Abstract
Carbohydrates account for about half of the molecular mass of the envelope glycoprotein gp120. This heavy glycosylation constitutes a strong defense mechanism for viral evasion. Nevertheless, the discovery of more than a dozen of glycan-dependent broadly HIV-neutralizing antibodies from HIV-infected individuals suggests that the defensive viral “glycan shield” can also serve as important targets of vaccines. A major issue in further characterization of the detailed structures of the neutralizing epitopes and the reconstitution of the epitopes for vaccine development is the heterogeneity of HIV glycosylation, which is difficult to control in recombinant glycoprotein production. Synthesis of defined carbohydrate antigens provides an important approach to epitope characterization and design of epitope-focused HIV vaccine. This chapter provides a brief description on the challenges in HIV vaccine development, the features of HIV glycosylation, and why synthesis of novel carbohydrate antigens represents a promising approach to HIV vaccine design.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- HIV vaccine
- Neutralizing antibody
- Carbohydrate antigen
- Epitope
- Glycosylation
- Synthetic antigen
- Glycopeptide
- Glycoprotein
Introduction
Vaccination is perhaps the most efficient means in controlling the global epidemic of life-threatening infectious diseases. This was exemplified by the eventual eradication of polio and smallpox, which once took millions of lives on the earth, and by the combat of common flu via vaccination. Thus, it was once expected that an HIV vaccine would follow closely behind upon the identification of the human immunodeficiency virus (HIV) as the cause of the acquired immunodeficiency syndrome, AIDS. However, despite tremendous efforts in the past three decades, an effective HIV vaccine capable of eliciting broadly neutralizing antibodies remains elusive. HIV is clearly an elegant virus that has evolved a number of defense mechanisms to evade host immune surveillance, including frequent mutation of the neutralizing epitopes, formation of oligomeric envelope spikes to occlude conserved epitopes, change of conformations from static to infectious states, and heavy glycosylation of the envelope. Each defense mechanism adds a new level of complexity and challenge for vaccine design (Burton et al. 2012). HIV has two envelope glycoproteins , gp120 and gp41, which form a trimeric complex of homodimer on the viral surface (Julien et al. 2013). The outer envelope glycoprotein gp120 is heavily glycosylated. Since the viral glycans are biosynthetically produced by the host glycosylation machinery, they resemble the structures of host cell-surface glycans and are generally viewed as “self.” Thus the heavy glycosylation protects the viral protein surface from immune recognition by forming a dense “glycan shield.” Nevertheless, the defense is not seamless. The discovery of 2G12, a broadly neutralizing antibody that exhibits its HIV-neutralizing activity by recognizing a novel glycan cluster on gp120, implicates that the host immune system can find a way to break the defense. More recently, a new class of glycan-dependent broadly neutralizing antibodies from HIV-infected individuals, including PG9, PG16, PGT121, and PGT128, has been identified, which target conserved glycopeptide epitopes located in the variable (V1V2 and V3) regions of gp120 (Walker et al. 2009, 2011). These novel glycan-active antibodies can neutralize various HIV clades with remarkable breadth and potency. These discoveries suggest that the defensive “glycan shield” could also be the Achilles’ heel of the virus and point to an exciting new direction in vaccine design. For this purpose, an essential first step is to characterize the glycan-associated neutralizing epitopes, which will serve as the template for epitope-focused immunogen design. In this pursuit, synthetic carbohydrate antigens have become an increasingly important tool that enables the characterization and reconstitution of the neutralizing epitopes to design an epitope-focused HIV vaccine.
Viral Carbohydrate Antigens as Unique Targets for Vaccine
A typical gp120 molecule usually carries more than 20 N-glycans, which account for about half of the molecular mass. This dense “glycan shield” constitutes a major defense mechanism for immune evasion, reducing the immunogenicity of the envelope and limiting antibody’s access to the viral protein antigens. The carbohydrates, particularly the high-mannose-type and the fucosylated complex-type N-glycans, also play an important role in promoting HIV-1 infection and transmission through their interactions with respective lectins such as DC-SIGN on dendritic cells or mannose-binding proteins on macrophages.
A notable feature of HIV glycosylation is the unusually high numbers of high-mannose-type N-glycans on the envelope glycoprotein gp120. Another important feature is the tendency for these N-glycans to form novel glycan clusters: the high-mannose glycans are usually clustering together to form novel oligomannose patches, while some complex-type N-glycans turn to form a complex-type glycan cluster on the envelope. Individual viral N-glycans are similar to host glycans, but the dense high-mannose clusters are rare on normal host glycoproteins and thus can be viewed as “nonself” for immune discrimination. In contrast to peptide-based neutralizing epitopes that are subject to frequent mutation and are usually less accessible on viral surface, the carbohydrate antigens are exposed to the immune system and their global presentation is well conserved. Moreover, conserved viral N-glycan and peptide domain can integrate to form unique glycopeptide epitopes. The identification of broadly neutralizing antibody 2G12, which binds a high-mannose glycan cluster on gp120, provides the first example of an antibody that exhibits its viral neutralizing activity by targeting the viral carbohydrate antigens. More recently, a new class of glycopeptide-dependent HIV-neutralizing antibodies was discovered, which show broad and potent neutralizing activities against all major primary HIV-1 strains (Walker et al. 2009, 2011). These include antibodies PG9 and PG16 that recognize glycopeptide epitopes in the V1V2 region and antibodies PGT121 and PGT128 that target novel glycopeptide epitopes in the V3 region. These new findings have reinforced the notion that HIV carbohydrate antigens represent novel targets for vaccine and have stimulated great interests in further characterization and reconstitution of the fine neutralizing epitopes for immunogen design.
Synthesis as a Novel Approach to Epitope Characterization and HIV Vaccine Design
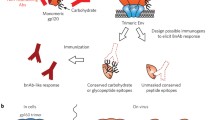
The discovery of more than a dozen of the glycan-dependent broadly neutralizing antibodies provides an exciting new template (novel epitope structures) for designing an effective HIV vaccine. However, deciphering the nature of those neutralizing epitopes, particularly the glycan specificity of the neutralizing antibodies, has been a challenging task. Mutational, biochemical, and crystal structural approaches have played essential roles in epitope mapping, which provides useful information on the residues and glycans that might be involved in antibody recognition, but further characterization of the glycan specificity has been complicated by the heterogeneity in glycosylation of gp120, due to the difficulties in controlling the structures of individual N-glycans at distinct sites. In the case of almost all the X-ray crystallographic studies related to gp120 structure and its complexes with antibodies, one has to truncate the heterogeneous N-glycans to a more unified state in order to obtain quality crystals, which will unfortunately lose the detailed structural information of the glycans involved in antibody recognition. To address this problem, an alternative approach is to design and synthesize structurally well-defined oligosaccharides and glycopeptides as mimics of the putative neutralizing epitopes, which are then optimized through antibody binding analysis, immunization, and redesign (Fig. 1). In particular, the synthetic approach could lead to reconstitution of the neutralizing epitopes, which can be directly incorporated in the design and synthesis of epitope-focused vaccine.
Synthesis of defined HIV carbohydrate antigens as an approach to epitope characterization and vaccine design. The structure of the envelope trimer was modeled and modified on the basis of the recently solved crystal structure (Julien et al. 2013)
Tremendous work has been carried in the design and synthesis of oligosaccharide clusters to approach the epitope of neutralizing antibody 2G12 (Wang 2013). These include the use of small molecule scaffolds such as monosaccharide, cholic acid, and cyclic peptide as templates to defined oligosaccharide clusters consisting of two to four high-mannose-type glycans (Fig. 2a–c), the use of a dendron core to display oligosaccharides as dendrimers (Fig. 2d), and the application of viruslike particles as a platform to have a highly ordered multivalent display (Fig. 2e). The synthetic studies, together with subsequent antibody binding and immunization analysis, have further revealed the requirement of a terminal Manα1,2Man subunit for 2G12 binding, the necessity of a well-configured oligomannose cluster for high-affinity antibody interaction, and the relationship between the immunogenicity and the nature of the N-glycans. Concurrent with the development of more sophisticated chemical and chemoenzymatic methods, chemists now can take the challenges of synthesizing complex glycopeptides and glycoproteins to decipher the nature of the more complex glycopeptide epitopes. This was exemplified by the synthesis of various homogeneous V1V2 glycopeptide s related to the neutralizing epitope of antibodies PG9 and PG16 (Fig. 2f; Alam et al. 2013; Amin et al. 2013). The synthesis of a library of homogeneous V1V2 glycopeptides carrying structurally well-defined N-glycans at the conserved glycosylation sites, together with binding studies, has revealed a clear structure-affinity relationship in terms of the nature and site of glycosylation in the glycopeptide epitope, which was not identified in previous structural and biochemical studies. Moreover, the synthetic study has also revealed a critical role of cyclization and/or dimerization of the V1V2 glycopeptides in significantly enhancing the affinity for the antibodies.
Structures of synthetic oligosaccharide clusters and glycopeptides as epitope mimics of antibodies 2G12, PG9, and PG16. (a) A galactopyranoside-based oligomannose cluster as 2G12 epitope mimic; (b) a cholic acid-based oligomannose cluster as 2G12 epitope mimic; (c) a cyclic peptide-based oligomannose cluster as 2G12 epitope mimic; (d) an oligomannose dendrimer as 2G12 epitope mimic; (e) a viruslike particle-based oligomannose cluster as 2G12 epitope mimic; (f) a synthetic V1V2 glycopeptide as the minimal epitope of antibody PG9
Conclusion
The discovery of a number of glycan-dependent HIV-neutralizing antibodies suggests that the defensive viral “glycan shield” can also serve as promising targets for HIV vaccines. In combination with mutagenesis and biochemical studies, synthesis has played an important role in further characterization and reconstitution of the neutralizing epitopes of the prototype antibody 2G12 and the glycopeptide-specific antibodies PG9 and PG16. It is expected that synthetic carbohydrate antigens will continue to play an increasingly important role in deciphering the glycopeptide-based epitopes of other glycan-dependent neutralizing antibodies such as the PGT121, PGT135, and PGT128 that appear to target more complex glycopeptide epitopes located in the V2 and V3 regions of gp120. Taken together, these combined synthetic and immunological studies will facilitate the design of a truly effective, epitope-focused HIV vaccine.
References
Alam SM, Dennison SM, Aussedat B, Vohra Y, Park PK, Fernandez-Tejada A, Stewart S, Jaeger FH, Anasti K, Blinn JH, Kepler TB, Bonsignori M, Liao HX, Sodroski JG, Danishefsky SJ, Haynes BF (2013) Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc Natl Acad Sci U S A 110:18214–18219
Amin MN, McLellan JS, Huang W, Orwenyo J, Burton DR, Koff WC, Kwong PD, Wang LX (2013) Synthetic glycopeptides reveal the glycan specificity of HIV-neutralizing antibodies. Nat Chem Biol 9:521–526
Burton DR, Ahmed R, Barouch DH, Butera ST, Crotty S, Godzik A, Kaufmann DE, McElrath MJ, Nussenzweig MC, Pulendran B, Scanlan CN, Schief WR, Silvestri G, Streeck H, Walker BD, Walker LM, Ward AB, Wilson IA, Wyatt R (2012) A blueprint for HIV vaccine discovery. Cell Host Microbe 12:396–407
Julien JP, Cupo A, Sok D, Stanfield RL, Lyumkis D, Deller MC, Klasse PJ, Burton DR, Sanders RW, Moore JP, Ward AB, Wilson IA (2013) Crystal structure of a soluble cleaved HIV-1 envelope trimer. Science 342:1477–1483
Walker LM et al (2009) Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289
Walker LM et al (2011) Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470
Wang LX (2013) Synthetic carbohydrate antigens for HIV vaccine design. Curr Opin Chem Biol 17:997–1005
Acknowledgments
We thank Joseph Lomino for the remodeling of the gp120 crystal structure. This work was supported by the National Institutes of Health (NIH grant AI101035).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Wang, LX. (2015). Synthetic Carbohydrate Antigens for HIV Vaccine Design. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_36
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_36
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences