Abstract
Sialic acids are nine-carbon acidic monosaccharides found in terminal position of glycan chains. The sialylation of cell surface glycoconjugates is frequently altered in cancers, resulting in the expression of sialylated tumor-associated carbohydrate antigens that are specific markers for this disease. Because sialylated glycans are involved in many biological processes, their expression by tumor cells is often associated with increased aggressiveness and metastatic potential of the tumors. The sialylated tumor-associated carbohydrate antigens are usually weakly expressed by healthy tissues and can be therefore used as immunotherapeutic targets for anticancer vaccines. This chapter summarizes the main tumor-associated sialylated structures and their role in cancer cells biology as well as their use for the development of anticancer vaccines.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Sialic acids are nine-carbon acidic monosaccharides found in terminal position of glycoconjugate glycan chains. The changes of glycosylation that occur during cell carcinogenesis lead to an increased sialylation pattern of the cells resulting in the expression of sialylated tumor-associated carbohydrate antigens (TACA) . First discovered in the 1970s using glycopeptides isolated from virus-transformed fibroblasts, these changes of cancer cells sialylation were observed on both glycoproteins and glycolipids from most cancer types. They were rapidly found associated with tumor growth and/or metastasis. Despite the vast amount of possible structures of glycans, only a few number of sialylated structures appear to be recurrently expressed by cancer cells. This review describes some of the best studied sialylated TACA and how they can alter the phenotype of cancer cells.
Expression of N-Glycolylneuraminic Acid in Cancers
N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) are the two major sialic acids in mammals. Neu5Gc is metabolized from Neu5Ac by the hydroxylation of its acetyl group catalyzed by the CMP-Neu5Ac hydroxylase (CMAH). Although detected in all mammals, CMAH gene is inactivated in humans, thus Neu5Gc is absent from normal human tissues (Varki 2010). However, Neu5Gc occurs in the glycan structures of many human tumors. Indeed, cancer cells metabolically incorporate exogenous Neu5Gc, originating from red meat and milk products diet. For humans, Neu5Gc-containing antigens are immunogenic and this may promote tumor growth. Low doses of anti-Neu5Gc antibodies sustain Neu5Gc-positive tumors in Neu5G-deficient mice by triggering a chronic inflammation that helped tumor growth. Anti-Neu5Gc-sialyl-Tn autoantibodies are identified as novel carcinoma serum biomarker. Neu5Gc is also associated to gangliosides (see section “Gangliosides”) such as Neu5Gc-GM3 and Neu5Gc-GM2 that are described as cancer-associated antigens and potential therapeutic targets.
α2,6-Sialylation of N-Acetyllactosamine
The α2,6-sialylation of N-acetyllactosamine (Galβ1-4GlcNAc) terminal sequences is very common in humans. Although widely expressed in normal tissues, it shows a dramatic increase in several cancers (Dall’Olio 2000). Two human β-galactoside α2,6-sialyltransferases have been cloned but the biosynthesis of Neu5Acα2-6Galβ1-4GlcNAc (Sia6LacNAc) is mainly achieved by ST6Gal I. An increased expression of ST6Gal I was reported in different types of cancer including gastric and colon cancers, choriocarcinoma, cervical carcinoma, and brain tumors from non-neuroectoderm origin. In liver and breast cancers, increased ST6Gal I expression is observed only in subgroups of patients. ST6Gal I expression was shown to be increased by the c-Ha-ras oncogene and high expression of Sia6LacNAc in ras-transformed rat fibroblasts correlates with invasive potential. On the other hand, ST6Gal I increases adhesion of colon and breast cancer cells to extracellular matrix and the overexpression of ST6Gal I also reduced invasiveness of colon and glioma cancer cell lines. Thus, α2,6-sialylation, though commonly observed in cancers, is of variable occurrence and phenotypic consequences, depending on the cancer type.
Sialyl-Tn Antigen
The sialyl-Tn antigen (Neu5Acα2-6GalNAc-Ser/Thr, sTn) is a truncated O-glycan resulting from a premature sialylation event. Sialyl-Tn is absent from healthy adult tissues but can be detected at various frequencies in almost all kinds of carcinomas including stomach, liver, pancreas, and colon (Julien et al. 2012). In breast cancer, sTn expression correlates with a poorly differentiated state and resistance to adjuvant therapy in node-positive patients. In colon cancer, sTn is expressed by most primary tumors and their respective metastases and correlates with poor prognosis.
ST6GalNAc I is catalyzing the synthesis of sTn in vivo. It has been proposed that COSMC mutation was necessary to provide the Tn acceptor substrate for ST6GalNAc I to synthesize sTn. However, transfection of ST6GalNAc I cDNA is sufficient to induce sTn expression in various breast cancer cell lines expressing core 1 and core 2 glycans, proving that ST6GalNAc I can compete with active Core1 β1,3-galactosyltransferase. sTn has been found carried by several glycoproteins on the cancer cell surface, from which only few have been identified so far: β1-integrin, MUC1, CD44, and MUC16. As a result sTn expression altered adhesion and migration of cancer cells in vitro, in a cell-line-dependent way.
The expression of sTn is often correlated with cancer progression (Julien et al. 2012). In breast cancer, mucin glycosylation undergoes a characteristic switch from the expression of core 2 structures to accumulation of T and sTn antigens (Cazet et al. 2010). The significance of sTn as a tumor marker and its association with increased malignancy suggested its use as a target for cancer immunotherapy (see section “Anticancer Vaccine Strategies Targeting Sialylated Antigens”).
Sialyl-Lewisx (sLex)
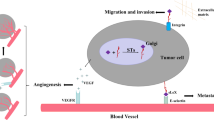
SLex is a tetrasaccharide epitope (Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAc-R) that can be found at the terminal position of a lactosamine chain built on glycolipids, O-glycans, or N-glycans framework. The lactosamine disaccharide is turned into sLex by first adding a sialic acid on the galactose with an α2,3-linkage and then adding a fucose on the GlcNAc residue. It thus requires the combined action of one sialyltransferase and one fucosyltransferase. Several isoforms exist for each enzyme (ST3Gal III, IV or VI, and FucT III to VII) that display various activities depending on the lactosamine framework and the cancer type. Consequently, sLex expression in cancer can arise from various genetic alterations and lead to different effects, depending on the cancer type or even the individual. However, some general features can be highlighted from this complicated picture. SLex is a marker of metastasis and poor prognosis in colorectal and prostatic carcinomas. Furthermore, sLex expression on colon and prostate cancer cells allows them to adhere to activated endothelial cells (HUVEC) under flow conditions. It is thought that sLex present on circulating tumor cells can serve as ligand for selectins and mediate cancer cells extravasation in vivo, mimicking the molecular process used by immune cells during inflammation. These discoveries have triggered research in the development of antagonists able to block sLex/selectins interactions, such as glycomimetics (Magnani and Ernst 2009) or heparan sulfate-derived molecules.
In breast cancer, sLex expression is associated with the hormonal status of the tumor and found more expressed in the more aggressive hormone-independent subtypes. However, sLex was not associated with metastasis or prognosis in this group. Instead, it was correlated with bone metastasis in the hormonal dependent tumor group. This illustrates the difficulty to generalize on the role of sialylated TACAs in cancers. Indeed, many parameters can be involved in their function in cancers, such as the nature of their framework (e.g., protein vs. lipid or O-glycans vs. N-glycans), their level of expression, or their subsequent modifications (e.g., O-acetylation, sulfation).
Gangliosides
Gangliosides are sialylated glycosphingolipids classified in four series (0-, a-, b-, and c-series) according to the number of sialic acids linked to lactosylceramide. Normal tissues express “simple” gangliosides from 0- and a-series, whereas “complex” gangliosides from b- and c-series are mainly restricted to the nervous system of healthy adults and reexpressed in several types of cancer including melanoma and brain tumors (Bobowski et al. 2012). The disialoganglioside GD3 is highly expressed in melanoma primary tumors and cell lines playing a central role in the maintenance of malignancy by promoting cell growth, migration, and invasive properties. Melanomas are also enriched in de-N-acetyl-GM3, GD2, 9-O-acetyl-GD3 (CDw60), and 9-O-acetyl-GD2, de-N-acetyl-GM3 being a marker of metastatic melanoma and promoting cell migration and invasion. GD3 is also overexpressed in brain tumors (Bobowski et al. 2012) and involved in the proliferative and invasive capacities of tumor cells. In neuroblastoma cells, the inhibition of GD3 expression reduces cell migration and metastatic potential. Similarly, the expression of GD3 increases tumorigenicity and invasion of glioma cells, whereas anti-GD3 mAb inhibits tumor growth. On the contrary, the proliferation of human neuroblastoma cells is reduced in the presence of GM3, GM1, GD1a, and GT1b via the inhibition of EGFR receptor phosphorylation, and GM3 also inhibits VEGF-induced angiogenesis of brain tumors. b-series gangliosides are also reexpressed in small-cell lung tumors (SCLC). In particular, GD2 is involved in the acquisition of the malignant phenotype of SCLC and sufficient to increase cell growth and invasion of SK-LC-17 cells, whereas GD2 inhibition by small interfering RNAs reduces cell proliferation and tumor growth in SCID mice, and anti-GD2 mAb inhibits cell growth and induces apoptosis of SCLC cells by a mechanism dependent of MEK/Erk and p38/MAPK signaling pathways. GD3, 9-O-acetyl-GD3, and 9-O-acetyl-GT3 are overexpressed in about 50 % of invasive ductal breast carcinoma (IDC) and N-glycolyl-GM3 is also detected in 100 % of grade II IDC. Recently, GD2 and GD3 were shown to be greatly increased in breast cancer stem cells and involved in mammosphere formation and cell motility.
Anticancer Vaccine Strategies Targeting Sialylated Antigens
TACAs have attracted strong interest for the development of anticancer immunotherapy achieved either by vaccination or passive immunization. Because of its pan-carcinoma expression associated with an adverse outcome, an anticancer vaccine towards the sTn epitope, named Theratope, was designed. In spite of the initial enthusiasm around anti-sTn immunotherapy, Theratope failed on Phase III clinical trial. Several other immunogens based on TACAs have been preclinically investigated, but so far none made it to the clinical trial level. Alternatively, mAbs targeting sialylated TACA including sTn, sLea/x, and gangliosides have been developed during the last decades and assessed in clinical trials for a variety of cancers with contrasted results. However, recent progress was obtained in antitumor therapy using anti-TACA mAb (Rabu et al. 2012). For example, numerous anti-GD2 mAbs have been developed for clinical use and tested on melanomas and in neuroblastomas. Follow-up analysis of patients indicated that immunotherapy with anti-GD2 chimeric mAb ch14.18 may prevent late relapses. Previously, a Phase III clinical trial treatment of neuroblastoma has shown that the addition of ch14.18 to standard treatment induced an increased event-free and overall survival in high-risk neuroblastoma. Different Phase I clinical trials assessing murine anti-Neu5Gc-GM3 have also shown efficient tumor cells killing in advanced melanoma, breast cancer, and lung cancer. A humanized anti-Neu5Gc-GM3 mAb was confirmed to exert antitumor activity in human breast carcinoma. Alternatively, investigators targeted the Neu5Gc-sustained inflammation using anti-idiotype mAb targeting Neu5Gc-containing gangliosides. For example, racotumomab induced a strong antimetastatic effect in tumor-bearing mice. A Phase II/III clinical trial showed a significant clinical benefit in the patients who were treated with the anti-idiotype vaccine together with anti-Lewis mAb.
Concluding Remarks
As reviewed, sialic acid containing glycoconjugates are expressed by most of cancer cells. They are crucially involved in tumor aggressiveness by promoting cell proliferation, migration, angiogenesis, and metastasis. They were discovered as tumor markers and are increasingly investigated as targets for anticancer immunotherapy. Improved knowledge of the biosynthetic mechanisms and biological function of sialylated glycans is now critical in order to provide new therapeutic approaches targeting cancer cells. New generation of anti-sialylated glycans mAbs should also be improved to increase tumor specificity and reduce toxicity against healthy tissues. The use of multi-antigens immunotherapy or mAbs which only bind to high-density antigens present on tumor cells, together with specific drug delivery, may be particularly useful in targeting tumor cells.
References
Bobowski M, Cazet A, Steenackers A et al (2012) Role of complex gangliosides in cancer progression. In: Pilar Rauter A, Lindhorst TK (eds) Carbohydrate chemistry: chemical and biological approaches, vol 37. RSC Publishing, Cambridge, pp 1–20
Cazet A, Julien S, Bobowski M et al (2010) Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res 12:204
Dall’Olio F (2000) The sialyl-alpha2,6-lactosaminyl-structure:biosynthesis and functional role. Glycoconj J 17:669–676
Julien S, Videira PA, Delannoy P (2012) Sialyl-Tn in cancer: (how) did we miss the target? Biomolecules 2:435–466
Magnani JL, Ernst B (2009) Glycomimetic drugs – a new source of therapeutic opportunities. Discov Med 8:247–252
Rabu C, McIntosh R, Jurasova Z et al (2012) Glycans as targets for therapeutic antitumor antibodies. Future Oncol 8:943–960
Varki A (2010) Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci U S A 107:8939–8946
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this entry
Cite this entry
Julien, S., Delannoy, P. (2015). Sialic Acid and Cancer. In: Taniguchi, N., Endo, T., Hart, G., Seeberger, P., Wong, CH. (eds) Glycoscience: Biology and Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54841-6_193
Download citation
DOI: https://doi.org/10.1007/978-4-431-54841-6_193
Received:
Accepted:
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54840-9
Online ISBN: 978-4-431-54841-6
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences