraflagellar transport proteins in ciliogenesis of photoreceptor cells. Biol Cell 103:449–466
Shu X, Fry AM, Tulloch B, Manson FD, Crabb JW, Khanna H, Faragher AJ, Lennon A, He S, Trojan P, Giessl A, Wolfrum U, Vervoort R, Swaroop A, Wright AF (2005) RPGR ORF15 isoform co-localizes with RPGRIP1 at centrioles and basal bodies and interacts with nucleophosmin. Hum Mol Genet 14:1183–1197
Singla V, Reiter JF (2006) The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313:629–633
Sommer JR, Wong F, Petters RM (2011) Phenotypic stability of Pro347Leu rhodopsin transgenic pigs as indicated by photoreceptor cell degeneration. Transgenic Res 20:1391–1395
Stenmark H (2009) Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol 10:513–525
Tam BM, Moritz OL, Hurd LB, Papermaster DS (2000) Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol 151:1369–1380
Tam BM, Moritz OL, Papermaster DS (2004) The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell 15:2027–2037
Tao B, Bu S, Yang Z, Siroky B, Kappes JC, Kispert A, Guay-Woodford LM (2009) Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J Am Soc Nephrol 20:2570–2580
Tsang WY, Bossard C, Khanna H, Peranen J, Swaroop A, Malhotra V, Dynlacht BD (2008) CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Dev Cell 15:187–197
van Reeuwijk J, Arts HH, Roepman R (2011) Scrutinizing ciliopathies by unraveling ciliary interaction networks. Hum Mol Genet 20:R149–157
Veltel S, Gasper R, Eisenacher E, Wittinghofer A (2008) The retinitis pigmentosa 2 gene product is a GTPase-activating protein for Arf-like 3. Nat Struct Mol Biol 15:373–380
Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K (2006) FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 103:18556–18561
Wang J, Morita Y, Mazelova J, Deretic D (2012) The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J 31:4057–4071
Wang WJ, Tay HG, Soni R, Perumal GS, Goll MG, Macaluso FP, Asara JM, Amack JD, Bryan Tsou MF (2013) CEP162 is an axoneme-recognition protein promoting ciliary transition zone assembly at the cilia base. Nat Cell Biol 15(6):591–601
Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A (2011) A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 22:3289–3305
Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK (2011) Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA 108:2759–2764
Williams DS (2008) Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vision Res 48:433–441
Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, Yoder BK, Leroux MR (2011) MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 192:1023–1041
Winkler BS, Boulton ME, Gottsch JD, Sternberg P (1999) Oxidative damage and age-related macular degeneration. Mol Vision (Online) 5:32
Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, Jackson PK (2011) An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 25:2347–2360
Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA (2005) Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol 12:879–885
Yang J, Liu X, Zhao Y, Adamian M, Pawlyk B, Sun X, McMillan DR, Liberman MC, Li T (2010) Ablation of whirlin long isoform disrupts the USH2 protein complex and causes vision and hearing loss. PLoS Genet 6:e1000955
Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA (2007) Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol 178:363–369
Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2:107–117
Zhang XM, Ellis S, Sriratana A, Mitchell CA, Rowe T (2004) Sec15 is an effector for the Rab11 GTPase in mammalian cells. J Biol Chem 279:43027–43034
Zhang H, Constantine R, Vorobiev S, Chen Y, Seetharaman J, Huang YJ, Xiao R, Montelione GT, Gerstner CD, Davis MW, Inana G, Whitby FG, Jorgensen EM, Hill CP, Tong L, Baehr W (2011) UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci 14:874–880
Zhao C, Malicki J (2011) Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. EMBO J 30:2532–2544
Zhao Y, Hong DH, Pawlyk B, Yue G, Adamian M, Grynberg M, Godzik A, Li T (2003) The retinitis pigmentosa GTPase regulator (RPGR)-interacting protein: subserving RPGR function and participating in disk morphogenesis. Proc Natl Acad Sci USA 100:3965–3970
Acknowledgments
I thank past and present laboratory members, particularly Jing Wang, for their valuable contributions to the studies described here and many colleagues for their stimulating discussions. Supported by the NIH grant EY-12421.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Deretic, D. (2014). Role and Mechanism of Ciliary Transport. In: Furukawa, T., Hurley, J., Kawamura, S. (eds) Vertebrate Photoreceptors. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54880-5_6
Download citation
DOI: https://doi.org/10.1007/978-4-431-54880-5_6
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54879-9
Online ISBN: 978-4-431-54880-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)