Abstract
Spinal cord ischemia is one of the most dreadful complications of thoracic and thoracoabdominal aortic surgery. The complex mechanisms of spinal cord perfusion under iatrogenic circumstances, such as surgery, are still not fully unraveled. Neuromonitoring during open and endovascular aortic surgery aims at assessing perioperative spinal cord function, allowing corrective measures in case of compromised spinal cord perfusion and thus reducing the incidence of postoperative spinal cord ischemia. The technique of motor evoked potentials is a reliable and highly accurate method to evaluate spinal cord integrity during thoracic and thoracoabdominal aortic repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Spinal cord
- Neuromonitoring
- Motor evoked potentials
- Magnetic stimulation
- Somatosensory evoked potentials
1 Introduction
Since more than two decades, spinal cord ischemia (SCI) after complex aortic surgery has increasingly become a prominent subject in vascular surgical conferences and publications. Research on prevention of spinal cord ischemia has led to the introduction of several perioperative protective measures, such as reimplantation of segmental arteries, hypothermia, extracorporeal circulation protocols, cerebrospinal fluid drainage, strict blood pressure management, and neuromonitoring. The main goal of neuromonitoring during and after open and endovascular thoracic and thoracoabdominal aortic surgery is to assess spinal cord function during the procedure and subsequently guide surgical and hemodynamic strategies to prevent spinal cord ischemia with ensuing neurological deficit. In the case of thoracoabdominal aortic aneurysm (TAAA) repair, there is abundant experience with two different neuromonitoring modalities: somatosensory evoked potentials (SSEPs) and motor evoked potentials (MEPs). This book chapter describes the background of spinal cord perfusion, both neuromonitoring techniques, and their clinical usefulness and applicability.
2 Spinal Cord Perfusion and Its Preservation
In general, spinal cord ischemic injury results from temporary or permanent interruption of spinal cord blood supply. Ischemic injury caused by temporary interruption of blood flow depends on its duration and degree of hypoperfusion, indicated by increased paraplegia rates after prolonged aortic cross-clamp times. Therefore, to understand the mechanisms and prevention of spinal cord ischemia, it is important to be familiar with the physiology of spinal cord perfusion and the pathological changes that develop during and after either aortic dissection or degenerative aneurysmal disease.
In summary, the anterior spinal artery and two posterolateral spinal arteries, supplemented by longitudinal and transverse anastomoses, provide the main arterial blood supply to the spinal cord. Large inflow vessels include the subclavian artery via the vertebral artery, the thyrocervical and costocervical trunks, the intercostal and lumbar arteries, and the hypogastric arteries through the lateral sacral and iliolumbar arteries: all with substantial regional and interindividual variability [1, 2]. Additionally, an extensive paraspinous arterial network is contributing to the collateral vasculature of the spinal cord [3].
Little is known about hemodynamics within the arterial and venous vessels of the spinal cord. Bidirectional and reversible currents of blood flow in the longitudinal arteries indicate the existence of watershed zones. Critical areas of vascular supply are specifically endangered if arterial inflow is diminished and the necessary collateral circulation provided by the anastomoses of the arterial pathways is inadequate. In addition to reduced arterial inflow during aortic repair, reversal of blood flow in the spinal arteries may cause steal phenomena with subsequent ischemic damage. Under pathological conditions, such as aortic cross-clamping and endografting, spinal cord autoregulation is disturbed or abolished [4, 5]. Gray and white matter blood supply then depends mainly on the local blood pressure. Finally, local spinal cord hemodynamics is significantly influenced by venous drainage. Elevated central venous pressure during and after aortic procedures might play a role in the development of spinal cord ischemia, especially in phases with a critically low arterial inflow.
Interpreting this anatomic and dynamic information, it is obvious that SCI is a multifactorial phenomenon in which individual (anatomical and physiological) components vary and interact differently between individuals. In patients with descending thoracic aortic aneurysm (DTAA) or thoracoabdominal aortic aneurysm (TAAA), the segmental artery supplying the Adamkiewicz artery (AKA) often is occluded by intraluminal aortic thrombus or aortic atherosclerotic wall changes. In fact, in patients with degenerative aneurysms, the majority of intercostal and lumbar arteries are occluded, whereas in post-dissection aneurysms, the majority of segmental vessels are open. Therefore, preoperative information about the location and patency of the feeding segmental artery can be important for operative planning and postoperative neurologic outcome. Computed tomography angiography and magnetic resonance angiography have been utilized to delineate the AKA and collateral circulation in case of segmental artery occlusion [6,7,8,9]. Using these techniques, it has become apparent how important the lower lumbar and pelvic arteries are with regard to collateral circulation, contributing to the spinal cord circulation in 16% and 8% of cases, respectively [10].
During open and endovascular aortic surgery, a variety of adjunctive measures can be performed to maintain adequate spinal cord perfusion and thereby reduce spinal cord ischemia, including distal aortic perfusion, cerebrospinal fluid (CSF) drainage, intercostal artery reconstruction, and neuron-protective adjuncts such as hypothermia and endorphin receptor blockage. Without assessment of spinal cord function perioperatively, however, the effects of these measures only become evident as the patient regains consciousness. In the case of paraplegia, this is obviously too late. Therefore assessment of spinal cord function and integrity during the procedure is of crucial importance, allowing interventions to prevent ischemia and paraplegia. Ideally, perioperative monitoring should be as reliable as possible, i.e., be sensitive enough to detect any relevant malfunction of the spinal cord but also as specific as possible to avoid unnecessary precautionary measures.
3 Motor Evoked Potential (MEP) Monitoring
Motor evoked potentials (MEPs) when applied during aortic repair are recorded from muscles following stimulation of the motor cortex, either electrically or magnetically. The amplitude of the MEP mainly reflects the number of functioning motor units, which are located in the anterior horn of the spinal cord. The spinal cord blood flow to the anterior horn of the spinal cord, e.g., the gray matter, is three times higher than to the white matter (dorsal column), and accordingly, the motor units in the anterior horn are extremely sensitive to hypoperfusion.
In our MEP monitoring protocol, the motor cortex is electrically stimulated by means of three silver/silver chloride surface electrodes fixed to the skull just posterior to the vertex. These electrodes are connected to the cathode of the stimulation device, whereas the anode is connected to three electrodes placed over the mastoids and the forehead, respectively. A series of five stimuli is administered with an interstimulus interval of 2 ms and strength of 500 V and 1.3–1.5 A each, the latter being dependent on the resistance at the interface between the stimulation electrode and the skin. Stimulation takes place every 1–5 min depending on the stage of the surgical procedure. The strong electric current will give rise to a rather nonspecific activation of the intracranial motor tract, including the motor cortex, as well as the pyramidal tract at brain stem level [11]. All action potentials traveling caudally along the pyramidal tract will be summarized at the motor neuron in the anterior horn of the spinal cord. The motor neuron, in turn, will activate the muscle fibers to which it is attached. Therefore, even though a series of stimuli is applied and several stages of the motor tract are stimulated, a single muscle contraction will be the result. The muscle twitch of the anterior tibial and the abductor pollicis brevis muscle is recorded with adhesive surface electrodes placed over the muscle belly. These electrodes pick up the sum of all action potentials that are involved in the contraction of the muscle fibers. Therefore, the recorded signal is actually a “compound muscle action potential” (CMAP), although the term CMAP is usually reserved for potentials caused by stimulation of peripheral nerves. See Fig. 79.1 for an outline of the stimulation and registration method.
The principle of electrically evoked motor potentials. For reasons of clarity, only the right side of the body is depicted. An electrical stimulator is connected to the skull with the cathode (black solid line) placed just behind the vertex and the anode (red solid line) at both mastoids and the forehead. Application of a stimulus to the skull will result in a series of action potentials traveling along the spinal cord (dashed black line). These action potentials will reach the motor neuron in the anterior horn of the spinal cord (open black circle) and activate the peripheral nerve (dotted black line), subsequently causing in a muscle twitch of the anterior tibial muscle and the abductor pollicis brevis muscle. The amplitude of the twitch is readily measurable. The cursors (1 and 2) automatically detect the lowest and highest value of the curve within a given time frame
Myogenic MEPs are only measurable in the presence of a muscle twitch. On the other hand, movement of the patient should be limited for obvious reasons. A partial muscle relaxation complies with both conditions. We apply a target of approximately 80% muscle relaxation; however, in practice, the extent of movement might lead to adjustment of this value. To monitor muscle relaxation, the right ulnar nerve is supramaximally stimulated at the wrist throughout the surgical procedure, and the resulting twitch of the abductor digiti quinti muscle is recorded. The amplitude of the CMAP at any given moment is compared with the first value obtained before any muscle relaxant (either atracurium, rocuronium, or vecuronium) is administered. Administration of the muscle relaxant is adjusted according to the CMAP values, and hence a constant level of neuromuscular blockade can be accomplished (Fig. 79.2).
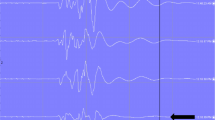
Illustration of a patient with uneventful MEP neuromonitoring. We use three different graphics; within all three the time is depicted on the x-axis. (a) On the left y-axis, the MEP amplitudes of the right leg (thick red line), the left leg (thick blue line), the right hand (thin red line), and the left leg (thin blue line) are depicted. On the right y-axis, the degree of muscle relaxation is given as the percentage of the CMAP of the abductor digiti quinti muscle compared to the value before muscle relaxation (green line). Note the parallel course of all five registrations throughout the entire monitoring procedure. Additionally, spontaneous fluctuations of the MEP amplitude between successive measurements are clearly visible, most obvious in the trace of the right hand. (b) On the left y-axis, the ratio of the amplitude of the MEP of each leg and the mean of the MEPs of both hands are given (red line right leg; blue line left leg). This representation of the data allows for rapid detection of MEP changes solely affecting the legs. Note that the steep rise and fall of the MEPs of the anterior tibial (TA) muscles at approximately 11:30 (see a) are practically invisible because the MEPs of the upper extremities showed the same changes that obviously were due to a marked variation in the degree of muscle relaxation (APB abductor pollicis brevis). (c) Same as a, except for the MEPs of the upper extremities, which are not displayed. Instead, the blood pressure as measured in the right arm (yellow line) and the right leg (purple line) is given on the right y-axis in mmHg. RR refers to blood pressure
Furthermore, the peroneal nerve is bilaterally stimulated in between the cortical stimulation. Not only does this measurement provide us with additional information on the degree of muscle relaxation, which might not be the same in all muscles measured, but it also gives information on the proper functioning of the recording system since the resulting CMAP of the anterior tibial muscle is measured with the same electrodes and cables that are used for the MEP responses. The amplitude values of all four MEPs as well as three CMAPs are manually inserted in a spreadsheet combined with the intra-arterially measured blood pressure values. In this way, the data can be displayed graphically during the surgical procedure, which improves the interpretation, especially with regard to the relationship between the different parameters (Fig. 79.2).
The anesthetic regimen has to be geared to be able to elicit myogenic MEPs. Isoflurane especially has a deleterious effect on measurement of MEPs [12]; in our protocol, sufentanil, etomidate, and midazolam are utilized during the induction of anesthesia, and during the surgical procedure, etomidate is replaced by ketamine.
3.1 Magnetic Stimulation
Transcranial electrical stimulation is too painful to be performed in a conscious patient. In contrast to an electrical field, a magnetic field is not substantially hindered by the structures protecting the brain from the outer world, such as the skull and meninges. A painless magnetic stimulus delivered by means of a coil placed on the head will easily reach, for example, the motor cortex. Therefore, the introduction of transcranial magnetic stimulation enables evaluation of the central motor pathways in the conscious neurological patient.
Magnetic brain stimulation during aortic surgery, however, offers no advantages as compared to electrical stimulation. On the contrary, it is more cumbersome since the stimulation device is much larger and more expensive. The stimulation coil is very bulky; it will cover almost the whole skull and is quite difficult to fixate, which will result in spontaneously variable MEP amplitudes. Moreover, the MEPs evoked by means of magnetic stimulation are more susceptible to anesthetic agents [13].
3.2 SSEP Monitoring
Somatosensory evoked potentials provide monitoring for the dorsal columns of the spinal cord. Usually, the tibial nerve is stimulated electrically by means of surface electrodes placed over the nerve at the medial malleolus. The cortical response is measured just posterior to the vertex, e.g., by means of silver/silver chloride cup electrodes. Since the amplitude of the response is at least one order of a magnitude smaller than the randomly occurring EEG activity, averaging of several stimuli is mandatory. Typically, stimuli are administered with a rate of approximately 3 per second (bilaterally alternating); approximately 100 responses are needed to establish a signal with a sufficient signal/noise ratio that will allow for a reliable interpretation. So, in practice, new information is provided every minute. In addition to the cranial recording, the responses at the knee (popliteal fossa) and at the level of the cervical spine (C5) can be recorded to allow for a differentiation between ischemia of the leg, the spinal cord, and the brain [14]. Furthermore, stimulation of the median nerve might allow for ruling out systemic influences being responsible for any SSEP change [15].
Regarding the spinal cord, SSEPs test the functional integrity of the dorsal column system, which exclusively consists of white matter, i.e., only axons and no neuronal cell nuclei nor synapses. This has major implications for the potential of SSEPs to guide the surgeon in preventing the patient from developing paraplegia. First, as described above, the spinal cord blood flow to the white matter is only approximately one-third of the flow to the gray matter, e.g., the motor neurons in the anterior horn, suggesting that the dorsal column system is less susceptible to spinal cord ischemia. Additionally, the low metabolic need of the dorsal column system is likely to be responsible for the fact that SSEP changes due to ischemia occur with a relatively long latency (approximately 7–18 min) [16]. Finally, the dorsal column system is supplied with the blood by a pair of two posterior spinal arteries, which might account for misinterpretations of the function of the motor tract since the latter is supplied by the anterior spinal artery. However, it should be kept in mind that in practice, all three spinal arteries resemble a collateral network rather than isolated arteries, with interindividual variability being the rule rather than the exception [17]. Nonetheless, all factors mentioned might have contributed to the main result of our study on the role of SSEPs in predicting outcome during TAAA repair, which showed that the use of SSEPs was a poor screening tool for the development of a neurological deficit [18].
3.3 Simultaneous MEP and SSEP Monitoring
Previously, several authors reported that SSEPs were inferior to MEPs in terms of correctly identifying spinal cord ischemia [18,19,20]. The simultaneous use of both SSEPs and MEPs might theoretically improve the sensitivity for detecting impending spinal cord damage. However, a more recent study could not confirm that a combined approach was superior to MEP alone in terms of preventing spinal cord ischemia [21]. The possible advantage of performing SSEP monitoring in case MEP monitoring is not functional depends on the reliability of the latter. In our series of MEP monitoring in almost 800 patients, we succeeded in recording myogenic MEPs in every patient, i.e., the measurement was 100% technically feasible. Moreover, the fact that in our experience the presence or absence of MEPs to the anterior tibial muscle at the end of the surgical procedure predicted the immediate neurological outcome (paraplegia) with a sensitivity and specificity of 100% leads to our opinion that introduction of another monitoring modality is not indicated.
4 MEP Neuromonitoring During Open Thoracoabdominal Aortic Surgery
Our surgical protocol in the European Vascular Center Aachen-Maastricht includes CSF drainage, spinal cord function monitoring with MEPs, distal aortic perfusion, intercostal artery reattachment, and, if necessary, systemic hypothermia. Neurophysiological assessment is done centrally in the Department of Neurophysiology in Maastricht, whereas thoracoabdominal aneurysm repair is performed in both locations, Aachen and Maastricht. Therefore, we have established an online Internet connection to transmit neuromonitoring signals from the patient in the operating room in Aachen to the neurophysiologist in Maastricht. Moreover, online telephone connection between both centers allows continuous exchange of information on MEPs and surgical interventions in case of MEP disturbances. Over the last five years, four other centers in Switzerland and Germany have joined our telemonitoring system and have since then treated more than 300 TAAA patients using remote MEP neuromonitoring [22].
In order to preserve spinal cord blood supply as long as possible, we try to clamp the thoracoabdominal aorta in sequential steps, especially in post-dissection aneurysms in which the majority of segmental vessels are patent. However, in many cases, the configuration of the aneurysm does not allow sequential clamping, and the aorta has to be excluded over a long distance. To determine a baseline level of the MEPs, measurements are first taken every 5 min before the aorta is cross-clamped and subsequently every minute after the clamping maneuver, while we wait and observe the MEPs during a period of 5 min. Following cross-clamping, decrease in MEP amplitude can occur, and elevating distal aortic pressure usually normalizes evoked potentials (Fig. 79.3). ECC is routinely established before aortic cross-clamping. In normotensive patients, the average distal aortic pressure during cross-clamping is normally maintained at about 60 mmHg. This perfusion pressure, however, may not be sufficient in particular patients having impaired collateral circulation (e.g., occlusion of hypogastric arteries) or patients with hypertension and concomitant vascular degeneration, especially when a relatively long segment of the lower descending aorta is cross-clamped. An inadequate perfusion pressure is reflected by an amplitude decrease of the MEPs compared to those measured before ECC was initiated. Thus, in these patients the distal perfusion pressure has to be adjusted accordingly. Moreover, increasing the systemic mean arterial pressure also contributes to improved spinal cord perfusion.
Influence of distal perfusion pressure on MEP amplitude of the lower extremities. On the left y-axis, the MEP amplitude is given in mV (red, right TA muscle; blue, left TA muscle), and on the right y-axis, the mean arterial blood pressure in the right brachial artery (yellow) and femoral artery (purple) is given in mmHg as well as the degree of muscle relaxation (green). The CMAP of the right abductor digiti quinti muscle is compared with the initial situation before intubation and expressed as a percent value. At the first red vertical bar (1), the aorta is still cross-clamped. The distal perfusion had to be stopped, causing a sharp decrease of blood pressure in the right femoral artery. Note that within 1 min, both MEPs of the lower extremities also showed a marked decrease, which was fully reversible after adequate perfusion was reestablished (second red vertical bar; 2)
When the MEPs of the anterior tibial muscle consistently decrease or even disappear (despite an adequate distal perfusion pressure) after clamping an aortic segment, revascularization of intercostal or lumbar arteries is mandatory. In case of rapid deterioration, we take the clamps off and cool the patient to 32 °C as an additional measure for neuron protection. Thereafter, the clamps are replaced, the aorta is opened, and relevant (back-bleeding) segmental arteries are identified. Depending on the quality of the aortic tissue and the progress of the work involved in aortic reconstruction, the surgeon has to decide whether selective bypass grafting or insertion of the segmental arteries by an onlay anastomosis is more practical. In general, intercostal or lumbar arteries in solid aortic tissue are reimplanted, whereas segmental arteries in fragile and mushy material will need selected grafts. Once the anastomoses are completed, perfusion of the segmental arteries can be reestablished by either using a selective perfusion catheter or by antegrade or (artificial) retrograde aortic blood flow. Our approach is to perfuse such selective grafts with a side branch of the extracorporeal circuit in order to provide blood to the cord as soon as possible. Depending on the duration of MEP impairment, restoration of the MEP amplitude may occur immediately or after some delay. If these surgical measures are effective, a partial or even complete restoration of the MEPs ought to be noted (Fig. 79.4), reflecting an improved blood flow within the collateral vascular network.
The amplitude of the MEP of both anterior tibial (TA) and abductor pollicis brevis (APB) muscles as well as the degree of muscle relaxation is given in panel (a). The ratio of the MEP of each TA muscle and the mean of both abductor pollicis brevis muscles are given in panel (b), whereas panel (c) displays the mean arterial blood pressure as measured in the right brachial and femoral artery. The first vertical red bar indicates aortic cross-clamping, which is followed by a complete loss of the MEPs of the TA muscle several minutes later. Shortly after reimplantation of four segmental arteries at T8/9 level (second vertical red bar), the MEPs of the lower extremities reappear. Possibly, reimplantation of another two segmental arteries (third vertical red bar) also contributes to a marked improvement, finally resulting in a complete normalization of the amplitude as compared with the amplitude of the abductor pollicis brevis muscle (b). Note the marked fluctuations of all MEP amplitudes in the beginning of the procedure, which perfectly follow the degree of muscle relaxation (a). Because this affects all MEPs, the ratio of leg and arm MEPs remains rather stable (b). Increasing the distal perfusion pressure immediately after cross-clamping could not prevent the loss of the leg MEPs (c)
In the most extreme case in which no ostia of intercostal and lumbar arteries are identifiable in the opened aortic segment, a local endarterectomy is necessary, which frequently unmasks back-bleeding segmental arteries. Since these arteries obviously play a role in the collateral circulation, we treat these with a selective graft, subsequently revascularizing the spinal cord. The aortic tissue around the intercostal arteries to be reimplanted should be excluded as much as possible in order to avoid aneurysm formation of the reattached button, indicating that the sutures should be as close as possible to the orifices of the segmental arteries. Small balloon occlusion catheters in the intercostal arteries not only stop back-bleeding but can also indicate the anatomic direction and course of the vessels that might be helpful in avoiding suturing through such an intercostal vessel (Fig. 79.5).
Example of MEP restitution after aortic wall endarterectomy and intercostal artery reattachment. The MEP amplitudes to all four extremities as well as the degree of muscle relaxation are displayed as in Fig. 79.2. (a) At approximately 15:14, the entire descending aorta was cross-clamped. Initially, no MEP changes occurred. First, the MEP response only to the left leg started to decrease, a finding that is often encountered in ischemia of the leg but only very rarely associated with spinal cord ischemia. With a delay of almost half an hour, the MEP of the right leg also showed a gradual decrease. After extensive search and aortic wall endarterectomy, two intercostal arteries at the level of T11/12 could be identified and revascularized by means of two polyester grafts. Blood supply of the spinal cord via these intercostal arteries was effective from 17:12 onward. With a latency of only a few minutes, this led to a recovery of the MEP of the right leg after 1 h of absence. The MEP of the left leg followed shortly thereafter; it had been absent for the strikingly long period of 1 h and 30 min. Finally, the original MEP amplitudes were achieved. Fluctuations of the MEP amplitudes due to an unstable degree of muscle relaxation are clearly visible. (b) Relating the MEP responses of the legs to those of the arms obviously allows for a better depiction of the relevant changes of the MEPs of the lower extremities
Another situation might occur on opening of the aneurysmal sac: severe back-bleeding from intercostal and lumbar arteries accompanied by a sudden decrease in the spinal cord perfusion pressure. As a result of the so-called steal effect, an immediate decrease or loss of MEPs can be noted, indicating critical spinal cord ischemia. In this case, it is important to control bleeding to restore the spinal cord perfusion. At this point, however, it is not clear whether revascularization of the back-bleeding arteries is necessary. Therefore, the authors prefer a temporary balloon occlusion of intercostal and/or lumbar arteries with three French Pruitt balloon catheters. Once back-bleeding is sufficiently controlled, the MEP amplitudes have to be monitored carefully. In this case, it can be helpful to further increase the distal perfusion pressure. A spontaneous recovery of the MEPs indicates that a steal effect was responsible for the observed MEP alterations and a particular revascularization is not necessary (Fig. 79.6). If the MEPs show a prolonged depression and do not adequately recover, the aforementioned immediate active surgical interventions are necessary to prevent irreversible spinal cord injury. After revascularization, full or partial recovery of the MEPs should then be noted. However, in a few patients, a persistent loss of the MEPs despite a technically successful revascularization was observed. This observation correlated with a postoperative paraplegia.
The MEP amplitudes of both TA and abductor pollicis brevis muscles as well as the degree of muscle relaxation are shown (a). In addition, the mean arterial blood pressure as measured in the right brachial and femoral artery is displayed (b). The first vertical red bar (1) indicates aortic cross-clamping excluding almost the entire descending aorta, which is followed by an immediate decrease of the MEP of the left TA muscle. Vessel wall inspection revealed back-bleeding of a segmental artery in the excluded segment. After preventing further back-bleeding by introducing a blocking catheter (2), an immediate restoration of the MEP amplitude of the left leg can be observed. Note the relatively high distal perfusion pressure during the decline of the MEP. The MEP of the right leg had already disappeared before and recovered only later after reimplantation of several segmental arteries, which may reflect a combination of peripheral and spinal cord ischemia
At the end of the surgery, neuromonitoring is terminated in our institution; however we are currently designing a postoperative MEP neuromonitoring protocol. Moreover, a recent publication demonstrated a beneficial effect of extended MEP monitoring in preventing delayed paraplegia after open thoracoabdominal aortic surgery [23].
5 MEP Neuromonitoring During Endovascular Thoracoabdominal Aortic Surgery
There is significantly fewer data on the use and value of neuromonitoring during endovascular treatment of DTAA or TAAA than during open surgery. Spinal cord monitoring during treatment of descending thoracic aortic aneurysms with TEVAR allows assessment of spinal cord function during the procedure, and, in cases where evoked potentials decreased significantly, the value of adequate blood pressure management was learned: increasing mean arterial pressure was rapidly followed by improved evoked potentials [24,25,26,27]. Moreover, neuromonitoring during TEVAR has been used to identify patients with the need for postoperative cerebrospinal fluid drainage. In our institution, routine CSF drainage is performed during the majority of TEVAR procedures (such as in cases of distal DTAA repair, long aortic length coverage, and previous abdominal aortic aneurysm repair), but neuromonitoring is not routinely used. Therefore, we apply strict postoperative blood pressure management maintaining mean arterial pressures above 85 mmHg during the first 24–48 h post TEVAR. In contrast, during endovascular branched or fenestrated repair of type I–III thoracoabdominal aortic aneurysms, we routinely apply neuromonitoring as a vital part of our strategy to prevent paraplegia by identifying high-risk patients with the need for a staged repair. Our protocol consists of general anesthesia, CSF drainage for 2–3 days postoperatively, and spinal cord function monitoring by measuring MEPs [28]. MEPs are measured as described previously in this chapter. In case of a branched (one or more) stent graft configuration, renal and visceral arteries are connected to the main stent graft with covered stents up to the last branch (usually the branch for the celiac artery). The last branch is subsequently occluded with a balloon for a period of 15 min during which MEPs are measured every minute. Blood pressure during this period is maintained stable. If MEPs do not significantly decrease, the last branch is connected to the visceral artery using a covered stent, as in the other branches. However, in case of a decrease of MEPs by more than 50%, the balloon is deflated, and the procedure is terminated leaving the last branch open, creating a type III endoleak (Fig. 79.7a) and maintaining aneurysm sac perfusion (Fig. 79.8).
(a) Schematic illustration of branched TEVAR before (left) and during balloon occlusion of the celiac branch (right). MEPs are adequate before but decrease significantly during balloon occlusion. In this scenario, the celiac branch is left open and will be connected in a secondary procedure. (b) Schematic illustration of fenestrated TEVAR. After exclusion of the aneurysm, the MEPs decrease significantly (left). MEPs recover after creation of a type III endoleak between stent graft components (right) [28]
MEP registration during branched repair of type II TAAA including bilateral iliac branch devices. The amplitude of the MEP registration at the level of the anterior tibial muscle (blue line: left leg / red line: right leg) is shown. The amplitude of the MEP of the pollicis brevis muscle is not shown. First, the MEPs of the right leg disappear due to peripheral ischemia during sheath occlusion of the femoral artery at insertion of the main devices and right iliac branched device (IBD) (first arrow). After the main devices and right IBD are deployed, leg perfusion is restored, and MEPs return to normal. The MEPs from the left leg exhibit an identical pattern during IBD deployment (second arrow). During connection of the left renal artery (arrow LRA), right renal artery (arrow RRA), and superior mesenteric artery (arrow SMA), the MEPs remain at normal level. However, during balloon occlusion of the celiac artery (CA), MEPs decrease by 60%. After balloon deflation, MEPs return to normal, and the celiac artery branch is left open
In case of a stent graft configuration with only fenestrations, a guide wire is left between one of the proximal stent grafts and the fenestrated stent graft. When, after stenting of all fenestrations and thereby excluding the aneurysm from the circulation, MEPs decrease by more than 50%, a type III endoleak is created by placing a balloon expandable stent between stent graft components (Fig. 79.7b) or by leaving open the contralateral limb of the bifurcated stent graft.
In cases of an open branch or another deliberately created type III endoleak, a second procedure to exclude aneurysm sac perfusion completely is planned after 4–6 weeks, depending on the physical status of the patient. The aneurysm is excluded by connecting the open branch with a covered stent, connecting the contralateral limb or by occluding a balloon expandable stent in the branch with a plug. Again, in all cases a test balloon occlusion is performed with continuous MEP monitoring (Fig. 79.9). Until now, we have not had any patients with significant (>50%) MEP changes during the secondary procedure.
MEP registration during the second stage of branched repair of type III TAAA. The amplitude of the MEP registration at the level of the anteriortibial muscle relative to the amplitude of the pollicis brevis muscle is displayed (blue line: left leg/red line: right leg). During balloon occlusion of the celiac artery branch (CA), MEPs stay unchanged. The procedure is finished by connecting the branch to the celiac artery (arrow CA)
The advantage of MEP neuromonitoring as part of our endovascular TAAA protocol is that it identifies patients at risk for spinal cord ischemia. The majority of patients does not exhibit MEP changes and can be treated in a one-stage procedure and are no longer at risk for rupture. In patients identified as high-risk for paraplegia, waiting for the second procedure is part of the protocol but comprises the risk of rupture during the time interval.
6 Conclusion
As paraplegia is clearly one of the most severe complications of open and endovascular DTAA and TAAA repair, a strict protocol for neuroprotection has to be followed. This protocol should include adjunctive procedures, such as extracorporeal circulation for distal aortic perfusion, CSF drainage, active blood pressure management, hypothermia, and neuromonitoring, which altogether contribute to improved clinical outcome.
Monitoring MEPs is a reliable technique to assess spinal cord function and integrity.
Altered aortic and peripheral circulation induced by cross-clamping or deployment of thoracic stent grafts during TAAA repair, causing spinal cord ischemia, can be detected by this technique, providing online information on spinal cord function, allowing adjunctive measures to correct or improve spinal cord perfusion, and identifying patients with a high risk for developing spinal cord ischemia in need for a staged procedure. The technique is highly reproducible and reliable. However, it will show its full potential only in an environment where the surgeon, anesthesiologist, perfusionist, and clinical neurophysiologist interact together while being fully aware of the necessity to approach the patient as a team.
References
Melissano G, Bertoglio L, Rinaldi E, Leopardi M, Chiesa R. An anatomical review of spinal cord blood supply. J Cardiovasc Surg. 2015;56:699–706.
Melissano G, Civilini E, Bertoglio L, Calliari F, Campos Moraes Amato A, Chiesa R. Angio-CT imaging of the spinal cord vascularisation: a pictorial essay. Eur J Vasc Endovasc Surg. 2010;39:436–40.
Etz CD, Kari FA, Mueller CS, Silovitz D, Brenner RM, Lin HM, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg. 2011;141:1020–8.
Bosmia AN, Hogan E, Loukas M, Tubbs RS, Cohen-Gadol AA. Blood supply to the human spinal cord: part I. Anatomy and hemodynamics. Clin Anat. 2015;28:52–64.
Bosmia AN, Tubbs RS, Hogan E, Bohnstedt BN, Denardo AJ, Loukas M, et al. Blood supply to the human spinal cord: part II. Imaging and pathology. Clin Anat. 2015;28:65–74.
Nijenhuis RJ, Jacobs MJ, Jaspers K, Reinders M, van Engelshoven JM, Leiner T, et al. Comparison of magnetic resonance with computed tomography angiography for preoperative localization of the Adamkiewicz artery in thoracoabdominal aortic aneurysm patients. J Vasc Surg. 2007;45:677–85.
Nijenhuis RJ, Jacobs MJ, Schurink GW, Kessels AG, van Engelshoven JM, Backes WH. Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg. 2007;45:71–7.
Yamada N, Takamiya M, Kuribayashi S, Okita Y, Minatoya K, Tanaka R. MRA of the Adamkiewicz artery: a preoperative study for thoracic aortic aneurysm. J Comput Assist Tomogr. 2000;24:362–8.
Yoshioka K, Niinuma H, Ohira A, Nasu K, Kawakami T, Sasaki M, et al. MR angiography and CT angiography of the artery of Adamkiewicz: noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. Radiographics. 2003;23:1215–25.
Jacobs MJ, de Mol BA, Elenbaas T, Mess WH, Kalkman CJ, Schurink GW, et al. Spinal cord blood supply in patients with thoracoabdominal aortic aneurysms. J Vasc Surg. 2002;35:30–7.
Rothwell J, Burke D, Hicks R, Stephen J, Woodforth I, Crawford M. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481:243–50.
Ubags LH, Kalkman CJ, Been HD. Influence of isoflurane on myogenic motor evoked potentials to single and multiple transcranial stimuli during nitrous oxide/opioid anesthesia. Neurosurgery. 1998;43:90–4.
Ubags LH, Kalkman CJ, Been HD, Koelman JH, Ongerboer de Visser BW. A comparison of myogenic motor evoked responses to electrical and magnetic transcranial stimulation during nitrous oxide/opioid anesthesia. Anesth Analg. 1999;88:568–72.
Achouh PE, Estrera AL, Miller CC 3rd, Azizzadeh A, Irani A, Wegryn TL, et al. Role of somatosensory evoked potentials in predicting outcome during thoracoabdominal aortic repair. Ann Thorac Surg. 2007;84:782–7.
Shahin GM, Hamerlijnck RP, Schepens MA, Ter Beek HT, Vermeulen FE, Boezeman EH. Upper and lower extremity somatosensory evoked potential recording during surgery for aneurysms of the descending thoracic aorta. Eur J Cardiothorac Surg. 1996;10:299–304.
Sloan TB, Jameson LC. Electrophysiologic monitoring during surgery to repair the thoraco-abdominal aorta. J Clin Neurophysiol. 2007;24:316–27.
Krauss WE. Vascular anatomy of the spinal cord. Neurosurg Clin N Am. 1999;10:9–15.
Meylaerts SA, Jacobs MJ, van Iterson V, De Haan P, Kalkman CJ. Comparison of transcranial motor evoked potentials and somatosensory evoked potentials during thoracoabdominal aortic aneurysm repair. Ann Surg. 1999;230:742–9.
Dong CC, MacDonald DB, Janusz MT. Intraoperative spinal cord monitoring during descending thoracic and thoracoabdominal aneurysm surgery. Ann Thorac Surg. 2002;74:S1873–6.
van Dongen EP, Schepens MA, Morshuis WJ, ter Beek HT, Aarts LP, de Boer A, et al. Thoracic and thoracoabdominal aortic aneurysm repair: use of evoked potential monitoring in 118 patients. J Vasc Surg. 2001;34:1035–40.
Keyhani K, Miller CC 3rd, Estrera AL, Wegryn T, Sheinbaum R, Safi HJ. Analysis of motor and somatosensory evoked potentials during thoracic and thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2009;49:36–41.
Greiner A, Mess WH, Schmidli J, Debus ES, Grommes J, Dick F, et al. Cyber medicine enables remote neuromonitoring during aortic surgery. J Vasc Surg. 2012;55:1227–32.
See RB, Awosika OO, Cambria RP, Conrad MF, Lancaster RT, Patel VI, et al. Extended motor evoked potentials monitoring helps prevent delayed paraplegia after aortic surgery. Ann Neurol. 2016;79:636–45.
Schurink GW, Nijenhuis RJ, Backes WH, Mess W, de Haan MW, Mochtar B, et al. Assessment of spinal cord circulation and function in endovascular treatment of thoracic aortic aneurysms. Ann Thorac Surg. 2007;83:S877–81.
Weigang E, Hartert M, Siegenthaler MP, Pitzer-Hartert K, Luehr M, Sircar R, et al. Neurophysiological monitoring during thoracoabdominal aortic endovascular stent graft implantation. Eur J Cardiothorac Surg. 2006;29:392–6.
Weigang E, Hartert M, Siegenthaler MP, Beckmann NA, Sircar R, Szabo G, et al. Perioperative management to improve neurologic outcome in thoracic or thoracoabdominal aortic stent-grafting. Ann Thorac Surg. 2006;82:1679–87.
ter Wolbeek C, Hartert M, Conzelmann LO, Peivandi AA, Czerny M, Gottardi R, et al. Value and pitfalls of neurophysiological monitoring in thoracic and thoracoabdominal aortic replacement and endovascular repair. Thorac Cardiovasc Surg. 2010;58:260–4.
Schurink GW, De Haan MW, Peppelenbosch AG, Mess W, Jacobs MJ. Spinal cord function monitoring during endovascular treatment of thoracoabdominal aneurysms: implications for staged procedures. J Cardiovasc Surg. 2013;54:S117–24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Austria, part of Springer Nature
About this chapter
Cite this chapter
Mees, B., Schurink, G.W., Peppelenbosch, N., Mess, W., Jacobs, M. (2019). Monitoring Spinal Cord Function in Open and Endovascular Treatment of Thoracoabdominal Aortic Pathologies. In: Stanger, O., Pepper, J., Svensson, L. (eds) Surgical Management of Aortic Pathology. Springer, Vienna. https://doi.org/10.1007/978-3-7091-4874-7_79
Download citation
DOI: https://doi.org/10.1007/978-3-7091-4874-7_79
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-4872-3
Online ISBN: 978-3-7091-4874-7
eBook Packages: MedicineMedicine (R0)