Abstract
Since the establishment of monoclonal antibody production using mouse hybridoma technology in the 1980s, there has been expanding progress and continuous technological improvement in the development of therapeutic antibodies. The chimeric, humanized, and fully human technologies broke through the immunogenicity issues of the first-generation mouse monoclonal antibodies and have led to the great success of therapeutic antibodies, such as rituximab, trastuzumab, cetuximab, and bevacizumab. As of 2013, more than 30 therapeutic antibodies had been approved for clinical use, and these antibodies represent a major new class of drugs. However, there still remain unmet needs for the improvement of the efficacy of these therapeutic antibodies. Based on the current understanding of the clinical mechanisms of several therapeutic antibodies, it has been demonstrated that the antibody constant region (Fc)-mediated effector function, especially antibody-dependent cellular cytotoxicity (ADCC), is important for improving the clinical outcome of therapeutic antibodies and that the Fc-linked oligosaccharide structure of the antibody dramatically influences ADCC. The present review focuses on the recent progress in the development of “glyco-engineered therapeutic antibodies,” which have an improved Fc-mediated effector function of ADCC. This is achieved by reducing the fucosylation level of the Fc-linked oligosaccharides. In 2012, the first non-fucosylated therapeutic antibody, mogamulizumab, was approved for the treatment of adult T-cell leukemia/lymphoma, and a new type anti-CD20, obinutuzumab, with a low level of the Fc fucosylation, was later approved in 2013. The glyco-engineered therapeutic antibodies have just started to be used in the clinical setting, and their use will continue to expand in various clinical areas.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
The clinical successes of recombinant humanized therapeutic antibodies have been shown by the improvement of overall survival and the time to disease progression in the treatment of human malignancies, such as breast, colon, and hematological cancer. Currently, more than 40 monoclonal therapeutic antibodies have been approved for clinical use. The initial technological breakthrough that triggered the expanding development of therapeutic antibodies was the technologies involved in reducing the immunogenicity derived from mouse antibody sequences. Chimeric antibodies, humanized antibodies, and fully human antibodies derived from phage display system or genetically engineered mice have been successfully generated to overcome the immunogenicity issues. The advantages of therapeutic antibodies over conventional small molecules are (i) a lower risk of off-targeting adverse effects due to the strict specificity for the target antigens, (ii) a long half-life in vivo (typically 2–3 weeks in human blood) through the neonatal Fc receptor (FcRn), and (iii) a capability of utilizing the host immune responses related to antibody effector function, such as antibody-dependent cellular cytotoxicity (ADCC ) and complement-dependent cytotoxicity (CDC). The in vivo physiological activity of therapeutic antibodies is mediated by two independent mechanisms, namely, the efficacy of the therapeutic antibodies resulting from their specificity for the target antigen (i.e., neutralization and apoptosis) and from biological activities referred to as the Fc-mediated antibody effector functions (ADCC and CDC).
In turn, the major downsides of these therapeutic antibodies are (i) their expensive production with sophisticated and fixed quality, (ii) poor penetration into tissues due to the high molecular weights of the antibodies (ca. 150 kDa), and (iii) the inability to target intracellular target components. Despite the approval of many agents for use in the clinical setting, antibody therapy still faces critical issues related to insufficient efficacy and the high cost of the production. The importance of ADCC for the clinical efficacy of therapeutic antibodies has become clear from the genetic analyses of leukocyte receptor (FcγR) polymorphisms in patients, and ADCC-enhancing technology focused on the antibody constant region (Fc) has been a focus of attention for developing next-generation therapeutic antibodies with improved efficacy. Fc engineering can be classified into two technological approaches: (i) introducing amino acid mutations and (ii) modifying Fc-linked oligosaccharide structures, and these latter glyco-engineered therapeutic antibodies have recently started to be approved for clinical use. We herein review the advances in the Fc oligosaccharide-modified therapeutic antibodies.
Basic Structure of Therapeutic Antibodies
Human immunoglobulin G (IgG) is a glycoprotein bearing N-linked oligosaccharides and is preferentially employed as a therapeutic agent among the five classes of human immunoglobulin (IgM, IgD, IgG, IgA, and IgE) due to its long half-life in blood and superior biological activities. To date, all licensed recombinant therapeutic antibodies have been of the IgG class. The basic structure of the IgG molecule consists of two immunoglobulin light chains and two immunoglobulin heavy chains in covalent and non-covalent association, thus forming three independent protein moieties connected through a flexible linker referred to as the hinge region (Fig. 1). Two of these moieties, both referred to as Fab regions, are identical, and each expresses a specific antigen-binding site. The third moiety, the Fc region, expresses the interaction sites for ligands that induce various biological activities referred to as “antibody effector functions,” such as ADCC and CDC.
