Abstract
The Rap proteins comprise a subfamily of the Ras-like small G-proteins, most closely related to Ras, which were originally found to antagonize Ras-induced cell transformation. Rap transduces extracellular stimuli to a variety of different processes, amongst others cell–matrix adhesion, cell–cell adhesion, and actin dynamics. Here, we present an overview of the mechanisms that regulate activation, including guanine nucleotide exchange factors and GTPase activating proteins responsible for the regulation. We discuss the various biological functions that are controlled by Rap proteins and the molecular mechanism used by Rap1 to induce these responses.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Rap Proteins
Rap proteins were first identified by Pizon and coworkers in a search for Ras-related genes in a human cDNA library (Pizon et al. 1988). Five Rap isoforms exist in humans: Rap1A, 1B, 2A, 2B, and 2C, which share approximately 50 % sequence identity with Ras and as such are most closely related to the classical Ras proteins. The Rap1 isoforms are identical in “the business end” of the molecule (Fig. 11.1, termed “switch 1” and “switch 2”), i.e., the region that changes conformation upon binding to GDP or GTP and that interacts with guanine nucleotide exchange factors (GEFs), GTPase activating proteins (GAPs), and effectors (Bos et al. 2007). Within this region, one amino acid difference exists between the Rap2 isoforms. Six amino acids within this region are different between Rap1 and Rap2, an important one is on position 39 (S in Rap1, F in Rap2), as this residue allows specific GEFs and effectors to bind to Rap2 (Miertzschke et al. 2007; Nonaka et al. 2008; Yaman et al. 2009). Most differences between the Rap isoforms reside within the C-terminal hypervariable region (HVR). This region determines the subcellular localization of the protein in three ways (Prior and Hancock 2012): (1) the last four residues constitute the CAAX-motif (C = cysteine, A = aliphatic, X = any amino acid). This CAAX-motif is recognized by either a farnesyltransferase or a geranylgeranyltransferase, which covalently attaches an isoprenoid (either farnesyl or geranylgeranyl) to the cysteine. These lipid modifications insert into lipid bilayers to target the protein to cell membranes. Rap1A, Rap1B, and Rap2B are geranylgeranylated, whereas Rap2A and Rap2C are farnesylated. (2) Rap1 proteins have a poly-basic region in the HVR that aid to membrane targeting. For instance, Rap1A contains two clusters of three lysine residues within the HVR, in which it differs subtly with Rap1B (Fig. 11.1). (3) In contrast, Rap2 proteins contain two cysteine residues that are palmityolated. Palmitoylation is dynamic and a driving force in recycling of small GTPases (Rocks et al. 2010). Although Rap proteins are found in the plasma membrane, intracellular positions have been identified as well (Berger et al. 1994; Hisata et al. 2007; Mochizuki et al. 2001).
Sequence alignment of the five Rap proteins. The amino acid sequence of Rap1A is indicated. Dashes indicate identical amino acids in the other isoforms. Red amino acids are different between Rap1 and Rap2. Green amino acids are different between Rap1 isoforms, whereas blue amino acids are different between Rap2 isoforms. Green boxes indicate the P-loop (important for nucleotide binding) and the Switch regions (important for GEF and effector binding). The yellow box indicates the hypervariable region (HVR) (important for localization), which includes the palmitoylatable cysteines and the CAAX-motif
Most research has focused on Rap1 as overexpression of Rap1, but not Rap2, suppresses Ras-induced cell transformation. This was shown in an elegant genetic screen by Noda and coworkers in 1989 (Kitayama et al. 1989). cDNAs were introduced into Ras transformed cells and selected for flat revertants. One of the hits was Kirsten Ras revertant 1 (Krev-1), identical to Rap1A. As Rap1 and Ras are very similar in their effector-binding region and effector proteins binding to Rap1 in pull-down assays invariably bind Ras as well, it was hypothesized that Rap interferes in Ras signaling by functioning as a decoy that traps Ras effectors in a nonfunctional compartment. Indeed, it was reported that Rap1 binds to and inhibits Raf1 (Cook et al. 1993; Nassar et al. 1995). In contrast, Rap1 was reported to be the dominant activator of B-Raf to positively regulate the ERK pathway (Vossler et al. 1997). Both these results were questioned by other groups, and the prevailing model is that the effect of Rap1 on the morphology of Ras-induced transformed cells is due to the ability of Rap1 to increase cell adhesion (Bos 2005). It should be noted that there is indeed evidence that Rap1 can influence the Raf/B-Raf-MEK-ERK pathway; however, whether this is a direct effect due to an interaction with Raf or B-Raf, or indirectly, for instance, due to the regulation of integrins, is still a matter of debate. Our own results point to an indirect effect (Zwartkruis et al. 1998).
Despite their similarities, Ras and Rap were early inventions in evolution (van Dam et al. 2011). Both proteins were already present in the last eukaryotic common ancestor. Rap1 and Rap2 deviate later during evolution. The presence of Rap in unicellular organisms further stresses a role of Rap proteins in the regulation of fundamental cellular events. Indeed, studies in yeast were critical in understanding the overall function of Rap1 proteins. The Rap1 ortholog in budding yeast, Bud1/Rsr1, is involved in bud site selection (Park et al. 1993; Chant et al. 1991). Spatial landmarks determine the position of a future bud adjacent to a budscar of a previous bud. Bud1 together with its GEF, Bud5, and GAP, Bud2, recognize the landmark and pass the signal on to recruit the actin cytoskeleton. This generated the hypothesis that Rap proteins are involved in the recognition of spatial cues to direct actin driven processes.
Also studies in Drosophila helped in understanding the function of Rap proteins. Disruption of Rap1 results in a dorsal closure defect suggesting an effect on cell migration (Asha et al. 1999). If the maternal contribution of Rap1 is disrupted as well, an early lethal effect is observed due to a failure of migration of the pole cells (early germ cells) during embryogenesis. Disruption of Rap1 in the wing results in a defect in DE-cadherin mediated cell-cell junctions (Knox and Brown 2002). Together, these studies point already to a function of Rap proteins in cell adhesion, migration, and the actin cytoskeleton. This is supported by the phenotype of Rap1 knockout mice. Rap1A−/− mice are viable and fertile, but cells isolated from these mice show defective integrin activation (Duchniewicz et al. 2006). Rap1B−/− mice show 85 % embryonic lethality due to excessive bleeding, probably attributable to weakening of the vessel wall (Chrzanowska-Wodnicka et al. 2005, 2008). By now, regulation of cell-cell adhesion, cell-matrix adhesion and actin dynamics confine the textbook functions of the Rap proteins in mammalian cells, the analysis of which has been performed along three main lines: the analysis of the molecular mechanism of Rap1 activation and inactivation, the analysis of the biological responses and the identification of the molecular mechanism, including the identification of the critical effects of Rap proteins. We will present the highlights of these studies.
2 Rap Activity Regulation
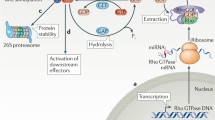
The first indication that Rap1 acts as a molecular switch came from work of Altschuler and coworkers who showed that cAMP activates Rap1 (Altschuler et al. 1995). They were using a classical labeling experiment, in which cells transfected with epitope-tagged Rap1 were labeled with 32P orthophosphate. After immunoprecipitation of Rap1, the ratio GDP/GTP was determined by thin layer chromatography. Activation measurements became much easier by the introduction of the pull-down assay by Franke and coworkers (1997), which employs the Rap1-binding domain of RalGDS as a probe to precipitate Rap1GTP, followed by western blotting. Using this technology it was shown that Rap1 is activated by a large variety of stimuli, suggesting that multiple GEFs or GAPs are involved in the regulation of Rap1 (Gloerich and Bos 2011). Indeed, many of these regulatory proteins were identified and molecular details on their regulation elucidated.
2.1 RapGEFs
GEFs activate small G-proteins by decreasing their affinity for nucleotides (Bos et al. 2007). As a result, GDP is replaced by the more abundant GTP. The catalytic domain of RapGEFs is the CDC25 Homology Domain (CDC25-HD), flanked by a stabilizing Ras Exchange Motif (REM). In addition, these GEFs have usually several other domains (Fig. 11.2) or posttranslational modifications that respond to extracellular signals and regulate either activity, localization, or both.
2.1.1 C3G
C3G (Crk SH3 domain-binding GEF) (RapGEF1) was the first RapGEF identified by Tanaka et al. (1994) and Matsuda et al. (1994), based on its possession of a for RasGEFs characteristic REM-CDC25-HD region and its binding to the SH3 domain of the adaptor protein Crk through a proline-rich sequence. The predominant target of C3G is Rap1. C3G is ubiquitously expressed and C3G knockout mice are early embryonic lethal indicating the importance of this GEF (Ohba et al. 2001). C3G confers an auto-inhibited conformation, as its activity can be greatly enhanced by removal of the N-terminal half (Ichiba et al. 1999). In cells, release of autoinhibition is conferred by phosphorylation of Y504 by kinases of the Src family, which in their turn are activated by a variety of stimuli.
2.1.2 Epacs
A real surprise was the discovery of Epac proteins by De Rooij et al. and Kawasaki et al. in 1998 (de Rooij et al. 1998; Kawasaki et al. 1998a), as these proteins turn out to be regulated by direct cAMP binding. At that time it was commonly accepted that protein kinase A (PKA) and some ion channels were the only targets of cAMP. The Epac proteins, Epac1 (RapGEF3) and Epac2 (RapGEF4), are characterized by the presence of either one (Epac1) or two (Epac2) cyclic nucleotide-binding domains. Furthermore, both isoforms contain a DEP domain, the catalytic REM-CDC25-HD tandem, and an RA domain (Gloerich and Bos 2010). Expression of both Epac1 and Epac2 is highly variable between cell types, with Epac1 amongst others high in endothelial cells, kidney, heart, brain, adipose tissue, and ovary and Epac2 in brain, adrenal glands and beta cells of the pancreas (Kawasaki et al. 1998a). Epac1 and Epac2 single and double knockout mice are viable with mild defects (Pereira et al. 2013), suggesting a modulatory role rather than a critical role in Rap1 regulation.
Structure analysis of both active and inactive Epac2 revealed that Epac is normally in a closed inactive conformation, in which the N-terminal (regulatory) region occludes the Rap-binding site (Rehmann et al. 2006). Upon binding of cAMP, a massive conformational change allows Rap to reach the catalytic helix (Rehmann et al. 2008). Interestingly, in addition to allosteric activation, cAMP induces the translocation of Epac1, but not Epac2, to the plasma membrane (Ponsioen et al. 2009). Recently, the molecular mechanism of this translocation was eluded. Binding of cAMP induces a conformation change in the DEP domain (Li et al. 2011), allowing it to bind to phosphatidic acid, which is enriched in the plasma membrane (Consonni et al. 2012). Hence, cAMP controls both Epac1 activity as well as plasma membrane localization. A diverse set of proteins binds Epac1 resulting in a variety of different membrane localizations. These include the ERM proteins (Ezrin, Radixin, Moesin), RanBP2 and β-arrestin (Gloerich et al. 2010, 2011; Mangmool et al. 2010). In all cases cAMP remains required for activation.
2.1.3 RasGRPs (CalDAG-GEFs)
RasGRP was first identified as a RasGEF having, in addition to the REM-CDC25-HD module, (calcium-binding) EF hands and a (DAG-binding) C1 domain (Ebinu et al. 1998). Subsequently, additional members of the family were found that were specific for Rap proteins, or have dual specificity (Kawasaki et al. 1998b; Yamashita et al. 2000). At that time these were renamed in CalDAG-GEFs, but the official name remains RasGRP for all protein members. Both DAG and calcium are under control of phospholipase C (PLC), which converts the lipid PI-4,5P2 to DAG and IP3, the latter being an inducer of calcium release from the endoplasmic reticulum. Structural data show that RasGRP forms dimers in which the catalytic core is shielded. Binding of calcium and DAG overcomes this autoinhibition (Iwig et al. 2013). Furthermore, DAG employs additional control on RasGRPs. First, it enhances the targeting of RasGRP to cell membranes (Ebinu et al. 1998). Second, it activates PKC, which phosphorylates RasGRP (Zheng et al. 2005). This phosphorylation event is important for RasGRP activity status, but not its localization, suggesting DAG controls both RasGRP activity as well as localization. Here, it is important to note some intriguing differences between the RasGRPs. RasGRP2 does not directly bind DAG and therefore escapes DAG-mediated control on its localization (Johnson et al. 2007). Hence, RasGRP2 is only subjected to DAG-PKC-mediated activation. Furthermore, the RasGRPs display specificity in the activation of substrates. RasGRP2 shows substrate specificity towards the Raps and does not activate other Ras-like G-proteins. Conversely, RasGRP1 does not activate Raps but does activate Ras isoforms (Kawasaki et al. 1998b). RasGRP3 promiscuously activates both Rap and Ras isoforms (Yamashita et al. 2000). Hence, RasGRP2 and 3 are of importance for Rap. Both these isoforms are under control of DAG, albeit to different extents. We have kept the CalDAG-GEF nomenclature (RasGRP2 is CalDAG-GEF1 and RasGRP3 CalDAG-GEF3) for these two RapGEFs.
2.1.4 PDZ-GEFs
PDZ-GEF1 (RapGEF2) and PDZ-GEF2 (RapGEF6) are two RapGEFs that at first notice appear highly similar to the Epacs. PDZ-GEFs contain the REM-CDC25-HD tandem and an RA domain in their C-terminal half and two cyclic nucleotide-binding domains and a PDZ domain in the N-terminal half (de Rooij et al. 1999). However, the cyclic nucleotide-binding domains lack critical residues for cAMP or cGMP binding, and indeed cyclic nucleotides cannot activate PDZ-GEF in vitro (Kuiperij et al. 2003; Pham et al. 2000). Although PDZ-GEFs exhibit an auto-inhibited state (de Rooij et al. 1999), the mechanism of its relieve is currently unclear. Additional control of PDZ-GEF can be conferred by interacting scaffolding proteins, phosphorylation, and degradation (Letschka et al. 2008; Magliozzi et al. 2013; Sakurai et al. 2006). Furthermore, active G-proteins can bind to the RA domain present in PDZ-GEF. The RA domain of PDZ-GEF1 binds active Rap1 (Liao et al. 2001), whereas the RA domain of PDZ-GEF2 binds active M-Ras (Yoshikawa et al. 2007). Recently, PDZ-GEF was found to interact with phosphatidic acid in the apical membrane of intestinal epithelial cells. This interaction resulted in the activation of Rap2A (Gloerich et al. 2012).
2.1.5 RasGEF1s
The RasGEF1 proteins RasGEF1A, B, and C comprise a relatively uncharacterized family of RapGEFs (Yaman et al. 2009). They contain the typical REM-CDC25-HD tandem, but other designated domains or regulatory sequences are lacking. So far experimental data on the RasGEFs have been restricted to in vitro measurements to test their activity towards various G-proteins. This revealed that the RasGEFs confer substrate specificity towards the Rap2 proteins (Yaman et al. 2009). In line with this, depletion of RasGEF1C enhances the barrier function of endothelial monolayers, similar to depletion of Rap2 (Pannekoek et al. 2013).
2.1.6 PLCε
PLCε is an atypical RapGEF in the sense that it bears multiple catalytically active domains (Jin et al. 2001; Lopez et al. 2001; Song et al. 2001). The N-terminus contains the REM-CDC25-HD module to catalyze Rap activation. The CDC25-HD is flanked by a PLC domain, which processes phospholipids. Furthermore, PLCε also contains an RA domain that can bind active Rap1 (Jin et al. 2001). Thus, PLCε induces Rap1 activation at places where Rap1 activity is already present, establishing a positive feedback system to enhance Rap activation.
2.2 RapGAPs
Rap inactivation by GTP hydrolysis occurs as one of the phosphates of GTP (gamma-phosphate) reacts with H2O to GDP and Pi (Bos et al. 2007). However, charges around the G-protein-bound GTP do not allow the correct orientation and polarization of the attacking H2O molecule, resulting in a very slow intrinsic GTPase activity of small G-proteins. GAPs generally function to coordinate the attacking H2O and neutralize the negative charge of the gamma-phosphate, thereby greatly enhancing the efficiency of the H2O attack on the gamma-phosphate (Bos et al. 2007).
It should be noted that inactivation is an essential step in the dynamic control of small GTPases. For instance, Rap1 enhances migration of Dictyostelium by inducing attachment to matrix. Efficient migration requires the formation of attachment at the leading front and release of cell attachment at the rear end. The latter is ensured by GAP-mediated inactivation of Rap1 (Jeon et al. 2007). Similarly, extravasation of tumor cells is blocked by both constitutive Rap1 activation and RapGAP overexpression, as these conditions confer either too strong or too weak adhesion to migrate through the vessel wall (Freeman et al. 2010).
2.2.1 RapGAPs
The function of Rap1GAP is still rather elusive. Rap1GAP, comprising several splice variants, contains a GAP domain that catalyzes GTP hydrolysis on Rap proteins and a GoLoco domain. The GoLoco domain was found to interact with Gα subunits of hetero-trimeric G-proteins as well as 14-3-3 (Jordan et al. 2005; Meng et al. 1999; Willard et al. 2007), but the precise function of this domain is still unclear as interactions were reported to be activating and inhibitory. Phosphorylation by cGMP-dependent kinases may activate Rap1GAP.
2.2.2 Spa1 and the SPARs
Spa1 and the related GAPs SPAR1, 2, and 3 are RapGAPs characterized by the presence of a C-terminal PDZ domain. PDZ domains are notorious protein–protein interaction domains that regulate spatial distribution of signaling. As such, Spa1 and the SPARs are also found to locate to various complexes under control of their PDZ domains. For instance, SPAR is localized to Ephrin receptors to induce Rap1-mediated de-attachment by Ephrins (Richter et al. 2007), and to dendritic spines to regulate spine morphology (Pak et al. 2001). Apart from localization, the SPARs are also regulated by protein degradation via multiple pathways, all of which function to manage sustained activity of Rap1 (Gloerich and Bos 2011).
2.2.3 Plexins
Plexins are cell surface receptors for the semaphorin family of guidance cues. Their cytosolic tail harbors a GAP domain that catalyzes inactivation of Rap G-proteins. When not bound to semaphorins, Plexins exist as monomers, the GAP domain of which is auto-inhibited. Plexins dimerize upon ligand binding, which induces release of auto-inhibition. Therefore, semaphorin molecules act as repulsive guidance cues by inducing Plexin-mediated Rap1 inactivation (Wang et al. 2012).
2.2.4 GAP1 Proteins and SynGAP
Analogous to the CalDAG-GEFs, which display dual specificity towards Ras and Rap, RasGAPs of the GAP1 family inactivate both Ras and Rap (Kupzig et al. 2006, 2009). These GAPs, termed RASA2 (GAP1m), RASA3 (GAP1IP4BP), RASA4 (CAPRI), and RASAL1, contain a central GAP domain which is flanked by two C2 domains at the N terminus and a PH domain at the C terminus (King et al. 2013). The dual specificity towards both Ras and Rap is surprising as RasGAPs and RapGAPs generally utilize different mechanisms: RasGAPs provide an arginine to reposition Gln61 in Ras to allow correct polarization of the attacking H2O. Rap proteins lack Gln61, but its function can be fulfilled by an asparagine that is provided by RapGAPs (Daumke et al. 2004). The dual specificity GAPs in essence function as RasGAPs by providing the arginine residue to facilitate Ras inactivation. In addition to that, dual specificity GAPs contain sequences outside the GAP domain that induce conformational changes in Rap that allow its Gln63 to aid in correct positioning of the attacking H2O, thereby allowing these GAPs to also catalyze Rap inactivation (Sot et al. 2010).
3 Rap Functions
Initially, the function of Rap1 in mammalian cells was studied by the introduction of a constitutively active Rap1 (RapV12), by the introduction of a dominant negative Rap1 (RapN17) or the introduction of a RapGAP. For instance, a key finding in elucidating Rap1 functioning was made in 1999, when overexpression of the RapGAP Spa1 was shown to prevent attachment of HeLa cells (Tsukamoto et al. 1999). Indeed subsequent analysis revealed that Rap1 is a key mediator of integrin-mediated cell adhesion and of cell–cell junction formation (Boettner and Van Aelst 2009; Pannekoek et al. 2009; Raaijmakers and Bos 2009). Many subsequent studies on the function of Rap1 were greatly helped by the development of an Epac-selective cAMP analogue, 8-pCPT-2′O-Me-cAMP (007) (Enserink et al. 2002). Using this analogue endogenous Epac and subsequently Rap proteins were selectively activated in cells, and biological responses could be identified and analyzed. As such, the Rap proteins have now been solidly implicated in regulating endothelial barrier function, epithelial cell–cell adhesion, homing of circulatory cells, neurite outgrowth, induction of cell polarity, and cardiac contraction. To this end, Rap controls the activity or localization effector proteins by binding to their RA, RBD, or B41/ERM domain, which are structurally similar domains (Kiel and Serrano 2006). The effectors of the Rap proteins can be classified in different groups: inducers of cell–matrix adhesion (RAPL, RIAM), inhibitors of Rho-mediated contraction (KRIT1, Radil, Rasip1, ARAP1, ARAP3, RA-RhoGAP) and activators of actin remodeling (TIAM1, Vav2, TNIK, NIK, MINK) (Fig. 11.3). Furthermore, the cell–cell adhesion scaffold AF6 and the lipid modifier (and RapGEF) PLCε are controlled by Rap1. We will discuss these effectors in light of the physiological effects of Rap1 (see Fig. 11.4 for an overview picture).
3.1 Endothelial Barrier Function
The endothelium is the inner lining of the vascular system. Its main function is to form a barrier between the blood and the tissues underlying the vasculature. The tightness of this barrier should be very dynamically regulated, as controlled passage of fluid, solutes, and even circulating cells should be allowed upon request of the tissue (Komarova and Malik 2010). Rap1 activation enhances the barrier function of the endothelium (Pannekoek et al. 2014). To this end, barrier tightening agents increase cAMP levels, which activate both Epac1 and PKA to enhance barrier function (Lorenowicz et al. 2008). Also in the absence of cAMP moderate levels of active Rap1 are maintained to ensure a certain amount of basal barrier function. This effect is controlled by PDZ-GEF (Pannekoek et al. 2011). PDZ-GEF1 is the main isoform, which is supported by the defective cell–cell adhesion and concomitant yolk sac vasculogenesis observed in PDZ-GEF1−/− mice (Kanemura et al. 2009; Wei et al. 2007). Furthermore, Rap1 is activated when hyperpermeability is induced to prevent excessive leakage and ensure rapid reestablishment of the barrier (Birukova et al. 2013). Recent advances suggest that Rap1 controls endothelial barrier by regulating tension on the actin cytoskeleton. To this end, various pathways have suggested, some of which may act in concert. Diminished tension on radial stress fibers is conferred by the Rap1 effectors KRIT1 (Glading et al. 2007; Stockton et al. 2010) or Rasip1/Radil (Post et al. 2013; Wilson et al. 2013; Xu et al. 2009). How KRIT1 regulates tension remains to be elucidated. Rasip1 and Radil control the RhoGAP ArhGAP29 to inhibit the tension pathway (Post et al. 2013; Xu et al. 2011). In contrast, Rap1 also induces tension in the actin cytoskeleton that runs along the cell–cell contact, a process mediated by Cdc42 (Ando et al. 2013). Hence, Rap1 simultaneously increases and decreases cytoskeletal tension depending on where the actin bundle is located. Together this renders cell–cell junction tightening. Interestingly, the Rap1-Rasip1/Radil-ArhGAP29 pathway also directs cell spreading, both in endothelial and in epithelial cells, due to the abovementioned relaxation of stress fibers (Post et al. 2013).
3.2 Epithelial Cell–Cell Adhesion
Similar to the endothelium, the epithelium is a cell layer that confers a barrier function to protect underlying tissue. The main difference between the two is that the epithelium is avascular, as the epithelium lines body cavities. Its barrier function depends on its location and can be very tight (e.g., skin) but also less tight (e.g., kidney). Rap1 is important for epithelial cell–cell adhesion. However, in contrast the endothelium, Rap1 effects on epithelial monolayers are only observed upon challenging of the monolayer: Rap1GAP overexpression prevents de novo formation of cell–cell junctions, but does not affect mature cell–cell junctions (Hogan et al. 2004). Similarly, effects of Rap1 siRNA could only be observed after replating the monolayer (Dube et al. 2008). Hence, Rap1 is required for epithelial cell–cell adhesion either during development or when the monolayer is challenged, but not for maintenance of cell–cell adhesion. To this end, Rap1 is activated by either C3G or PDZ-GEF2. Overexpression of dominant negative C3G prevents de novo junction formation, similar to Rap1GAP overexpression (Hogan et al. 2004). Here, the junctions lack E-cadherin, suggesting C3G/Rap1 functions in the recruitment of E-cadherin during junction formation. Depletion of PDZ-GEF2 has a milder effect: junctional E-cadherin levels are 80 % of their control counterparts and the junction appears zipper-like, indicative of high tension (Dube et al. 2008). Interestingly, these PDZ-GEF2 effects are differentially phenocopied by depletion of Rap1 isoforms: Rap1A depletion induces the zipper-like morphology, but does not affect the E-cadherin levels. The reverse goes for depletion of Rap1B, which affects E-cadherin levels but not junction morphology. It remains to be determined how these effects are controlled by Rap1. The junctional scaffold AF6 and the small G-protein Cdc42 have been suggested to control cell–cell adhesion downstream of Rap1, specifically considering E-cadherin levels (Fukuyama et al. 2005; Hogan et al. 2004; Hoshino et al. 2005). How these effectors cooperate and how the zipper phenotype is conferred remains to be elucidated.
3.3 Adhesion of Circulatory Cells
Circulatory cells like lymphocytes and platelets are non-adherent but can be rapidly induced to adhere when the body requires an immune response (lymphocytes) or blood clot formation (platelets) (Varga-Szabo et al. 2008; von Andrian and Mackay 2000). This adhesion relies on multistep adhesion cascades, in which activation of Rap1 ensures strong integrin-mediated adhesion to extracellular matrix. For platelets it has been shown that weak adhesion or prothrombotic factors impinge on CalDAG-GEF1 to induce activation of Rap1 and concomitant strong, integrin-mediated adhesion (Bernardi et al. 2006; Stefanini et al. 2009). Indeed, CalDAG-GEF1−/− mice display defective integrin-dependent platelet aggregation (Crittenden et al. 2004). Using model cell lines it is suggested that active Rap1 at the plasma membrane functions by recruiting RIAM, which induces the high affinity conformation of αIIbβ3 integrins via its binding protein Talin (Han et al. 2006; Lee et al. 2009). As a result, the platelets will form a meshwork with soluble fibrinogen fibers to form the blood clot. Additional control on this process is ensured by Rap1GAP. Nitric oxide prevents blood clot formation and it can do so by inducing phosphorylation of the platelet-specific isoform of Rap1GAP, thereby restricting Rap1 activation and concomitant integrin activation (Danielewski et al. 2005; Schultess et al. 2005).
Just as platelets, lymphocytes can be induced to adhere via a multistep adhesion cascade, thereby allowing lymphocyte adhesion at secondary lymphoid organs, where they screen for antigen, but also to allow strong adhesion between lymphocyte and an antigen presenting cell (APC). The latter relies on engagement of the T-cell receptor (TCR), which induces Rap1 activation via the GEFs C3G, CalDAG-GEF, and PDZ-GEF (Boussiotis et al. 1997; Katagiri et al. 2004b; Letschka et al. 2008). Why and how these three GEFs cooperate is unknown. Next, activated Rap1 engages with its effector RAPL to induce both clustering and activation of the LFA-1 integrin, thereby securing strong adhesion between lymphocyte and APC (Katagiri et al. 2003, 2004a).
Much less is known about adhesion of lymphocytes at secondary lymphoid organs. Here, reversible adhesion of lymphocytes is induced by chemokines, which impinge on CalDAG-GEF1 (Ghandour et al. 2007). However, the requirement of CalDAG-GEF1 for lymphocyte adhesion appears restricted to certain integrins, so other GEFs might contribute to regulate other integrins. The same holds for events downstream of Rap1: RAPL is required for stable arrest of lymphocytes. However, RAPL is not required for initial arrest, whereas Rap1 is (Ebisuno et al. 2010). Hence, other Rap1 effectors are at play here as well. RIAM would be the prime candidate, as it controls integrin activation downstream of Rap1. Indeed, RIAM depletion inhibits lymphocyte adhesion (Lafuente et al. 2004). However, as opposed to its function in platelet adhesion, RIAM does not require its Talin-binding domain to induce lymphocyte adhesion (Menasche et al. 2007). Instead, the central RA and PH domain are sufficient. These domains bind the adaptor protein SKAP1, which, together with its binding partners ADAP and SLP-76, is required for Rap1 translocation (Kliche et al. 2006; Patzak et al. 2010). Furthermore, RIAM has been suggested to induce Rap1 activation by CalDAG-GEF1 by mobilizing intracellular calcium (Patsoukis et al. 2009). Apparently, the function of RIAM in Rap1 signaling may be more complex than solely being an effector in integrin signaling.
3.4 Neurological Functions
Rap1 is implicated in two aspects of neuronal functioning. First, Rap1 controls the extension of neurites, which will become the axon and dendrites (Anneren et al. 2000). Second, once a neurite establishes a synaptic contact, Rap1 controls synaptic transmission (Imamura et al. 2003). Little mechanistic data are known about the latter. However, Rap1 functioning in neurite extension is better understood. Activation of Rap1 is conferred by different inputs, which impinge on Epac2, C3G, and PDZ-GEF1 (Hisata et al. 2007; Liu et al. 2008; Radha et al. 2008). Intriguingly, the latter two seem to cooperate in maintaining Rap1 activity upon neurotrophin receptor stimulation by activating Rap1 at early endosomes (C3G) and late endosomes (PDZ-GEF) (Hisata et al. 2007). Guidance cues that either attract or repel the extending neurite also impinge on Rap1 activity, most notably via RapGAPs: Ephrins induce retraction by recruiting SPAR to the activated receptor (Richter et al. 2007), whereas Semaphorins bind to and induce dimerization of Plexins, thereby activating the GAP domain in the cytosolic tail of the Plexins (Wang et al. 2012). The mode by which Rap1 mediates neurite extension is clear-cut: it prevents retraction by inactivating Rho-mediated contraction. RA-RhoGAP and ARAP3 are effectors that harbor RhoGAP activity, thereby directly relaying Rap1 activation towards Rho inactivation (Jeon et al. 2010a, b; Yamada et al. 2005). In a different setting, the sequential activation of Rap1B and CDC42 determines the fate of an axon. This fate is regulated by selective protection for degradation of Rap1B in the future axon (Schwamborn et al. 2007; Schwamborn and Puschel 2004).
3.5 Polarity
Polarity occurs when a polarization cue induces an uneven distribution of proteins over the cell. By inducing cell adhesion to matrix or other cells, Rap can facilitate polarization. However, Rap can also directly induce polarization. Front-rear polarity induced by Rap1 is observed in migrating lymphocytes (Shimonaka et al. 2003) and neutrophils (Carbo et al. 2010) and apical-basal polarity induction by Rap1 is seen in hepatocytes during canalicular network formation (Fu et al. 2011) and in the endothelium upon VE-cadherin engagement (Lampugnani et al. 2010). In terms of mechanisms, the contribution of Rap2 to apical-basal polarity of the intestinal epithelium is best understood (Gloerich et al. 2012). Here, induction of the polarity kinase Lkb1 induces translocation of PDZ-GEF to the apical membrane by increasing the membrane levels of phosphatidic acid, which functions as a direct anchor for PDZ-GEF. PDZ-GEF activates Rap2A, which induces translocation of its effector TNIK, its target MST4, and the MST4 substrate Ezrin. Next, activated Ezrin induces actin remodeling that results in the formation of a brush border. As such, a designated Rap2 signaling pathway couples induction of polarity to the polarized distribution of the actin cytoskeleton (Gloerich et al. 2012).
3.6 Cardiac Contraction
Cardiac myocytes are specialized muscle cells, the contraction of which functions to pump blood within the heart ventricles into the circulation. Contraction of cardiac myocytes relies on the electrically evoked release of calcium from the sarcoplasmatic reticulum. This release of calcium can be enhanced by cAMP via Epac1/Rap1. Activated Rap1 binds to the RA domain of PLCε, which induces the generation of DAG and IP3. These second messengers activate PKC to induce CaMKII-mediated phosphorylation of the ryanodine receptor, thereby inducing calcium release from the sarcoplasmic reticulum (Oestreich et al. 2009). As mentioned above, PLCε also harbors a CDC25-HD that can activate Rap1. This domain is also required for cardiac contraction. Here, Rap1 activated PLCε establishes a positive feedback loop to sustain Rap1 activity and cardiac contraction (Oestreich et al. 2009; Pereira et al. 2007).
Apart from directly inducing signaling to enhance excitation-contraction coupling, Rap1 also facilitates coordinated contraction of neighboring cells. This process is regulated by gap junctions, which allow the excitatory signal to be transduced to neighboring cells. The formation of gap junctions is facilitated by the presence of adherens junctions, which are induced by Epac1/Rap1 (Somekawa et al. 2005). Indeed, Rap1 increases cell–cell contact levels of Connexin43, which is the main constituent of gap junctions in cardiac myocytes (Somekawa et al. 2005). Furthermore, the aforementioned pathway Epac1/Rap1/PLCepsilon/PKC also increases phosphorylation of Connexin43, thereby further contributing to coordinated contraction (Duquesnes et al. 2010).
4 Concluding Remarks
The Rap1 protein, which originally was identified based on its capacity to revert Ras-induced cell transformation, is now known as a Ras-like G-protein that functions via its own independent signaling pathways. Rap1 is regulated by GEFs and GAP that are spatially localized in the cell and respond to a variety of extracellular stimuli. Molecular details of several downstream pathways have been revealed and the emerging picture is that Rap proteins are molecular switches that in most pathways, but not exclusively, impinge on processes that are linked to the actin cytoskeleton. Indeed, Rap1 modulates Rho GTPases, like Rho, Rac, and Cdc42, as well as adhesion molecules, like integrins and cadherins, which are connected to the actin cytoskeleton. How are Rap1 proteins directing all these different functions? One could envision two models. The first is that depending on the cellular context and the activating signal, Rap1 mediates a localized event that results in a single response. The second is that activation of Rap1 results directly in a pleotropic effect, with multiple endpoints. The activation of Epac by the selective agonist 007 frequently results in pleiotropic effects (increased adhesion, tightening of junctions, inhibition of migration), suggesting that multiple pathways are activated. Indeed, the Radil/Rasip1-ArhGAP29 pathway mediates Epac1-induced cell spreading, but not Epac1-induced integrin-mediated adhesion in the same cell. In endothelial cells, Epac1 activation simultaneously inhibits Rho and activates CDC42, most likely through different pathways. In contrast to this multifaceted pathway, Rap2 employs a straightforward linear pathway to control intestinal epithelial cell brush border formation. Most likely, Rap proteins serve diverse roles in signaling, one as an upstream controller of diverse (actin-driven) processes, like the dynamic regulation of cell–cell and cell–matrix adhesion, and one that is more restricted to one directional event, like possibly the formation of brush borders, the determination of axon fate, and cardiac contraction.
In the past two decades we have obtained a fairly good picture on the function of Rap proteins and the molecular mechanism of several processes. It turned out to be one of the best model systems to study spatial and temporal control of signaling, with GEFs that are regulated by second messengers and that translocate to the site of action, with effectors that are spatially localized and activated after Rap activation, and with protein domains that change conformation to interact with spatial cues. However, much remains to be explored and many questions remain. For instance, relatively simple, why is Rap2A uniquely involved in brush border formation, whereas the other Rap proteins are expressed? More complicated is the integration of all Rap signaling events amongst each other and with other signaling events, which requires a systems biology approach. With 007 as a unique single trigger, and high-end single cell analyses, these latter studies can be done. In addition, the Rap signaling pathway controls many processes that are underlying disease, like endothelial cell leakage, cardiac contraction, blood cell, and platelet adhesion. Our knowledge of the Rap1 signaling pathway requires translation into the clinic. In our opinion selective activation of the Rap1 signaling pathway may be more beneficial than inhibition, e.g., to inhibit vessel leakage. 007 can be a lead compound, but specific activators for PDZ-GEF may be worth to develop.
References
Altschuler DL, Peterson SN, Ostrowski MC, Lapetina EG (1995) Cyclic AMP-dependent activation of Rap1b. J Biol Chem 270(18):10373–10376
Ando K, Fukuhara S, Moriya T, Obara Y, Nakahata N, Mochizuki N (2013) Rap1 potentiates endothelial cell junctions by spatially controlling myosin II activity and actin organization. J Cell Biol 202(6):901–916
Anneren C, Reedquist KA, Bos JL, Welsh M (2000) GTK, a Src-related tyrosine kinase, induces nerve growth factor-independent neurite outgrowth in PC12 cells through activation of the Rap1 pathway. Relationship to Shb tyrosine phosphorylation and elevated levels of focal adhesion kinase. J Biol Chem 275(37):29153–29161
Asha H, de Ruiter ND, Wang MG, Hariharan IK (1999) The Rap1 GTPase functions as a regulator of morphogenesis in vivo. EMBO J 18(3):605–615
Berger G, Quarck R, Tenza D, Levy-Toledano S, de Gunzburg J, Cramer EM (1994) Ultrastructural localization of the small GTP-binding protein Rap1 in human platelets and megakaryocytes. Br J Haematol 88(2):372–382
Bernardi B, Guidetti GF, Campus F, Crittenden JR, Graybiel AM, Balduini C, Torti M (2006) The small GTPase Rap1b regulates the cross talk between platelet integrin alpha2beta1 and integrin alphaIIbbeta3. Blood 107(7):2728–2735
Birukova AA, Tian X, Tian Y, Higginbotham K, Birukov KG (2013) Rap-afadin axis in control of Rho signaling and endothelial barrier recovery. Mol Biol Cell 24(17):2678–2688
Boettner B, Van Aelst L (2009) Control of cell adhesion dynamics by Rap1 signaling. Curr Opin Cell Biol 21(5):684–693
Bos JL (2005) Linking Rap to cell adhesion. Curr Opin Cell Biol 17(2):123–128
Bos JL, Rehmann H, Wittinghofer A (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129(5):865–877
Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM (1997) Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science 278(5335):124–128
Carbo C, Duerschmied D, Goerge T, Hattori H, Sakai J, Cifuni SM, White GC II, Chrzanowska-Wodnicka M, Luo HR, Wagner DD (2010) Integrin-independent role of CalDAG-GEFI in neutrophil chemotaxis. J Leukoc Biol 88(2):313–319
Chant J, Corrado K, Pringle JR, Herskowitz I (1991) Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell 65(7):1213–1224
Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC 2nd (2005) Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest 115(3):680–687
Chrzanowska-Wodnicka M, Kraus AE, Gale D, White GC II, Vansluys J (2008) Defective angiogenesis, endothelial migration, proliferation, and MAPK signaling in Rap1b-deficient mice. Blood 111(5):2647–2656
Consonni SV, Gloerich M, Spanjaard E, Bos JL (2012) cAMP regulates DEP domain-mediated binding of the guanine nucleotide exchange factor Epac1 to phosphatidic acid at the plasma membrane. Proc Natl Acad Sci USA 109(10):3814–3819
Cook SJ, Rubinfeld B, Albert I, McCormick F (1993) RapV12 antagonizes Ras-dependent activation of ERK1 and ERK2 by LPA and EGF in Rat-1 fibroblasts. EMBO J 12(9):3475–3485
Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM (2004) CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med 10(9):982–986
Danielewski O, Schultess J, Smolenski A (2005) The NO/cGMP pathway inhibits Rap 1 activation in human platelets via cGMP-dependent protein kinase I. Thromb Haemost 93(2):319–325
Daumke O, Weyand M, Chakrabarti PP, Vetter IR, Wittinghofer A (2004) The GTPase-activating protein Rap1GAP uses a catalytic asparagine. Nature 429(6988):197–201
de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396(6710):474–477
de Rooij J, Boenink NM, van Triest M, Cool RH, Wittinghofer A, Bos JL (1999) PDZ-GEF1, a guanine nucleotide exchange factor specific for Rap1 and Rap2. J Biol Chem 274(53):38125–38130
Dube N, Kooistra MR, Pannekoek WJ, Vliem MJ, Oorschot V, Klumperman J, Rehmann H, Bos JL (2008) The RapGEF PDZ-GEF2 is required for maturation of cell-cell junctions. Cell Signal 20(9):1608–1615
Duchniewicz M, Zemojtel T, Kolanczyk M, Grossmann S, Scheele JS, Zwartkruis FJ (2006) Rap1A-deficient T and B cells show impaired integrin-mediated cell adhesion. Mol Cell Biol 26(2):643–653
Duquesnes N, Derangeon M, Metrich M, Lucas A, Mateo P, Li L, Morel E, Lezoualc’h F, Crozatier B (2010) Epac stimulation induces rapid increases in connexin43 phosphorylation and function without preconditioning effect. Pflugers Arch 460(4):731–741
Ebinu JO, Bottorff DA, Chan EY, Stang SL, Dunn RJ, Stone JC (1998) RasGRP, a Ras guanyl nucleotide- releasing protein with calcium- and diacylglycerol-binding motifs. Science 280(5366):1082–1086
Ebisuno Y, Katagiri K, Katakai T, Ueda Y, Nemoto T, Inada H, Nabekura J, Okada T, Kannagi R, Tanaka T, Miyasaka M, Hogg N, Kinashi T (2010) Rap1 controls lymphocyte adhesion cascade and interstitial migration within lymph nodes in RAPL-dependent and -independent manners. Blood 115(4):804–814
Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL (2002) A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4(11):901–906
Franke B, Akkerman JW, Bos JL (1997) Rapid Ca2 + -mediated activation of Rap1 in human platelets. EMBO J 16(2):252–259
Freeman SA, McLeod SJ, Dukowski J, Austin P, Lee CC, Millen-Martin B, Kubes P, McCafferty DM, Gold MR, Roskelley CD (2010) Preventing the activation or cycling of the Rap1 GTPase alters adhesion and cytoskeletal dynamics and blocks metastatic melanoma cell extravasation into the lungs. Cancer Res 70(11):4590–4601
Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM (2011) Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA 108(4):1403–1408
Fukuyama T, Ogita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M, Takai Y (2005) Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem 280(1):815–825
Ghandour H, Cullere X, Alvarez A, Luscinskas FW, Mayadas TN (2007) Essential role for Rap1 GTPase and its guanine exchange factor CalDAG-GEFI in LFA-1 but not VLA-4 integrin mediated human T-cell adhesion. Blood 110(10):3682–3690
Glading A, Han J, Stockton RA, Ginsberg MH (2007) KRIT-1/CCM1 is a Rap1 effector that regulates endothelial cell cell junctions. J Cell Biol 179(2):247–254
Gloerich M, Bos JL (2010) Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol 50:355–375
Gloerich M, Bos JL (2011) Regulating Rap small G-proteins in time and space. Trends Cell Biol 21(10):615–623
Gloerich M, Ponsioen B, Vliem MJ, Zhang Z, Zhao J, Kooistra MR, Price LS, Ritsma L, Zwartkruis FJ, Rehmann H, Jalink K, Bos JL (2010) Spatial regulation of cyclic AMP-Epac1 signaling in cell adhesion by ERM proteins. Mol Cell Biol 30(22):5421–5431
Gloerich M, Vliem MJ, Prummel E, Meijer LA, Rensen MG, Rehmann H, Bos JL (2011) The nucleoporin RanBP2 tethers the cAMP effector Epac1 and inhibits its catalytic activity. J Cell Biol 193(6):1009–1020
Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL (2012) Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol 14(8):793–801
Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH (2006) Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol 16(18):1796–1806
Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y (2007) Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J Cell Biol 178(5):843–860
Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y (2004) Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol 24(15):6690–6700
Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y (2005) Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem 280(25):24095–24103
Ichiba T, Hashimoto Y, Nakaya M, Kuraishi Y, Tanaka S, Kurata T, Mochizuki N, Matsuda M (1999) Activation of C3G guanine nucleotide exchange factor for Rap1 by phosphorylation of tyrosine 504. J Biol Chem 274(20):14376–14381
Imamura Y, Matsumoto N, Kondo S, Kitayama H, Noda M (2003) Possible involvement of Rap1 and Ras in glutamatergic synaptic transmission. Neuroreport 14(9):1203–1207
Iwig JS, Vercoulen Y, Das R, Barros T, Limnander A, Che Y, Pelton JG, Wemmer DE, Roose JP, Kuriyan J (2013) Structural analysis of autoinhibition in the Ras-specific exchange factor RasGRP1. eLife 2:e00813
Jeon TJ, Lee DJ, Lee S, Weeks G, Firtel RA (2007) Regulation of Rap1 activity by RapGAP1 controls cell adhesion at the front of chemotaxing cells. J Cell Biol 179(5):833–843
Jeon CY, Kim HJ, Lee JY, Kim JB, Kim SC, Park JB (2010a) p190RhoGAP and Rap-dependent RhoGAP (ARAP3) inactivate RhoA in response to nerve growth factor leading to neurite outgrowth from PC12 cells. Exp Mol Med 42(5):335–344
Jeon CY, Kim HJ, Morii H, Mori N, Settleman J, Lee JY, Kim J, Kim SC, Park JB (2010b) Neurite outgrowth from PC12 cells by basic fibroblast growth factor (bFGF) is mediated by RhoA inactivation through p190RhoGAP and ARAP3. J Cell Physiol 224(3):786–794
Jin TG, Satoh T, Liao Y, Song C, Gao X, Kariya K, Hu CD, Kataoka T (2001) Role of the CDC25 homology domain of phospholipase Cepsilon in amplification of Rap1-dependent signaling. J Biol Chem 276(32):30301–30307
Johnson JE, Goulding RE, Ding Z, Partovi A, Anthony KV, Beaulieu N, Tazmini G, Cornell RB, Kay RJ (2007) Differential membrane binding and diacylglycerol recognition by C1 domains of RasGRPs. Biochem J 406(2):223–236
Jordan JD, He JC, Eungdamrong NJ, Gomes I, Ali W, Nguyen T, Bivona TG, Philips MR, Devi LA, Iyengar R (2005) Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 activation through G alpha(o/i)-triggered proteasomal degradation of Rap1GAPII. J Biol Chem 280(12):11413–11421
Kanemura H, Satoh T, Bilasy SE, Ueda S, Hirashima M, Kataoka T (2009) Impaired vascular development in the yolk sac and allantois in mice lacking RA-GEF-1. Biochem Biophys Res Commun 387(4):754–759
Katagiri K, Maeda A, Shimonaka M, Kinashi T (2003) RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat Immunol 4(8):741–748
Katagiri K, Ohnishi N, Kabashima K, Iyoda T, Takeda N, Shinkai Y, Inaba K, Kinashi T (2004a) Crucial functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol 5(10):1045–1051
Katagiri K, Shimonaka M, Kinashi T (2004b) Rap1-mediated lymphocyte function-associated antigen-1 activation by the T cell antigen receptor is dependent on phospholipase C-gamma1. J Biol Chem 279(12):11875–11881
Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM (1998a) A family of cAMP-binding proteins that directly activate Rap1. Science 282(5397):2275–2279
Kawasaki H, Springett GM, Toki S, Canales JJ, Harlan P, Blumenstiel JP, Chen EJ, Bany IA, Mochizuki N, Ashbacher A, Matsuda M, Housman DE, Graybiel AM (1998b) A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc Natl Acad Sci USA 95(22):13278–13283
Kiel C, Serrano L (2006) The ubiquitin domain superfold: structure-based sequence alignments and characterization of binding epitopes. J Mol Biol 355(4):821–844
King PD, Lubeck BA, Lapinski PE (2013) Nonredundant functions for Ras GTPase-activating proteins in tissue homeostasis. Sci Signal 6(264):re1
Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M (1989) A ras-related gene with transformation suppressor activity. Cell 56(1):77–84
Kliche S, Breitling D, Togni M, Pusch R, Heuer K, Wang X, Freund C, Kasirer-Friede A, Menasche G, Koretzky GA, Schraven B (2006) The ADAP/SKAP55 signaling module regulates T-cell receptor-mediated integrin activation through plasma membrane targeting of Rap1. Mol Cell Biol 26(19):7130–7144
Knox AL, Brown NH (2002) Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 295(5558):1285–1288
Komarova Y, Malik AB (2010) Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72:463–493
Kuiperij HB, de Rooij J, Rehmann H, van Triest M, Wittinghofer A, Bos JL, Zwartkruis FJ (2003) Characterisation of PDZ-GEFs, a family of guanine nucleotide exchange factors specific for Rap1 and Rap2. Biochim Biophys Acta 1593(2–3):141–149
Kupzig S, Deaconescu D, Bouyoucef D, Walker SA, Liu Q, Polte CL, Daumke O, Ishizaki T, Lockyer PJ, Wittinghofer A, Cullen PJ (2006) GAP1 family members constitute bifunctional Ras and Rap GTPase-activating proteins. J Biol Chem 281(15):9891–9900
Kupzig S, Bouyoucef-Cherchalli D, Yarwood S, Sessions R, Cullen PJ (2009) The ability of GAP1IP4BP to function as a Rap1 GTPase-activating protein (GAP) requires its Ras GAP-related domain and an arginine finger rather than an asparagine thumb. Mol Cell Biol 29(14):3929–3940
Lafuente EM, van Puijenbroek AA, Krause M, Carman CV, Freeman GJ, Berezovskaya A, Constantine E, Springer TA, Gertler FB, Boussiotis VA (2004) RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev Cell 7(4):585–595
Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, Dejana E (2010) CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci 123(Pt 7):1073–1080
Lee HS, Lim CJ, Puzon-McLaughlin W, Shattil SJ, Ginsberg MH (2009) RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences. J Biol Chem 284(8):5119–5127
Letschka T, Kollmann V, Pfeifhofer-Obermair C, Lutz-Nicoladoni C, Obermair GJ, Fresser F, Leitges M, Hermann-Kleiter N, Kaminski S, Baier G (2008) PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood 112(12):4617–4627
Li S, Tsalkova T, White MA, Mei FC, Liu T, Wang D, Woods VL, Cheng XD (2011) Mechanism of Intracellular cAMP Sensor Epac2 Activation cAMP-induced conformational changes identified by amide hydrogen/deuterium exchange mass spectrometry (DXMS). J Biol Chem 286(20):17889–17897
Liao Y, Satoh T, Gao X, Jin TG, Hu CD, Kataoka T (2001) RA-GEF-1, a guanine nucleotide exchange factor for Rap1, is activated by translocation induced by association with Rap1*GTP and enhances Rap1-dependent B-Raf activation. J Biol Chem 276(30):28478–28483
Liu C, Takahashi M, Li Y, Song S, Dillon TJ, Shinde U, Stork PJ (2008) Ras is required for the cyclic AMP-dependent activation of Rap1 via Epac2. Mol Cell Biol 28(23):7109–7125
Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW (2001) A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem 276(4):2758–2765
Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL (2008) PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol 87(10):779–792
Magliozzi R, Low TY, Weijts BG, Cheng T, Spanjaard E, Mohammed S, van Veen A, Ovaa H, de Rooij J, Zwartkruis FJ, Bos JL, de Bruin A, Heck AJ, Guardavaccaro D (2013) Control of epithelial cell migration and invasion by the IKKbeta- and CK1alpha-mediated degradation of RAPGEF2. Dev Cell 27(5):574–585
Mangmool S, Shukla AK, Rockman HA (2010) beta-Arrestin-dependent activation of Ca(2+)/calmodulin kinase II after beta(1)-adrenergic receptor stimulation. J Cell Biol 189(3):573–587
Matsuda M, Hashimoto Y, Muroya K, Hasegawa H, Kurata T, Tanaka S, Nakamura S, Hattori S (1994) CRK protein binds to two guanine nucleotide-releasing proteins for the Ras family and modulates nerve growth factor-induced activation of Ras in PC12 cells. Mol Cell Biol 14(8):5495–5500
Menasche G, Kliche S, Chen EJ, Stradal TE, Schraven B, Koretzky G (2007) RIAM links the ADAP/SKAP-55 signaling module to Rap1, facilitating T-cell-receptor-mediated integrin activation. Mol Cell Biol 27(11):4070–4081
Meng JW, Glick JL, Polakis P, Casey PJ (1999) Functional interaction between G alpha(z) and Rap1GAP suggests a novel form of cellular cross-talk. J Biol Chem 274(51):36663–36669
Miertzschke M, Stanley P, Bunney TD, Rodrigues-Lima F, Hogg N, Katan M (2007) Characterization of interactions of adapter protein RAPL/Nore1B with RAP GTPases and their role in T cell migration. J Biol Chem 282(42):30629–30642
Mochizuki N, Yamashita S, Kurokawa K, Ohba Y, Nagai T, Miyawaki A, Matsuda M (2001) Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411(6841):1065–1068
Nassar N, Horn G, Herrmann C, Scherer A, McCormick F, Wittinghofer A (1995) The 2.2 A crystal structure of the Ras-binding domain of the serine/threonine kinase c-Raf1 in complex with Rap1A and a GTP analogue. Nature 375(6532):554–560
Nonaka H, Takei K, Umikawa M, Oshiro M, Kuninaka K, Bayarjargal M, Asato T, Yamashiro Y, Uechi Y, Endo S, Suzuki T, Kariya K (2008) MINK is a Rap2 effector for phosphorylation of the postsynaptic scaffold protein TANC1. Biochem Biophys Res Commun 377(2):573–578
Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV (2009) Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem 284(3):1514–1522
Ohba Y, Ikuta K, Ogura A, Matsuda J, Mochizuki N, Nagashima K, Kurokawa K, Mayer BJ, Maki K, Miyazaki J, Matsuda M (2001) Requirement for C3G-dependent Rap1 activation for cell adhesion and embryogenesis. EMBO J 20(13):3333–3341
Pak DTS, Yang SY, Rudolph-Correia S, Kim E, Sheng M (2001) Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron 31(2):289–303
Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL (2009) Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta 1788(4):790–796
Pannekoek WJ, van Dijk JJ, Chan OY, Huveneers S, Linnemann JR, Spanjaard E, Brouwer PM, van der Meer AJ, Zwartkruis FJ, Rehmann H, de Rooij J, Bos JL (2011) Epac1 and PDZ-GEF cooperate in Rap1 mediated endothelial junction control. Cell Signal 23(12):2056–2064
Pannekoek WJ, Linnemann JR, Brouwer PM, Bos JL, Rehmann H (2013) Rap1 and Rap2 antagonistically control endothelial barrier resistance. PLoS One 8(2):e57903
Pannekoek WJ, Post A, Bos JL (2014) Rap1 signaling in endothelial barrier control. Cell Adhes Migr 8(2)
Park HO, Chant J, Herskowitz I (1993) BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365(6443):269–274
Patsoukis N, Lafuente EM, Meraner P, Kim J, Dombkowski D, Li L, Boussiotis VA (2009) RIAM regulates the cytoskeletal distribution and activation of PLC-gamma1 in T cells. Sci Signal 2(99):ra79
Patzak IM, Konigsberger S, Suzuki A, Mak TW, Kiefer F (2010) HPK1 competes with ADAP for SLP-76 binding and via Rap1 negatively affects T-cell adhesion. Eur J Immunol 40(11):3220–3225
Pereira L, Metrich M, Fernandez-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Benitah JP, Lezoualc'h F, Gomez AM (2007) The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol 583(Pt 2):685–694
Pereira L, Cheng H, Lao DH, Na L, van Oort RJ, Brown JH, Wehrens XH, Chen J, Bers DM (2013) Epac2 mediates cardiac beta1-adrenergic-dependent sarcoplasmic reticulum Ca2+ leak and arrhythmia. Circulation 127(8):913–922
Pham N, Cheglakov I, Koch CA, de Hoog CL, Moran MF, Rotin D (2000) The guanine nucleotide exchange factor CNrasGEF activates ras in response to cAMP and cGMP. Curr Biol 10(9):555–558
Pizon V, Chardin P, Lerosey I, Olofsson B, Tavitian A (1988) Human cDNAs rap1 and rap2 homologous to the Drosophila gene Dras3 encode proteins closely related to ras in the ‘effector’ region. Oncogene 3(2):201–204
Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K (2009) Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol 29(10):2521–2531
Post A, Pannekoek WJ, Ross SH, Verlaan I, Brouwer PM, Bos JL (2013) Rasip1 mediates Rap1 regulation of Rho in endothelial barrier function through ArhGAP29. Proc Natl Acad Sci USA 110(28):11427–11432
Prior IA, Hancock JF (2012) Ras trafficking, localization and compartmentalized signalling. Semin Cell Dev Biol 23(2):145–153
Raaijmakers JH, Bos JL (2009) Specificity in Ras and Rap signaling. J Biol Chem 284(17):10995–10999
Radha V, Rajanna A, Gupta RK, Dayma K, Raman T (2008) The guanine nucleotide exchange factor, C3G regulates differentiation and survival of human neuroblastoma cells. J Neurochem 107(5):1424–1435
Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL (2006) Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature 439(7076):625–628
Rehmann H, Arias-Palomo E, Hadders MA, Schwede F, Llorca O, Bos JL (2008) Structure of Epac2 in complex with a cyclic AMP analogue and RAP1B. Nature 455(7209):124–127
Richter M, Murai KK, Bourgin C, Pak DT, Pasquale EB (2007) The EphA4 receptor regulates neuronal morphology through SPAR-mediated inactivation of Rap GTPases. J Neurosci 27(51):14205–14215
Rocks O, Gerauer M, Vartak N, Koch S, Huang ZP, Pechlivanis M, Kuhlmann J, Brunsveld L, Chandra A, Ellinger B, Waldmann H, Bastiaens PI (2010) The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell 141(3):458–471
Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N (2006) MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell 17(2):966–976
Schultess J, Danielewski O, Smolenski AP (2005) Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood 105(8):3185–3192
Schwamborn JC, Puschel AW (2004) The sequential activity of the GTPases Rap1B and Cdc42 determines neuronal polarity. Nat Neurosci 7(9):923–929
Schwamborn JC, Muller M, Becker AHM, Puschel AW (2007) Ubiquitination of the GTPase Rap1B by the ubiquitin ligase Smurf2 is required for the establishment of neuronal polarity. EMBO J 26(5):1410–1422
Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T (2003) Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol 161(2):417–427
Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N (2005) Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res 97(7):655–662
Song C, Hu CD, Masago M, Kariyai K, Yamawaki-Kataoka Y, Shibatohge M, Wu D, Satoh T, Kataoka T (2001) Regulation of a novel human phospholipase C, PLCepsilon, through membrane targeting by Ras. J Biol Chem 276(4):2752–2757
Sot B, Kotting C, Deaconescu D, Suveyzdis Y, Gerwert K, Wittinghofer A (2010) Unravelling the mechanism of dual-specificity GAPs. EMBO J 29(7):1205–1214
Stefanini L, Roden RC, Bergmeier W (2009) CalDAG-GEFI is at the nexus of calcium-dependent platelet activation. Blood 114(12):2506–2514
Stockton RA, Shenkar R, Awad IA, Ginsberg MH (2010) Cerebral cavernous malformations proteins inhibit Rho kinase to stabilize vascular integrity. J Exp Med 207(4):881–896
Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T, Nagashima K et al (1994) C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc Natl Acad Sci USA 91(8):3443–3447
Tsukamoto N, Hattori M, Yang H, Bos JL, Minato N (1999) Rap1 GTPase-activating protein SPA-1 negatively regulates cell adhesion. J Biol Chem 274(26):18463–18469
van Dam TJ, Bos JL, Snel B (2011) Evolution of the Ras-like small GTPases and their regulators. Small GTPases 2(1):4–16
Varga-Szabo D, Pleines I, Nieswandt B (2008) Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol 28(3):403–412
von Andrian UH, Mackay CR (2000) T-cell function and migration. Two sides of the same coin. N Engl J Med 343(14):1020–1034
Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ (1997) cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell 89(1):73–82
Wang Y, He H, Srivastava N, Vikarunnessa S, Chen YB, Jiang J, Cowan CW, Zhang X (2012) Plexins are GTPase-activating proteins for Rap and are activated by induced dimerization. Sci Signal 5(207):ra6
Wei P, Satoh T, Edamatsu H, Aiba A, Setsu T, Terashima T, Kitazawa S, Nakao K, Yoshikawa Y, Tamada M, Kataoka T (2007) Defective vascular morphogenesis and mid-gestation embryonic death in mice lacking RA-GEF-1. Biochem Biophys Res Commun 363(1):106–112
Willard FS, Low AB, McCudden CR, Siderovski DP (2007) Differential G-alpha interaction capacities of the GoLoco motifs in Rap GTPase activating proteins. Cell Signal 19(2):428–438
Wilson CW, Parker LH, Hall CJ, Smyczek T, Mak J, Crow A, Posthuma G, De Maziere A, Sagolla M, Chalouni C, Vitorino P, Roose-Girma M, Warming S, Klumperman J, Crosier PS, Ye W (2013) Rasip1 regulates vertebrate vascular endothelial junction stability through Epac1-Rap1 signaling. Blood 122(22):3678–3690
Xu K, Chong DC, Rankin SA, Zorn AM, Cleaver O (2009) Rasip1 is required for endothelial cell motility, angiogenesis and vessel formation. Dev Biol 329(2):269–279
Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O (2011) Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 20(4):526–539
Yamada T, Sakisaka T, Hisata S, Baba T, Takai Y (2005) RA-RhoGAP, Rap-activated Rho GTPase-activating protein implicated in neurite outgrowth through Rho. J Biol Chem 280(38):33026–33034
Yaman E, Gasper R, Koerner C, Wittinghofer A, Tazebay UH (2009) RasGEF1A and RasGEF1B are guanine nucleotide exchange factors that discriminate between Rap GTP-binding proteins and mediate Rap2-specific nucleotide exchange. FEBS J 276(16):4607–4616
Yamashita S, Mochizuki N, Ohba Y, Tobiume M, Okada Y, Sawa H, Nagashima K, Matsuda M (2000) CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J Biol Chem 275(33):25488–25493
Yoshikawa Y, Satoh T, Tamura T, Wei P, Bilasy SE, Edamatsu H, Aiba A, Katagiri K, Kinashi T, Nakao K, Kataoka T (2007) The M-Ras-RA-GEF-2-Rap1 pathway mediates tumor necrosis factor-alpha dependent regulation of integrin activation in splenocytes. Mol Biol Cell 18(8):2949–2959
Zheng Y, Liu H, Coughlin J, Zheng J, Li L, Stone JC (2005) Phosphorylation of RasGRP3 on threonine 133 provides a mechanistic link between PKC and Ras signaling systems in B cells. Blood 105(9):3648–3654
Zwartkruis FJ, Wolthuis RM, Nabben NM, Franke B, Bos JL (1998) Extracellular signal-regulated activation of Rap1 fails to interfere in Ras effector signalling. EMBO J 17(20):5905–5912
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Pannekoek, WJ., Bos, J.L. (2014). Rap Signaling. In: Wittinghofer, A. (eds) Ras Superfamily Small G Proteins: Biology and Mechanisms 1. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1806-1_11
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1806-1_11
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1805-4
Online ISBN: 978-3-7091-1806-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)