Abstract
Because of their unique morphology, turtles have raised profound questions as to their evolutionary origin. In striking contrast to the body plan of other tetrapods, the shoulder girdle of turtles sits inside the rib cage, which comprises the dorsal shell, or carapace. By this topological change of the skeletal elements, the carapace has been regarded as an example of evolutionary novelty that violates the ancestral body plan of tetrapods. In this chapter, we first overview the phylogenetic positioning of turtles, and then review how turtles evolved their unique body plan. In brief, three points have been clarified by recent studies. (1) Turtles have birds/crocodilians (or archosaurians) affinity of evolutionary origin. (2) During embryogenesis, the turtle also establishes the vertebrate basic body plan, as in other vertebrates, followed by the late developmental stages of generating turtle-specific structures, such as folding of the lateral body wall to make the apparent inside-out topology of shoulder girdle against ribs. (3) One of the causal factors of folding appears to be the concentric growth of carapacial margin, which involves an ancestral gene expression cascade in a new location. These reports allow us to hypothesize the stepwise, not necessarily saltatory, evolution of turtles, consistent with the recent finding of a transitional fossil animal, Odontochelys, that did not have the carapace but already possessed the plastron.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
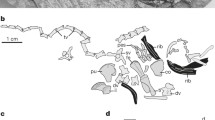
Turtles have long been regarded not only as the most primitive extant amniotes but also as animals that evolved abruptly without any intermediate morphology. Their skull does not possess any temporal fenestrae (representing the anapsid state), a character that was once hypothesized to be a hallmark of basal amniotes and their ancestral amphibians (Romer 1956; reviewed by Tsuji and Müller 2009). The trunk of turtles, on the other hand, shows an extensively derived feature. The turtle shell is composed of dorsal and ventral moieties; the ventral moiety is referred to as a plastron, consisting of nine dermal elements corresponding to clavicle, interclavicle, and gastralia in other amniotes (Fig. 23.1b–d). The dorsal shell, termed the carapace, comprises the thoracic vertebrae, ribs, and dermal bones surrounding the axial skeleton (Fig. 23.1a). In many turtle species, these bony shells are covered by keratinous scutes, whereas in some species, such as the soft-shelled turtles, the scutes and the peripheral dermal bones are lost.
The turtle shell and body plan of turtles. a, b Turtle shell. a Dorsal (left) and ventral (right) views of the carapacial skeleton of Chinemys reevesii. The carapace is formed by ribs (r), vertebrae (v), and dermal bones arranged peripherally. b Dorsal view of the plastron. Epiplastron Fig. 23.1 (continued) (epi) is homologized with clavicle, entplastron (ent) with interclavicle, and other dermal bones with gastralia. c, d Comparison of body plan between other amniotes (c) and turtles (juvenile of Chinese soft-shelled turtle, Pelodiscus sinensis) (d). Note that scapula (sc) and coracoid (cor) are outside of the ribs in other amniotes and inside the ribs in turtles. ca carapace, c clavicle, cos costal plate, g gastralia, hyo hyoplastron, hypo hypoplastron, ic interclavicle, neu neural plate, nu nuchal plate, pe peripheral plate, pl plastron, py pygal plate, r dorsal ribs, spy suprapygal plate, v dorsal vertebrae, xiphi xiphiplastron (figure modified from Nagashima et al. 2012a)
The outstanding feature in the turtle flank is not the shell itself, but the resultant body plan of turtles (Burke 2009). Turtle ribs, rather than growing ventrally, grow laterally to form the carapace, and the uniqueness of the turtles is that their shoulder girdle composed of scapula and coracoid is housed inside the ribcage. This inside-out morphology appears to have been established by violating the basic rules of the vertebrate body plan, thus regarded as a typical example of evolutionary novelties (Hall 1998; Rieppel 2001; Gilbert et al. 2001, 2008).
Lack of any transitional patterns to explain this turtle-specific topological change, as well as the absence of intermediate fossils, have led biologists to assume that turtles emerged by saltatory evolution (reviewed by Nagashima et al. 2012a). In this section, we overview the evolutionary origin of turtles by introducing studies aimed to clarify the phylogenetic position of turtles, followed by studies focused on the body plan evolution of turtles.
2 Phylogenetic Position of Turtles
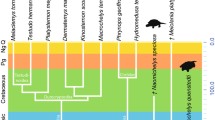
When and where did turtles come into being? Historically, three major hypotheses have been proposed for the phylogenetic origin of turtles. Largely based on the skull morphology, the earliest hypothesis relegated turtles to early-diverged reptiles, called anapsids, located basal to Diapsida (Tsuji and Müller 2009; Kuratani et al. 2011). Meanwhile, with almost every accessible element from egg, embryo, and adult morphology, Rieppel and de Braga (1996) proposed the turtle as a sister group with the lizard–snake–tuatara (Lepidosauria) clade (Fig. 23.2). As the third hypothesis, first by molecular phylogenetic analysis with rRNA (Hedges et al. 1990), and other molecular studies (Caspers et al. 1996; Crawford et al. 2012), it was alleged that turtles are closely related to a lineage including crocodilians and birds (Archosauria) (Fig. 23.2). What confused researchers was that controversy arose even among the molecular-based approaches. Based on the existence or absence of miRNAs, Lyson et al. (2012) reported that the turtles are a sister group of Lepidosauria. For this study, however, Hedges (2012) draws cautionary attention that the phylogenetic analysis using miRNA is yet to be established to obtain significant results. Finally, recent studies that reported the draft genome sequences of turtles (Pelodiscus sinensis and Chelonia mydas, by Wang et al. 2013; Chelonia picta, by Shaffer et al. 2013), robustly supported the archosaurian affinity of turtles (Fig. 23.2).
The common ancestor of turtles arose around 267.9–248.3 million years ago, splitting from archosaurians. Molecular phylogenetic analysis based on genome-wide data (1,113 orthologous genes from 12 vertebrate species) supported that turtles are a sister group of archosaurians. Molecular clock analysis, calibrated with fossil records (white dots in each branching node) supported that the split occurred around 267.9–248.3 million years ago, the period that overlaps or followed shortly after the Permian extinction event (Chen and Benton 2012). The black ellipses on the nodes indicate the 95 % credibility intervals of the estimated posterior distributions of the divergence times (figure modified from Wang et al. 2013)
These molecular phylogenetic studies are already making some impact on paleontological study, and some paleontologists are reexamining the morphological characters to reconcile morphological data with molecular results (Rieppel 2000). For example, morphology of the vormer (Damiani and Modesto 2001), temporal region (Müller 2003), and carotid circulation (Müller et al. 2011) are suggested not to support a turtle–anapsids relationship. The presence of a laterosphenoid ossification in a basal turtle is proposed to unite turtles to Archosauria (Bhullar and Bever 2009). In the archosauromorph lineage, Merck (1997) found the Euryapsida (Helveticosaurus, Sauropterygia, and Ichthyosauria)–Thalattosauria clade, to which turtles are suggested to be a sister group (Rieppel and de Braga 1996).
Although the ancestor did not possess the complete dorsal shell such as that in existing turtles, the oldest known fossil turtle, Odontochelys (Li et al. 2008), showed that the turtle ancestor already existed 220 million years ago. Consistent with this oldest fossil record, Wang et al. (2013), based on their genome-wide dataset including two turtle species (Chinese soft-shelled turtle and green sea turtle), estimated that the common ancestor of turtles already existed before the emergence of Odontochelys. According to the estimate based on genome-wide analysis, turtles split from the lineage of archosaurians at around 267.9–248.3 million years ago. Interestingly, the period coincides with the one of the largest mass extinction events on this planet, called the Permian-Triassic extinction event (Chen and Benton 2012). However, whether this extinction event has a certain role in the evolution of the turtle ancestor awaits further investigation.
3 Body Plan Development and Evolution of Turtles
How did turtles evolve their unique body plan after splitting from the archosaurians (the group consisting of birds/crocodilians)? Substantial contribution has been made to this question by recent comparative embryonic studies.
Despite the uniqueness of the turtle body plan, recent studies clarified that turtles also follow the general rule for embryonic evolution of vertebrates, or the developmental hourglass model (Fig. 23.3) (Duboule 1994; Irie and Kuratani 2011; Wang et al. 2013), to produce their unique body. The model explains that vertebrate embryos pass through the conserved bottleneck-like period, the period that shows the basic vertebrate body plan (called the phylotypic period), and then specialize afterward. Meanwhile, earlier to, or later than, this phylotypic period, divergent characteristics appear among different species. Actually, the midembryonic stages of turtles and chicken (e.g., stage TK11 of turtle and stage HH16 of chicken) appear somewhat more similar to each other than the earlier and later embryonic stages (Fig. 23.4). Furthermore, quantitative evidence was obtained from the study that took advantage of whole embryonic gene expression profiles (Wang et al. 2013), showing that maximal similarity between turtle and chicken embryos appears in the pharyngula stages (Fig. 23.4; red dashed circles). Direct inference of this observation suggests that turtles, similar to other vertebrates, develop their body first by establishing the vertebrate basic body plan, and then modify the developmental trajectory to obtain the turtle-specific morphological patterns. Actually, the first sign of a turtle-specific character, the carapacial ridge (CR; Figs. 23.4 and 23.5) (Burke 1989), appears after this phylotypic period (see following section). These studies as a whole tell us that the turtle body plan evolved by adding major changes to the embryonic stages after the vertebrate phylotypic period during evolution. Actually, Wang et al. reported that genes that potentially explain the turtle-specific features, such as genes involved in ossification, extracellular matrix reorganization, and collagen, show increasing expression only in the later phase of turtle embryogenesis (Wang et al. 2013).
Turtles also follow the developmental hourglass model during embryogenesis. The hourglass model (right), first proposed by Duboule (1994), explains that vertebrate embryogenesis is rather divergent during early and late stages whereas midembryonic stages show maximum similarity both in morphology and in whole embryonic gene expression profile. The bottleneck period, called the vertebrate phylotypic period, becomes the source of the vertebrate basic body plan found among adult vertebrates. The horizontal width of the hourglass model represents the evolutionary divergence among vertebrate embryos, and embryogenesis flows upward in this drawing. Recent molecular studies (Irie and Kuratani 2011; Wang et al. 2013) identified that pharyngular embryos are the stages that show most conservation among vertebrates
External appearances of chicken and Chinese soft-shelled turtle embryos. Soft-shelled turtle embryogenesis shows a rather different morphology of the gastrula but soon converges to show similar morphology with the chicken at around the pharyngula stage. Actually, chicken stage HH 16 (Hamburger and Hamilton 1951) and turtle stage TK 11 (Tokita and Kuratani 2001) show the most similar gene expression profile compared to other developmental stages (dashed circles). These embryos show striking similarity in morphology as well, despite the fact that these two species split more than 250 million years ago, with almost twice the time for embryogenesis. White arrows show the direction and relative length of time needed for embryogenesis. Size of embryos not to scale
Finally, the reason why turtles still adhere to the conservation of the vertebrate phylotypic period is not clear; however, some researchers attribute this to a particularly complex signaling network working in this embryonic period (e.g., Hox colineality, interdependent molecular signals: Duboule 1994; Raff 1996).
4 Carapacial Ridge
As mentioned earlier, the CR appears after the phylotypic period as a longitudinal ridge on the lateral aspect of the flank of turtle embryos (Fig. 23.5). The CR forms the leading edge of the developing carapace, and functions to make the turtle-specific rostrocaudally expanded pattern of ribs through accelerated growth of the carapacial margin (Figs. 23.5 and 23.6) (Burke 1989, 1991; Nagashima et al. 2007).
A scheme representing trunk development in the chicken (left) and turtle (right). Plates at both lateral ends are transverse views; those in the middle columns are lateral view. From top to bottom, development proceeds as follows. Top: Both animals have nearly identical morphology at an early developmental stage. Note that turtle ribs (r) are morphologically shorter than those of chicken but grow along the the muscle plate (mp) as do chicken ribs. Middle and bottom: Chicken development proceeds without a major change in morphology from the initial state. The folding process occurring in the late developmental stage of turtles does not change the topological relationship between the ribs, muscle plate, and shoulder girdle from that at the beginning of development. Note that only the body folding is different between the animals. Shaded domain (r′) in transverse view of turtles (bottom) represents undeveloped ribs in the lateral body wall, which are expected to be found along the muscle plate as are those in chicken. ax axial domain, h humerus, lbw lateral body wall, nt neural tube, v vertebrae (figure modified from Nagashima et al. 2012a)
Morphologically, the embryonic body is composed of the dorsomedially located axial part and the ventrolaterally lateral body wall. The dermal mesenchyme of the former is derived from somites and that of the latter from the somatic mesoderm. The CR develops at the ventrolateral edge of the axial domain and delineates a boundary between the two kinds of dermal mesenchyme with its ventral edge through its development, which indicates the uniqueness of the structure among amniote embryos, because such a structure does not appear in other amniote embryos (Figs. 23.5 and 23.6) (reviewed by Kuratani et al. 2011).