A schematic diagram of a human I g G1 molecule and its oligosaccharide structure. Human IgG1 is composed of two identical heavy chains and two identical light chains that are in covalent and non-covalent association to form three independent protein moieties connected through the hinge region. Human IgG1 has two N-linked oligosaccharides in the CH2 domain in the Fc region. The general structure of human IgG1 N-linked oligosaccharides is that of a complex and is characterized by a mannosyl-chitobiose core GlcNAc (□) and mannose (○), with or without bisecting GlcNAc (■), core fucose (Δ), galactose (●), and sialic acid (□)
These ligands include three structurally homologous cellular Fc receptor types (FcγRI, FcγRII, FcγRIII), the C1q component of complement, and the neonatal Fc receptor (FcRn). The Fc region is a homodimer comprising covalently disulfide-bonded hinge regions; the CH2 domains are glycosylated through the covalent attachment of oligosaccharides at asparagine 297 (Asn-297) and non-covalently paired CH3 domains. The oligosaccharide attached to the Fc region of human serum IgG is of the biantennary complex type, and it has a basic common trimannosyl core structure composed of pentasaccharides in the presence or absence of a core fucose , a bisecting N-acetylglucosamine (GlcNAc), and terminal galactose and sialic acid (Fig. 1). This structure gives rise to heterogeneity with a mixture of 30 or more glycoforms.
Most of the therapeutic IgG antibodies on the market are produced in rodent cell cultures using CHO, mouse myeloma NS0 and SP2/0, and mouse hybridomas as host cells. The majority of the glycoforms of the antibody products from these rodent cells are also of the biantennary complex type, comparable to the G0F (agalactosyl core-fucosylated) and the G1F (mono-galactosyl core-fucosylated) glycoforms present in human serum IgG, although a minor population of other glycoforms, such as the high-mannose type, has also been marginally detected. Importantly, both human serum IgG1 and recombinant therapeutic antibodies are fucosylated. Unlike the oligosaccharides attached to other glycoproteins, the antibody oligosaccharides linked to the Fc region via Asn-297 are integral to the protein portion of the Fc and form multiple non-covalent interactions with the CH2 domains, which has been clearly observed in the crystal structure analysis of human IgG1. Although it has been revealed that the IgG1 effector functions of CDC and ADCC are mediated via the binding of the hinge and the adjacent CH2 domains of the Fc region to either the complement or the FcγR on effector cells, such as NK cells and activated macrophages, the generation of the essential Fc tertiary conformation for binding to these ligands is known to depend on the presence of the oligosaccharides attached to the CH2 domains. The effector mechanisms mediated via FcγRI, FcγRII, FcγRIII, and C1q are severely compromised or abrogated in the aglycosylated or deglycosylated forms of IgG1, despite the fact that the antigen binding activity is identical to that of the glycosylated forms. These complexities, the considerable heterogeneity of the Fc oligosaccharides and the unique oligosaccharide structure integrated into the protein, have long prevented the discovery that a subtle change in the core fucosylation of the Fc oligosaccharides greatly affects the antibody effector function of ADCC.
Non-fucosylated Antibodies as Second-Generation Therapeutic Antibodies
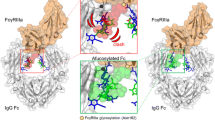
Non-fucosylated forms of human IgGs with Fc biantennary complex-type oligosaccharides are observed as a natural component in normal human serum, although the majority of the IgGs are fucosylated. In early 2000s, several groups have found that the lack of fucose, but not other sugar components such as terminal galactose and bisecting N-acetylglucosamine, greatly augments ADCC of human IgG1, which is a consequence of increased FcγRIIIa binding capacity of the Fc with non-fucosylated oligosaccharides (Shields et al. 2002; Shinkawa et al. 2003). A crystal structure analysis revealed that the ADCC enhancement by non-fucosylated IgG1s is attributable to a subtle conformational change in a limited region of IgG1-Fc, and the high affinity of non-fucosylated antibodies for FcγRIII is mediated by interactions formed between the carbohydrate at FcγRIII Asn-162 and regions of the Fc that are only accessible when the Fc oligosaccharide is non-fucosylated (Ferrara et al. 2011; Mizushima et al. 2011). Among the effector functions of therapeutic antibodies, ADCC has recently been clinically demonstrated to be an important mechanism of action of anticancer antibodies. Thus, non-fucosylated IgG1s with enhanced ADCC are expected to show superior clinical efficacy as second-generation therapeutic antibodies.
The advantages of non-fucosylated IgG1s include the ability to achieve therapeutic activity against tumor cells that express low levels of antigen and triggering high effector function in NK cells with a low-affinity FcγRIIIa allotype for the IgGs. Moreover, the ADCC enhancement induced by fucose removal has also been shown to be applicable to all human IgG subclasses and Fc-fusion molecules. Importantly, non-fucosylated IgG1s have a much higher binding affinity for FcγRIIIa than fucosylated human serum IgG, which is a preferential characteristic that will allow them to overcome the competition of human serum IgG for the binding of therapeutic IgG1 to FcγRIIIa on NK cells, and which have exhibited high ADCC in human whole blood (Iida et al. 2009). These biological features of non-fucosylated antibodies are expected to lead to the various clinical benefits (Table 1).
Production Systems for Non-fucosylated Antibodies
As described above, the importance of ADCC as a mode of action of anti-cancer antibodies has been illustrated by the significant correlation between the clinical outcomes and patient FcγRIII functional polymorphisms. This correlation was first demonstrated for the anti-CD20 treatment of lymphomas in the early 2000s (Cartron et al. 2002), followed by similar observations in solid tumors being treated with anti-HER2 (Musolino et al. 2008) and anti-EGFR (Bibeau et al. 2009) antibodies. This clinical evidence has stimulated pharmaceutical companies and biotech companies to exert extensive efforts to develop ADCC-enhanced antibodies as next-generation therapeutic antibodies.
A major issue in the industrialization of the production of non-fucosylated antibodies is the establishment of stable manufacturing processes. Because the enhanced ADCC of non-fucosylated antibodies can be inhibited by the contaminating fucosylated antibody molecules through competition for binding to antigens on the target, the establishment of production systems for non-fucosylated therapeutic antibodies with high purity is particularly important. For this purpose, efforts have been made to generate mammalian host cells (e.g., CHO, SP2/0 and NS0) that are engineered to produce glycoproteins lacking the core fucose. It is known that α-1,6 fucosyltransferase (FUT8) is the sole enzyme in mammalian cells which is responsible for the core fucosylation of N-glycan. By knocking out the FUT8 gene in CHO cells, Kyowa Hakko Kirin has established a robust system for the production of completely non-fucosylated antibodies (Potelligent® technology; Yamane-Ohnuki et al. 2004). The removal of the core fucose from N-glycan is also achieved by adding bisecting GlcNAc to the N-linked oligosaccharides, which is catalyzed by β1,4 N-acetylglucosaminyltransferase III (GnTIII). Roche/GlycArt has established engineered CHO cells overexpressing the GnTIII gene for the production of non-fucosylated antibodies (GlycoMabTM technology; Ferrara et al. 2006). Alternative expression systems that utilize yeast, duckweed, and moss have also been explored, because these organisms inherently lack the capacity for core α1,6-fucosylation, although these host systems are less well validated for the production of therapeutic proteins. Because yeast and plant cells add carbohydrate structures in a manner that is totally different from that of mammalian cells, the modification of multiple enzymes is required in these systems. In yeast, the mannose transferase gene should be replaced with a mannosidase gene to avoid a highly mannosylated glycoform, which causes rapid clearance in humans. In the case of plant cells, α1,3-fucosyl transferase and xylose transferase were deleted, in addition to introducing galactosyl transferase.
Clinical Applications of Non-fucosylated Antibodies
Since the first discovery of ADCC enhancement by defucosylation, almost 20 glyco-engineered antibodies have entered clinical trials (Table 2). Of these, two antibodies have been successfully approved so far. The first success was mogamulizumab (Ishida et al. 2012), an anti-CCR4 humanized antibody armed with non-fucosylated oligosaccharides by Kyowa Hakko Kirin’s Potelligent® technology, followed by the recent approval of obinutuzumab (Goede et al. 2014), a second-generation non-fucosylated anti-CD20 antibody developed by Roche/GlycArt. Mogamulizumab has been approved for relapsed or refractory CCR4-positive adult T-cell leukemia/lymphoma (ATL) in Japan in 2012 and is also under global development in clinical trials for untreated ATL, cutaneous T-cell lymphoma (CTCL), and peripheral T-cell lymphoma (PTCL). In addition to mogamulizumab, multiple Potelligent® antibodies are currently under evaluation in various disease areas, including benralizumab (MEDI-563: an anti-IL-5Ra IgG1; Laviolette et al. 2013) for phase III trials for asthma, MEDI-551 (an anti-CD19 IgG1; Ward et al. 2011) for phase II lymphomas and other antibodies are in earlier stages of development.
