Abstract
Mammalian inner ear has limited regenerative ability, and functional recovery does not occur after damage. However, recent studies indicated that the cells within the inner ear have the characteristics of stem cells, namely, capacity for self-renewal and pluripotency. Since the specific markers for inner ear stem cells have not been found, several methods have been used to detect inner ear stem cells, including sphere-forming assay, fluorescence-activated cell sorting (FACS), side population study, and analysis of slow-cycling cells or Wnt signaling in the inner ear. The potential candidates of cochlear stem cells are the supporting cells, the cells at lesser epithelial ridge (LER), the cells at greater epithelial ridge (GER), and the tympanic border cells. The number of stem cells in the inner ear is estimated to be very low and is reported to decrease dramatically with maturation. It is necessary to elucidate the regulatory mechanisms of inner ear stem cells, clarify the reasons behind the quiescence of inner ear stem cells, and identify the causative factors that influence the decrease in the number of inner ear stem cells with maturation, in order to facilitate future regeneration therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Stem cells are defined by their capacity for self-renewal, the ability to give rise to new stem cells, and pluripotency, which is the ability to differentiate into more than one type of cells. Although mammalian inner ear has limited ability to regenerate, several groups have reported the existence of stem cells within the mammalian inner ear.
Since specific markers for inner ear stem cells have not been found, several methods have been used to detect inner ear stem/progenitor cells so far. The major approach involves the in vitro generation of cell-derived floating spheres. Using this technique, mammalian inner ears were reported to contain cells that had the ability to proliferate clonally and to differentiate into several types of cells, including hair cells in vitro [1–4].
Fluorescence-activated cell sorting (FACS) was also used to isolate specific type of cells and analyze their stemness potential [5–10].
The location of inner ear stem cells is very important in order to analyze their environmental regulatory mechanism. However, dissociation distorts the microanatomy of the inner ear, making it difficult to determine the precise location of stem cells. To overcome this disadvantage of dissociation-based study, an in vivo study was performed using the slow-cycling nature of stem cells [11].
Several groups analyzed the cells expressing Wnt target genes and identified stem/progenitor cells in the cochleae by using both in vivo and in vitro models [8–10].
In this section, we would like to review these studies reporting stem/progenitor cells within the mammalian inner ear.
2 Inner Ear Stem Cells
It has been reported that damaged mammalian postnatal vestibular organs generate new hair cells (see Part IV “Hair Cell Regeneration”). Li et al. [1] first demonstrated that cells from an adult mouse utricle sensory epithelium contained cells that displayed characteristic features of stem cells. These cells grew clonally and formed floating spheres in vitro. In addition, they had the capacity for self-renewal, and they gave rise to immature spheres that expressed various genetic markers of immature developing inner ears, including nestin, Pax2, bone morphogenetic protein (BMP)-4, and BMP-7. Sphere-derived cells differentiated into cells of all three layers, ectodermal, endodermal, and mesodermal linage, which is the main characteristics of stem cells. Using the spheres, cells were generated with hair-cell phenotype, positive for myosin7a, with espin- and F-actin-positive stereociliary bundle-like structure.
Although regeneration was reported to occur in vestibules, the mammalian organ of Corti in the inner ear does not regenerate in vivo after damage. Therefore, it is noteworthy that White et al. [5] showed that mammalian cochlear supporting cells contained cells that had the ability to transdifferentiate into hair cells. They purified neonatal cochlear supporting cells by fluorescence-activated cell sorting (FACS) from transgenic mice expressing green fluorescent protein (GFP) under the control of the p27kip1 promoter. p27kip1 protein is a cyclin-dependent kinase inhibitor that functions as an inhibitor of cell cycle progression. It is first expressed in the primordial organ of Corti and is downregulated during subsequent hair-cell differentiation, but it persists at high levels in differentiated supporting cells of the mature organ of Corti [12]. The GFP expression was observed in different supporting cells, including interphalangeal cells, pillar cells, Deiters’ cells, and Hensen’s cells. They cultured purified p27kip1-GFP-positive supporting cells with periotic mesenchymal cells and found that they retained the ability to divide and transdifferentiate into myosin 6-positive hair cells in vitro. This suggested that mammalian cochlear supporting cells possessed at least progenitor capabilities. They reported that, among the different types of supporting cells, pillar and Hensen’s cells were found to have greater potential to form hair cells by using p75NGFR+ as their marker in FACS purification.
Zhang et al. [3] isolated greater epithelial ridge (GER) cells from neonatal rat cochleae enzymatically and mechanically. GER cells formed proliferative spheres, and they had the ability to generate myosin7a-positive hair cells and p27kip1-positive supporting cells. Therefore, they considered GER cells as targets for regenerative therapy of the inner ear.
Oshima et al. [4] isolated sphere-forming stem cells from early postnatal mouse inner ear and analyzed their gene expression and efficiency for sphere formation. They showed that spheres from the organ of Corti and vestibular sensory epithelial cells expressed multiple hair-cell markers including myosin7a and espin, and expressed functional ion channels, reminiscent of nascent hair cells. They reported that the capacity for sphere formation of cells in the mouse cochleae decreased sharply during the second and third postnatal weeks, which was much faster than that of the vestibular organs.
Savary et al. [6] performed side population (SP) analysis, using Abcg2/Bcrp1 as one of the markers for stem/progenitor cells. The SP phenotype has been used to isolate putative stem cell populations. It is based on the unique ability to efflux Hoechst dye in an ATP-binding cassette (ABC) transporter-dependent manner [13, 14]. Abcg2/Bcrp1 is a member of the ATP-biding cassette family of cell-surface transporter proteins, and it is considered to be one of the stem cell markers [15]. Savary et al. reported that Abcg2 transporter was expressed in supporting cells with other stem/progenitor cell markers, nestin and musashi1, in the postnatal mouse cochleae. They purified SP supporting cells by FACS and found that the SP cells differentiated into the colony expressing myosin7a or p27kip1 in vitro. Therefore, they concluded that supporting cells should be regarded as hair-cell progenitors.
Sinkkonen et al. [7] used antibodies to cell-surface proteins to label dissociated cells of the neonatal organ of Corti. They purified different cell types by FACS analysis and found that CD326+/CD146low/CD271low cells in lesser epithelial ridge (LER) and supporting cells gave rise to more myosin7a-positive cells in vitro than in the other non-sensory epithelial cells. Cells at LER in rat cochlea were also reported to have the ability to form spheres and differentiate into myosin7a-positive cells by Zhai et al. [2].
Taniguchi et al. [11] identified slow-cycling cells, one of the characteristics of stem cells, in the mouse cochleae in vivo. Stem cells normally proliferate at a slow rate in mature organs [16]. The so-called label-retaining cells, or slow-cycling cells, of the skin and prostate have been recognized as stem cells [17, 18]. They used the exogenous proliferation marker 5-bromo-2′-deoxyuridine (BrdU) in combination with the endogenous proliferation marker Ki-67 and identified tympanic border cells, located beneath the basilar membrane, as slow-cycling cells of the mouse cochlea in vivo. Immunohistochemical analysis indicated that these cells stained positive for immature cell marker nestin. The number of slow-cycling cells in the tympanic border cells decreased dramatically in about two weeks after birth as the cochlea matured. This decrease coincides with other reports on inner ear stem cells [4].
Wnt signaling plays a critical role in regulating tissue homeostasis, including the maintenance of somatic stem cells [19]. Several groups have been studying Wnt target genes in the inner ear to detect inner ear stem/progenitor cells.
Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) is one of the Wnt target genes. Chai et al. [8] and Shi et al. [9] reported that they purified Lgr5+ supporting cells from postnatal mouse cochlea by FACS and that they formed spheres and differentiated into myosin7a-positive hair cells in vitro.
Jan et al. [10] reported that they found transient but robust Wnt signaling and proliferation in tympanic border cells during the first 3 postnatal weeks. They used the Axin2lacZ reporter mouse, as Axin2 is a downstream target and feedback inhibitor of Wnt pathway, whose active signaling marked endogenous stem cells in many tissues [20]. In vivo lineage tracing showed that a subset of hair cells and supporting cells was derived postnatally from Axin2-expressing tympanic border cells. In vitro, Axin2lacZ cells formed clonal colonies and differentiated into hair-cell-like (myosin7a positive) and supporting cell-like (e.g., sox2 positive) cells. They concluded that Axin2-positive tympanic border cells had the potential to act as precursors of sensory epithelial cells.
3 Conclusion
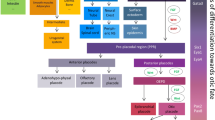
In summary, although the inner ear has limited ability to regenerate, there is evidence indicating the existence of inner ear stem cells. Potential candidates of stem cells are supporting cells [2, 4, 5, 7–9], cells at LER [2, 4, 7], cells at GER [3], and tympanic border cells [10, 11] (Fig. 30.1). The total number of them in the inner ear is estimated to be very small and reported to decrease as the inner ear matures [4].
The microenvironment surrounding the stem cells is called as niche [21]. It has a significant role in the maintenance of stem cells and enables them to keep proliferate and differentiate into mature cells. However, the niche for inner ear stem cells is unknown. It is necessary to elucidate the regulatory mechanisms of inner ear stem cells, clarify what keeps the inner ear stem cells in quiescent condition, and identify the reason for the decrease in the number of inner ear stem cells with maturation. If these questions are addressed to activate inner ear stem cells in vivo, it will be a great step in regeneration therapy.
References
Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9(10):1293–9.
Zhai S, Shi L, Wang BE, Zheng G, Song W, Hu Y, et al. Isolation and culture of hair cell progenitors from postnatal rat cochleae. J Neurobiol. 2005;65(3):282–93. doi:10.1002/neu.20190.
Zhang Y, Zhai SQ, Shou J, Song W, Sun JH, Guo W, et al. Isolation, growth and differentiation of hair cell progenitors from the newborn rat cochlear greater epithelial ridge. J Neurosci Meth. 2007;164(2):271–9. doi:10.1016/j.jneumeth.2007.05.009.
Oshima K, Grimm CM, Corrales CE, Senn P, Martinez Monedero R, Géléoc GS, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi:10.1007/s10162-006-0058-3.
White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–7.
Savary E, Hugnot JP, Chassigneux Y, Travo C, Duperray C, Van De Water T, et al. Distinct population of hair cell progenitors can be isolated from the postnatal mouse cochlea using side population analysis. Stem Cells. 2007;25(2):332–9.
Sinkkonen ST, Chai R, Jan TA, Hartman BH, Laske RD, Gahlen F, et al. Intrinsic regenerative potential of murine cochlear supporting cells. Sci Rep. 2011;1:26. doi:10.1038/srep00026.
Chai R, Kuo B, Wang T, Liaw EJ, Xia A, Jan TA, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A. 2012;109(21):8167–72. doi:10.1073/pnas.1202774109.
Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32(28):9639–48. doi:10.1523/JNEUROSCI.1064-12.2012.
Jan TA, Chai R, Sayyid ZN, van Amerongen R, Xia A, Wang T, et al. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development. 2013;140(6):1196–206. doi:10.1242/dev.087528.
Taniguchi M, Yamamoto N, Nakagawa T, Ogino E, Ito J. Identification of tympanic border cells as slow-cycling cells in the cochlea. PLoS One. 2012;7(10):e48544.
Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–90.
Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806.
Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7(9):1028–34. doi:10.1038/nm0901-1028.
Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells. 2002;20(1):11–20. doi:10.1634/stemcells.20-3-274.
Quesenberry P, Levitt L. Hematopoietic stem cells (second of three parts). N Engl J Med. 1979;301(15):819–23. doi:10.1056/NEJM197910113011505.
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102(4):451–61.
Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. J Cell Biol. 2002;157(7):1257–65.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi:10.1146/annurev.cellbio.20.010403.113126.
Zeng YA, Nusse R. Wnt proteins are self-renewal factors for mammary stem cells and promote their long-term expansion in culture. Cell Stem Cell. 2010;6(6):568–77. doi:10.1016/j.stem.2010.03.020.
Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Taniguchi, M., Yamamoto, N. (2014). Inner Ear Stem Cells. In: Ito, J. (eds) Regenerative Medicine for the Inner Ear. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54862-1_30
Download citation
DOI: https://doi.org/10.1007/978-4-431-54862-1_30
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54861-4
Online ISBN: 978-4-431-54862-1
eBook Packages: MedicineMedicine (R0)