Abstract
The study of the mechanical processes that occur within the aorta—both healthy and diseased—from an engineering perspective is rapidly progressing. Understanding the exact biomechanical processes that underlie aortic disease, such as aortic aneurysm, dissection, and rupture, is a critical supplement to clinical studies of the natural history of these diseases. Engineering analysis promises to enhance management and timing of surgical intervention. In this chapter we discuss the current understanding of the mechanics of the thoracic aorta and thoracic aortic aneurysm based on in vivo and ex vivo studies. We address also the clinical application of engineering insights and their significance for surgical decision-making.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The aorta is a vital organ of the human body. This tubular structure delivers oxygen-enriched blood to all organs and tissues of the human body. However, unlike one would imagine, the aorta is much more than a hollow, passive conduit for transportation of a fluid. The aorta is a living organ with a unique anatomy, complex intrinsic biology, and sophisticated mechanical properties [1] that permit its active “playing a game of catch” with the stroke volume.
Aneurysms of the aorta are currently the 19th most common cause of death in the United States, causing approximately 13,000 deaths annually [2], a number that in all likelihood markedly underestimates the true prevalence of aorta-related deaths, which are often misdiagnosed as heart attacks. The nature of aneurysm disease makes it “silent” in the absolute majority of affected individuals. Most affected patients do not know that they are harboring a growing aortic aneurysm in their chest or abdomen [1]. The first presentation of this disease may be devastating, either producing an instant death or resulting in a complication that is likely to produce death—such as rupture or dissection—unless treated emergently in a specialized center [3]. Therefore, timely identification of individuals with an aortic aneurysm and preemptive extirpation are key to preventing deaths related to this condition.
Current guidelines for surgical treatment of thoracic aortic aneurysm recommend elective surgical resection of ascending aortic aneurysms before they reach 5.5 cm (for descending aortic aneurysms before they reach 6.0 cm) [4]. These intervention criteria have been developed largely based on detailed examination of the natural history of the disease and the risks of dissection, rupture, and death of aortas reaching a certain size [5,6,7]. These intervention criteria were recently further refined to account for differences in a person’s height and weight. The risks of adverse events were calculated in relation to body surface area, providing a more accurate estimate of the actual risk in a specific patient [8]. Similarly, guidelines on abdominal aortic aneurysm recommend preventive surgical/endovascular treatment at 5.0 cm for females and 5.5 cm for males [9].
However, it is becoming increasingly apparent that estimation of risk of aortic dissection or rupture purely based on a size criterion is, although good, far from perfect [10]. Currently, establishment of the genetic nature of thoracic and abdominal aortic aneurysm is burgeoning [11,12,13]. The aorta in patients with certain specific genetic mutations can behave more malignantly and unpredictably, so that the general size criteria may not be applicable. Likewise, bioengineering understanding of aortic disease is also advancing rapidly, permitting understanding of the mechanical processes that occur within the aorta, both healthy and diseased. Understanding the mechanics of the aortic wall, which may vary from individual to individual, is critical for further refinement of the surgical intervention criteria.
The mechanics and mechanobiology of abdominal aortic aneurysm have been studied in detail and reported in an excellent review article by Humphrey and Holzapfel [14]. In this chapter we describe the current understanding of the mechanics of the thoracic aorta and thoracic aortic aneurysm (TAA). We explore the potential clinical application of engineering parameters to surgical decision-making.
2 Normal Anatomy, Structure, and Histology of the Aortic Wall
The histologic structure of the thoracic aorta is similar to other types of blood vessels and is described in detail in earlier chapters of this textbook. We focus on the main histologic components that are critical for understanding the mechanics of the aortic wall.
The aortic wall consists of three main layers: the internal layer, tunica intima; the medial layer, tunica media; and the outer layer, tunica adventitia [1]:
-
Tunica Intima—accounts for approximately 5% of the wall thickness [15] and consists of a single layer of flattened endothelial cells, lying on a basal lamina (internal elastic lamina). The endothelial cells play multiple important functions, including barrier, blood clotting, inflammatory, tone regulatory, reparatory, and other functions. The subendothelial layer contains elastic fibers and type I collagen fibrils, fibroblasts, and myointimal cells (cells that structurally resemble smooth muscle cells) [16, 17]. The intimal layer is supported by an internal elastic lamina that lies between the intimal and medial layers.
-
Tunica Media—is the largest layer of the aortic wall and accounts for approximately 77–80% of the wall thickness [15]. It consists of concentric layers of smooth muscle cells (43%), collagen (30%), elastin (23%), and glycosaminoglycans and proteoglycans (4%) [15]. According to the general classification of blood vessels, the aorta is considered to be an elastic artery [16, 17] (together with the brachiocephalic trunk, the common carotid, subclavian and common iliac arteries, etc.) since elastic fibers predominate in this layer. Individual elastic fibers (0.1–1.0 μm in diameter) fuse to produce lamellae of elastic material. The media has a layered structure, in which layers of elastic lamellae alternate with interlamellar smooth muscle cells and collagen [16]. Such regular repetition of these medial elements allows us to define a “lamellar unit,” first described by Wolinsky and Glagov [18], which consists of two layers of elastic lamellae (surrounded by proteins of the extracellular matrix, including collagen, adhesion molecules, and glycosaminoglycans/proteoglycans) and one smooth muscle cell in between them (Fig. 10.1) [19, 20]. It is estimated that the ascending aorta has anywhere from 53 to 78 lamellar units [19, 21], while the abdominal aorta has approximately 28 medial lamellar units [21]. Blood flow within the aorta is highly pulsatile, which is why the medial layer, with its layers of elastic lamellae, is designed to sustain and smooth out the blood flow despite the pulsatile cardiac output [16].
-
Tunica Adventitia—accounts for approximately 15–18% of the wall thickness [15] and is formed from general connective tissue and includes blood vessels that supply the aorta (vasa vasorum) and nerves that innervate the aorta (nervi vasorum). Interestingly, studies have shown that in the thoracic aorta, the outer parts of the medial layer also contain the vasa vasorum, which contrasts with the medial layer of the abdominal aorta, which is completely avascular [21]. The adventitial layer can vary in thickness but is primarily quite thin. Despite the adventitia being so thin, it is highly respected by surgeons, who feel it is the “strength layer” of the aorta, essential for secure suturing of aortic tissues [1, 3].
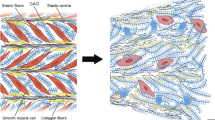
Electron micrograph of a single lamellar unit in the medial layer of the aortic wall showing a VSMC lying between two layers of elastin fibers and surrounded by ECM proteins containing microfibrils and proteoglycans. Lamellar units are intercalated by collagen bundles. The lamellar unit represents the basic structural and functional unit of the aortic wall. Abbreviations: ECM extracellular matrix, VSMC vascular smooth muscle cell (Reprinted with permission from [20])
3 Engineering Definitions and Characteristics
In mechanics, “strain” is defined as a change in size (e.g., length) produced by a given force.
“Stress” is defined as the force applied per cross-sectional area of the aortic wall (force/area unit). There are two types of stress that the aorta experiences:
-
Tensile stress (wall stress)—an applied force, for arteries primarily dependent on the arterial geometry and the hemodynamic load (blood pressure). Tensile stress can be circumferential, radial, and longitudinal.
-
Shear stress—is the dragging frictional force created by blood flowing against the inner surface of the aortic wall. Shear stress is dependent on the arterial geometry, the characteristics of blood flow, and the viscosity of the blood.
The result of stress that is applied to the aortic wall is strain—a change in aortic size. Stated briefly, stress is applied and strains results.
Other important engineering characteristics that are used to describe biomechanical properties of the aorta include:
-
Distensibility—the capacity of the aortic wall to dilate during changes in intraluminal pressure. Vessel wall distensibility is directly related to elastin and collagen content and their structural characteristics.
-
Elastic modulus (stress/strain relationship)—quantification of how much force is needed to produce a specific change in size. The stress/strain relationship describes the amount of strain (deformation) that elastin and collagen can absorb in response to a specific stress (pressure).
4 Assessing the Mechanics of the Aortic Wall
Since the geometry of the aorta plays an important role in determining the mechanical properties of the aorta, accurate imaging modalities are vital to enable bioengineering calculations and models. Recent advances in computed tomography (CT) and magnetic resonance imaging (MRI) have substantially enhanced the accuracy and resolution of aortic imaging, thus making biomechanical analysis and modeling considerably more accurate. In addition, new MR angiography methods have expanded the use of MR angiography beyond purely anatomical information toward quantitative hemodynamic analyses [22]. On the other hand, ex vivo studies using uniaxial [23] and biaxial [24, 25] stretch models of explanted aortic tissue (both healthy and aneurysmal) have contributed to understanding the tensile limits of the aorta. Computational fluid dynamics [26], fluid-solid interactions [27], and finite element analysis [28] are all computational models that are becoming increasingly more sophisticated due to advancements in imaging techniques.
5 In Vivo Evaluation of the Aortic Wall Mechanics Via Epiaortic Echocardiography
At our institution, we have utilized epiaortic echocardiography to assess the mechanical properties of the ascending aorta in patients undergoing open-heart surgery [29, 30]. The measurements are conducted after median sternotomy, and pericardiotomy is performed (before arterial or venous cannulation) by using an echocardiographic probe in a sterile sheath together with a cushion constructed from a surgical glove finger filled with normal saline to act as an interface between the probe and the aorta (Fig. 10.2).
Epiaortic echocardiography. The echo probe is placed directly on the aorta at the time of surgery, with an interposed fluid-filled interface (the tip of a sterile glove) (Reprinted with permission from [1])
Epiaortic echocardiography makes it possible to measure six specific parameters that are needed to determine the important mechanical properties of the aorta (Fig. 10.3):
-
Aortic diameter (systolic and diastolic)
-
Aortic wall thickness (systolic and diastolic)
-
Aortic blood pressure (systolic and diastolic)
The six parameters needed to calculate the important engineering characteristics of the aorta: aortic diameter (d), in systole and diastole; aortic wall thickness (t), in systole and diastole; and blood pressure (BP), in systole and diastole (Reproduced with permission from [1])
Based on these six measurements, a complete mechanical profile of the aorta can be constructed, including the following engineering characteristics: aortic wall distensibility, aortic wall stress, and the incremental elastic modulus (stress/strain relationship).
5.1 Distensibility
Intraoperative measurements done via epiaortic echocardiography have shown that as the aorta enlarges in diameter, the distensibility of the aortic wall significantly decreases (Fig. 10.4). For example, distensibility values for the ascending aortas greater than 5.0 cm in diameter are 1.45 ± 0.38 mm Hg−1, while the distensibility of the normal ascending aortas and ascending aortic aneurysms smaller than 4.0 cm was significantly greater (2.499 ± 0.49 and 3.02 ± 0.60 mm Hg−1, respectively). By 6 cm aortic diameter, the distensibility values are extremely low (0.81 ± 0.32 mm Hg−1), suggesting that the severely enlarged aorta becomes essentially a rigid, inelastic, and nondistensible tube [29]. As a consequence of such loss of elasticity, the force generated by the left ventricle in systole can no longer be dissipated in expanding the aorta.
Distensibility values in normal aortas and aortic aneurysms of different diameters. Distensibility of ascending aortic aneurysms decreases rapidly as diameter increases to very low values at dimensions greater than 6 cm (Reproduced with permission from [29])
5.2 Wall Stress
Since the aneurysmal aortic wall does not possess normal elasticity properties, the full force of cardiac contraction is translated into wall stress. Therefore, the enlarged aorta demonstrates very high wall tension. The relationship between wall stress and size of the ascending aorta is shown in Fig. 10.5. The mean wall stress for the normal-sized aortas is approximated at 92.51 ± 6.35 kPa. However, as the size of the aorta increases, the wall stress also increases in a step-wise exponential manner. Ascending aortas with a diameter greater than 6.0 cm have a mean wall stress of 376 ± 146.6 kPa. This figure was recorded at a systolic blood pressure of 100 mm Hg. Furthermore, at a mathematically extrapolated systolic blood pressure of 200 mm Hg, the wall stress value for a 6 cm aorta is 857 ± 291 kPa [29]. Such blood pressure, although uncommon in a controlled environment of an operating room, is quite common in real life and corresponds to episodes of strenuous physical activity [31] and emotional stress [32] or a hypertensive crisis of other etiology. From previous studies we know that the ultimate tensile strength of the aorta is about 800 to 1000 kPa [23, 33]. It is interesting to note that a 6 cm aorta at a blood pressure of 200 mm Hg is exceeding (or “flirting” with) the ultimate tensile limits of the aorta, which can lead to rupture of the aortic wall [1, 10, 29].
In vivo mechanical properties of human ascending aorta. Exponential relationship between wall stress and aneurysm size in ascending aortic aneurysms. The red columns represent a blood pressure of 100 mm Hg, and the blue columns represent a blood pressure of 200 mm Hg. The lines at 800–1000 kPa represent the range of maximum tensile strength of the human aorta (Reproduced with permission from [10])
5.3 Elastic Modulus
Similarly, the elastic modulus values for aortic aneurysms (1.93 ± 0.88 MPa) were significantly greater than those in the normal aorta (1.18 ± 0.21 MPa), while large aneurysms (greater than 5 cm in diameter) had even greater values (3.56 ± 0.88 MPa), suggesting that large ascending aortic aneurysms have already been stretched to their limits [29].
5.4 Confluence of Clinical and Bioengineering Findings
Remarkably, these intraoperative bioengineering measurements showed that the mechanical properties of the human aorta deteriorate dramatically at precisely the same diameter—6 cm—that studies on the natural history of thoracic aortic aneurysms [5,6,7,8, 34] had identified as critical. Studies of the mechanical properties of the thoracic aorta provide independent engineering justification for our clinical intervention criteria. At 6 cm, the aorta is no longer distensible and thus becomes essentially a non-distensible rigid tube manifesting malignant behavior such as rupture or dissection [1, 10, 29, 35].
6 Ex Vivo Biaxial Stretch Mechanics of the Thoracic Aorta
There are limited data on the mechanical properties of the aneurysmal ascending aortic wall subjected to planar biaxial testing, as most studies of this type have relied on uniaxial deformation of aortic wall specimen. However, in real life the aortic wall deforms in both longitudinal and circumferential directions, which is why testing the aortic samples in biaxial deformation is important for complete understanding of the mechanical behavior of the aortic wall. Dr. Wei Sun and colleagues have developed a technique for biaxial testing of samples of aneurysmal aortas that are obtained intraoperatively during aneurysm resection [24, 25], illustrated in Fig. 10.6 and Video 10.1 (see online supplementary material).
Illustration of a representative testing sample and its orientation with respect to the excised region of ascending aortic aneurysm. C circumferential, L longitudinal (Reprinted with permission from [25])
In collaborative experiments with Dr. Sun, we aimed to perform a predictive biomechanical analysis of ascending aortic tissue samples to assess the risk of rupture. Once again we were able to demonstrate a close correlation of engineering findings with the natural behavior of the aortas in our patients. By combining the uniaxial and biaxial testing techniques, we were able to plot the ultimate tensile strength values (found in uniaxial experiments) into equations modeled from biaxial stretch experiments [24, 25, 36]. This analysis gave us an exact prediction of when aortic dissection or rupture would occur in a particular patient (Fig. 10.7). Please note that a patient would experience an ascending aortic dissection at a blood pressure of 168 mm Hg when the aorta is 5.29 cm in diameter. Rupture would have occurred at a diameter of 5.47 cm and a blood pressure of 263 mm Hg [24, 36]. Similarly to the biomechanical studies performed in vivo, these experiments correlate well with our clinical recommendation to perform prophylactic surgery on patients with ascending aortic aneurysm before they reach the 5.5 cm size.
Panel a: engineering analysis combining ultimate tensile strength from uniaxial testing with mathematic modeling from biaxial testing permits “prediction” of exact sizes at which rupture and dissection would have occurred (Video 10.1) (Reprinted with permission from [36]). Panel b: note that the predicted dissection and rupture points for the patient illustrated in panel a fall exactly in the size range for adverse events (arrow) found in our previous clinical studies (Reproduced with permission from [5])
Bicuspid aortic valve [37, 38] and a bovine aortic arch anomaly [39, 40] have been associated with increased risk of aortic dilation and aortic dissection. Studies of the mechanical properties of human ascending aortic specimens conducted by our group via planar biaxial testing did not reveal a significant difference of the mechanical properties of the aortas with or without these conditions [24, 25]. On the other hand, a recent study by Forsell et al. [41] that compared the mechanical properties of the aneurysmal ascending aorta from patients with trileaflet (TAV) and bicuspid aortic (BAV) valves found that the BAV ascending aorta was significantly stiffer than the TAV aorta. The authors attributed this to increased collagen-related stiffness [41]. This study used uniaxial tensile strength measurements, which were input into a finite element model, although the sample size was relatively small (13 BAV and 11 TAV patients). The authors suggest that the mechanism of aneurysm formation is clearly distinct between the BAV and TAV patients and involves differences in collagen turnover and composition [41,42,43].
7 Measuring Aortic Wall Shear Stress Using 4D PC-MRI
Most recently, high-resolution, time-resolved, three-dimensional (3D), three-directional velocity encoded, radially undersampled phase-contrast MR sequence (4D PC-MRI) (Video 10.2 in online supplementary materials) has been used to estimate aortic wall shear stress in non-aneurysmal ascending aortas [44, 45] (with and without BAV) [46] as well as in patients with ascending aortic dilatation [45, 47] and aortic dissection [48].
In normal, non-aneurysmal aortas, the time-averaged wall shear stress was found to be greatest on the inner and outer curvature of the ascending aorta and lowest on the left and right walls [44]. At the same time, in patients with a dilated ascending aorta, the peak systolic wall shear stress in the ascending aorta and aortic arch was significantly lower than in healthy controls [45]. The same study also found that the incidence of alterations in blood flow patterns, such as the presence of vortex and supra-physiologic helical flow, was significantly increased in the setting of a dilated ascending aorta. The strength of the ascending aortic supra-physiologic-helix and vortex formation increased directly with an increase in ascending aortic diameters [45]. In contrast, during diastole, the wall shear stress in the setting of ascending aortic dilatation is increased (compared to non-dilated aortas) [47]. Interestingly, a study that compared quantitative flow metrics in patients with normal-size ascending aorta with or without BAV (mean aortic size in these groups was 2.8 ± 0.8 cm and 2.2 ± 0.6 cm, respectively) found reduced wall shear stress in the BAV group [46]. Thus, the authors raise the question of whether a dilated ascending aorta causes deranged flow, or, alternatively, whether deranged flow (through a BAV) causes the aorta to dilate [46]. Clearly further understanding of the cumulative changes in aortic wall strain and wall shear stress will enhance our understanding of the mechanics and pathophysiology of aortic dilatation.
8 Biomechanics of Aortic Dissection
Aortic dissection is the most common lethal complication of an ascending aortic aneurysm. A dissection is the splitting of the layers of the aorta that, by conventional understanding, usually starts with a tear in the intimal layer, which then propagates and dissects (splits) the medial layer of the aortic wall. As a result, a pressurized false lumen is created that is separated from the true lumen of the aorta by the “dissection flap” [1, 3]. From a biomechanical perspective, the aortic dissection is a separation or delamination of the elastic layers of the degenerated aortic wall that occurs when the hemodynamic loads exerted on the aneurysmal wall exceed the bonding forces that normally hold the layers together [49]. To date, the exact mechanics involved in the development of an aortic dissection are not completely clear, although several studies have shed light on the possible underlying mechanisms [50,51,52,53,54,55].
One recent study evaluated the delamination strength of the aneurysmal ascending aorta, in patients with BAV or TAV, in comparison with a normal-size (non-aneurysmal) aorta. The authors showed that aneurysmal aortas had significantly lower delamination strength than non-aneurysmal aortas. The same study also showed that the intimal half of the delaminated/dissected aorta is significantly weaker than the outer half that is attached to the adventitia [49]. Humphrey et al. [56] proposed a new hypothesis of initiation and propagation of aortic dissection (Fig. 10.8). Their hypothesis suggests that the pooling of glycosaminoglycans and proteoglycans may contribute to an initial delamination within the media due to a combined localized loss of tensile strength, genesis of stress concentrations, and increased swelling pressures, which in turn could promote dissections in the thoracic aorta [56, 57]. This hypothesis also challenges the generally accepted concept that the dissection starts with a tear in the intima and the false lumen is then generated under the action of blood pressure. This novel hypothesis suggests that the dissection might start with an initial delamination process of the media, which in turn propagates proximally or distally, subsequently creating an intimal tear. Such pooling of glycosaminoglycans and proteoglycans could be related to the dysregulation of transforming growth factor beta (TGF-β) [57,58,59]. The hypothesis is that aortic smooth muscle cells increase their production of glycosaminoglycans and proteoglycans in response to increased TGF-β activity [56, 57].
This comparative illustration summarizes the hypothesis that pools of glycosaminoglycans/proteoglycans initiate the dissection from within. Panels a and b: sections of the medial layer of the human ascending aorta stained with Alcian blue (which stains glycosaminoglycans blue) for both a normal (panel a) and an aneurysmal (panel b) aortic wall. (Reproduced with permission from [75]). Panel c: Schematic drawing of a normal aortic medial lamellar unit consisting of paired elastic laminae (at the top and bottom) with an embedded smooth muscle cell (SMC) as well as collagen fibers, adhesion molecules (e.g., fibronectin), and glycosaminoglycans (GAGs) (not to scale). Shown, too, are thin “radially oriented” elastic fibers (i.e., elastin and associated microfibrils, predominately fibrillin-1) that may provide direct mechanical connections between the elastic laminae and smooth muscle cell and thus a mechanosensory capability beyond the typical cytoskeletal (CSK)—integrin—extracellular matrix (fibronectin/collagen) axis. It is possible that negatively charged GAGs sequester water and may thereby contribute a normal intralamellar pressure that could help maintain the thin elastic fibers in tension. Panel d: This schematic drawing depicts a localized accumulation of GAGs, on the right side of the medial lamellar unit, which results in an increased swelling pressure, which in turn helps to separate the elastic laminae and possibly disrupt connections between the SMCs and either thin elastic fibers or the collagenous matrix. Such effects could initiate a local delamination and/or altered mechanosensitive cellular response leading to dysregulated wall homeostasis (Panels c and d are reprinted with permission from [57])
9 The Aging Aorta
It is well known that as the aorta ages, it becomes stiffer, thus losing its elastic properties and, subsequently, dilates [60]. Both of these phenomena are most pronounced in the proximal aorta [61]. The histologic aging changes that occur in the aorta are listed in Table 10.1 [62]. Such age-related remodeling affects the intimal layer, which becomes thicker due to accumulation of disorganized collagen [28]. However, there is evidence to suggest that aging also affects the medial layer of the aorta—the elastic lamellae fracture [63] and thin [60]. As a result the lamellae become separated by increasing amounts of material that is not load-bearing. All these factors contribute to stiffening of the aorta and its dilation [60]. Interestingly, some studies have shown that young females have more compliant arteries than men, while older females tend to have much stiffer arteries than age-matched male individuals [64, 65].
Previous studies have shown that in youth the abdominal aorta dilates approximately 10% with every heartbeat [66], which is primarily accomplished via stretching of the elastin. Our own studies have shown that the ascending aorta expands by 9.2% between diastole and systole in young male patients, and this value decreases with age to about 5.6% in male patients of advanced age [67]. This implies that the elastin, the half-life of which is several decades, might be subject to the so-called material fatigue, a term used in engineering science that explains why physical materials exposed to repetitive cycles of bending or stretch can fracture or break. As O’Rourke states in his review of aging changes to the aorta [60, 61], the phenomenon of material fatigue is often overlooked by many biologists who concentrate on living cells, whose components are turned over on a regular basis. Thus, it is possible that principles of fatigue and fracture might very well apply to elastin, as well as to all nonliving material structures in the human body [68, 69].
Multiple studies have investigated the effects of aging on the properties of the ascending aorta based on in vivo imaging diagnostic studies (echocardiography, CT, MRI), which provide additional evidence to support the phenomena of stiffening, dilatation, and elongation that occur in the aging aorta [70,71,72,73,74]. Our group conducted a study [67] to investigate the age-dependent in vivo mechanical properties of the ascending aorta in males (all individuals had no known aortopathies) by analyzing images of multiphase CT scans taken at different time points with 3D reconstructions. We assessed the following mechanical characteristics under physiological loading conditions: circumferential strain, pressure-strain modulus, and wall tension. Our measurements showed a significant negative linear correlation between the peak circumferential strain and patient age (Fig. 10.9a), as well as significant positive linear correlations between the mean diameter of the ascending aorta, systolic wall tension, and pressure-strain modulus and the patient age (Fig. 10.9b–d) [67]. The mean pressure-strain modulus was found to be significantly less for young individuals (30–49 years), compared with those of middle (50–59 years) and advanced age (60–79 years). Pressure-strain modulus was significantly less in normotensive individuals (systolic blood pressure < 140 mm Hg) than in hypertensive individuals (systolic blood pressure ≥ 140 mm Hg) (Fig. 10.10) [67].
Raw (a) peak circumferential ascending aortic strain, (b) mean diameter, (c) systolic wall tension, and (d) pressure-strain modulus data plotted versus age with linear regression lines (Reprinted with permission from [67])
Comparison of the mean pressure-strain modulus value between (a) patients in each of the three age groups and (b) normotensive and hypertensive patients (Reprinted with permission from [67])
10 Conclusions
The biomechanics of the aortic wall are complex due to multiple structural and functional factors, which are being investigated through ex vivo mechanical testing and in vivo modeling using data from high-quality imaging studies. At the same time, it is fascinating to see how well the engineering findings correlate with the empirically observed clinical data on the natural behavior of aortic aneurysm. Such confluence of data increases the confidence of the findings and provides stronger evidence for the accepted data-driven guidelines for management of thoracic aortic disease.
Looking forward, we feel that the potential to analyze accurately the hemodynamic forces and biomechanical properties of the thoracic aortic wall that potentially contribute to aneurysm, growth, dissection, and rupture will be useful in optimizing management strategy for each individual patient. The appropriate time for surgical intervention will be more precisely determined. Together with rapidly advancing knowledge of the genetic causes of thoracic aortic aneurysm and dissection, detailed and accurate determination of the biomechanical properties of the aortic wall will enable the physician to establish and execute personalized care for each individual patient.
References
Elefteriades JA. Thoracic aortic aneurysm: reading the enemy’s playbook. Curr Probl Cardiol. 2008;33:203–77.
WISQARS leading causes of death reports, 1999–2007. 2014. http://webappa.cdc.gov/sasweb/ncipc/leadcaus10.html. Accessed 25 Oct 2014.
Ziganshin BA, Elefteriades JA. Thoracic aortic disease. In: Stergiopoulos K, Brown DL, editors. Evidence-based cardiology consult. First ed. London: Springer-Verlag; 2014. p. 331–53.
Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55:e27–9.
Coady MA, Rizzo JA, Hammond GL, et al. What is the appropriate size criterion for resection of thoracic aortic aneurysms? J Thorac Cardiovasc Surg. 1997;113:476–91. discussion 489-91
Coady MA, Rizzo JA, Goldstein LJ, Elefteriades JA. Natural history, pathogenesis, and etiology of thoracic aortic aneurysms and dissections. Cardiol Clin. 1999;17:615–35.
Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–80.
Davies RR, Gallo A, Coady MA, et al. Novel measurement of relative aortic size predicts rupture of thoracic aortic aneurysms. Ann Thorac Surg. 2006;81:169–77.
Chaikof EL, Brewster DC, Dalman RL, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50:880–96.
Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. J Am Coll Cardiol. 2010;55:841–57.
Elefteriades JA, Pomianowski P. Practical genetics of thoracic aortic aneurysm. Prog Cardiovasc Dis. 2013;56:57–67.
Pomianowski P, Elefteriades JA. The genetics and genomics of thoracic aortic disease. Ann Cardiothorac Surg. 2013;2:271–9.
Golledge J, Kuivaniemi H. Genetics of abdominal aortic aneurysm. Curr Opin Cardiol. 2013;28:290–6.
Humphrey JD, Holzapfel GA. Mechanics, mechanobiology, and modeling of human abdominal aorta and aneurysms. J Biomech. 2012;45:805–14.
Iliopoulos DC, Kritharis EP, Giagini AT, Papadodima SA, Sokolis DP. Ascending thoracic aortic aneurysms are associated with compositional remodeling and vessel stiffening but not weakening in age-matched subjects. J Thorac Cardiovasc Surg. 2009;137:101–9.
Standring S, Borley NR, Collins P, et al. Smooth muscle and the cardiovascular and lymphatic systems. In: Standring S, Gray H, editors. Gray’s anatomy: the anatomical basis of clinical practice. 40th ed. Edinburgh: Churchill Livingstone/Elsevier; 2008. p. 127–43.
Young B, O’Dowd G, Woodford P. Circulatory system. In: Young B, O’Dowd G, Woodford P, editors. Wheater’s functional histology: a text and colour atlas. Sixth ed. London: Churchill Livingstone; 2014. p. 144–58.
Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111.
Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular matrix of the human aortic media: an ultrastructural histochemical and immunohistochemical study of the adult aortic media. Anat Rec. 2000;258:1–14.
El-Hamamsy I, Yacoub MH. Cellular and molecular mechanisms of thoracic aortic aneurysms. Nat Rev Cardiol. 2009;6:771–86.
Wolinsky H. Comparison of medial growth of human thoracic and abdominal aortas. Circ Res. 1970;27:531–8.
Francois CJ. Advances in CT and MR technology. Perspect Vasc Surg Endovasc Ther. 2012;24:128–36.
Vorp DA, Schiro BJ, Ehrlich MP, Juvonen TS, Ergin MA, Griffith BP. Effect of aneurysm on the tensile strength and biomechanical behavior of the ascending thoracic aorta. Ann Thorac Surg. 2003;75:1210–4.
Martin C, Sun W, Pham T, Elefteriades J. Predictive biomechanical analysis of ascending aortic aneurysm rupture potential. Acta Biomater. 2013;9:9392–400.
Pham T, Martin C, Elefteriades J, Sun W. Biomechanical characterization of ascending aortic aneurysm with concomitant bicuspid aortic valve and bovine aortic arch. Acta Biomater. 2013;9:7927–36.
Canstein C, Cachot P, Faust A, et al. 3D MR flow analysis in realistic rapid-prototyping model systems of the thoracic aorta: comparison with in vivo data and computational fluid dynamics in identical vessel geometries. Magn Reson Med. 2008;59:535–46.
Humphrey JD, Taylor CA. Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng. 2008;10:221–46.
Weisbecker H, Pierce DM, Regitnig P, Holzapfel GA. Layer-specific damage experiments and modeling of human thoracic and abdominal aortas with non-atherosclerotic intimal thickening. J Mech Behav Biomed Mater. 2012;12:93–106.
Koullias G, Modak R, Tranquilli M, Korkolis DP, Barash P, Elefteriades JA. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J Thorac Cardiovasc Surg. 2005;130:677–83.
Modak RK, Koullias GJ, Govindarajulu US, Tranquilli M, Barash PG, Elefteriades JA. Ascending aortic aneurysms: asymmetrical differences in aortic cross-sectional wall motion detected by epiaortic echocardiography. J Cardiothorac Vasc Anesth. 2010;24:776–9.
Elefteriades JA, Hatzaras I, Tranquilli MA, et al. Weight lifting and rupture of silent aortic aneurysms. JAMA. 2003;290:2803.
Hatzaras IS, Bible JE, Koullias GJ, Tranquilli M, Singh M, Elefteriades JA. Role of exertion or emotion as inciting events for acute aortic dissection. Am J Cardiol. 2007;100:1470–2.
Garcia-Herrera CM, Atienza JM, Rojo FJ, et al. Mechanical behaviour and rupture of normal and pathological human ascending aortic wall. Med Biol Eng Comput. 2012;50:559–66.
Davies RR, Goldstein LJ, Coady MA, et al. Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg. 2002;73:17–27.
Ziganshin BA, Elefteriades JA. Yale milestones in reading the playbook of thoracic aortic aneurysms. Conn Med. 2012;76:589–98.
Elefteriades JA, Habel N, Sun W, Sang AX, Kuzmik GA. The aortic wall: four questions and insights. J Thorac Cardiovasc Surg. 2013;145:S130–4.
Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370:1920–9.
Friedman T, Mani A, Elefteriades JA. Bicuspid aortic valve: clinical approach and scientific review of a common clinical entity. Expert Rev Cardiovasc Ther. 2008;6:235–48.
Hornick M, Moomiaie R, Mojibian H, et al. ‘Bovine’ aortic arch – a marker for thoracic aortic disease. Cardiology. 2012;123:116–24.
Dumfarth J, Plaikner M, Krapf C, et al. Bovine aortic arch: predictor of entry site and risk factor for neurologic injury in acute type a dissection. Ann Thorac Surg. 2014;98:1339–46.
Forsell C, Bjorck HM, Eriksson P, Franco-Cereceda A, Gasser TC. Biomechanical properties of the thoracic aneurysmal wall: differences between bicuspid aortic valve and tricuspid aortic valve patients. Ann Thorac Surg. 2014;98:65–71.
Wagsater D, Paloschi V, Hanemaaijer R, et al. Impaired collagen biosynthesis and cross-linking in aorta of patients with bicuspid aortic valve. J Am Heart Assoc. 2013;2:e000034.
Phillippi JA, Green BR, Eskay MA, et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2014;147:1056–64.
Frydrychowicz A, Stalder AF, Russe MF, et al. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. J Magn Reson Imaging. 2009;30:77–84.
Burk J, Blanke P, Stankovic Z, et al. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J Cardiovasc Magn Reson. 2012;14:84.
Barker AJ, Lanning C, Shandas R. Quantification of hemodynamic wall shear stress in patients with bicuspid aortic valve using phase-contrast MRI. Ann Biomed Eng. 2010;38:788–800.
Bieging ET, Frydrychowicz A, Wentland A, et al. In vivo three-dimensional MR wall shear stress estimation in ascending aortic dilatation. J Magn Reson Imaging. 2011;33:589–97.
Clough RE, Waltham M, Giese D, Taylor PR, Schaeffter T. A new imaging method for assessment of aortic dissection using four-dimensional phase contrast magnetic resonance imaging. J Vasc Surg. 2012;55:914–23.
Pasta S, Phillippi JA, Gleason TG, Vorp DA. Effect of aneurysm on the mechanical dissection properties of the human ascending thoracic aorta. J Thorac Cardiovasc Surg. 2012;143:460–7.
Tam AS, Sapp MC, Roach MR. The effect of tear depth on the propagation of aortic dissections in isolated porcine thoracic aorta. J Biomech. 1998;31:673–6.
MacLean NF, Dudek NL, Roach MR. The role of radial elastic properties in the development of aortic dissections. J Vasc Surg. 1999;29:703–10.
Sommer G, Gasser TC, Regitnig P, Auer M, Holzapfel GA. Dissection properties of the human aortic media: an experimental study. J Biomech Eng. 2008;130:021007.
Karmonik C, Bismuth J, Shah DJ, Davies MG, Purdy D, Lumsden AB. Computational study of haemodynamic effects of entry- and exit-tear coverage in a DeBakey type III aortic dissection: technical report. Eur J Vasc Endovasc Surg. 2011;42:172–7.
Nathan DP, Xu C, Gorman JH 3rd, et al. Pathogenesis of acute aortic dissection: a finite element stress analysis. Ann Thorac Surg. 2011;91:458–63.
Beller CJ, Labrosse MR, Thubrikar MJ, Robicsek F. Finite element modeling of the thoracic aorta: including aortic root motion to evaluate the risk of aortic dissection. J Med Eng Technol. 2008;32:167–70.
Humphrey JD. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-beta. J Vasc Res. 2013;50:1–10.
Roccabianca S, Ateshian GA, Humphrey JD. Biomechanical roles of medial pooling of glycosaminoglycans in thoracic aortic dissection. Biomech Model Mechanobiol. 2014;13:13–25.
Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46:119–37.
Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16.
O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13.
O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12:329–41.
Gallagher PJ, van der Wal AC. Blood vessels. In: Mills SE, editor. Histology for pathologists. Third ed. Philadelphia: Lippincott Williams & Wilkins; 2007. p. xi, 1272 p.
Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–29.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Clinical appraisal of arterial stiffness: the Argonauts in front of the Golden Fleece. Heart. 2006;92:1544–50.
Waddell TK, Dart AM, Gatzka CD, Cameron JD, Kingwell BA. Women exhibit a greater age-related increase in proximal aortic stiffness than men. J Hypertens. 2001;19:2205–12.
Boutouyrie P, Laurent S, Benetos A, Girerd XJ, Hoeks AP, Safar ME. Opposing effects of ageing on distal and proximal large arteries in hypertensives. J Hypertens. 1992;10:S87–91.
Martin C, Sun W, Primiano C, McKay R, Elefteriades J. Age-dependent ascending aorta mechanics assessed through multiphase CT. Ann Biomed Eng. 2013;41:2565–74.
Rigby BJ. Effect of cyclic extension on the physical properties of tendon collagen and its possible relation to biological ageing of collagen. Nature. 1964;202:1072–4.
Broom ND. Fatigue-induced damage in glutaraldehyde-preserved heart valve tissue. J Thorac Cardiovasc Surg. 1978;76:202–11.
Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1:739–48.
Wolak A, Gransar H, Thomson LE, et al. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age, gender, and body surface area. JACC Cardiovasc Imaging. 2008;1:200–9.
Redheuil A, Yu WC, Wu CO, et al. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–26.
Rose JL, Lalande A, Bouchot O, et al. Influence of age and sex on aortic distensibility assessed by MRI in healthy subjects. Magn Reson Imaging. 2010;28:255–63.
Mirea O, Maffessanti F, Gripari P, et al. Effects of aging and body size on proximal and ascending aorta and aortic arch: inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J Am Soc Echocardiogr. 2013;26:419–27.
Borges LF, Touat Z, Leclercq A, et al. Tissue diffusion and retention of metalloproteinases in ascending aortic aneurysms and dissections. Hum Pathol. 2009;40:306–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
1 Electronic Supplementary Material
Illustration of the biaxial stretch experiments with samples of the thoracic aorta (MOV 19780 kb)
The use of 4-D MRI to assess flow parameters within the aorta (Video courtesy of Northwestern University, Department of Diagnostics Image) (MOV 14682 kb)
Rights and permissions
Copyright information
© 2019 Springer-Verlag GmbH Austria, part of Springer Nature
About this chapter
Cite this chapter
Ziganshin, B.A., Elefteriades, J.A. (2019). Mechanics of the Thoracic Aortic Wall. In: Stanger, O., Pepper, J., Svensson, L. (eds) Surgical Management of Aortic Pathology. Springer, Vienna. https://doi.org/10.1007/978-3-7091-4874-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-7091-4874-7_10
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-4872-3
Online ISBN: 978-3-7091-4874-7
eBook Packages: MedicineMedicine (R0)