Abstract
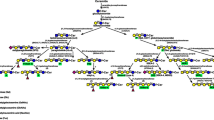
Forssman heterophilic glycolipid antigen (GalNAcαl-3GalNAcβl-3Galαl-4Galβ1-4G1c-Cer) is a member of the globo series glycosphingolipid family and is formed by the addition of GalNAc in αl,3-linkage to the terminal GalNAc residue of globoside (globotetraosylceramide). This reaction is catalyzed by globoside α-1,3-N-acetylgalactosaminyltransferase 1 (GBGT1). This enzyme is also called Forssman glycolipid synthase (FS). The canine FS cDNA was cloned from an MDCK-cell cDNA library using an expression cloning method (Haslam and Baenziger 1996). The isolated FS shows 42 % identity in the amino acid sequence to the histo-blood group A and B transferases (Yamamoto et al. 1990) and 35 % identity to the α1,3-galactosyltransferase (Joziasse et al. 1989). The A or B transferases transfer GalNAc or Gal in αl,3-linkage to the histo-H acceptor, respectively, and the α3 galactosyltransferase transfers Gal to the terminal Galβ1-4GlcNAc structure on glycoproteins as well as glycolipids. The close sequence identity and the similar enzyme reaction suggested that these glycosyltransferase genes have the same evolutionary origin (Haslam and Baenziger 1996). Furthermore, in humans, all these related glycosyltransferase genes are located on chromosome 9q34, supporting the hypothesis that they arose by gene duplication and subsequent divergence (Joziasse et al. 1992; Yamamoto et al. 1995; Xu et al. 1999), although the human FS gene encodes nonfunctional FS protein (Xu et al. 1999).
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Glycosyltransferase Gene
- Liquid Scintillation Technique
- Forssman Glycolipid
- Urinary Epithelial Cell
- GalNAc Transferase
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Forssman heterophilic glycolipid antigen (GalNAcαl-3GalNAcβl-3Galαl-4Galβ1-4G1c-Cer) is a member of the globo series glycosphingolipid family and is formed by the addition of GalNAc in αl,3-linkage to the terminal GalNAc residue of globoside (globotetraosylceramide). This reaction is catalyzed by globoside α-1,3-N-acetylgalactosaminyltransferase 1 (GBGT1). This enzyme is also called Forssman glycolipid synthase (FS). The canine FS cDNA was cloned from an MDCK-cell cDNA library using an expression cloning method (Haslam and Baenziger 1996). The isolated FS shows 42 % identity in the amino acid sequence to the histo-blood group A and B transferases (Yamamoto et al. 1990) and 35 % identity to the α1,3-galactosyltransferase (Joziasse et al. 1989). The A or B transferases transfer GalNAc or Gal in αl,3-linkage to the histo-H acceptor, respectively, and the α3 galactosyltransferase transfers Gal to the terminal Galβ1-4GlcNAc structure on glycoproteins as well as glycolipids. The close sequence identity and the similar enzyme reaction suggested that these glycosyltransferase genes have the same evolutionary origin (Haslam and Baenziger 1996). Furthermore, in humans, all these related glycosyltransferase genes are located on chromosome 9q34, supporting the hypothesis that they arose by gene duplication and subsequent divergence (Joziasse et al. 1992; Yamamoto et al. 1995; Xu et al. 1999), although the human FS gene encodes nonfunctional FS protein (Xu et al. 1999).
Databanks
IUBMB enzyme nomenclature: E.C.2.4.1.88
Globoside Alpha-1,3-N-acetylgalactosaminyltransferase 1 (GBGT1)
Species | Gene symbol | GenBank accession number | UniProt ID | PDB accession number |
|---|---|---|---|---|
Homo sapiens | GBGT1 | NM_021996 | Q8N5D6 | N/A |
Mus musculus | Gbgt1 | NM_139197 | Q8VI38 | N/A |
Canis familiaris | GBGT1 | NM_001003193 | Q95158 | N/A |
Gallus gallus | GBGT1 | NM_001030683 | Q5ZLK4 | N/A |
Name and History
In 1911, Forssman discovered that rabbits injected with a suspension of kidney tissue from guinea pig or horse, but not from cow or rat, produce antibodies that hemolyze sheep erythrocytes (Forssman 1911). This heterophilic antigen is present in a variety of species including mouse and dog (Forssman positive), but not in others such as human and other primates (Forssman negative) (Buchbinder 1935). The Forssman antigen is one of the most potent haptenic glycosphingolipids (Papirmeister and Mallette 1955). Its structure was proven to be GalNAcαl-3GalNAcβ1-3Galαl-4Galβ1-4Glcβ1-Cer (Siddiqui and Hakomori 1971). Forssman glycolipid synthase (FS), which is recommended to call Globoside α-1,3-N-acetylgalactosaminyltransferase 1(EC 2.4.1.88), catalyzes the transfer of N-acetylgalactosamine from UDP-GalNAc to the terminal GalNAc residue of globoside in α1,3-linkage. FS activity has been demonstrated in various mammalian tissues (Kijimoto et al. 1974; Taniguchi et al. 1981).
In 1996, the canine GBGT1 gene cDNA encoding FS was cloned through an expression cloning method using a monoclonal antibody against Forssman antigen (Haslam and Baenziger 1996). The isolated gene exhibited sequence homology to the previously cloned GGTA1 gene for α1,3-galactosyltransferase (Joziasse et al. 1989) and the ABO genes encoding the histo-blood group A and B transferases (Yamamoto et al. 1990). Later, other α1,3-galactosyltransferase family members, the A3GALT2 gene encoding isoglobotriaosylceramide (iGb3) synthase (Keusch et al. 2000) and the GT6m5, GT6m6, and GT6m7 genes encoding putative proteins (Turcot-Dubois et al. 2007), were identified.
Structure
Canine, mouse, and human FS consist of 347 amino acids (Haslam and Baenziger 1996; Elliott et al. 2003; Xu et al. 1999). The amino acid sequences predict a type II transmembrane topology as do all other Golgi-resident glycosyltransferases. FS, histo-blood group A and B glycosyltransferases, α1,3-galactosyltransferase, and iGb3 synthase are categorized in the GT6 family in the CAZy glycosyltransferase database (http://www.cazy.org/GlycosylTransferases.html). These enzymes are retaining glycosyltransferases that catalyze a reaction in which the anomeric configuration of the monosaccharide in the donor substrate (ain UDP-sugars) is retained in the product (Boix et al. 2002). The 3D structure of FS is predicted based on that of α1,3-galactosyltransferase and histo-blood group A and B transferases (Boix et al. 2001; Boix et al. 2002; Heissigerova et al. 2003), although FS has never been crystallized.
Enzyme Activity Assay and Substrate Specificity
A typical enzyme reaction is performed in a 100-μl reaction mixture containing 100 mM MES (pH 6.7), 10 mM MnCl2, 5 μM UDP-[3H]GalNAc, 20 μM globoside, and membrane extract as an enzyme source (Haslam and Baenziger 1996). After incubation at 37 °C for 2 h, the reaction is terminated by the addition of 1 ml ice-cold water containing EDTA. Glycolipid products are separated from unincorporated sugar nucleotide using a Sep-Pak C18 cartridge. The glycolipid fraction is then applied onto a TLC plate and developed with authentic Forssman glycolipid. The radioactivity corresponding to the standard glycolipid is determined by fluorography or a liquid scintillation technique. A unique assay method using anti-Forssman antibody is also employed (Taniguchi et al. 1982).
Canine spleen FS has a pH optimum at 6.7–6.9 and requires Mn2+ (Taniguchi et al. 1982). Studies on substrate specificity for canine spleen FS indicate that the enzyme recognizes GalNAcβ1-3Gal-R structure (Taniguchi et al. 1982). The recombinant canine FS did not act on the histo-H acceptor, N-acetyllactosamine, LacCer, globotriaosylceramide, or GM3 (Haslam and Baenziger 1996). In addition, it had no galactosyltransferase activity toward globoside, indicating that FS and SSEA-3 (Galβ1-3GalNAcβ1-3Galαl-4Galβ1-4Glcβ1-Cer) synthase are distinct enzymes (Haslam and Baenziger 1996).
Preparation
Forssman glycolipid synthase was purified over 3,500-fold in a 4 % yield from a Triton X-100 extract of canine spleen microsomes by affinity chromatography on globoside acid-agarose (Taniguchi et al. 1982). The purified enzyme preparation showed two major bands with apparent molecular weights of 56,000 and 66,000 on SDS-PAGE under reduced conditions. Since these proteins are too large for the 347 amino acid protein predicted from the cloned cDNA (Haslam and Baenziger 1996), the genuine Forssman glycolipid synthase might have been hidden behind the co-purified proteins.
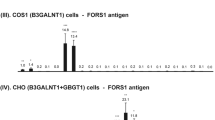
Recombinant canine FS was obtained from COS-1 cells transfected with the pFS-7 plasmid (Haslam and Baenziger 1996). The cDNA-introduced COS cells are homogenized by sonication. After the removal of nuclear fractions, membrane fractions are solubilized with 1 % Triton X-100 and used as an enzyme source.
Biological Aspects
Forssman glycolipid is expressed in a tissue-specific and developmentally regulated fashion in many mammals (Willison and Stern 1978). However, a biological function for Forssman glycolipid has not been identified. Unlike many other mammalian species, humans do not normally produce Forssman glycolipid but produce the precursor globoside, suggesting that human tissues lack Forssman synthase activity. Although the human FS gene is indeed expressed ubiquitously and generates a highly homologous protein with the canine enzyme, the gene product shows no αl,3-GalNAc transferase activity (Xu et al. 1999). Very recently, molecular basis of the human Forssman glycolipid negativity was elucidated by comparing the FS protein sequences between the Forssman-positive and Forssman-negative species (Yamamoto et al. 2012). In the human GBGT1 gene, two common missense mutations, G230S and Q296R, were identified. The reversion of these two mutations restored the GalNAc transferase activity to synthesize Forssman antigen in vitro. These findings confirmed the belief that human cells do not synthesize Forssman antigen and indicates that Forssman glycolipid is dispensable in terms of physiological functions. Since human FS retains 83 % amino acid sequence identity with the canine orthologue and is expressed widely in human tissues, the human FS may have another as yet unknown biochemical function, which is different from the GalNAc transferase activity.
Knockout and Transgenic Mice
Neither knockout mice nor transgenic mice of FS have been reported.
Human Disease
Glycosphingolipids serve as receptors for several pathogenic organisms (Karlsson 1989). Therefore, differences in glycolipid expression between species contribute to the tropism of many infectious pathogens for their hosts. The globo series glycosphingolipids have been reported to be an attachment site for bacteria, viruses, and bacterial toxins (Strömberg et al. 1990; Brown et al. 1993; Lingwood 1993; Jacewicz et al. 1994). For example, a canine Escherichia coli uropathogenic isolate cannot cling to human urinary epithelial cells, but expression of canine FS therein enables adherence by the same pathogen (Xu et al. 1999). Hence, the absence of active FS is thought to be advantageous for humans to evade infection. However, expression of canine and murine FS reduce the Shiga toxin (Stx) susceptibility in Vero cells through the decrease of the Stx receptor, globotriaosylceramide content, while human FS has no effect on it (Elliott et al. 2003). In this case, inactivation of the FS gene is supposed to be disadvantageous for humans. Mutual evolutionary pressure from host-microbial interactions may have contributed to diversity in glycolipid expression among species (Xu et al. 1999).
Despite the absence of FS activity in normal human tissues, Forssman antigen is detected in human tumors (Kawanami 1972; Hakomori et al. 1977; Yoda et al. 1980; Mori et al. 1982; Fredman 1993). Although the molecular mechanism of how Forssman glycolipid emerges in human tumors is unknown, somatic changes may enable human cells to produce Forssman glycolipid (Yamamoto et al. 2012). Some factors may activate the dormant FS in cancer tissues. Alternatively, other GalNAc transferases that are activated in tumors may synthesize Forssman glycolipid as a result of the looseness of substrate specificity. Future studies will be directed to this issue.
Future Perspectives
The CAZY GT6 gene family members are found in bird, fish, and amphibian genomes as well as mammalian. The number and type of GT6 genes vary widely from species to species, even within phylogenetically close groups (Turcot-Dubois et al. 2007). Moreover, some of the GT6 gene family members are inactivated, as seen in the case of human FS and α-1,3-galactosyltransferase. Thus, individual GT6 genes have expanded and contracted by recurrent duplications and deletions during vertebrate evolution (Turcot-Dubois et al. 2007). This birth and death evolution model seems to apply to gene families involved in interactions with the environment, including gene families of the immune system and the sensory system. The biological roles of the GT6 family members in the interaction between organisms and their environment are interesting open questions.
References
Boix E, Swaminathan GJ, Zhang Y, Natesh R, Brew K, Acharya KR (2001) Structure of UDP complex of UDP-galactose:b-galactoside a-1,3-galactosyltransferase at 1.53-A resolution reveals a conformational change in the catalytically important C terminus. J Biol Chem 276:48608–48614
Boix E, Zhang Y, Swaminathan GJ, Brew K, Acharya KR (2002) Structural basis of ordered binding of donor and acceptor substrates to the retaining glycosyltransferase, a-1,3-galactosyltransferase. J Biol Chem 277:28310–28318
Brown KE, Anderson SM, Young NS (1993) Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science 262:114–117
Bushbinder L (1935) Heterophile phenomena in immunology. Arch Pathol 19:841–880
Elliott SP, Yu M, Xu H, Haslam DB (2003) Forssman synthetase expression results in diminished shiga toxin susceptibility: a role for glycolipids in determining host-microbe interactions. Infect Immun 71:6543–6552
Forssman J (1911) Die Herstellung hochwertiger spezifischer Schafhämolysine ohne Verwendung von Schafblut. Ein Beitrag zur Lehre von heterologer Antikörpebildung. Biochem Z 37:78–115
Fredman P (1993) Glycosphingolipid tumor antigens. Adv Lipid Res 25:213–234
Hakomori S, Wang SM, Young WW Jr (1977) Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with “A-like antigen” in human cancer. Proc Natl Acad Sci USA 74:3023–3027
Haslam DB, Baenziger JU (1996) Expression cloning of Forssman glycolipid synthase: a novel member of the histo-blood group ABO gene family. Proc Natl Acad Sci USA 93:10697–10702
Heissigerova H, Breton C, Moravcova J, Imberty A (2003) Molecular modeling of glycosyltransferases involved in the biosynthesis of blood group A, blood group B, Forssman, and iGb3 antigens and their interaction with substrates. Glycobiology 13:377–386
Jacewicz MS, Mobassaleh M, Gross SK, Balasubramanian KA, Daniel PF, Raghavan S, McCluer RH, Keusch GT (1994) Pathogenesis of Shigella diarrhea. XVII. A mammalian cell membrane glycolipid, Gb3, is required but not sufficient to confer sensitivity to Shiga toxin. J Infect Dis 169:538–546
Joziasse DH, Shaper JH, Van den Eijnden DH, Van Tunen AJ, Shaper NL (1989) Bovine alpha-1-3-galactosyltransferase: isolation and characterization of a cDNA clone. Identification of homologous sequences in human genomic DNA. J Biol Chem 264:14290–14297
Joziasse DH, Shaper NL, Kim D, Van den Eijnden DH, Shaper JH (1992) Murine alpha 1,3-galactosyltransferase. A single gene locus specifies four isoforms of the enzyme by alternative splicing. J Biol Chem 267:5534–5541
Karlsson KA (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem 58:309–350
Kawanami J (1972) The appearance of Forssman hapten in human tumor. J Biochem 72:783–785
Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU (2000) Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isogloboglycosphingolipids. J Biol Chem 275:25308–25314
Kijimoto S, Ishibashi T, Makita A (1974) Biosynthesis of Forssman hapten from globoside by α-N-acetylgalactosaminyltransferase of guinea pig tissues. Biochem Biophys Res Commun 56:177–184
Lingwood CA (1993) Verotoxins and their glycolipid receptors. Adv Lipid Res 25:189–211
Mori E, Mori T, Sanai Y, Nagai Y (1982) Radioimmuno-thin-layer chromatographic detection of Forssman antigen in human carcinoma cell lines. Biochem Biophys Res Commun 108:926–932
Papirmeister B, Mallette MF (1955) The isolation and some properties of the Forssman hapten from sheep erythrocytes. Arch Biochem Biophys 57:94–105
Siddiqui B, Hakomori S (1971) A revised structure for the Forssman glycolipid hapten. J Biol Chem 246:5766–5769
Stromberg N, Marklund BI, Lund B, lIver D, Hamers A, Gaastra W, Karlsson KA, Normark S (1990) Host-specificity of uropathogenic Escherichia coli depends on differences in binding specificity to Gal alpha-I-4-Gal-containing isoreceptors. EMBO J 9:2001–2010
Taniguchi N, Yokosawa N, Narita M, Mitsuyama T, Makita A (1981) Expression of Forssman antigen synthesis and degradation in human lung cancer. J Natl Cancer Inst 67:577–583
Taniguchi N, Yokosawa N, Gasa S, Makita A (1982) UDP-N-acetylgalactosamine:globosideα-3-N-acetylgalactosa-minyltransferase. Purification, characterization, and some properties. J Biol Chem 257:10631–10637
Turcot-Dubois AL, Le Moullac-Vaidye B, Despiau S, Roubinet F, Bovin N, Le Pendu J, Blancher A (2007) Long-term evolution of the CAZY glycosyltransferase 6 (ABO) gene family from fishes to mammals – a birth and death evolution model. Glycobiology 17:516–528
Willison KR, Stern PL (1978) Expression of a Forssman antigenic specificity in the preim-plantation mouse embryo. Cell 14:785–793
Xu H, Storch T, Yu M, Elliott SP, Haslam DB (1999) Characterization of the human Forssman synthetase gene. An evolving association between glycolipid synthesis and host-microbial interactions. J Biol Chem 274:29390–29398
Yamamoto F, Marken J, Tsuji T, White T, Clausen H, Hakomori S (1990) Cloning and characterization of DNA complementary to human UDP-GaINAc: Fucα1-2Galα1-3GalNAc transferase (histoblood group A transferase) mRNA. J Biol Chem 265:1146–1151
Yamamoto F, McNeill PD, Hakomori S (1995) Genomic organization of human histoblood, group ABO genes. Glycobiology 5:51–58
Yamamoto M, Cid E, Yamamoto F (2012) Molecular genetic basis of the human Forssman glycolipid antigen negativity. Sci Rep 2:975
Yoda Y, Ishibashi T, Makita A (1980) Isolation, characterization, and biosynthesis of Forssman antigen in human lung and lung carcinoma. J Biochem 88:1887–1890
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this entry
Cite this entry
Honke, K. (2014). Globoside Alpha-1,3-N-Acetylgalactosaminyltransferase 1 (GBGT1). In: Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54240-7_55
Download citation
DOI: https://doi.org/10.1007/978-4-431-54240-7_55
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54239-1
Online ISBN: 978-4-431-54240-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences