Abstract
Isoglobotriaosylceramide or isogloboside 3 (iGb3) has been the subject of intense research by numerous laboratories and has provoked lively debate in the literature since it was suggested to be the endogenous ligand involved in thymic selection of a subset of natural killer T cells (iNKT) in both mice and humans. iGb3 is the first member of the isoglobo-series glycosphingolipids and is synthesized by alpha 1,3-galactosyltransferase 2.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Isoglobotriaosylceramide or isogloboside 3 (iGb3) has been the subject of intense research by numerous laboratories and has provoked lively debate in the literature since it was suggested to be the endogenous ligand involved in thymic selection of a subset of natural killer T cells (iNKT) in both mice and humans. iGb3 is the first member of the isoglobo-series glycosphingolipids and is synthesized by alpha 1,3-galactosyltransferase 2.
Databanks
IUBMB enzyme nomenclature: EC 2.4.1.87
Alpha 1,3-galactosyltransferase 2, pseudogene (A3GALT2P)
Species | Gene symbol | GenBank accession number | UniProt ID | PDB accession number |
|---|---|---|---|---|
Mus musculus | A3galt2 | NC_000070.6 | Q3V1N9 | N/A |
Rattus norvegicus | A3galt2 | NC_005104.3 | A0A4Z3 | N/A |
Homo sapiens | A3GALT2P | NG_012483 | N/A | N/A |
Name and History
Alpha 1,3-galactosyltransferase 2, also referred to as isoglobotriaosylceramide synthase or iGb3 synthase (iGb3S), catalyses the transfer of galactose (Gal) to lactosylceramide (Lac-Cer) to form iGb3 (Galα1,3Galβ1,4Glc-Cer). Here we will refer to this galactosyltransferase as iGb3S.
The glycosphingolipid iGb3 was originally chemically identified in rat spleen (Stoffyn et al. 1973b). Subsequently iGb3 has been found in dog intestine (Sung and Sweeley 1979), non-epithelial cells of the small intestine of the rat (Angstrom et al. 1982b; Breimer et al. 1982), in the small intestine of the cat (Teneberg et al. 2004), and more recently in the dorsal root ganglion (Speak et al. 2007), thymus (Milland et al. 2006; Li et al. 2009; Porubsky et al. 2012), and dendritic cells (Li et al. 2009) of the mouse. Furthermore, a fucosylated form of iGb3, Fucα1,2Galα1,3Gal®1,4Glc-Cer, has been identified in the pig (Slomiany et al. 1974; Diswall et al. 2007; Puga Yung et al. 2012) and the rat large intestine (Hansson et al. 1980). In addition, novel branched or hybrid-type variants of the isogloboside series have also been identified in rat (Hansson et al. 1987), horse (Yamamoto et al. 1999), and salmon (Niimura 2006).
Other glycosyltransferases can utilize iGb3 as a substrate to produce other glycosphingolipids. The next member of the isogloboside series, iGb4 GalNAcα1,3Galα1,3Galβ1,4Glc-Cer also known as cytolipin R, has been identified in the small intestine of the rat (Angstrom et al. 1982a; Breimer et al. 1982), rat lymphosarcoma (Laine et al. 1972), and in the rat kidney (Siddiqui et al. 1972) as well as in the thymus and dendritic cells of the mouse (Li et al. 2009).
The cDNA clone encoding iGb3S was originally isolated from a rat placental cDNA library by expression cloning using Chinese hamster ovary (CHO) cells (Keusch et al. 2000). Other members of the isogloboside series, iGb4 and iGb5, are produced in transfected CHO cells (Keusch et al. 2000). Mouse iGb3S has also been isolated from GGTA1−/−thymus mRNA (Milland et al. 2006).
Structure
The amino acid sequence of iGb3S assigns it to the Family 6 of glycosyltransferases [as defined by the Glycosyltransferase Family Server of the CAZy (carbohydrate-active enzymes) database] that are all retaining enzymes that transfer either αGal or αGalNAc. Other members of this family include the α1,3-galactosyltransferase 1 (GGTA1), the A and B blood group glycosyltransferases, and the Forssman synthase (Milland and Sandrin 2006). iGb3S is a type II integral membrane protein of 339 amino acids in the rat (Keusch et al. 2000) and 370 amino acids in the mouse (Milland et al. 2006). The difference in size is due to the cytoplasmic domain of the mouse being atypically long for a glycosyltransferase, having an insertion of 31 amino acids before the conserved 38RAKKR42 flanking the cytoplasmic domain (Milland et al. 2006). The transmembrane domain of the rat is one residue larger (19 amino acids) than the mouse. The C-terminal catalytic domain, containing the essential DVD sequence, has 91 % amino acid identity between these species, with a conserved pattern of cysteine residues (Keusch et al. 2000; Milland et al. 2006). In both rat and mouse, the iGb3S coding regions are encoded by five exons with conserved exon and intron lengths and consensus sequences at the exon-intron junctions (Milland et al. 2006).
Enzyme Activity Assay and Substrate Specificity
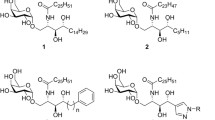
An in vitro assay for this enzyme was developed to examine substrate specificity using transfected cell lysates and supernatants (Keusch et al. 2000). The reaction buffer was 100 mM sodium cacodylate pH 6.8, 0.5 % Triton X-110, 5 mM ATP, 250 μM UDP-Gal, UDP-[3H]Gal, 15 mM MnCl2, and protease inhibitors in a final volume of 100 μl. Cell lysates/supernatants and acceptor substrates were incubated at 37 °C for 90 min to overnight, before reverse-phase chromatography using Sep-Pak C18 cartridges and analysis by TLC. Using this assay, iGb3S was able to utilize Lac-Cer, Gal-Cer, and Gb3 to produce Galα1,3Galβ1,4Glc-Cer (iGb3), Galα1,3Gal-Cer, and Galα1,3Galα1,4Galβ1,4Glc-Cer, respectively (Keusch et al. 2000) (See Fig. 12.1).
Transfection of the rat iGb3S into CHOP cells (Chinese hamster ovary cells transformed with polyoma large T antigen) results in the synthesis of poly-α1,3Gal glycolipids (Taylor et al. 2003). Two types of poly-α1,3Gal glycosphingolipids have been identified chemically in the rat (Angstrom et al. 1982a, b; Breimer et al. 1982; Ariga et al. 1989) and cat (Teneberg et al. 2004), found either on Gb3 or iGb3 (Fig. 12.1), and it is highly likely that these are produced by iGb3S. For Gb3 the number of α1,3Gal moieties identified ranges from 1 to 5 (Angstrom et al. 1982a, b; Breimer et al. 1982; Ariga et al. 1989), and for iGb3, one α1,3Gal has been identified (Teneberg et al. 2004), although the data from Taylor suggest up to five additional α1,3Gal can be added to iGb3 (Taylor et al. 2003). In addition, globoside-like terminated forms (terminating in GalNAcβ1,3) of the poly-α1,3Gal glycolipids have also been identified (Angstrom et al. 1982a; Breimer et al. 1982) (Fig. 12.2).
Preparation
Unlike other glycosyltransferases, protocols to purify iGb3S from tissues have not been developed. However, iGb3 has been synthesized in vitro using lactosylceramide and microsomes isolated from rat spleen cells (Stoffyn et al. 1973a), rat bone marrow cells (Stoffyn et al. 1973a), and rat kidney cells (Stoffyn et al. 1974). More recently, a recombinant form of mouse iGb3S has been produced in insect cells and used to synthesize iGb3 in vitro (Zhou et al. 2004).
Biological Aspects
Interest in a potential biological role for iGb3 arose from the observation that a glycosphingolipid isolated from the marine sponge Agelas mauritianus, α-galactosylceramide (α-Gal-Cer), was a potent agonist for NKT cells in a CD1d-dependent manner in both mice and humans (Kawano et al. 1997; Brossay et al. 1998). However, as this glycosphingolipid is not produced in mammals, the physiological relevance was unclear. Subsequently, several glycosphingolipids with terminal αGal were examined for their ability to activate iNKT cells. This resulted in iGb3 being proposed to be the main endogenous ligand responsible for iNKT cell development and self-recognition in both mice and humans (Zhou et al. 2004). However, this was initially challenged by two publications (Porubsky et al. 2007; Speak et al. 2007). Using a highly sensitive HPLC assay, Speak et al. failed to detect iGb3 in mouse or human thymus (Speak et al. 2007). Whereas Porubsky et al. more strongly challenged the significance of iGb3 in mouse iNKT cell development by reporting normal numbers of NKT cells in the thymus of iGb3−/−mice (Porubsky et al. 2007).
Membrane bound glycolipids are known to be attachment sites for bacteria and bacterial toxins (Karlsson 1989), and products of iGb3S are no exceptions: enterohemorrhagic Escherichia coli have been shown to bind to Galα1,3Galα1,3Galβ1,4Glc-Cer, but not Galα1,3Galα1,4Galβ1,4Glc-Cer isolated from cat small intestine (Teneberg et al. 2004).
Knockout Mouse and Transgenic Mice
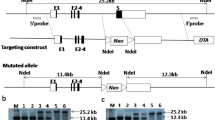
iGb3 synthase knockout (iGb3S−/−) mice have been generated by targeting the coding sequence of exon 5 and replacing this with a neomycin selection cassette in C57BL/6 ES cells (Porubsky et al. 2007). Homozygous iGb3S−/−mice grow and breed normally, with no evident signs of developmental or behavioral defects (Porubsky et al. 2007). Furthermore, these mice had normal iNKT cells numbers (in the thymus, spleen, and liver) and TCR Vb usage. In addition, iNKT cells and dendritic cells from either iGb3S−/−or wild-type mice responded to α-Galcerin in an identical manner (Porubsky et al. 2007). These data strongly suggest that iGb3 is not the endogenous ligand for iNKT cell selection.
To date there are no reports regarding iGb3S transgenic mice.
Human Disease
The issue of iGb3 expression in humans is of major importance to both xenotransplantation and NKT cell biology. If humans express iGb3S, iGb3 lipid on transplanted pig tissues would not pose a problem, as tissue would not be recognized as foreign. Conversely, if humans do not express functional iGb3S, then expression of iGb3 on pig cells could lead to NKT cell activation resulting in destruction of the xenograft. Although it has been suggested that human thymus express extremely low levels of iGb4 (and therefore by inference iGb3) (Li et al. 2008), extensive analysis of several human tissues (heart, lung, kidney, spleen, and thymus) failed to detect spliced iGb3S mRNA (Christiansen et al. 2008). Indeed, expression of chimaeric molecules, containing the catalytic domain of human iGb3S with the remaining portion from rat iGb3S (cytoplasmic tail to stalk region), were unable to synthesize iGb3 (Christiansen et al. 2008). Furthermore, site-directed mutagenesis used to analyze which amino acid(s) contributed to the loss of function showed that substitution of rat Y252N resulted in the complete elimination of iGb3, whereas L187P showed a significant reduction (typically 70–95 %). Reverse mutation of the nonfunctional chimaeric human iGb3S to their rat equivalents with either point mutation alone (i.e., P187L or N252Y), or in combination (P187L+N252Y), did not lead to a gain of function, implying that human iGb3S must have other mutations that are important for its inactivation (Christiansen et al. 2008). Thus, even if human iGb3S was expressed at either the mRNA or protein level, it would be nonfunctional due to several mutations that differentiate the human enzyme from its functional counterpart in the rat, and therefore, human A3GALT2P is a non-processed pseudogene.
Future Perspectives
The potential role of iGb3 as the principle endogenous ligand for iNKT cell development and function in mice and, albeit indirectly, in humans still represents one of the most important and controversial issues in the iNKT cell field. A fundamental question that remains to be answered is if iGb3 is expressed in humans due to an unidentified functional allele, does this have any significance in iNKT biology? Another intriguing question is the molecular basis for the unique characteristic of iGb3S to utilize multiple substrates with different anomeric configurations.
Further Reading
-
Christiansen et al. 2008: First publication to show that humans lack iGb3 due to the absence of functional iGb3S.
-
Keusch et al. 2000: First report on cloning of iGb3S.
-
Milland et al. 2006: Demonstrated the molecular basis for Galα(1,3)Gal expression in animals with a deletion of the GGTA1 gene.
-
Porubsky et al. 2007: Demonstrated normal iNKT cell development in iGb3S knockout mice.
-
Speak et al. 2007: Described lack of iGb3 in human thymus.
-
Zhou et al. 2004: Suggested that iGb3 was the main endogenous ligand responsible for iNKT cell development and self -recognition in both mice and humans.
References
Angstrom J, Breimer ME, Falk KE, Hansson GC, Karlsson KA, Leffler H (1982a) Chemical characterization of penta-, hexa-, hepta-, octa-, and nonaglycosylceramides of rat small intestine having a globoside-like terminus. J Biol Chem 257:682–688
Angstrom J, Breimer ME, Falk KE, Hansson GC, Karlsson KA, Leffler H, Pascher I (1982b) Structural characterization of glycolipids of rat small intestine having one to eight hexoses in a linear sequence. Arch Biochem Biophys 213:708–725. doi:0003-9861(82)90601-4 [pii]
Ariga T, Suzuki M, Yu RK, Kuroda Y, Shimada I, Inagaki F, Miyatake T (1989) Accumulation of unique globo-series glycolipids in PC 12 h pheochromocytoma cells. J Biol Chem 264:1516–1521
Breimer ME, Hansson GC, Karlsson KA, Leffler H (1982) Glycosphingolipids of rat tissues. Different composition of epithelial and nonepithelial cells of small intestine. J Biol Chem 257:557–568
Brossay L, Chioda M, Burdin N, Koezuka Y, Casorati G, Dellabona P, Kronenberg M (1998) CD1d-mediated recognition of an alpha-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med 188:1521–1528
Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, Godfrey DI, Sandrin MS (2008) Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol 6:e172. doi:07-PLBI-RA-3601 [pii] 10.1371/journal.pbio.0060172
Diswall M, Angstrom J, Schuurman HJ, Dor FJ, Rydberg L, Breimer ME (2007) Studies on glycolipid antigens in small intestine and pancreas from alpha1,3-galactosyltransferase knockout miniature swine. Transplantation 84:1348–1356. doi:10.1097/01.tp.0000287599.46165.15 00007890-200711270-00018 [pii]
Hansson GC, Karlsson KA, Thurin J (1980) Glycolipids of rat large intestine. Characterization of a novel blood group B-active tetraglycosylceramide absent from small intestine. Biochim Biophys Acta 620:270–280. doi:0005-2760(80)90208-8 [pii]
Hansson GC, Bouhours JF, Angstrom J (1987) Characterization of neutral blood group B-active glycosphingolipids of rat gastric mucosa. A novel type of blood group active glycosphingolipid based on isogloboside. J Biol Chem 262:13135–13141
Karlsson KA (1989) Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem 58:309–350. doi:10.1146/annurev.bi.58.070189.001521
Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M (1997) CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science 278:1626–1629
Keusch JJ, Manzella SM, Nyame KA, Cummings RD, Baenziger JU (2000) Expression cloning of a new member of the ABO blood group glycosyltransferases, iGb3 synthase, that directs the synthesis of isoglobo-glycosphingolipids. J Biol Chem 275:25308–25314. doi:10.1074/jbc.M002629200 M002629200 [pii]
Laine R, Sweeley CC, Li YT, Kisic A, Rapport MM (1972) On the structure of cytolipin R, a ceramide tetrahexoside hapten from rat lymphosarcoma. J Lipid Res 13:519–524
Li Y, Teneberg S, Thapa P, Bendelac A, Levery SB, Zhou D (2008) Sensitive detection of isoglobo and globo series tetraglycosylceramides in human thymus by ion trap mass spectrometry. Glycobiology 18:158–165. doi:cwm129 [pii] 10.1093/glycob/cwm129
Li Y, Thapa P, Hawke D, Kondo Y, Furukawa K, Hsu FF, Adlercreutz D, Weadge J, Palcic MM, Wang PG, Levery SB, Zhou D (2009) Immunologic glycosphingolipidomics and NKT cell development in mouse thymus. J Proteome Res 8:2740–2751. doi:10.1021/pr801040h
Milland J, Sandrin MS (2006) ABO blood group and related antigens, natural antibodies and transplantation. Tissue Antigens 68:459–466. doi:TAN721 [pii] 10.1111/j.1399-0039.2006.00721.x
Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS (2006) The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3galactosyltransferase gene. J Immunol 176:2448–2454. doi:176/4/2448 [pii]
Niimura Y (2006) Structural analysis of a unique hybrid-type ganglioside with isoglobo-, neolacto-, and ganglio-core from the gills of the Pacific salmon (Oncorhynchus keta). Carbohydr Res 341:2669–2676. doi:S0008-6215(06)00367-3 [pii] 10.1016/j.carres.2006.07.016
Porubsky S, Speak AO, Luckow B, Cerundolo V, Platt FM, Grone HJ (2007) Normal development and function of invariant natural killer T cells in mice with isoglobotrihexosylceramide (iGb3) deficiency. Proc Natl Acad Sci USA 104:5977–5982. doi:0611139104 [pii] 10.1073/pnas.0611139104
Porubsky S, Speak AO, Salio M, Jennemann R, Bonrouhi M, Zafarulla R, Singh Y, Dyson J, Luckow B, Lehuen A, Malle E, Muthing J, Platt FM, Cerundolo V, Grone HJ (2012) Globosides but not isoglobosides can impact the development of invariant NKT cells and their interaction with dendritic cells. J Immunol 189:3007–3017. doi:jimmunol.1201483 [pii] 10.4049/jimmunol.1201483
Puga Yung GL, Li Y, Borsig L, Millard AL, Karpova MB, Zhou D, Seebach JD (2012) Complete absence of the alphaGal xenoantigen and isoglobotrihexosylceramide in alpha1,3galactosyltransferase knock-out pigs. Xenotransplantation 19:196–206. doi:10.1111/j.1399-3089.2012.00705.x
Siddiqui B, Kawanami J, Li YT, Hakomori S (1972) Structures of ceramide tetrasaccharides from various sources: uniqueness of rat kidney ceramide tetrasaccharide. J Lipid Res 13:657–662
Slomiany BL, Slomiany A, Horowitz MI (1974) Characterization of blood-group-H-active ceramide tetrasaccharide from hog-stomach mucosa. Eur J Biochem 43:161–165
Speak AO, Salio M, Neville DC, Fontaine J, Priestman DA, Platt N, Heare T, Butters TD, Dwek RA, Trottein F, Exley MA, Cerundolo V, Platt FM (2007) Implications for invariant natural killer T cell ligands due to the restricted presence of isoglobotrihexosylceramide in mammals. Proc Natl Acad Sci USA 104:5971–5976. doi:0607285104 [pii] 10.1073/pnas.0607285104
Stoffyn P, Stoffyn A, Hauser G (1973a) Structure of trihexosylceramide biosynthesized in vitro. J Biol Chem 248:1920–1923
Stoffyn P, Stoffyn A, Hauser G (1973b) Structure of trihexosylceramide isolated from rat spleen. Biochim Biophys Acta 306:283–286. doi:0005-2760(73)90233-6 [pii]
Stoffyn A, Stoffyn P, Hauser G (1974) Structure of trihexosylceramide biosynthesized in vitro by rat kidney galactosyltransferase. Biochim Biophys Acta 360:174–178
Sung SS, Sweeley CC (1979) The structure of canine intestinal trihexosylceramide. Biochim Biophys Acta 575:295–298. doi:0005-2760(79)90031-6 [pii]
Taylor SG, McKenzie IF, Sandrin MS (2003) Characterization of the rat alpha(1,3)galactosyltransferase: evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1,3)Gal by two separate glycosylation pathways. Glycobiology 13:327–337. doi:10.1093/glycob/cwg030
Teneberg S, Angstrom J, Ljungh A (2004) Carbohydrate recognition by enterohemorrhagic Escherichia coli: characterization of a novel glycosphingolipid from cat small intestine. Glycobiology 14:187–196. doi:10.1093/glycob/cwh015
Yamamoto H, Iida-Tanaka N, Kasama T, Ishizuka I, Kushi Y, Handa S (1999) Isolation and characterization of a novel Forssman active acidic glycosphingolipid with branched isoglobo-, ganglio-, and neolacto-series hybrid sugar chains. J Biochem 125:923–930
Zhou D, Mattner J, Cantu C 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786–1789. doi:1103440 [pii] 10.1126/science.1103440
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this entry
Cite this entry
Christiansen, D., Mouhtouris, E., Sandrin, M.S. (2014). Alpha 1,3-Galactosyltransferase 2, Pseudogene (A3GALT2P). In: Taniguchi, N., Honke, K., Fukuda, M., Narimatsu, H., Yamaguchi, Y., Angata, T. (eds) Handbook of Glycosyltransferases and Related Genes. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54240-7_119
Download citation
DOI: https://doi.org/10.1007/978-4-431-54240-7_119
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54239-1
Online ISBN: 978-4-431-54240-7
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences