Abstract
An auditory brainstem implant (ABI) is an electrical device that stimulates the cochlear nucleus to provide auditory sensation. The ABI was originally developed for deaf patients with bilateral vestibular schwannomas associated with neurofibromatosis type 2 (NF2), but candidacy for an ABI has recently been extended to non-NF2 populations, including patients with congenital inner ear and/or internal auditory canal (IAC) malformations, severe cochlear ossification after meningitis or fracture of the cochlea, trauma-induced cochlear nerve disruption, and advanced otosclerosis. Different causes of hearing loss are associated with different hearing outcomes for ABIs, but the peripheral stimulation provided by cochlear implants (CIs) seems to result in better outcomes than central stimulation of the auditory neural system with ABIs. However, if cochlear implantation is contraindicated or fails to provide sufficient auditory sensation, auditory brainstem implantation may be the only solution. Since neural input and trophic support from spinal ganglion neurons (SGNs) to neurons in the cochlear nucleus are important for maturation or maintenance of neural circuits in the cochlear nucleus, auditory brainstem implantation in combination with preceding cochlear implantation and/or approaches for SGN regeneration or prevention of SGN degeneration might improve ABI outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Auditory brainstem implant
- Cochlear implant
- Cochlear nerve deficiency
- Cochlear nucleus
- Inner ear malformation
- Internal auditory canal
- Neurofibromatosis type 2
- Spiral ganglion neuron
- Vestibular schwannoma
1 Introduction
An auditory brainstem implant (ABI) is an electrical device that stimulates the cochlear nucleus to provide auditory sensation. The ABI was originally developed for deaf patients with bilateral vestibular schwannomas associated with neurofibromatosis type 2 (NF2). The first ABI with a single electrode was implanted by William House in 1979. In the 1990s, the single-channel implant was replaced by a multichannel implant that improved ABI-aided auditory performance. At first, candidacy for auditory brainstem implantation was limited to individuals diagnosed with NF2, but an increasing number of non-NF2 patients have received an ABI since 2002, when Colletti reported the first pediatric ABI cases with congenital malformations of the inner ear and internal auditory canal [1].
The fundamental mechanisms of an ABI are similar to those of a cochlear implant (CI). The ABI electrically stimulates the surface of the cochlear nucleus by a paddle-shaped electrode array, while the CI stimulates the spiral ganglion neurons (SGNs) through an electrode array inserted into the scala tympani. The cochlear nucleus is composed of the dorsal cochlear nucleus (DCN) and the ventral cochlear nucleus (VCN), with the VCN being further divided into anteroventral (AVCN) and posteroventral (PCVN) regions. The auditory nerve fibers, which are axons of the SGNs, branch to innervate these three subnuclei. Tonotopic organization is observed in each subnucleus [2]. In contrast to the linear tonotopic organization along the basal-apical axis in the cochlea, the tonotopy in the cochlear nucleus has a 3-dimensional organization in which the characteristic frequency changes in a vertical direction to the surface of the brainstem [3, 4]. Theoretically, the electrode array of the CI can reproduce the cochlear linear tonotopic organization, but the most commonly used surface electrode array of the ABI has difficulty reproducing the 3-dimensional tonotopic map of the cochlear nucleus, especially its vertical component [4]. To overcome this problem, an investigational penetrating ABI (PABI) having 2 arrays, a 12-electrode surface array plus a 10-electrode array with vertically penetrating needle microelectrodes, was developed to stimulate the cochlear nucleus in a more 3-dimensional manner [5]. The results of PABI were discouraging because less than 25 % of the penetrating electrodes resulted in auditory sensations, and the penetrating electrode array did not improve speech perception when compared with the use of a surface electrode array alone. The penetrating electrodes did contribute to a lower threshold, increased pitch range, and higher selectivity [5]. These results imply that, in addition to differences in tonotopic organization, we should focus on other functional differences between the cochlea and the cochlear nucleus. Inhibitory interneurons in the cochlear nucleus sharpen the neural representation of auditory stimuli by lateral inhibition [6]. Direct stimulation of neurons in the cochlear nucleus using ABI might disturb this lateral inhibition. Consistent with the theoretical disadvantages of ABI, several studies reported that the hearing outcomes of cochlear implantation are usually better than those of auditory brainstem implantation, as described below [7–9]. Auditory brainstem implantation is, however, the only effective treatment to restore hearing in deaf patients if cochlear implantation is contraindicated or unsuccessful due to lesions in the inner ear and/or internal auditory canal (IAC).
2 Indications for Auditory Brainstem Implant
The ABI was originally designed to restore hearing in deaf patients with NF2. More recently, the ABI has been used in patients without NF2 who are not candidates for a CI or who have failed to receive benefits from a CI. Candidacy for an ABI is categorized as patients with and without NF2, and the latter category is further divided into congenital and acquired groups. Etiologies of deafness differ between the congenital and acquired non-NF2 groups: congenital inner ear and/or IAC malformations in the congenital non-NF2 group and meningitis-related ossification, trauma, and severe otosclerosis in the acquired non-NF2 group [10]. As mentioned in a 2011 consensus statement developed from outcomes in 61 non-NF2 children with ABIs [9], it is possible to use the ABI to stimulate the auditory system in a majority of patients with severe inner ear and/or IAC malformations, but widespread application of the ABI in prelingually deaf children would not be appropriate until longer-term effects and safety of pediatric auditory brainstem implantation have been established.
2.1 Patients with Neurofibromatosis Type 2
Neurofibromatosis type 2 is an autosomal dominant disorder characterized by the development of multiple nervous system tumors, especially schwannomas. Loss-of-function mutation of the NF2 gene, which codes for the tumor suppressor protein called merlin, is reported to cause NF2 [10]. Diagnostic prevalence of NF2 is 1 in 100,000 people and its penetrance is almost 100 % by 60 years of age. Bilateral vestibular schwannomas are observed in 90–95 % of patients with NF2, who usually develop hearing loss that becomes noticeable between 20 and 30 years of age [10]. Unlike a unilateral non-NF2 vestibular schwannoma, vestibular schwannomas associated with NF2 tend to diffusely infiltrate adjacent nerves. The cochlear nerve is often sacrificed or severely damaged during the removal of the vestibular schwannoma, especially when the size of the tumor is large. In these patients, CI-mediated stimulation does not reach the brainstem and an ABI is the only solution to restore hearing. Radiotherapy for the vestibular schwannoma can precede the implantation of an ABI, but some reports have suggested that delayed deterioration of hearing outcomes after auditory brainstem implantation might be associated with radiotherapy-induced damage to the cochlear nucleus [9].
2.2 Congenitally Deaf Children
Congenitally deaf children have severe inner ear and/or IAC malformations that are associated with insufficient space for electrode insertion and hypoplasia of the cochlear branch of the vestibulocochlear nerve (VCN), also referred to as cochlear nerve deficiency (CND). Patients with the following inner ear and IAC malformations are potential candidates for an ABI: (1) Michel aplasia (complete labyrinthine aplasia), (2) cochlear aplasia, (3) common cavity deformity, (4) incomplete partition type 1, (5) narrow IAC (NIAC), and (6) hypoplasia of bony cochlear nerve canal (HBCNC), also called cochlear aperture aplasia or hypoplasia [9]. Among these malformations, only bilateral Michel aplasia is a definite indication for auditory brainstem implantation because there is no labyrinth available for cochlear implantation [8]. Patients with cochlear aplasia, NIAC, HBCNC, and isolated CND are usually good candidates for an ABI, but it should be noted that radiographic evaluations cannot exclude candidacy for cochlear implantation in these groups. Jeong and Kim reported two cases of patients with cochlear aplasia who showed favorable speech perception abilities with a CI. In these cases, computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated the absence of a cochlea; nevertheless, clear auditory brainstem responses (ABRs) were electrically evoked using electrodes inserted in the malformed labyrinth [12]. Common cavity and incomplete partition type I malformations are sometimes associated with CND [13], but CI-mediated intraoperative electrically evoked auditory brainstem response (EABR) testing elicited an obvious evoked wave V in patients with these malformations [14, 15]. These results suggest that auditory nerve fibers are distributed in the malformed inner ear and transmit CI-mediated auditory signals to the central auditory system. In patients with NIAC, Song et al. demonstrated that the presence or absence of the VCN in preoperative imaging did not always correlate with the VCN status identified during auditory brainstem implantation, suggesting that the sensitivity of MRI is not high enough for a precise diagnosis of a hypoplastic VCN or cochlear nerve. Indications for auditory brainstem implantation in the population with inner ear or IAC malformations are under discussion, and more detailed information will be provided later in this chapter.
2.3 Acquired Deafness in Patients Without Neurofibromatosis Type 2
This category includes (1) severe cochlear ossification after meningitis or fracture of the cochlea, (2) trauma-induced cochlear nerve disruption, and (3) advanced otosclerosis [9, 10]. Among these, bilateral cochlear nerve disruption after trauma is a definite indication for auditory brainstem implantation because CI-mediated auditory signals cannot be transmitted to the auditory brainstem in individuals with this type of damage. Severe cochlear ossification and advanced otosclerosis may be indications for auditory brainstem implantation if a CI with the appropriate electrode arrays (e.g., the Contour and Double array electrode manufactured by Cochlear or the Compressed or Split electrode produced by MED-EL) failed to provide satisfactory outcomes or could not be inserted.
3 Surgical Procedures for Auditory Brainstem Implantation
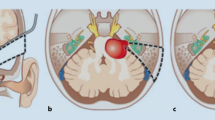
In NF2 patients, the disc electrode is placed on the surface of the cochlear nucleus complex on the dorsolateral surface of the brainstem, immediately rostral to the pontomedullary junction, through a standard translabyrinthine approach following removal of the vestibular schwannoma. The cochlear nucleus is composed of the DCN and the VCN. As shown in Fig. 18.1, both the DCN and VCN are located adjacent to the lateral recess of the fourth ventricle near the foramen of Luschka. The cochlear nucleus has few visible surface landmarks; therefore, the surrounding structures, such as the root entry point of cranial nerve VIII, cranial nerve VII, the choroid plexus, cranial nerve IX, and the tela choroidea, are important guides for placing the electrode array accurately on the surface of the cochlear nucleus. Many surgeons prefer to place the electrode array of the ABI within the lateral recess to stimulate the DCN and the intraventricular part of the VCN (Fig. 18.1). In addition to the anatomical landmarks surrounding the cochlear nucleus, intraoperative monitoring of EABR using ABI-mediated stimuli is used to correctly place the electrode array on the cochlear nucleus. In non-NF2 patients, neurosurgeons prefer the retrosigmoid approach to the translabyrinthine approach because it is more familiar to them and the labyrinth and auditory nerve can be preserved.
(a) Cross section of pontomedullary junction at level of lateral recess of the fourth ventricle (4th Vent.). DCN (Dorsal Coch. Nucl.) and VCN (Ventral Coch. Nucl.) are located adjacent to the lateral recess (Lat. Recess) of the fourth ventricle. DCN underlies a prominence, while VCN is more deeply imbedded in brainstem and does not produce as great a prominence on surface of brainstem. (b) Retrosigmoid view. Flocculus has been elevated to expose junction of vestibulocochlear nerve with side of brainstem at pontomedullary junction. Foramen of Luschka (For. Luschka), which is positioned dorsal to glossopharyngeal nerve (CNIX), is partially covered by choroid plexus. (c) Choroid plexus has been retracted rostrally to expose dorsal cochlear nucleus sitting in floor of lateral recess. Ventral cochlear nucleus is positioned in area between lateral edge of dorsal cochlear nucleus and junction of vestibulocochlear nerve (CN VIII) with brainstem [16]
4 Complications Specific to Auditory Brainstem Implantation
Compared to standard cochlear implantation, stimulation of nonauditory neurons, electrode migration, and cerebrospinal fluid (CSF) leakage are listed as ABI-specific complications. Up to 42 % of multichannel ABI users suffered from nonauditory sensations, including nausea, tingling in the throat, jittering of the visual field, and shoulder contraction, that were probably caused by activation of the vagal nerve, glossopharyngeal nerve, flocculus of the cerebellum, and accessory nerve, respectively [17, 18]. Incorrect positioning of the ABI electrode array is usually associated with nonauditory sensations. Accurate placement of the electrode array on the surface of the cochlear nucleus is essential in auditory brainstem implantation; however, a large tumor often compresses the pontomedullary junction and creates difficulty in identification of the landmarks surrounding the cochlear nucleus. Changing the program of the speech processor, including deactivating the responsible electrodes and decreasing the amplitude of electrical stimuli, is usually effective to reduce these aversive ABI-mediated symptoms [17, 19]. Unlike the CI with an electrode array that is inserted into the cochlea, the electrode array of the ABI is placed on the surface of the brainstem with an unstable fixation. The fixation of the electrode array has been improved by the development of silicone backing, Dacron mesh, and nonelastic wire, but migration and dislocation of the electrode array still occur, especially in NF2 patients with large tumors compressing the brainstem. In these patients, the shape and position of the brainstem may change after tumor resection, which probably increases the risk for postoperative migration of the electrode array. Migration of the electrode array is usually associated with nonauditory sensations and deterioration of auditory perception; accordingly, these manifestations suggest the necessity of evaluating the position of the electrode array using high-resolution CT. The electrode lead of the ABI penetrates the meningeal dura and CSF may leak from the subarachnoid space to the mastoid air cells or the subcutaneous space along the electrode lead, regardless of the surgical procedure and choice of translabyrinthine or retrosigmoid approach [20, 21]. The percentage of CSF leakage in the ABI population is reported to range from 3.3 to 11 % [17, 19]. CSF leakage is usually controlled conservatively with or without lumbar drainage, but revision surgery is sometimes necessary.
5 Hearing Outcomes for Auditory Brainstem Implantation
During the last two decades, several groups have published the results of auditory brainstem implantation in NF2 and non-NF2 patients [17, 18, 21–24]. In general, hearing outcomes were lower in ABI recipients compared with outcomes for patients with CIs, but ABI-aided audiological performance varied widely according to the etiologies of deafness. As Colletti et al. established following long-term observation of 80 ABI adults with or without NF2 (Fig. 18.2) [23], trauma-induced cochlear nerve disruption and cochlear ossification were associated with favorable ABI outcomes and open-set speech discrimination scores greater than 50 %, while subjects with NF2 and auditory neuropathy spectrum disorder (ANSD) showed only limited improvement of auditory performance, even though the majority of these patients had ABI-aided benefits in daily life, particularly in combination with lip reading. Patients with congenital inner ear malformations showed moderate ABI-aided speech discrimination scores that were better than those in the NF2 patients, but worse than those in patients with trauma-induced cochlear nerve disruption and cochlear ossification. Considering that both populations (NF2 patients and patients with cochlear nerve disruption and cochlear ossification) suffered from postlingual deafness, the limited benefit of ABI in patients with NF2, which was also reported by other studies [17, 22, 25], should be related to NF2-specific problems such as direct damage to the cochlear nucleus caused by chronic tumor compression on the brainstem, as well as surgical procedures for tumor removal. ANSD is diagnosed by (1) absent or abnormal ABR and (2) present otoacoustic emissions and/or cochlear microphonics. Several reported etiologies causing sensorineural hearing loss meet these criteria, suggesting heterogeneity among patients with ANSD. Several studies have demonstrated that ANSD is highly associated with CND in the congenitally deaf population [26, 27]. In patients with an otoferlin-encoding (OTOF) gene mutation, synaptic release from inner hair cells is primarily affected, whereas demyelination and axonal loss may be responsible for hearing loss in systemic neurodegenerative diseases such as Charcot-Marie-Tooth disease. In Colletti’s study, there was no description of the etiologies about ANSD; therefore, the exact reason for poor ABI-aided hearing outcomes in patients with ANSD was unclear. These results demonstrated that hearing loss of different etiologies was associated with varied hearing outcomes following auditory brainstem implantation.
Improvement of open-set speech discrimination scores (% correct) in patients with different causes of hearing loss after brainstem implantation [23]. NF2, neurofibromatosis type 2; ANSD, auditory neuropathy spectrum disorder; trauma, head trauma
6 Auditory Brainstem Implantation Compared with Cochlear Implantation
Previous studies showed that peripheral stimulation with CIs usually results in better outcomes than central stimulation of the auditory neural system using an ABI, suggesting that cochlear implantation should be tried before ABI whenever possible [10]. Utilization of the simple linear tonotopic organization in the cochlea and the natural sound processing mechanisms in the auditory nerves and neurons in the cochlear nucleus might contribute to favorable outcomes for cochlear implantation. The relatively high probability of ABI-related complications, including nonauditory stimulation, electrode migration, and CSF leakage, requires surgeons to carefully consider the indications for auditory brainstem implantation. As discussed previously, patients with Michel deformity or sacrifice of the cochlear nerve during tumor resection are definitely ABI candidates [8–10]. Trauma-induced bilateral cochlear nerve disruption is also an indication for auditory brainstem implantation, although this situation is unlikely to occur without the subject incurring fatal damage to the brain [8]. In patients with cochlear ossification and advanced otosclerosis, cochlear implantation may be followed by placement of an ABI [8]. For patients with cochlear aplasia or absence of the cochlear nerve or VCN, a more comprehensive analysis would be required to conclude the advantage of ABI. Due to the limitations of spatial resolution, high-resolution CT and MRI cannot exclude the possibility that a small number of cochlear nerve fibers innervate the inner ear, indicating the necessity for other functional evaluations. Since there are only a few reported cases of patients undergoing cochlear implantation followed by auditory brainstem implantation on the same side [28], the evidence for the advantages of an ABI compared with a CI for speech perception has not been fully established. In clinical application, a CI might be recommended for the initial operation in these patients. If the CI fails to provide sufficient auditory sensation, then an ABI may be the only solution. It should be noted that the efficacy of auditory brainstem implantation may decrease after the critical period for development of auditory perception if effective auditory stimulation is not available during the first several years after birth. Therefore, prelingually deaf children should undergo implantation of an ABI as early as possible if an ABI is indicated [9]. To resolve the dilemma of the need for careful decision-making regarding the indications for ABI and the importance of brainstem implantation at an early age, electrophysiological evaluation of the auditory system might be effective. As described in Chap. 15, intraoperative CI-mediated EABR testing may be useful to predict outcomes of CI [29]; thus, electrophysiological examination may be an effective method to determine the necessity for an ABI during cochlear implantation.
7 Future Prospects
As described above, different causes of hearing loss were associated with different hearing outcomes following implantation of an ABI [23]. Since the ABI directly stimulates the cochlear nucleus, ABI outcomes should be influenced by the severity of pathological changes in the cochlear nucleus. Compared with SGNs, neurons in the cochlear nucleus are less susceptible to loss of hair cells, but transneuronal loss of neurons in the cochlear nucleus occurs when the cochlea is damaged during an early period of development [30]. Interestingly, prior to exposure to normal environmental stimuli during the developmental period, spontaneous neural firing is observed in the central auditory system. Similar to other sensory systems, these spontaneous neural activities are thought to facilitate maturation of auditory neurons and their synapses [31]. Therefore, the congenital hypoplasia of SGNs that is associated with CND can attenuate synaptic input from the cochlear nerve to the neurons in the cochlear nucleus, which may lead to disorganization or immature development of neuronal connectivity in the cochlear nucleus. In this respect, CI-mediated intracochlear stimulation preceding auditory brainstem implantation may be effective to maximize the use of the remaining SGNs and promote development of the central auditory system, even if a CI would provide only limited auditory sensation. As discussed in Chaps. 28 and 30, the combination of a CI with the SGN regeneration or extension of SGN afferent dendrites by pharmacological or genetic methods can increase the responsiveness of SGNs to CI-mediated electrical stimuli, which in turn increases synaptic input from the cochlear nerve to the cochlear nucleus.
Even after ABI surgery, it is possible that the remaining SGNs contribute to maintaining the functions of the cochlear nucleus. It is well known that a synaptic vesicle contains trophic factors in addition to neurotransmitters [32, 33]. Spontaneous synaptic release from the axon terminals of the remaining cochlear nerve fibers does not contain auditory information, but co-transmitted trophic factors can promote survival of neurons or maintain the dendritic protrusion in the cochlear nucleus. CI-mediated electrical stimuli showed some trophic effects to SGNs [34], but a part of these effects may be induced indirectly by stimulation of glia and residual hair cells around the afferent dendrites of SGNs, rather than by direct activation of the SGNs. In the same way, ABI-mediated electrical stimuli can elicit synaptic release from the axon terminal of the residual SGNs, which may have positive effects to maintain the functions of the cochlear nucleus. Taking these results into consideration, both SGN regeneration and prevention of SGN degeneration may show some benefit to improve ABI outcomes, even though ABIs bypass the cochlear nerve to directly stimulate the cochlear nucleus. In patients with NF2, the cochlear nerve is often severely damaged. The cochlear nerve and the modiolus of the cochlear are accessible during removal of the tumor; therefore, pharmacological, cell biological, and gene therapy approaches to the remaining SGNs or cochlear nerve may be applicable during surgery.
References
Colletti V, Carner M, Fiorino F, Sacchetto L, Miorelli V, Orsi A, et al. Hearing restoration with auditory brainstem implant in three children with cochlear nerve aplasia. Otol Neurotol. 2002;23:682–93.
Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci. 2009;12(6):711–7. doi:10.1038/nn.2332.
Luo F, Wang Q, Farid N, Liu X, Yan J. Three-dimensional tonotopic organization of the C57 mouse cochlear nucleus. Hear Res. 2009;257(1–2):75–82. doi:10.1016/j.heares.2009.08.002.
Rauschecker JP, Shannon RV. Sending sound to the brain. Science. 2002;295(5557):1025–9. doi:10.1126/science.1067796.
Otto SR, Shannon RV, Wilkinson EP, Hitselberger WE, McCreery DB, Moore JK, et al. Audiologic outcomes with the penetrating electrode auditory brainstem implant. Otol Neurotol. 2008;29(8):1147–54. doi:10.1097/MAO.0b013e31818becb4.
Roberts MT, Trussell LO. Molecular layer inhibitory interneurons provide feedforward and lateral inhibition in the dorsal cochlear nucleus. J Neurophysiol. 2010;104(5):2462–73. doi:10.1152/jn.00312.2010.
Vincenti V, Pasanisi E, Guida M, Di Trapani G, Sanna M. Hearing rehabilitation in neurofibromatosis type 2 patients: cochlear versus auditory brainstem implantation. Audiol Neurootol. 2008;13(4):273–80. doi:10.1159/000115437.
Merkus P, Lella FD, Trapani GD, Pasanisi E, Beltrame MA, Zanetti D, et al. Indications and contraindications of auditory brainstem implants: systematic review and illustrative cases. Eur Arch Otorhinolaryngol. 2013. doi:10.1007/s00405-013-2378-3.
Sennaroglu L, Colletti V, Manrique M, Laszig R, Offeciers E, Saeed S, et al. Auditory brainstem implantation in children and non-neurofibromatosis type 2 patients: a consensus statement. Otol Neurotol. 2011;32(2):187–91. doi:10.1097/MAO.0b013e318206fc1e.
Sennaroglu L, Ziyal I. Auditory brainstem implantation. Auris Nasus Larynx. 2012;39(5):439–50. doi:10.1016/j.anl.2011.10.013.
Asthagiri AR, Parry DM, Butman JA, Kim HJ, Tsilou ET, Zhuang Z, et al. Neurofibromatosis type 2. Lancet. 2009;373(9679):1974–86. doi:10.1016/S0140-6736(09)60259-2.
Jeong SW, Kim LS. Cochlear implantation in children with cochlear aplasia. Acta Otolaryngol. 2012;132(9):910–5. doi:10.3109/00016489.2012.675627.
Giesemann AM, Kontorinis G, Jan Z, Lenarz T, Lanfermann H, Goetz F. The vestibulocochlear nerve: aplasia and hypoplasia in combination with inner ear malformations. Eur Radiol. 2011. doi:10.1007/s00330-011-2287-z.
Cinar BC, Atas A, Sennaroglu G, Sennaroglu L. Evaluation of objective test techniques in cochlear implant users with inner ear malformations. Otol Neurotol. 2011;32(7):1065–74. doi:10.1097/MAO.0b013e318229d4af.
Yamazaki H, Naito Y, Fujiwara K, Moroto S, Yamamoto R, Yamazaki T, et al. EABR-based evaluation of the spatial distribution of auditory neuronal tissue in common cavity deformities. Otol Neurotol. 2014;in press.
Abe H, Rhoton AL, Jr. Microsurgical anatomy of the cochlear nuclei. Neurosurgery. 2006;58(4):728–39; discussion −39. doi:10.1227/01.NEU.0000204870.83778.A1.
Otto SR, Brackmann DE, Hitselberger WE, Shannon RV, Kuchta J. Multichannel auditory brainstem implant: update on performance in 61 patients. J Neurosurg. 2002;96(6):1063–71. doi:10.3171/jns.2002.96.6.1063.
Vincent C, Zini C, Gandolfi A, Triglia JM, Pellet W, Truy E, et al. Results of the MXM Digisonic auditory brainstem implant clinical trials in Europe. Otol Neurotol. 2002;23(1):56–60.
Kanowitz SJ, Shapiro WH, Golfinos JG, Cohen NL, Roland Jr JT. Auditory brainstem implantation in patients with neurofibromatosis type 2. Laryngoscope. 2004;114(12):2135–46. doi:10.1097/01.mlg.0000149447.52888.f6.
Colletti V, Shannon RV, Carner M, Veronese S, Colletti L. Complications in auditory brainstem implant surgery in adults and children. Otol Neurotol. 2010;31(4):558–64. doi:10.1097/MAO.0b013e3181db7055.
Choi JY, Song MH, Jeon JH, Lee WS, Chang JW. Early surgical results of auditory brainstem implantation in nontumor patients. Laryngoscope. 2011;121(12):2610–8. doi:10.1002/lary.22137.
Sanna M, Di Lella F, Guida M, Merkus P. Auditory brainstem implants in NF2 patients: results and review of the literature. Otol Neurotol. 2012;33(2):154–64. doi:10.1097/MAO.0b013e318241bc71.
Colletti V, Shannon R, Carner M, Veronese S, Colletti L. Outcomes in nontumor adults fitted with the auditory brainstem implant: 10 years' experience. Otol Neurotol. 2009;30(5):614–8. doi:10.1097/MAO.0b013e3181a864f2.
Sennaroglu L, Ziyal I, Atas A, Sennaroglu G, Yucel E, Sevinc S, et al. Preliminary results of auditory brainstem implantation in prelingually deaf children with inner ear malformations including severe stenosis of the cochlear aperture and aplasia of the cochlear nerve. Otol Neurotol. 2009;30(6):708–15. doi:10.1097/MAO.0b013e3181b07d41.
Schwartz MS, Otto SR, Brackmann DE, Hitselberger WE, Shannon RV. Use of a multichannel auditory brainstem implant for neurofibromatosis type 2. Stereotactic Funct Neurosurg. 2003;81(1–4):110–4.
Huang BY, Roche JP, Buchman CA, Castillo M. Brain stem and inner ear abnormalities in children with auditory neuropathy spectrum disorder and cochlear nerve deficiency. AJNR Am J Neuroradiol. 2010;31(10):1972–9. doi:10.3174/ajnr.A2178.
Levi J, Ames J, Bacik K, Drake C, Morlet T, O'Reilly RC. Clinical characteristics of children with cochlear nerve dysplasias. Laryngoscope. 2013;123(3):752–6. doi:10.1002/lary.23636.
Colletti L, Wilkinson EP, Colletti V. Auditory brainstem implantation after unsuccessful cochlear implantation of children with clinical diagnosis of cochlear nerve deficiency. Ann Otol Rhinol Laryngol. 2013;122(10):605–12.
Walton J, Gibson WP, Sanli H, Prelog K. Predicting cochlear implant outcomes in children with auditory neuropathy. Otol Neurotol. 2008;29(3):302–9. doi:10.1097/MAO.0b013e318164d0f6.
Shepherd RK, Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol. 2001;6(6):305–18.
Marrs GS, Spirou GA. Embryonic assembly of auditory circuits: spiral ganglion and brainstem. J Physiol. 2012;590(Pt 10):2391–408. doi:10.1113/jphysiol.2011.226886.
Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4(1):47–52. doi:10.1016/j.coph.2003.08.001.
Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Progr Neurobiol. 2002;66(3):161–90.
Mitchell A, Miller JM, Finger PA, Heller JW, Raphael Y, Altschuler RA. Effects of chronic high-rate electrical stimulation on the cochlea and eighth nerve in the deafened guinea pig. Hear Res. 1997;105(1–2):30–43.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Yamazaki, H. (2014). Auditory Brainstem Implant. In: Ito, J. (eds) Regenerative Medicine for the Inner Ear. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54862-1_18
Download citation
DOI: https://doi.org/10.1007/978-4-431-54862-1_18
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54861-4
Online ISBN: 978-4-431-54862-1
eBook Packages: MedicineMedicine (R0)