Although the precise molecular mechanism involved in CR development is yet to be clarified, some studies have provided intriguing insights. Through a subtractive cDNA screening method, Kuraku et al. (2005) identified four genes specifically expressed in the CR, which include cellular retinoic acid-binding protein (Crabp)-I, Sp-5, lymphocyte enhancer factor (Lef)-1, and Apcdd-1. All these genes are components of, or are related to, the canonical Wnt signaling pathway (Kuratani et al. 2011). Actually, localization of β-catenin in nuclei of the CR epidermis (Kuraku et al. 2005) and arrest of CR formation after the inhibition of Lef-1 activity suggest that the Lef-1/β-catenin complex is involved in CR development as a transcriptional activator of the signal cascade (Nagashima et al. 2007).
Recently, based on comprehensive in situ hybridization screening that took advantages of turtle genomes, one of the upstream factors, Wnt 5a expression, was discovered in the CR mesenchyme (Wang et al. 2013). The reason why the subtractive cDNA method between the CR and the lateral body wall failed to detect this gene is that the gene was also expressed in the body wall. As another upstream molecule, hepatocyte growth factor (HGF) expressed at the vicinity of the CR is suggested; inactivation of HGF function leads to degradation of the CR (Kawashima-Ohya et al. 2011). Consistent with this, carcinoma studies have found regulation of the canonical Wnt pathway by HGF (Nelson and Nusse 2004). In chicken and mouse embryos, expressions of the orthologous genes are not observed at the corresponding site, reconfirming the novel nature of the CR. Many of the genes are commonly expressed in the limb bud of the amniote embryos including turtles, indicating that some of the gene cascade functioning in limb development would be secondarily recruited to invent the CR (Kuratani et al. 2011; also see Gilbert et al. 2001, 2008).
5 Positional Change of Ribs and Scapula
One of the differences between turtle ribs and those of other amniotes is the relative lengths of the ribs: the turtle ribs are morphologically shorter than those of other amniotes, because turtle ribs are arrested in the axial domain, never penetrating into the lateral body wall as in many other amniotes (axial arrest of the ribs; Fig. 23.6) (Burke 1989; Nagashima et al. 2007; Kuratani et al. 2011).
As the cause for the truncation of turtle ribs, a turtle-specific expression pattern of Hox genes and unique features of Myf5 have been proposed (Ohya et al. 2005, 2006; Nagashima et al. 2012a, b). Especially, transcriptional factor Myf5 is involved not only in myogenic activity but also in inductive activity of ribs (Nagashima et al. 2012b), which would explain both rib truncation and the characteristic meager development of muscle plates, elongated myotomes, in turtles (Nagashima et al. 2005).
At first glance, turtle ribs appear to take different trajectories from those in other amniotes because they grow laterally and superficially. The turtle ribs, however, are along the muscle plate as are chicken and mouse ribs (Fig. 23.6), reflecting that the ribs are induced by the muscle plate. Hence, it is convincing to suppose that if turtles would have long ribs, they would be found along the muscle plate in the lateral body wall, indicating that the muscle plate can be regarded as “the latent ribcage.” The shoulder girdle of turtles initially develops rostral to the ribs and outside the muscle plate or “the latent ribcage” as that in other amniotes (Fig. 23.6). Although chicken and mouse development proceed without much modification of this pattern, in turtles, the CR renders some of the rostral ribs fanned out rostrally to cover the scapula caudodorsally (Fig. 23.6). During this process, the shoulder girdle remains outside the muscle plate, which is now severely folded inward in the lateral body wall (Fig. 23.6). Thus, turtles change the spatial relationship between the ribs and shoulder girdle by folding the lateral body wall inward after skeletal development, and this process does not alter the body plan of amniotes (Nagashima et al. 2009).
These developmental findings highlight and fill the saltatory evolutionary gap once believed to be present in turtle evolution; namely, axial arrest, fanned-out expansion of ribs, and encapsulation of the scapula would have occurred in the ancestral animals of turtles successively. Illustrating this, a previously unknown fossil, Odontochelys, has intermediate morphology linking turtles and the ancestral animals (Li et al. 2008). This animal did not have a complete carapace but did have the plastron. The ribs were already arrested axially but did not show a flabellate pattern, so the scapula was still situated rostral to the ribs. This pattern is reminiscent of the morphological pattern of turtle embryos before the folding process. Thus, morphogenesis of Odontochelys would have been completed at this developmental stage and would not have acquired developmental programs to expand the ribs and to encase the shoulder girdle. Turtle evolution would have been achieved by secondarily adding the folding process in the late developmental phase of Odontochelys-like ancestral animals (Nagashima et al. 2009).
6 Perspective
Our studies have suggested that stepwise changes of the developmental program have caused the evolution of turtles. By comparative genomics as well as analyses of gene regulations, it could become possible in the near future to ascertain the net elements that are truly relevant to the modification of the animal body plan, especially through construction of a turtle-like developmental phenocopy by means of functional assays on model animals. By understanding the creation of animals such as turtles, which apparently violate the developmental constraints specific to vertebrates, we will be able eventually to correlate the DNA sequence and evolving morphology of animals. For this reason, turtles potentially provide very intriguing and promising aspects for the study of evolutionary developmental biology.
References
Bhullar B-AS, Bever GS (2009) An archosaur-like laterosphenoid in early turtles (Reptilia: Pantestudines). Breviora 518:1–11
Burke AC (1989) Development of the turtle carapace: implications for the evolution of a novel bauplan. J Morphol 199:363–378
Burke AC (1991) The development and evolution of the turtle body plan. Inferring intrinsic aspects of the evolutionary process from experimental embryology. Am Zool 31:616–627
Burke AC (2009) Turtles……again. Evol Dev 11:622–624
Caspers GJ, Reinders GJ, Leunissen JA, Wattel J, de Jong WW (1996) Protein sequences indicate that turtles branched off from the amniote tree after mammals. J Mol Evol 42:580–586
Chen Z-Q, Benton MJ (2012) The timing and pattern of biotic recovery following the end-Permian mass extinction. Nat Geosci 5:375–383
Crawford NG, Faircloth BC, McCormack JE, Brumfield RT, Winker K, Glenn TC (2012) More than 1000 ultraconserved elements provide evidence that turtles are the sister group of archosaurs. Biol Lett 8:783–786
Damiani RJ, Modesto JP (2001) The morphology of the pareiasaurian vomer. N Jb Geol Paläont Mh 7:423–434
Duboule D (1994) Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate bauplan and the evolution of morphologies through heterochrony. Development (Camb) 1994:135–142
Gilbert SF, Loredo GA, Brukman A, Burke AC (2001) Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev 3:47–58
Gilbert SF, Cebra-Thomas JA, Burke AC (2008) How the turtle gets its shell. In: Wyneken J, Godfrey MH, Bels V (eds) Biology of turtles. CRC, Boca Raton, pp 1–16
Hall BK (1998) Evolutionary developmental biology, 2nd edn. Chapman & Hall, London
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88:49–92
Hedges SB (2012) Amniote phylogeny and the position of turtles. BMC Biol 10:64
Hedges SB, Moberg KD, Maxson LR (1990) Tetrapod phylogeny inferred from 18S and 28S ribosomal RNA sequences and a review of the evidence for amniote relationships. Mol Biol Evol 7:607–633
Irie N, Kuratani S (2011) Comparative transcriptome analysis reveals vertebrate phylotypic period during organogenesis. Nat Commun 2:248
Kawashima-Ohya Y, Narita Y, Nagashima H, Usuda U, Kuratani S (2011) Hepatocyte growth factor is crucial for development of the carapace in turtles. Evol Dev 13:260–268
Kuraku S, Usuda R, Kuratani S (2005) Comprehensive survey of carapacial ridge-specific genes in turtle implies co-option of some regulatory genes in carapace evolution. Evol Dev 7:3–17
Kuratani S, Kuraku S, Nagashima H (2011) Evolutionary developmental perspective for the origin of the turtles: the folding theory for the shell based on the developmental nature of the carapacial ridge. Evol Dev 13:1–14
Li C, Wu X, Rieppel O, Wang L, Zhao L (2008) An ancestral turtle from the Late Triassic of southwestern China. Nature (Lond) 45:497–501
Lyson TR, Sperling EA, Heimberg AM, Gauthier JA, King BL et al (2012) MicroRNAs support a turtle + lizard clade. Biol Lett 8:104–107
Merck JW (1997) A phylogenetic analysis of the euryapsid reptiles. Unpublished Ph.D. dissertation, University of Texas at Austin
Müller J (2003) Early loss and multiple return of the lower temporal arcade in diapsid reptiles. Naturwissenschaften 90:473–476
Müller J, Sterli J, Anquetin J (2011) Carotid circulation in amniotes and its implications for turtle relationships. N Jb Geol Paläont Abh 261:289–297
Nagashima H, Uchida K, Yamamoto K, Kuraku S, Usuda R, Kuratani S (2005) Turtle-chicken chimera: an experimental approach to understanding evolutionary innovation in the turtle. Dev Dyn 232:149–161
Nagashima H, Kuraku S, Uchida K, Ohya YK, Narita Y, Kuratani S (2007) On the carapacial ridge in turtle embryos: its developmental origin, function, and the chelonian body plan. Development (Camb) 134:2219–2226
Nagashima H, Sugahara F, Takechi M, Ericsson R, Kawashima-Ohya Y, Narita Y, Kuratani S (2009) Evolution of the turtle body plan by the folding and creation of new muscle connections. Science 325:193–196
Nagashima H, Kuraku S, Uchida K, Kawashima-Ohya Y, Narita Y, Kuratani S (2012a) Body plan of turtles: an anatomical, developmental and evolutionary perspective. Anat Sci Int 87:1–13
Nagashima H, Kuraku S, Uchida K, Kawashima-Ohya Y, Narita Y, Kuratani S (2012b) Origin of the turtle body plan: the folding theory to illustrate turtle-specific developmental repatterning. In: Brinkman DB, Holroyd PA, Gardner JD (eds) Morphology and evolution of turtles: origin and early diversification. Springer, Dordrecht
Nelson WJ, Nusse R (2004) Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483–1487
Ohya YK, Kuraku S, Kuratani S (2005) Hox code in embryos of Chinese soft-shelled turtle Pelodiscus sinensis correlates with the evolutionary innovation in the turtle. J Exp Zool 304B:107–118
Ohya YK, Usuda R, Kuraku S, Nagashima H, Kuratani S (2006) Unique features of Myf-5 in turtles: nucleotide deletion, alternative splicing and unusual expression pattern. Evol Dev 8:415–423
Raff A (1996) The shape of life: genes, development, and the evolution of animal form. University of Chicago Press, Chicago
Rieppel O (2000) Turtles as diapsid reptiles. Zool Scr 29:199–212
Rieppel O (2001) Turtles as hopeful monsters. Bioessays 23:987–991
Rieppel O, de Braga M (1996) Turtles as diapsid reptiles. Nature (Lond) 384:453–455
Romer AS (1956) Osteology of the reptiles. University of Chicago Press, Chicago
Shaffer HB, Minx P, Warren DE, Shedlock AM, Thomson RC, Valenzuela N et al (2013) The western painted turtle genome, a model for the evolution of extreme physiological adaptations in a slowly evolving lineage. Genome Biol 14:R28
Tokita M, Kuratani S (2001) Normal embryonic stages of the Chinese soft-shelled turtle Pelodiscus sinensis. Zool Sci 18:705–715
Tsuji LA, Müller J (2009) Assembling the history of the Parareptilia: phylogeny, diversification, and a new definition of the clade. Fossil Rec 12:71–81
Wang Z, Pascual-Anaya J, Zadissa A, Li W, Niimura Y, Huang Z et al (2013) The draft genomes of soft-shell turtle and green sea turtle yield insights into the development and evolution of the turtle-specific body plan. Nat Genet. doi:10.1038/ng.2615
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Irie, N., Nagashima, H., Kuratani, S. (2014). The Turtle Evolution: A Conundrum in Vertebrate Evo-Devo. In: Kondoh, H., Kuroiwa, A. (eds) New Principles in Developmental Processes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54634-4_23
Download citation
DOI: https://doi.org/10.1007/978-4-431-54634-4_23
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54633-7
Online ISBN: 978-4-431-54634-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)