Obinutuzumab (GA101), an anti-CD20 IgG1 possessing non-fucosylated oligosaccharides with bisecting GlcNAc produced by Roche/GlycArt’s GlycoMabTM technology, has recently been approved by the US FDA for treating previously untreated CLL, based on the fact that a significant improvement is obtained in the PFS in comparison to rituximab in a phase III study in combination with chemotherapy. However, contrary to the success of obinutuzumab, imgatuzumab (GA201; Delord et al. 2014), another GlycoMab antibody targeting the EGFR, failed to meet its primary endpoint in a phase II trial in colorectal cancer patients, and Roche has announced the discontinuation of the development of this antibody.
Lessons from the clinical trials of non-fucosylated antibodies described above, particularly those from mogamulizumab and obinutuzumab, have led us to conclude that enhancing ADCC is clinically effective at least for hematological targets. It is likely that hematological target cells, including both malignant and normal immune cells, can be smoothly accessed by both antibody and effector cells (mainly NK cells). On the other hand, the proof of concept of this technology in solid tumors is still under evaluation; the key for success would lie in the establishment of a methodology to select the right patients and right dose, including the improvement of animal models, in which a considerable species-related difference in the immune systems exist, and the exploration of clinical biomarkers based on the biological mechanism underlying the enhanced ADCC.
Abbreviations
- ADCC :
-
Antibody-dependent cellular cytotoxicity
- CDC :
-
Complement-dependent cytotoxicity
- CHO :
-
Chinese hamster ovary
- Fc :
-
Antibody constant region
- FcγRIIIa :
-
Human Fcγ-receptor IIIa
- I g G :
-
Immunoglobulin G
- NK cell :
-
Natural killer cell
References
Bibeau F, Lopez-Crapez E, Di Fiore F et al (2009) Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol 27:1122–1129
Cartron G, Dacheux L, Salles G et al (2002) Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99:754–758
Delord JP, Tabernero J, García-Carbonero R et al (2014) Open-label, multicentre expansion cohort to evaluate imgatuzumab in pre-treated patients with KRAS-mutant advanced colorectal carcinoma. Eur J Cancer 50:496–505
Ferrara C, Brünker P, Suter T et al (2006) Modulation of therapeutic antibody effector functions by glycosylation engineering: influence of Golgi enzyme localization domain and co-expression of heterologous beta1, 4-N-acetylglucosaminyltransferase III and Golgi alpha-mannosidase II. Biotechnol Bioeng 93:851–861
Ferrara C, Grau S, Jäger C et al (2011) Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A 108:12669–12674
Goede V, Fischer K, Busch R et al (2014) Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 370:1101–1110
Iida S, Kuni-Kamochi R, Mori K et al (2009) Two mechanisms of the enhanced antibody-dependent cellular cytotoxicity (ADCC) efficacy of non-fucosylated therapeutic antibodies in human blood. BMC Cancer 9:58
Ishida T, Joh T, Uike N et al (2012) Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol 30:837–842
Laviolette M, Gossage DL, Gauvreau G et al (2013) Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol 132:1086–1096
Mizushima T, Yagi H, Takemoto E (2011) Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells 16:1071–1080
Musolino A, Naldi N, Bortesi B et al (2008) Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 26:1789–1796
Shields RL, Lai J, Keck R et al (2002) Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J Biol Chem 277:26733–26740
Shinkawa T, Nakamura K, Yamane N et al (2003) The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem 278:3466–3473
Ward E, Mittereder N, Kuta E et al (2011) A glycoengineered anti-CD19 antibody with potent antibody-dependent cellular cytotoxicity activity in vitro and lymphoma growth inhibition in vivo. Br J Haematol 155:426–437
Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M et al (2004) Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng 87:614–622
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Niwa, R., Shitara, K., Satoh, M. (2015). Glyco-engineered Therapeutic Antibodies as a Second-Generation Antibody Therapy. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_196
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_196
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences