Abstract
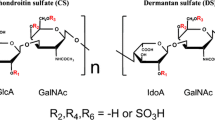
Chondroitin/dermatan sulfate contains GlcAβ1-3GalNAc(4, 6-bissulfate)β1-4 or IdoA α1-3GalNAc(4, 6-bissulfate)β1-4 repeating units at varying proportion depending on the biological sources of the glycosaminoglycans. Chondroitin sulfate (CS) containing this repeating unit was initially found in the cartilage of squid and named as CS-E. CS-E was found in vertebral cells and tissues such as mast cells or brain. Dermatan sulfate (DS) containing this repeating unit was found in mammalian organs such as liver, kidney, or spleen. N-Acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) is a sole enzyme that transfers sulfate to position 6 of GalNAc 4-sulfate residues of CS/DS and produces GlcAβ1-3GalNAc(4, 6-bissulfate)β1-4 or IdoA α1-3GalNAc(4, 6-bissulfate)β1-4 repeating units.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Chondroitin Sulfate
- Granule Cell Layer
- Dermatan Sulfate
- External Granule Cell Layer
- Internal Granule Cell Layer
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Chondroitin/dermatan sulfate contains GlcAβ1-3GalNAc(4, 6-bissulfate)β1-4 or IdoA α1-3GalNAc(4, 6-bissulfate)β1-4 repeating units at varying proportion depending on the biological sources of the glycosaminoglycans. Chondroitin sulfate (CS) containing this repeating unit was initially found in the cartilage of squid and named as CS-E. CS-E was found in vertebral cells and tissues such as mast cells or brain. Dermatan sulfate (DS) containing this repeating unit was found in mammalian organs such as liver, kidney, or spleen. N-Acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST) is a sole enzyme that transfers sulfate to position 6 of GalNAc 4-sulfate residues of CS/DS and produces GlcAβ1-3GalNAc(4, 6-bissulfate)β1-4 or IdoA α1-3GalNAc(4, 6-bissulfate)β1-4 repeating units.
Databanks
NC-IUBMB enzyme classification: EC 2.8.2.33
Carbohydrate (N-acetylgalactosamine 4-sulfate 6-O) sulfotransferase 15 (CHST15)
Species | Gene symbol | GenBank accession number | UniProt ID | PDB accession number |
|---|---|---|---|---|
Homo sapiens | CHST15 | NM_015892 | Q7LFX5 | N/A |
Mus musculus | Chst15 | NM_029935 | Q91XQ5 | N/A |
Rattus norvegicus | Chst15 | NM_173310 | Q8CHI9 | N/A |
Todarodes pacificus | GALNAC4S6ST | AB292855 | Q8WTN9 | N/A |
Name and History
Sulfotransferase activity that transfers sulfate to position 6 of GalNAc 4-sulfate residues was found in hen oviduct (Harada et al. 1967), squid cartilage (Habuchi et al. 1971), quail oviduct (Nakanishi et al. 1981), and human serum (Inoue et al. 1986) and purified to homogeneity from the squid cartilage (Ito and Habuchi 2000). A partial nucleotide sequence of squid GalNAc4S-6ST was revealed from the amino acid sequences of the purified enzyme. The sequence of squid GalNAc4S-6ST was used for the search of human database. The identified nucleotide sequence of human GalNAc4S-6ST was found to be nearly identical to the sequence of hBRAG isolated from Nalm-6 cells, a human pre-B cell line (Ohtake et al. 2001; Verkoczy et al. 1998), but deduced amino acid sequences of these proteins are different from each other because three missense mutations and two frameshift mutations were present in hBRAG. It has not been determined whether hBRAG protein retains the enzyme activity. A full nucleotide sequence of squid GalNAc4S-6ST was obtained by 5′- and 3′-RACE (Yamaguchi et al. 2007).
Structure
Both human and squid GalNAc4S-6ST are type II transmembrane proteins composed of 561 and 425 amino acid residues, respectively (Ohtake et al. 2001; Yamaguchi et al. 2007). There are five and seven N-glycosylation sites in human and squid GalNAc4S-6ST, respectively, and only one of these sites was conserved between the two species. Unlike squid enzyme, human GalNAc4S-6ST contains a long cytoplasmic domain composed of 81 amino acid residues. Both purified squid GalNAc4S-6ST and the recombinant human GalNAc4S-6ST expressed in COS-7 cells showed a broad protein band on SDS-PAGE, and the protein band was converted to a sharp one with lower molecular mass after removal of N-glycans by the digestion with N-glycosidase F, indicating that some of the N-glycosylation sites were actually N-glycosylated.
Enzyme Activity Assay and Substrate Specificity
GalNAc4S-6ST transfers sulfate to position 6 of GalNAc(4SO4) residue of CS/DS (Ito and Habuchi 2000; Ohtake et al. 2001) and chondroitin sulfate-derived oligosaccharides (Ito and Habuchi 2000; Ohtake et al. 2001, 2003; Yamaguchi et al. 2007).
In the assay of GalNAc4S-6ST, [35S]PAPS is used as a donor and CS-A is used as an acceptor (Ito and Habuchi 2000; Ohtake et al. 2001). After incubation, the sulfated glycosaminoglycan was isolated with ethanol precipitation and gel chromatography on Sephadex G-25 (Fast Desalting column, Amersham Pharmacia Biotech), and radioactivity was counted. To determine the activity toward oligosaccharides, the sulfated products were isolated with Superdex 30 gel chromatography (Habuchi et al. 1997). For determining the position of sulfate transferred to CS-A, 35S-labeled glycosaminoglycans were digested with chondroitinase ACII. The radioactive products formed after the enzymatic digestion were separated with HPLC using a Whatman Partisil-10 SAX column.
Human GalNAc4S-6ST transferred sulfate to position 6 of the nonreducing terminal and internal GalNAc(4SO4) residues contained in CS-A and DS (Ohtake et al. 2001). Nonreducing terminal GalNAc(4SO4) residues involved in a unique sequence found in CS-A, GalNAc(4SO4)-GlcA(2SO4)-GalNAc(6SO4), were efficiently sulfated by human GalNAc4S-6ST to form GalNAc(4, 6-SO4)-GlcA(2SO4)-GalNAc(6SO4) (Ohtake et al. 2003). CS-E synthesized by a recombinant human GalNAc4S-6ST showed binding activity toward midkine (Zou et al. 2003). DS that had been sulfated by a recombinant zebra fish GalNAc4S-6ST gained reactivity toward an antibody, GD3G7 (Purushothaman et al. 2007).
Squid GalNAc4S-6ST transferred sulfate to position 6 of GalNAc(4SO4) residues located mainly in the internal region of CS-A and DS (Ito and Habuchi 2000), and sulfation of the nonreducing terminal GalNAc(4SO4) residue in CS-A occurs when the GalNAc(4SO4) residue is located in the unique sequence, GalNAc(4SO4)-GlcA(2SO4)-GalNAc(6O4) (Yamaguchi et al. 2007). Unlike human GalNAc4S-6ST, squid GalNAc4S-6ST transferred sulfate to GalNAc(4SO4) residues included in a tetrasaccharide sequence, GlcA-GalNAc(4SO4)-GlcA(2SO4)-GalNAc(6SO4), present in shark cartilage CS-C and CS-D to form the E-D hybrid tetrasaccharide sequence, GlcA-GalNAc(4,6-SO4)-GlcA(2SO4)-GalNAc(6SO4) (Yamaguchi et al. 2007). CS-E synthesized by the purified squid GalNAc4S-6ST and a recombinant squid GalNAc4S-6ST showed binding activity toward midkine (Zou et al. 2003) and inhibition of thrombin activity in the absence of antithrombin III (Numakura et al. 2010), respectively.
Preparation
Squid GalNAc4S-6ST was purified from the extracts of squid cartilage with protamine precipitation, chromatography on Matrex Gel Red A, Heparin-Sepharose CL-6B, and 3′, 5′-ADP-agarose (Ito and Habuchi 2000). The purified GalNAc4S-6ST showed a broad protein band with a molecular mass of 63 kDa in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Human GalNAc4S-6ST has been expressed in COS-7 cells (Ohtake et al. 2001) or insect cells (Zou et al. 2003). Mouse GalNAc4S-6ST was expressed in COS-7 cells (Ohtake et al. 2008). Squid GalNAc4S-6ST has been expressed in COS-7 cells (Yamaguchi et al. 2007) and in insect cells (Numakura et al. 2010). Zebra fish GalNAc4S-6ST was expressed in COS-7 cells (Purushothaman et al. 2007).
Biological Aspects
Human GalNAc4S-6ST message was widely expressed in various human tissues including peripheral blood leukocyte, spleen, fetal spleen, lymph node, lung, stomach, and various brain tissues (Ohtake S, Habuchi O, unpublished data). Mouse GalNAc4S-6ST message was expressed strongly in cerebellum and decreased in the order of cerebrum, heart, kidney, liver, and lung (Ohtake-Niimi et al. 2010). Expression of GalNAc4S-6ST during early embryonic development of mouse was studied by whole mount in situ hybridization (Salgueiro et al. 2006). GalNAc4S-6ST was expressed in the anterior visceral ectoderm at stage E5.5, and the expression was restricted to the embryonic endoderm at E7.0. From E8.0 to E10.0, the expression was detected in the forebrain, branchial arches, gut tubes (hindgut, midgut, and foregut), vitelline veins and arteries, and splanchnopleure layer.
Expression of GalNAc4S-6ST determined by real-time RT-PCR analysis and CS-E unit both decreased during postnatal development of mouse cerebellum. In situ hybridization revealed that the level of GalNAc4S-6ST mRNA decreased in Purkinje cells, granular cells in the external granular layer, and inhibitory interneurons during development (Ishii and Maeda 2008a). The CS chains of the neocortex of mouse embryos contained significant amount of CS-E unit. GalNAc4S-6ST mRNA was expressed in the ventricular and subventricular zones, and the expression increased during late embryonic development. Knockdown of GalNAc4S-6ST resulted in the disturbed migration of cortical neurons (Ishii and Maeda 2008b). Immunohistochemical analysis of early-cultured neurons indicated that CS epitopes were accumulated in the focal contacts present in axons and cell bodies. Knockdown of GalNAc4S-6ST induced the formation of multiple axons in hippocampal neurons (Nishimura et al. 2010). Spot and Bonhoeffer stripe assays using astrocyte-conditioned media from siRNA-treated rat astrocytes showed a significant decrease in inhibition of attachment and neurite extensions of rat embryonic day 18 cortical neurons when compared to untreated and TGF α-treated astrocytes (Karumbaiah et al. 2011). In situ hybridization showed a wide expression of GalNAc4S-6ST transcript in the postnatal mouse brain except at postnatal day 7, where strong expression was observed in the external granule cell layer in the cerebellum. The expression switched from the external to internal granule cell layer with development (Purushothaman et al. 2007). CSPGs from the brain of GalNAc4S-6ST KO mice showed significant loss of inhibitory activity on neurite outgrowth of dissociated dorsal root ganglion neurons compared with CSPGs purified from wild-type mouse brain (Brown et al. 2012)
Bone marrow-derived mast cells (BMMCs) contain CS-E. When the bone marrow cells differentiate to BMMCs, level of chondroitin 4-sulfotransferase-1 (C4ST-1) and GalNAc4S-6ST messages increased, whereas chondroitin 6-sulfotransferase-1 (C6ST-1) message decreased. In the extract of BMMCs, enzyme activity of GalNAc4S-6ST and C4ST-1 was detected but C6ST-1 activity was not (Ohtake et al. 2008). Expression of C4ST-1 and GalNAc4S-6ST messages increased during maturation of bone marrow cells into CTMC-like BMMCs and MMC-like BMMCs (Duelli et al. 2009).
Knockout Mouse and Transgenic Mice
GalNAc4S-6ST-null mice were generated (Ohtake-Niimi et al. 2010). GalNAc4S-6ST-null mice were born normally and fertile. In GalNAc4S-6ST-null mice, GalNAc(4,6-SO4) residues in CS and DS disappeared completely, indicating that GalNAc4S-6ST should be a sole enzyme responsible for the synthesis of GalNAc(4,6-SO4) residues in both CS and DS. Bone marrow-derived mast cells (BMMCs) derived from GalNAc4S-6ST-null mice contained CS without GlcA-GalNAc(4,6-SO4) units. Tryptase and carboxypeptidase A activities of BMMCs derived from GalNAc4S-6ST-null mice were significantly lower than those activities of BMMCs derived from wild-type mice, although mRNA expression of these mast cell proteases was not altered. These observations suggest that CS containing GalNAc(4,6-SO4) residues in BMMCs may contribute to retain the active proteases in the granules of BMMCs
Human Disease
GalNAc4S-6ST was shown to express in colorectal cancer cells. In 57.5 % of 40 patients, expression of GalNAc4S-6ST mRNA was increased in cancer tissues compared with paired normal mucosa (Ito et al. 2007).
Future Perspectives
GalNAc4S-6ST is a sole enzyme that is able to synthesize CS/DS with GalNAc(4,6-SO4) residues (highly sulfated CS/DS). Several studies have provided evidence that the highly sulfated CS/DS plays important roles in the neural system. Analysis of GalNAc4S-6ST-null mice under the whole-body level or tissue/cellular level might uncover new roles of the highly sulfated CS/DS in the neural system. The highly sulfated CS/DS is present in various mouse organs such as liver, kidney, and spleen. Function of highly sulfated CS/DS in these organs remains to be studied.
Further Reading
-
Ito and Habuchi (2000): Purification of GalNAc4S-6ST from squid cartilage.
-
Ohtake et al. (2001): Cloning and characterization of human GalNAc4S-6ST.
-
Ohtake-Niimi et al. (2010): Generation and characterization of GalNAc4S-6ST-null mice.
-
Yamaguchi et al. (2007): Cloning and characterization of squid GalNAc4S-6ST.
References
Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC (2012) A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc Natl Acad Sci USA 109:4768–4773
Duelli A, Rönnberg E, Waern I, Ringvall M, Kolset SO, Pejler G (2009) Mast cell differentiation and activation is closely linked to expression of genes coding for the serglycin proteoglycan core protein and a distinct set of chondroitin sulfate and heparin sulfotransferases. J Immunol 183:7073–7083
Habuchi O, Yamagata T, Suzuki S (1971) Biosynthesis of the acetylgalactosamine 4,6-disulfate unit of squid chondroitin sulfate by transsulfation from 3′-phosphoadenosine 5′-phosphosulfate. J Biol Chem 246:7357–7365
Habuchi O, Suzuki Y, Fukuta M (1997) Sulfation of sialyl lactosamine oligosaccharides by chondroitin 6-sulfotransferase. Glycobiology 7:405–412
Harada T, Shimizu S, Nakanishi Y, Suzuki S (1967) Enzymatic transfer of sulfate from 3′-phosphoadenosine 5′-phosphosulfate to uridine diphosphate N-acetylgalactosamine 4-sulfate. J Biol Chem 242:2288–2290
Inoue H, Otsu K, Suzuki S, Nakanishi Y (1986) Difference between N-acetylgalactosamine 4-sulfate 6-O-sulfotransferases from human serum and squid cartilage in specificity toward the terminal and interior portion of chondroitin sulfate. J Biol Chem 261:4470–4475
Ishii M, Maeda N (2008a) Spatiotemporal expression of chondroitin sulfate sulfotransferases in the postnatal developing mouse cerebellum. Glycobiology 18:602–614
Ishii M, Maeda N (2008b) Oversulfated chondroitin sulfate plays critical roles in the neuronal migration in the cerebral cortex. J Biol Chem 283:32610–32620
Ito Y, Habuchi O (2000) Purification and characterization of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase from the squid cartilage. J Biol Chem 275:34728–34736
Ito Y, Watanabe M, Nishizawa T, Omachi T, Kobayashi T, Kasama S, Habuchi O, Nakayama J (2007) The utility of formalin-fixed and paraffin-embedded tissue blocks for quantitative analysis of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase mRNA expressed by colorectal cancer cells. Acta Histochem Cytochem 40:53–59
Karumbaiah L, Anand S, Thazhath R, Zhong Y, McKeon RJ, Bellamkonda RV (2011) Targeted downregulation of N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase significantly mitigates chondroitin sulfate proteoglycan-mediated inhibition. Glia 59:981–996
Nakanishi Y, Shimizu M, Otsu K, Kato S, Tsuji M, Suzuki S (1981) A terminal 6-sulfotransferase catalyzing a synthesis of N-acetylgalactosamine 4,6-bissulfate residue at the nonreducing terminal position of chondroitin sulfate. J Biol Chem 256:5443–5449
Nishimura K, Ishii M, Kuraoka M, Kamimura K, Maeda N (2010) Opposing functions of chondroitin sulfate and heparan sulfate during early neuronal polarization. Neuroscience 169:1535–1547
Numakura M, Kusakabe N, Ishige K, Ohtake-Niimi S, Habuchi H, Habuchi O (2010) Preparation of chondroitin sulfate libraries containing disulfated disaccharide units and inhibition of thrombin by these chondroitin sulfates. Glycoconj J 27:479–486
Ohtake S, Ito Y, Fukuta M, Habuchi O (2001) Human N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase cDNA is related to human B cell recombination activating gene-associated gene. J Biol Chem 276:43894–43900
Ohtake S, Kimata K, Habuchi O (2003) A unique nonreducing terminal modification of chondroitin sulfate by N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase. J Biol Chem 278:38443–38452
Ohtake S, Kondo S, Morisaki T, Matsumura K, Kimata K, Habuchi O (2008) Expression of sulfotransferases involved in the biosynthesis of chondroitin sulfate E in the bone marrow derived mast cells. Biochim Biophys Acta 1780:687–695
Ohtake-Niimi S, Kondo S, Ito T, Kakehi S, Ohta T, Habuchi H, Kimata K, Habuchi O (2010) Mice deficient in N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase are unable to synthesize chondroitin/dermatan sulfate containing N-acetylgalactosamine 4, 6-bissulfate residues and exhibit decreased protease activity in bone marrow-derived mast cells. J Biol Chem 285:20793–20805
Purushothaman A, Fukuda J, Mizumoto S, ten Dam GB, van Kuppevelt TH, Kitagawa H, Mikami T, Sugahara K (2007) Functions of chondroitin sulfate/dermatan sulfate chains in brain development. Critical roles of E and iE disaccharide units recognized by a single chain antibody GD3G7. J Biol Chem 282:19442–19452
Salgueiro A-M, Filipe M, Belo J-A (2006) N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase expression during early mouse embryonic development. Int J Dev Biol 50:705–708
Verkoczy LK, Marsden PA, Berinstein NL (1998) HBRAG, a novel B cell lineage cDNA encoding a type II transmembrane glycoprotein potentially involved in the regulation of recombination activating gene 1 (RAG1). Eur J Immunol 28:2839–2853
Yamaguchi T, Ohtake S, Kimata K, Habuchi O (2007) Molecular cloning of squid N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase and synthesis of a unique chondroitin sulfate containing E-D hybrid tetrasaccharide structure by the recombinant enzyme. Glycobiology 17:1365–1376
Zou P, Zou K, Muramatsu H, Ichihara-Tanaka K, Habuchi O, Ohtake S, Ikematsu S, Sakuma S, Muramatsu T (2003) Glycosaminoglycan structures required for strong binding to midkine, a heparin binding growth factor. Glycobiology 13:35–42
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this entry
Cite this entry
Habuchi, O. (2014). Carbohydrate (N-Acetylgalactosamine 4-Sulfate 6-O) Sulfotransferase 15 (CHST15). In: Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54240-7_44
Download citation
DOI: https://doi.org/10.1007/978-4-431-54240-7_44
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54239-1
Online ISBN: 978-4-431-54240-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences