Abstract
Over the last 50 years, dengue has been a growing public health challenge. Half of the World’s population lives in areas of risk; Latin America and the Caribbean (LAC) are not exceptions. Aedes aegypti, the main vector of dengue in the Americas, is widely spread, and autochthonous transmission of dengue virus (DENV) has been documented in all American countries except for Chile and Uruguay. In 2013, the largest epidemic of dengue in the history of the Americas accounted for a total of 2.3 million reported cases, 37,898 of them severe, including 1,318 deaths.
Despite different and enormous efforts, prevention and control programs have not been effective enough to halt dengue in the LAC region. Difficulties to implement and accomplish successful dengue control programs lie in diverse realms. Some of these relate to the biology of DENV: other to social and environmental factors favoring vector density and other to health systems, like the appropriate entomology or epidemiologic surveillance systems and access to medical services to detect and confirm clinical cases. Implementing multisectoral approaches of vector control has been particularly challenging. Complex dynamics of all such determinants, within and between countries, lead to the continuous increase of dengue incidence in the region, resulting in major health and economic burden.
In this chapter we summarize the epidemiology of dengue in LAC, compare it to that in other regions, and discuss existing evidence supporting the urgent need for developing and implementing integrated multisectoral strategies to effectively control dengue in this region.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Virus, Vector, and Disease
Dengue virus (DENV) is a single-stranded RNA Flavivirus that exists as four closely related but antigenically different serotypes (DENV 1–4). Besides the genome, the main components of the virus are a capsid, membrane envelope glycoproteins, and seven nonstructural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. These nonstructural proteins are related to viral replication and pathogenesis (Halstead 2008; World Health Organization 2009; Guzman and Harris 2015).
Phylogenetic analyses, based on partial or complete genomic sequences, have defined several DENV genotypes and allowed better understanding of genetic diversity, mechanisms of pathogenesis, transmission dynamics, and epidemic potential of the different DENV strains. For instance, some genotypes of DENV 2 and DENV 3 from the Americas are known to be less virulent than Asian genotypes of the same serotype (Holmes and Twiddy 2003; Weaver and Vasilakis 2009; Halstead 2007). Despite such differences, all serotypes and genotypes of DENV can produce epidemics and are associated with similar syndromes in humans, including severe clinical presentations (Halstead 2007; Messina et al. 2014; Costa et al. 2012; Ramirez et al. 2010). However, complex virus–vector–human interactions, transmission patterns in varying environments, and accumulation of human susceptibility underscore the need to continue investigating to further understand dengue epidemic dynamics, and to inform the design and implementation of more effective control strategies (Halstead 2008; Carrington and Simmons 2014; Tapia-Conyer et al. 2012; Tapia-Conyer et al. 2009; San Martin 2014b).
Although mosquitoes of both Aedes aegypti and Aedes albopictus species are competent vectors of DENV, A. aegypti is the main vector in the Americas (World Health Organization 2012). After it was imported from Africa or Asia many years ago, through travel and commerce (Holmes and Twiddy 2003; Halstead 2008; Brathwaite Dick et al. 2012), the Aedes genus has expanded its global span over the last 50 years, reaching all continents except the Antarctica. Spread of Aedes in America has been followed by a 30-fold increase in the incidence of dengue disease in the region, including large urban outbreaks (Halstead 2008; Tapia-Conyer et al. 2009; World Health Organization 2009; Gomez-Dantes et al. 2011; World Health Organization 2012).
Aedes mosquitos are endophilic; thus they feed on human blood and mostly breed in water containers of diverse sizes and types, in and around homes. Factors favoring vector breeding are common in most Latin American countries (Quintero et al. 2014; Halstead 2008; World Health Organization 2009). Aedes has high vectorial capacity, is well adapted to urban environments, and is mostly active during daylight hours (Halstead 2008). Increasing urbanization and climate change have been identified as the main drivers of geographic expansion into wider habitats and dispersion of the dengue vectors (Morin et al. 2013; Colon-Gonzalez et al. 2013; Gubler 2011). Prolonged rainy seasons and humidity have been also implicated as major factors of vector spread into milder climates and higher altitudes, leading to increased incidence of dengue and its emergence or reemergence in previously unaffected regions over the last few decades (Halstead 2008; Jansen and Beebe 2010; Lambrechts et al. 2011; Colon-Gonzalez et al. 2013).
Some authors have argued against climate change as a prominent determinant of dengue dispersion by pointing that increased urbanization, population growth, and the use of open containers for storing domestic water have promoted proliferation of Aedes within the immediate environment of humans, as has been documented in some dry areas in Australia (Jansen and Beebe 2010; Morin et al. 2013). Nonetheless, such demographic trends and behaviors may, in turn, be partially stimulated by climate change (Alirol et al. 2011; Gubler 2011; Morin et al. 2013; Kyle and Harris 2008).
The clinical spectrum of dengue disease ranges from asymptomatic infection to dengue fever (DF), severe hemorrhagic fever (DHF), and shock (DSS). Dengue disease, either mild or severe, resembles other infectious diseases presenting as febrile exanthema: yellow fever, chikungunya, leptospirosis, and rickettsial infections, among many others. DHF characterizes by fever, bleeding diathesis, and plasma leakage; a small proportion of cases can also progress to DSS with increased probability of fatal outcomes, but the crude estimated case fatality rate (CFR) for dengue fever is usually much lower than 1 % in most countries (World Health Organization 2009; Halstead 2008, 2007; Huy et al. 2013).
The risk of DHF increases with sequential infections by different DENV serotypes (Guzman and Harris 2015; Halstead 2012; Wahala and Silva 2011; Guzman et al. 1990; Whitehorn and Simmons 2011). Some evidence suggests that hemorrhagic risk grows larger after exposure to the second serotype of DENV than it does after further heterotypic infections beyond the second (Gibbons et al. 2007). Nonetheless, viral genetics and specific serotype infection sequences may also play a role in the pathogenesis of DHF (OhAinle et al. 2011).
An intricate network of cellular, tissular, and systemic processes links dengue immune response to its pathogenesis (Halstead 2007; Lai et al. 2008; Halstead et al. 2010; Whitehorn and Simmons 2011; Wahala and Silva 2011; Halstead 2012; Guzman and Harris 2015). Severity of infection relates to viral biology (Holmes and Twiddy 2003; Guzman and Harris 2015), host factors (Loke et al. 2001; Perez et al. 2010; Xavier-Carvalho et al. 2013; Guzman and Harris 2015), or a combination of both along with specific features of the immune response to subsequent dengue infections (Guzman and Harris 2015; Halstead 2012; Whitehorn and Simmons 2011; Wahala and Silva 2011).
Primary dengue infection induces neutralizing antibodies and results in lifelong protection against subsequent infections with the homologous serotype, but only transitory protection to heterologous serotypes (Halstead 2007; Whitehorn and Simmons 2011). Induction of strong cross-reactive non-neutralizing antibodies can enhance disease during infection by DENV of a different serotype, favoring the entry of virions into monocytes, macrophages, and immature and mature dendritic cells. Such enhancement of viral replication results in high viremia and elevated concentrations of pro-inflammatory and immunomodulatory cytokines (Halstead et al. 2010; Whitehorn and Simmons 2011; Guzman and Harris 2015).
Modeling studies have suggested that population immunity to each DENV serotype is shaped even at small geographic scales, such that spaciotemporal dependence in homotypic individual immunity occurs within large neighborhoods in dense urban settings (Salje et al. 2012; Rodriguez-Barraquer et al. 2014). Deeper understanding of the immunological response to dengue at individual and population levels is crucial to enlighten dengue vaccine development, estimate disease risk, and predict transmission dynamics across populations (Rodriguez-Barraquer et al. 2013).
Laboratory diagnosis of dengue includes virus isolation, serology, and detection by molecular testing (Guzman and Harris 2015; Guzman et al. 2010). The sensitivity of each test highly depends on the timing of clinical sample collection after the onset of fever. No specific antiviral treatment exists so clinical case management is mainly supportive. Early recognition of severe disease by monitoring hemorrhagic and shock development is paramount to reduce fatality in severe cases (Guzman et al. 2010; World Health Organization 2009).
Since 1975, the World Health Organization (WHO) has convened expert committees for drafting various technical guidelines on standard practices for laboratory diagnostics and clinical management. These guidelines, updated in 1986 and 1997, were used to classify dengue disease in three mutually exclusive sets: DF, DHF, and DSS. Such discrete classification aimed to help healthcare workers identify severe cases of disease (World Health Organization 1986). Over time, using these guidelines around the world evidenced the challenges of differentiating clinical expressions of intermediate level of severity and led to confusion regarding appropriate conduct in triage, treatment, and reporting. Most countries adapted the guidelines to their own needs (Rigau-Pérez 2006; Alexander et al. 2011).
WHO responded by revisiting the Guidelines trying to overcome these classification issues and its consequences on both clinical care and public health. A new version, published in 2009, reorganized signs and symptoms attributed to dengue in three levels of assumed incremental severity: Dengue, Dengue with Warning Signs, and Severe Dengue. Several countries have validated this severity-based classification and use it to guide standard medical interventions (World Health Organization 2009). Whereas some investigators deem the new classification more appropriate for preventing dengue casualties, others argue that using it may contribute to overwhelming hospitals by admitting mild cases and, therefore, potentially impair the quality of clinical case management (Srikiatkhachorn et al. 2011; Barniol et al. 2011; Lima et al. 2013; Horstick et al. 2012). Therefore, they suggest revising the 2009 WHO Case Definition to achieve more accurate identification of syndromes within the spectrum of dengue disease (Narvaez et al. 2011; Halstead 2013a).
2 Dengue Situation in the Twenty-First-Century Latin America and Caribbean
The recorded history of introduction, expansion and resultant epidemics of dengue in the LAC region goes back to seventeenth century. However the lack of etiologic confirmation and its clinical resemblance to other VBD, such as chikungunya, Yellow Fever, and other infectious diseases, make it hard to define the actual origin of dengue in the region (Brathwaite Dick et al. 2012). The initial isolation DENV occurred in 1943–1944 and the first diagnostic laboratory test was made available soon after, helping confirmation of endemic and epidemic transmission (Sabin and Schlesinger 1945). As it has been recently described, the history of dengue in LAC has four distinct phases: (1) DENV introduction, from 1600 to 1946; (2) a plan for the eradication of Aedes aegypti from 1947 to 1970; (3) reinfestation, from 1971 to 2000; and (4) increased dispersion of Aedes and dengue virus circulation since 2001 (Brathwaite Dick et al. 2012).
After World War II, the Yellow Fever control campaign, jointly implemented by the Pan American Health Organization (PAHO) and countries in the region, contributed to substantial reduction of Aedes aegypti populations and significantly decreased dengue infections in the Americas. Unfortunately, some countries were unable to eradicate the vector before the campaign was discontinued in early 1970s. One decade later, dengue incidence was on the rise and reached pre-campaign numbers by 1995 (Brathwaite Dick et al. 2012), making clear that mosquito density is a key element of dengue transmission and vector control programs are essential to mitigate dengue and other diseases sharing the same vector.
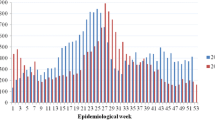
Estimating and comparing the burden of dengue infection across countries is challenged by regional heterogeneity in access to medical care, design and performance of surveillance systems, and availability of laboratory infrastructure for etiologic confirmation, among other issues (Badurdeen et al. 2013; Beatty et al. 2010). Despite these differences, it has been evident that dengue transmission increased in almost all countries in the Americas (Tapia-Conyer et al. 2009; San Martin 2014b) (Fig. 1), and currently, only Uruguay and Continental Chile remain free of dengue transmission (PanAmerican Health Organization 2014a). Since the year 2000, outbreaks of significant magnitude been reported: (Fig. 2).
Total number of cases of dengue (clinical cases) reported to Pan American Health Organization by Latin America and Caribbean Region, 2005–2013 (Source Adapted from number of reported cases of dengue and dengue hemorrhagic fever in the Americas, by country, years 2005–2013 (http://www.paho.org/hq/index.php?option=com_topics&view=article&id=1&Itemid=40734&lang=en) Access 10 Oct 2014)
Main outbreaks reported to Pan American Health Organization, 2005–2013 (Adapted from number of reported cases of dengue and dengue hemorrhagic fever in the Americas, by country, years 2005–2013 (http://www.paho.org/hq/index.php.option=com_topics&view=article&id=1&Itemid=40734&lang=en) Access 10 Oct 2014)
Several countries in LAC have reported large outbreaks (San Martin 2014a), supporting the view of progressive rise in dengue incidence due to one or several serotypes, either emerging or reintroduced: in 2000 Ecuador (DENV 2 and DENV 3) and Paraguay (DENV 1); in 2001 Peru; in 2002 (DENV 1–4) Honduras Venezuela, Colombia, and Brazil (DENV 1–4, mainly DENV–3); in 2005 Costa Rica (DENV 1 and reintroduction of DENV 2); in 2006 and 2007 Paraguay (DENV 3); in 2007–2008 Brazil (DENV 1, 2, and 3, mainly DENV 2); in 2009 Bolivia (DENV 1, 2, and 3), Argentina (DENV 1), Mexico (DENV 1 and some DENV 2), and Nicaragua (DENV 3); and in 2010 Colombia (DEV 1–4 mainly DENV 2), Venezuela, Honduras (DEV 1–4), and Brazil (DENV 1–4, mainly DENV 4). The Caribbean was the subregion most affected by outbreaks in 2010 such as in Guadeloupe with incidence of 1,289.99 × 100,000 inhabitants (DEND-1) using confirmed cases. In 2011, Brazil (DEV-1–4) and Paraguay were highly affected (DENG-1–3); in 2012, El Salvador (DENV-1–3), Nicaragua (DEV-1–3), Bolivia (DENV-2), Brazil (DENV-1–4), Mexico (DENV-1 and 2, occasional cases of DEV-3 and 4), Paraguay (DENV-2 and 4), Dominican Republic (DENV-2), and Puerto Rico (DENV-1–4); and finally in 2013, Mexico (DENV-1 and 2, occasional cases of DEV-3 and 4), Costa Rica (DENV-1–3), Nicaragua, Colombia, and Brazil (DEV-1–4), French Guiana, Puerto Rico, Dominican Republic, and Paraguay (DENV-1, 2, and 4) (PanAmerican Health Organization 2014b).
Although countries report transmission of more than one DENV serotype per year, the relative caseload associated with each one is inconsistently assessed because some of these serotypes are more prevalent than others even within the same country, the rate of dispersion varies among serotypes, or their transmission and predominance is confined to specific areas within countries, perhaps alternating along successive years (Dirección General de Epidemiologia and Secretaría de Salud 2014; Dantes et al. 2014; Tumioto et al. 2014; Vázquez-Pichardo et al. 2011; Gutierrez et al. 2011) (Fig. 4).
The largest epidemic cycle of dengue in the history of the Americas was reported in 2013, encompassing 2.3 million cases, 37,898 severe, and including 1,318 deaths (San Martin 2014b). The four DENV serotypes circulate in the region, with cycling periods of predominance of one or two serotypes in some countries and sustained co-circulation in others (San Martin 2014b; Nunes et al. 2014; Messina et al. 2014; Torres-Galicia 2014; Vázquez-Pichardo et al. 2011; Alvarez et al. 2006). Nonetheless, the estimated CFR of dengue has declined in the LAC, being the lowest of any WHO region (0.055 %) (PanAmerican Health Organization 2014b). Use of the 2009 dengue severity classification and efforts to train physicians on the early detection and appropriate treatment of severe dengue cases may have contributed to this outcome. Unfortunately, social and health inequalities, precarious sanitation, and water scarcity are still present in LAC and counter the potential effectiveness of dengue control programs (Tapia-Conyer et al. 2012; Gomez-Dantes et al. 2011).
In some countries such as Mexico, dengue transmission in certain geographic areas is not interrupted along the year, producing focal or regional outbreaks, some of them very large and prolonged in time, particularly in highly populated urban areas (e.g., Guadalajara, Jalisco in 2009 and Mérida, Yucatán in 2010–2011). Furthermore, the estimated incidence in municipalities or states is largely heterogeneous even within the same state region in the country (Fig. 3) (Dantes et al. 2014; Hernandez-Avila et al. 2013; Dirección General de Epidemiologia and Secretaría de Salud 2012).
Differential dengue transmission within geographic areas in Mexico, 2009–2012. Dengue cases have been reported in 30 of the 32 Mexican states. Georeferenced case reporting is an inter-operative system between the National Epidemiologic Surveillance Platform and the Geographical Information System (Dengue-GIS) (Source Hernandez-Avila (2013))
In the last few decades, countries in the LAC region have strived to develop dengue surveillance systems, including expanded laboratory capacities, to better understand the local epidemiology of dengue and to shape up prevention and control interventions. However, several challenges remain in developing effective dengue surveillance (Horstick and Morrison 2014; PanAmerican Health Organization 2014b; Messina et al. 2014; Badurdeen et al. 2013). This task has faced some challenges such as the need of well-designed and properly implemented surveillance systems, including electronic reporting to speed transference of core data.
A crucial step in establishing a sustainable capacity for prevention and control is developing, attracting, and retaining competent epidemiologist with advanced skills in quantitative data analysis and comprehensive training on interpreting primary surveillance information in context with scientific evidence (Subramanian et al. 2013). These are the professionals who can feed decision-makers with integrated knowledge on near real-time trends of dengue epidemics.
Geographic information systems and a solid laboratory network infrastructure help characterizing and confirming cases (PanAmerican Health Organization 2014b; Hernandez-Avila et al. 2013; Oliveira et al. 2013; Badurdeen et al. 2013). A successful example of these tools was established in Mexico in 2008. A web-based, geographically enabled dengue integral surveillance system (Dengue-GIS) was developed for the nation-wide collection, integration, analysis and reporting of geo-referenced epidemiologic, entomologic, and control interventions data (Hernandez-Avila et al. 2013). This system was a joint effort of the National Institute of Public Health investigators, vector control program personnel and Epidemiological surveillance programs of the Secretary of Health authorities. This system provides geographical detail evidence to plan, implement and evaluate dengue control activities (Figs. 4 and 5).
Proportional distribution of dengue serotypes detected by surveillance in Mexico, 1995–2011. Dengue virological surveillance in Mexico has been conducted since 1995; however the special national dengue epidemiological surveillance system with defined proportion of virological characterization started in 2008. (Source Dengue Epidemiological Surveillance, 1995–2011)
National epidemiologic surveillance platform and the geographical information system (Dengue-GIS) application for dengue control program activities. Dengue-GIS screenshot describing distribution of probable cases and case clusters (high transmission areas) during the CDC weeks 22–24 2012 in Linares City, Nuevo Leon. Locations of entomological survey activities (squares) and dengue fever cases accumulation in space and time (ellipses) represent the graphical output of the cluster detection algorithm. Two areas, one very close to downtown Linares and the other to the west of the city, show three intersecting ellipses; this indicates that transmission occurred uninterrupted during the 3-week period shown. Pictures presented here were modified to translate the text presented to English; the actual Dengue-GIS is an all-Spanish language system. doi:10.1371/journal.pone.0070231.g004 (SourceHernandez-Avila (2013))
Estimating the magnitude of disease burden at local and regional scales, describing spatiotemporal trends of dengue transmission, and assessing the occurrence of severe disease are key elements of knowledge that would help better design, planning, and implementation of policies and programs aiming to prevent and control dengue (Nagao et al. 2008). Unfortunately, many surveillance systems in the region currently limit their scope to accounting the caseload and, at most, describe basic clinical characteristics of suspect or laboratory-confirmed cases. This reduced approach to surveillance is both uninformative and sensitive to wide imprecision. Overestimation of the observed number of cases ensues when sensitive case definitions—such as clinical, probable, or suspected cases—are used, which can contribute to unnecessarily overwhelming prevention and control interventions. On the other hand, less sensitive, more specific definitions—such as laboratory confirmed cases—would lead to the underestimation of disease burden and potentially stimulate dismissal from public health authorities.
Comprehensive public health risk assessment is necessary to attain better preparedness, including predictive modeling of human disease, vector dynamics, public health interventions and healthcare availability and access. Semiquantitative risk stratification helps to predict areas of high probabilities for enhanced transmission and can guide focal interventions (PanAmerican Health Organization 2014b; Rodriguez-Barraquer et al. 2014; Badurdeen et al. 2013; Brady et al. 2012; Beatty et al. 2010).
The total annual cost of dengue in the Americas has been estimated at US$2.1 billion with a range of $1–4 billion with temporary variation (Shepard et al. 2011). In addition, the analysis of DALYs exhibits 36 % lost in Brazil, 28 % in the Andean region, and 21 % in Central America and Mexico. Another study analyzed cost in dengue ambulatory and hospitalized patients in Asia and the Americas (Suaya et al. 2009), and the average illness lasted 11.9 days for ambulatory patients and 11.0 days for hospitalized cases, 5.6 days of lost school for students, and 9.9 work days per lost work average per dengue episode. These studies did not include costs of vector control programs.
3 Dengue Control Programs
Aedes density is the main factor driving dengue transmission and vector control programs are essential to mitigate dengue disease, similar to other VBD. Aedes Aegypti proliferates in all kinds of toys, bottles, and devices that can hold water, which are commonly found in patios and other domiciliary environments. Thus, the successes of Aedes control programs are focused to eliminate vectors and to implement barriers to house colonization or to contact with humans (bednets) (Jansen and Beebe 2010; World Health Organization 2009; Ballenger-Browning and Elder 2009). Steps must be taken to sustain the elimination of mosquito habitats, such as preventing access of the vector to breeding containers, to eliminate or control vector young stages (larval), or to kill the adult vector using insecticides or biological control agents (PanAmerican Health Organization 2014b). Every step involves different determinants in dynamic complicated interactions: individual, domestic, and cultural behavior, community participation, economical and social conditions, climate and local ecology, appropriated and coordinated plan, and excellent communication at all levels. Aedes control methods (San Martin 2014b; World Health Organization 2009) must include environmental management to hamper with the access and breeding of the mosquitos by improving water supply and storage systems and waste management. This approach must be implemented in occupied and vacant buildings, public areas—parks, gardens, cemeteries, schools, etc.
Simultaneously, access and promotion of the use of individual and household barrier methods, including protective clothing to avoid mosquito biting, repellents, window and door screens, and bednets, are warranted. Chemical controls with larvicides or adulticides complement vector abatement (Torres-Estrada and Rodiles-Cruz Ndel 2013; Tapia-Conyer et al. 2012; World Health Organization 2009). Insecticide-treated materials (curtains and bednets) in combination with vector control methods have also been proposed as individual protection measures (Jones et al. 2014; Tapia-Conyer et al. 2012; Ballenger-Browning and Elder 2009; Kroeger et al. 2006). A number of entomological indices have been proposed for entomologic surveillance systems and to lead the monitoring and impact evaluation of vector control programs (World Health Organization 2009; Ballenger-Browning and Elder 2009; Gomez-Dantes et al. 2011).
Most public health systems in the LAC region have relied on governmental action for guide and operate surveillance, vector control and health promotion activities, but a core quandary is how to boost community participation in a consistent partnership framework that optimizes the impact of transmission mitigation measures (Tapia-Conyer et al. 2012). The theoretical advantages of coordinated interdisciplinary and intergovernmental approaches are encouraging, but there is need to populate the repertoire of hard evidence on the return on the investment vis-à-vis vertical and fragmented public health programs (World Health Organization 2009).
Integrated programs are expensive and complex to manage, as they require accurate information to focus activities and keep track of progress. Competing agendas of partners and sponsors may challenge the political and financial sustainability of joint ventures. Therefore vector control programs are mostly driven by routine entomologic surveillance and disease-specific surveillance programs that frequently target the most immediate outcomes, such as vector density and frequency of clinical and confirmed cases in specific areas. Disperse control interventions and missing opportunities for collaboration between political authorities across national boundaries or subnational entities, such as states or municipalities, are a reminder that infectious disease recognizes no borders (PanAmerican Health Organization 2014b). Accurate surveillance, transparent data exchange, and concerted public health action are the fundamentals of effective preparedness and response against dengue and most public health challenges (World Health Organization 2008).
Since 2001, PAHO established a reference frame to develop interdisciplinary approach for dengue prevention and control. The Integrated Management Strategy for Dengue Prevention and Control (IMS-dengue), launched in 2003, presents a model of six components: epidemiology, entomology, healthcare, laboratory, social communication, and environment (San Martin and Brathwaite-Dick 2007). By the end of the first decade of twenty-first century, 19 countries in the LAC region had implemented the IMS—dengue approach (Argentina, Bolivia, Brazil Colombia, Costa Rica, Chile, Ecuador, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, Paraguay, Peru, Puerto Rico, the Dominican Republic, Uruguay, and Venezuela). Four subnational IMS—dengue plans have also been established in Central America, the Andean Sub Region, MERCOSUR, and the Caribbean.
Despite encouraging signs of progress, the LAC region has reported a progressive increasing number of dengue cases. Some of this trend needs to disentangle the effects of improved surveillance and reporting of all forms of dengue cases, not just severe dengue (PanAmerican Health Organization 2014b; San Martin 2014b). In 2010, WHO launched the Global Strategy for the Prevention and Control of Dengue 2012–2020, aiming to reduce 50 % of dengue mortality and 25 % of morbidity by 2020 (World Health Organization 2012). The Strategy stems on five technical components (Table 1): (1) Diagnosis and case management; (2) Integrated surveillance and outbreak preparedness; (3) Sustainable vector control; (4) Future vaccine implementation; and (5) Basic operational and implementation research. The successful implementation of this global strategy requires integrated action, strong advocacy and effective resource mobilization, vigorous partnerships, effective coordination and collaboration, transparent communication, sustainable capacity building, and rigorous monitoring and evaluation.
In May 2014, PAHO analyzed dengue prevention and control in the Americas (PanAmerican Health Organization 2014b) by reviewing regional experiences in surveillance, detection, diagnosis, management, treatment, and prevention. Lessons learned by countries in recent years along with current scientific and operational evidence and best practices on dengue prevention and control conformed this comprehensive assessment. The Organization presented a standard set of recommendations to the regional programs for dengue control in the Americas and pointed at ways to identify research opportunities and fill gaps in the IMS-Dengue Strategy.
Experts and country representatives concluded that implementation of this strategy provided with a solid instrument for preventing and controlling dengue, which can be adapted to the needs and capacities of each country. Mexico IMS-Dengue include seven components: (1) Health promotion; (2) Social, community, intra-, and intersectoral participation; (3) Epidemiological and entomological surveillance; (4) Laboratory diagnosis by the state public health laboratory; (5) Patient care; (6) Control of health risks; and (7) Chemical vector control. Brazil pointed that decentralization of the health system allowed surveillance and vector control activities to be expanded across the country. Several countries in the Americas have implemented an outbreak control system that uses risk stratification and integrated actions to optimize the management of material and human resources in dengue prevention and control.
Dengue outbreaks are increasing in frequency and are associated with social and economic disruption and overwhelming of health services, many times with adverse political consequences due to mass media magnification. Dengue control programs need strengthening at different levels. While financial and human resources are in the need to support program actions, it is also clear that more operational research is needed to better understand how to mobilize community participation and how to achieve effective intersectoral and multilevel government control actions. There is an urgent need to improve early disease and vector surveillance with effective risk communication and vector control activities.
4 Dengue Vaccines
Due to the continuous increase of dengue cases, the high social and economic costs associated with its health effects, and the proven difficulty to accomplish successfully control programs, developing a dengue vaccine has become a global priority. Dengue vaccine development needs some particular considerations: first, the vaccine needs to protect effectively against the four serotypes simultaneously, and second, the vaccine needs to be safe and not to enhance severe disease manifestations associated with antibody-dependent enhancement associated with infection with a second serotype that may lead to severe disease manifestations, as it happens with natural dengue infections; thus a vaccine that induces protection in an early period of time might later increase the risk for enhanced disease, impact of previous immunity due to primary infections at early ages, among many others (Yauch and Shresta 2014; del Angel and Reyes-del Valle 2013; Halstead 2013b).
Several DENV vaccines are under development: live-attenuated, inactivated, recombinant subunit, viral vectored, and DNA vaccines. The most advanced studies are focused on live-attenuated developments; some of them are in phase II and III trials (Yauch and Shresta 2014; Thisyakorn and Thisyakorn 2014). Only one candidate vaccine is reaching phase III clinical trials, a live-attenuated chimeric containing dengue structural genes inserted into the infectious cDNA backbone of a yellow fever vaccine virus strain 17D (manufacture by Sanofi-Pasteur). The results of the phase 2b study of this vaccine showed good immunogenicity for all four serotypes (Sabchareon et al. 2012). However the global vaccine efficacy was 30.2 % (95 % confidence interval [CI]: 13.4–56.6) with wide differences by serotype: better for DENV 3 and 4, less for DENV 1, and no efficacy against DENV 2. In addition, the vaccine was well tolerated with no safety issues after 2 years of follow-up. The same vaccine has been evaluated in large phase III trials in Asia and Latin America.
The Asia arm of the study including 10,275 children (Capeding et al. 2014) estimated 56.6 % (95 % CI: 43.8–66.4) vaccine efficacy against confirmed dengue and, again, differences between serotypes are found with efficacy of 50 % (95 % CI: 24.6–66.8) for DENV 1, 35 % (95 % CI: −9.2–61.0) for DENV 2, 78.3 % (95 % CI: 52.9–90.8) for DENV 3, and 73.5 % (95 % CI: 54.5–87.0) for DENV 4. Vaccine efficacy against DENV 2 in this study is still significantly lower than the other serotypes and the CI is reaching no efficacy, similarly to the 2b trial.
In this study, the vaccine appears safe and well tolerated in the short term. Some other results showed that almost two-thirds of subjects in the trial were seropositive for dengue at baseline by microneutralization assay, and the proportion of seropositivity increased with age. Vaccine efficacy seems to be higher for participants who were seropositive for dengue than for those who were seronegative. The authors describe differences in DENV 2 genotypes in the vaccine components in comparison to those circulating during the trial, as a possible explanation of the lack of vaccine efficacy against this serotype. The increased efficacy observed in the phase III trial is obviously associated with more diversity of DENV serotypes circulating in different countries than the predominant DENV 2 cases detected in the 2b trial performed in only one specific area. In addition, the investigators pointed high vaccine efficacy against dengue hemorrhagic fever and clinically important reductions in severe disease and hospital admissions. These findings of vaccine efficacy on severe disease are encouraging, but we must keep in mind that the numbers for these conclusions are limited (Capeding et al. 2014).
The Latin American trial enrolled a total of 20,875 children aged 9–16 years from dengue endemic areas of Brazil, Colombia, Mexico, Honduras, and Puerto Rico (Villar et al. 2014), and the results demonstrated global vaccine efficacy of 60.8 % (95 % CI: 52.0–68.0). Differences in serotype vaccine efficacy were also found, with estimated mean values of 50.3 % (95 % CI: 29.1–65.2) for DENV 1, 42.3 % (95 % CI: 14.0–61.1) for DENV-2, 74.0 % (95 % CI: 61.9–82.4) for DENV 3, and 77.7 % (95 % CI: 60.2–88.0) for DENV 4. This study further showed differential vaccine efficacy for dengue serotypes: it is good for DENV 3 and 4, less for DENV 1, and significantly lower for DENV 2. In addition, vaccine efficacy seems to be better in the population previously immune to dengue. The vaccine was also safe and well tolerated and again vaccine efficacy seems to be higher in those children with seropositive dengue baseline status.
There is no doubt that the results of this live-attenuated chimeric dengue vaccine are good news for a first line of dengue vaccine development. Approximately 60 % global vaccine efficacy with no safety concerns after 25 months follow-up of disease enhancement is a great achievement for dengue control due to the magnitude of the disease transmission. The possibility of 50–60 % reduction of diseases, and even more on severe cases, would be very beneficial. Because dengue pathogenesis is mostly associated with subsequent infections (Thomas 2015), it will be important to define if the safety findings are sustained through longer period of time than the 25 months follow-up to date and to further characterize waning immunity. Furthermore -before its population use- more information is needed regarding the vaccine efficacy to prevent symptomatic and asymptomatic diseases and severe manifestations of disease associated with secondary infections (Guzman and Harris 2015).
However, other key issues (World Health Organization and Experts and S. A. G. O 2012) need to be considered by country public health decisions makers before introducing a dengue vaccine in the current immunization programs, either with the current available product or any with any other future development dengue vaccine program with the current available product or any other vaccine in progress (Mahoney 2014; Douglas et al. 2013; Live Dengue Vaccines Technical Consultation Reporting et al. 2013). As previously discussed, if transmission of one predominant or combined serotypes often occurred within and among countries and some of these serotypes may last for several years, then vaccine efficacy may be different and even lower if DENV 1 and 2 are predominant. There are still gaps in further understanding specific dynamic introduction and reintroduction and timing of each or combined serotypes in specific regions.
Some studies have reported cost-effectiveness of dengue vaccination studies including transmission and a different range of clinical vaccine efficacy (Durham et al. 2013). Interestingly, the findings exhibited that it is necessary to reach 82 % of population vaccination to reach herd immunity, and with 70 % vaccine efficacy, vaccination may be cost-effective with a price up to US $534 (95 % CI: $369–1008) per vaccinated individual and cost saving up to $204 (95 % CI: $39–678). If the vaccine efficacy is only 30 %, cost-effectiveness could be achieved to a cost of $237 (95 % CI: $159–512) with savings of $93 (95 % CI: $15–368).
Further cost-effectives studies per different region may include efficacy between serotypes in addition to mathematical models to address how a partial efficacious dengue vaccine would be expected to behave in a certain period of time, in different geographic areas, even within a country (Shepard et al. 2011). In regards of implementation of dengue vaccination, on one side, there is no question that dengue is a public health priority, in terms of the magnitude of the disease burden and associated costs and prevention with vaccine is an excellent option. Vaccine prices currently not known; in a costing exercise prices for public sector were fixed at US$15 ($10–20); with these figures, it has been estimated that dengue immunization programs for Colombia and Brazil’s will require an investment from public sector of US $2,400 million over 5 years.
As it happens in many of LAC, decision makers will need to analyze and prioritize use of funds for several public health problems, for example, investing in environmental improvements like water sanitation to reduce mosquito breeding sites, or strengthening vector control and surveillance systems, which may produce a greater benefit at lower cost. The vector control programs cannot be discontinued, not even think to be decreased, because the time and limit to reach dengue-vaccinated population proportion to have an impact in dengue transmission is not known. This is now more relevant when Chikungunya currently emerging in the Americas, a disease that shares vector and clinical presentation, imposes additional burden on established vector control programs (San Martin 2014b).
Furthermore, funding for a new vaccine program must carefully consider direct and indirect costs to assure the extra needs in human resources and infrastructure (World Health Organization and Experts and S. A. G. O 2012), not only for the specific new vaccine program but also for the National Vaccine Program of each country. This is a crucial step of programmatic and budgetary rationale to avoid jeopardizing global vaccine coverage already accomplished. After dengue vaccine introduction, epidemiological surveillance, including hospitalized severe cases, must be extensively improved, not only to further evaluate the global vaccination strategy impact but also to assure possible risk for enhanced disease after longer periods than the so far good safety results of this candidate vaccine.
Other concerns related to the capacity of immunization programs to successfully introduce any new vaccine is to deliver it over the long term. This will vary from country to country. However, given the large size of the target population and number of doses required, this is certainly an important point to consider.
5 Conclusion
The persistence problems related to escalating dengue disease spread in the Latin America and Caribbean region, vector control problems, absence of specific treatment, and the status of vaccine development impose a need for further development of research in several aspects of dengue disease: stronger epidemiological and entomological surveillance systems, basic immunology and virus genetic characterization, pathogenesis, vaccine development, fill the gaps to further understand specific dynamic introduction, and reintroduction and timing of serotypes per regions using specific modeling studies, among others.
Current available data suggest caution in the introduction of dengue vaccine; although disease is recognized as a public health priority, vaccine should be introduced with appropriate safety controls and economical evaluations for LAC. More evidence-based actions and integrated commitment are required to reinforce our battle against dengue.
Abbreviations
- CFR:
-
Case fatality rate
- CI:
-
Confidence interval
- DENV:
-
Dengue virus
- DHF:
-
Dengue hemorrhagic fever
- DF:
-
Dengue fever
- DSS:
-
Dengue shock syndrome
- LAC:
-
Latin America and the Caribbean
- PAHO:
-
Pan American Health Organization
- WHO:
-
World Health Organization
References
Alexander N, Balmaseda A, Coelho IC, Dimaano E, Hien TT, Hung NT, Janisch T, Kroeger A, Lum LC, Martinez E, Siqueira JB, Thuy TT, Villalobos I, Villegas E, Wills B, European Union, W. H. O. S. D. S. G. (2011) Multicentre prospective study on dengue classification in four South-East Asian and three Latin American countries. Trop Med Int Health 16:936–948
Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L (2011) Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis 11:131–141
Alvarez M, Rodriguez-Roche R, Bernardo L, Vazquez S, Morier L, Gonzalez D, Castro O, Kouri G, Halstead SB, Guzman MG (2006) Dengue hemorrhagic fever caused by sequential dengue 1-3 virus infections over a long time interval: Havana epidemic, 2001–2002. Am J Trop Med Hyg 75:1113–1117
Badurdeen S, Valladares DB, Farrar J, Gozzer E, Kroeger A, Kuswara N, Ranzinger SR, Tinh HT, Leite P, Mahendradhata Y, Skewes R, Verrall A, European Union, W. H. O. S. I. S. G. (2013) Sharing experiences: towards an evidence based model of dengue surveillance and outbreak response in Latin America and Asia. BMC Public Health 13:607
Ballenger-Browning KK, Elder JP (2009) Multi-modal Aedes aegypti mosquito reduction interventions and dengue fever prevention. Trop Med Int Health 14:1542–1551
Barniol J, Gaczkowski R, Barbato EV, Da Cunha RV, Salgado D, Martinez E, Segarra CS, Sandoval EBP, Mishra A, Laksono IS, Lum LCS, Martinez JG, Nunez A, Balsameda A, Allende I, Ramirez G, Dimaano E, Thomacheck K, Akbar NA, Ooi EE, Villegas E, Hien TT, Farrar J, Horstick O, Kroeger A, Jaenisch T (2011) Usefulness and applicability of the revised dengue case classification by disease: multi-centre study in 18 countries. BMC Infect Dis 11:106
Beatty ME, Stone A, Fitzsimons DW, Hanna JN, Lam SK, Vong S, Guzman MG, Mendez-Galvan JF, Halstead SB, Letson GW, Kuritsky J, Mahoney R, Margolis HS, Asia, P. and Americas Dengue Prevention Boards Surveillance Working, G. (2010) Best practices in dengue surveillance: a report from the Asia-Pacific and Americas dengue prevention boards. PLoS Negl Trop Dis 4:E890
Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, Moyes CL, Farlow AW, Scott TW, Hay SI (2012) Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 6:E1760
Brathwaite Dick O, San Martin JL, Montoya RH, Del Diego J, Zambrano B, Dayan GH (2012) The history of dengue outbreaks in the Americas. Am J Trop Med Hyg 87:584–593
Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, Pitisuttithum P, Thisyakorn U, Yoon I-K, Van Der Vliet D, Langevin E, Laot T, Hutagalung Y, Frago C, Boaz M, Wartel TA, Tornieporth NG, Saville M, Bouckenooghe A (2014) Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet 384:1358–1365
Carrington LB, Simmons CP (2014) Human to mosquito transmission of dengue viruses. Front Immunol 5:290
Colon-Gonzalez FJ, Fezzi C, Lake IR, Hunter PR (2013) The effects of weather and climate change on dengue. PLoS Negl Trop Dis 7:E2503
Costa RL, Voloch CM, Schrago CG (2012) Comparative evolutionary epidemiology of dengue virus serotypes. Infect Genet Evol 12:309–314
Dantes HG, Farfan-Ale JA, Sarti E (2014) Epidemiological trends of dengue disease in Mexico (2000–2011): a systematic literature search and analysis. PLoS Negl Trop Dis 8:E3158
Del Angel RM, Reyes-Del Valle J (2013) Dengue vaccines: strongly sought but not a reality just yet. PLoS Pathog 9, E1003551
Dirección General De Epidemiologia and Secretaría De Salud (2014) Panorama Epidemiológico Del Dengue, Semana 49. Dirección General De Epidemiología, Mexico City
Douglas DL, Deroeck DA, Mahoney RT, Wichmann O (2013) Will dengue vaccines be used in the public sector and if so, how? Findings from an 8-country survey of policymakers and opinion leaders. PLoS Negl Trop Dis 7:E2127
Durham DP, Ndeffo Mbah ML, Medlock J, Luz PM, Meyers LA, Paltiel AD, Galvani AP (2013) Dengue dynamics and vaccine cost-effectiveness in Brazil. Vaccine 31:3957–3961
Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP Jr, Srikiatkhachorn A (2007) Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg 77:910–913
Gomez-Dantes H, San Martin JL, Danis-Lozano R, Manrique-Saide P, De Grupo D (2011) Integrated prevention and control strategy for dengue in Mesoamerica. Salud Publica Mex 53(Suppl 3):S349–S357
Gubler DJ (2011) Dengue, urbanization and globalization: the unholy trinity of the 21(st) century. Trop Med Health 39:3–11
Gutierrez G, Standish K, Narvaez F, Perez MA, Saborio S, Elizondo D, Ortega O, Nunez A, Kuan G, Balmaseda A, Harris E (2011) Unusual dengue virus 3 epidemic in Nicaragua, 2009. PLoS Negl Trop Dis 5:E1394
Guzman MG, Harris E (2015). Dengue. Lancet 31:453-65
Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L (1990) Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am J Trop Med Hyg 42:179–184
Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martinez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW (2010) Dengue: a continuing global threat. Nat Rev Microbiol 8:S7–S16
Halstead SB (2007) Dengue. Lancet 370:1644–1652
Halstead SB (2008) Dengue virus-mosquito interactions. Annu Rev Entomol 53:273–291
Halstead SB (2012) Controversies in dengue pathogenesis. Paediatr Int Child Health 32(Suppl 1):5–9
Halstead SB (2013a) Dengue: the syndromic basis to pathogenesis research. Inutility of the 2009 who case definition. Am J Trop Med Hyg 88:212–215
Halstead SB (2013b) Identifying protective dengue vaccines: guide to mastering an empirical process. Vaccine 31:4501–4507
Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM (2010) Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis 10:712–722
Hernandez-Avila JE, Rodriguez MH, Santos-Luna R, Sanchez-Castaneda V, Roman-Perez S, Rios-Salgado VH, Salas-Sarmiento JA (2013) Nation-wide, web-based, geographic information system for the integrated surveillance and control of dengue fever in Mexico. PLoS One 8, E70231
Holmes E, Twiddy S (2003) The origin, emergence and evolutionary genetics of dengue virus. Infect Genet Evol 3:19–28
Horstick O, Morrison AC (2014) Dengue disease surveillance: improving data for dengue control. PLoS Negl Trop Dis 8:E3311
Horstick O, Farrar J, Lum L, Martinez E, San Martin JL, Ehrenberg J, Velayudhan R, Kroeger A (2012) Reviewing the development, evidence base, and application of the revised dengue case classification. Pathog Glob Health 106:94–101
Huy NT, Van Giang T, Thuy DH, Kikuchi M, Hien TT, Zamora J, Hirayama K (2013) Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis 7:E2412
Jansen CC, Beebe NW (2010) The dengue vector Aedes aegypti: what comes next. Microbes Infect 12:272–279
Jones CH, Benitez-Valladares D, Guillermo-May G, Dzul-Manzanilla F, Che-Mendoza A, Barrera-Perez M, Selem-Salas C, Chable-Santos J, Sommerfeld J, Kroeger A, O’dempsey T, Medina-Barreiro A, Manrique-Saide P (2014) Use and acceptance of long lasting insecticidal net screens for dengue prevention in Acapulco, Guerrero, Mexico. BMC Public Health 14:846
Kroeger A, Lenhart A, Ochoa M, Villegas E, Levy M, Alexander N, Mccall PJ (2006) Effective control of dengue vectors with curtains and water container covers treated with insecticide in Mexico and Venezuela: cluster randomised trials. BMJ 332:1247–1252
Kyle JL, Harris E (2008) Global spread and persistence of dengue. Annu Rev Microbiol 62:71–92
Lai CY, Tsai WY, Lin SR, Kao CL, Hu HP, King CC, Wu HC, Chang GJ, Wang WK (2008) Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved Residues at the fusion loop of domain II. J Virol 82:6631–6643
Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA 108:7460–7465
Lima FR, Croda MG, Muniz DA, Gomes IT, Soares KR, Cardoso MR, Tauro RL, Croda J (2013) Evaluation of the traditional and revised world health organization classifications of dengue cases in Brazil. Clinics 68:1299–1304
Live Dengue Vaccines Technical Consultation Reporting, G, Bentsi-Enchill AD, Schmitz J, Edelman R, Durbin A, Roehrig JT, Smith PG, Hombach J, Farrar J (2013) Long-term safety assessment of live attenuated tetravalent dengue vaccines: deliberations from a who technical consultation. Vaccine 31:2603–2609
Loke H, Bethell DB, Phuong CX, Dung M, Schneider J, White NJ, Day NP, Farrar J, Hill AV (2001) Strong HLA class I–restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis 184:1369–1373
Mahoney R (2014) The introduction of new vaccines into developing countries. V: will we lose a decade or more in the introduction of dengue vaccines to developing countries? Vaccine 32:904–908
Messina JP, Brady OJ, Scott TW, Zou C, Pigott DM, Duda KA, Bhatt S, Katzelnick L, Howes RE, Battle KE, Simmons CP, Hay SI (2014) Global spread of dengue virus types: mapping the 70 year history. Trends Microbiol 22:138–146
Morin CW, Comrie AC, Ernst K (2013) Climate and dengue transmission: evidence and implications. Environ Health Perspect 121:1264–1272
Nagao Y, Svasti P, Tawatsin A, Thavara U (2008) Geographical structure of dengue transmission and its determinants in Thailand. Epidemiol Infect 136:843–851
Narvaez F, Gutierrez G, Perez MA, Elizondo D, Nunez A, Balmaseda A, Harris E (2011) Evaluation of the traditional and revised who classifications of dengue disease severity. PLoS Negl Trop Dis 5:E1397
Nunes MR, Palacios G, Faria NR, Sousa EC Jr, Pantoja JA, Rodrigues SG, Carvalho VL, Medeiros DB, Savji N, Baele G, Suchard MA, Lemey P, Vasconcelos PF, Lipkin WI (2014) Air travel is associated with intracontinental spread of dengue virus serotypes 1-3 in Brazil. PLoS Negl Trop Dis 8:E2769
Ohainle M, Balmaseda A, Macalalad AR, Tellez Y, Zody MC, Saborio S, Nunez A, Lennon NJ, Birren BW, Gordon A, Henn MR, Harris E (2011) Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med 3:114ra128
Oliveira MA, Ribeiro H, Castillo-Salgado C (2013) Geospatial analysis applied to epidemiological studies of dengue: a systematic review. Rev Bras Epidemiol 16:907–917
PanAmerican Health Organization (2014a) Number of reported cases of dengue and severe dengue (Sd) in the Americas, by country. Washington, DC. Available: http://www.paho.org/hq/index.php?option=com_docman&task=doc_download&Itemid=&gid=28455&lang=en
PanAmerican Health Organization (2014b) State of the art in the prevention and control of dengue in the Americas. Washington, DC
Perez AB, Sierra B, Garcia G, Aguirre E, Babel N, Alvarez M, Sanchez L, Valdes L, Volk HD, Guzman MG (2010) Tumor necrosis factor-alpha, transforming growth factor-beta1, and interleukin-10 gene polymorphisms: implication in protection or susceptibility to dengue hemorrhagic fever. Hum Immunol 71:1135–1140
Quintero J, Brochero H, Manrique-Saide P, Barrera-Perez M, Basso C, Romero S, Caprara A, De Lima Cunha JC, Beltran-Ayala E, Mitchell-Foster K, Kroeger A, Sommerfeld J, Petzold M (2014) Ecological, biological and social dimensions of dengue vector breeding in five urban settings of Latin America: a multi-country study. BMC Infect Dis 14:38
Ramirez A, Fajardo A, Moros Z, Gerder M, Caraballo G, Camacho D, Comach G, Alarcon V, Zambrano J, Hernandez R, Moratorio G, Cristina J, Liprandi F (2010) Evolution of dengue virus type 3 genotype III in Venezuela: diversification, rates and population dynamics. Virol J 7:329
Rigau-Pérez JG (2006) Severe dengue: the need for new case definitions. Lancet Infect Dis 6:297–302
Rodriguez-Barraquer I, Mier-Y-Teran-Romero L, Burke DS, Cummings DA (2013) Challenges in the interpretation of dengue vaccine trial results. PLoS Negl Trop Dis 7:E2126
Rodriguez-Barraquer I, Buathong R, Iamsirithaworn S, Nisalak A, Lessler J, Jarman RG, Gibbons RV, Cummings DA (2014) Revisiting Rayong: shifting seroprofiles of dengue in Thailand and their implications for transmission and control. Am J Epidemiol 179:353–360
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567
Sabin AB, Schlesinger RW (1945) Production of immunity to dengue with virus modified by propagation in mice. Science 101:640–642
Salje H, Lessler J, Endy TP, Curriero FC, Gibbons RV, Nisalak A, Nimmannitya S, Kalayanarooj S, Jarman RG, Thomas SJ, Burke DS, Cummings DA (2012) Revealing the microscale spatial signature of dengue transmission and immunity in an urban population. Proc Natl Acad Sci USA 109:9535–9538
San Martin JL (2014a) Brotes recientes de Dengue en Latinoamérica: Lecciones aprendidas. Bucaramanga, Colombia
San Martin JL (2014b) Situación Epidemiológica Del Dengue. Bucaramanga, Colombia
San Martin JL, Brathwaite-Dick O (2007) Integrated strategy for dengue prevention and control in the region of the Americas. Rev Panam Salud Publica 21:55–63
Dirección General De Epidemiologia and Secretaría De Salud (2012) Perfil Epidemiológico Del Dengue En México. Mexico City
Shepard DS, Coudeville L, Halasa YA, Zambrano B, Dayan GH (2011) Economic impact of dengue illness in the Americas. Am J Trop Med Hyg 84:200–207
Srikiatkhachorn A, Rothman AL, Gibbons RV, Sittisombut N, Malasit P, Ennis FA, Nimmannitya S, Kalayanarooj S (2011) Dengue–how best to classify it. Clin Infect Dis 53:563–567
Suaya JA, Shepard DS, Siqueira JB, Martelli CT, Lum LC, Tan LH, Kongsin S, Jiamton S, Garrido F, Montoya R, Armien B, Huy R, Castillo L, Caram M, Sah BK, Sughayyar R, Tyo KR, Halstead SB (2009) Cost of dengue cases in eight countries in the Americas and Asia: a prospective study. Am J Trop Med Hyg 80:846–855
Subramanian RE, Herrera DG, Kelly PM (2013) An evaluation of the global network of field epidemiology and laboratory training programmes: a resource for improving public health capacity and increasing the number of public health professionals worldwide. Hum Resour Health 11:45
Tapia-Conyer R, Mendez-Galvan JF, Gallardo-Rincon H (2009) The growing burden of dengue in Latin America. J Clin Virol 46(Suppl 2):S3–S6
Tapia-Conyer R, Mendez-Galvan J, Burciaga-Zuniga P (2012) Community participation in the prevention and control of dengue: the patio limpio strategy in Mexico. Paediatr Int Child Health 32(Suppl 1):10–13
Thisyakorn U, Thisyakorn C (2014) Latest developments and future directions in dengue vaccines. Ther Adv Vaccines 2:3–9
Thomas SJ (2015) Preventing dengue – Is the possibility now a reality? N Engl J Med 372:172–173
Torres-Estrada JL, Rodiles-Cruz Ndel C (2013) Design and evaluation of a ovitrap for monitoring and control of Aedes aegypti, dengue fever vector. Salud Publica Mex 55:505–511
Torres-Galicia I (2014) Dengue en México: análisis de dos décadas. Gaceta Médica De México 150:122–127
Tumioto GL, Gregianini TS, Dambros BP, Cestari BC, Alves Nunes ZM, Veiga AB (2014) Laboratory surveillance of dengue in Rio Grande do Sul, Brazil, from 2007 to 2013. PLoS One 9, E104394
Vázquez-Pichardo M, Rosales-Jiménez C, Núñez-León A, Rivera-Osorio P, Cruz-Hernández SDL, Ruiz-López A, González-Mateos S, López-Martínez I, Rodríguez-Martínez JC, López-Gatell H, Alpuche-Aranda C (2011) Serotipos De Dengue En México Durante 2009 Y 2010. Boletín Médico Del Hospital Infantil De México 68:103–110
Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, Rey LC, Dietze R, Luz K, Rivas E, Montoya MC, Supelano MC, Zambrano B, Langevin E, Boaz M, Tornieporth N, Saville M, Noriega F, The, C. Y. D. S. G. (2014) Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med
Wahala WM, Silva AM (2011) The human antibody response to dengue virus infection. Viruses 3:2374–2395
Weaver SC, Vasilakis N (2009) Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol 9:523–540
Whitehorn J, Simmons CP (2011) The pathogenesis of dengue. Vaccine 29:7221–7228
World Health Organization (1986) Dengue hemorrhagic fever: diagnosis, treatment and control. Geneva
World Health Organization (2008) International health regulations 2005. Geneva.
World Health Organization (2009) Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva
World Health Organization (2012) Global strategy for dengue prevention and control. Geneva
World Health Organization and Experts, S. A. G. O (2012) Principles and considerations for adding a vaccine into a national immunization programme. Geneva
Xavier-Carvalho C, Gibson G, Brasil P, Ferreira RX, De Souza Santos R, Goncalves Cruz O, De Oliveira SA, De Sa Carvalho M, Pacheco AG, Kubelka CF, Moraes MO (2013) Single nucleotide polymorphisms in candidate genes and dengue severity in children: a case-control, functional and meta-analysis study. Infect Genet Evol 20:197–205
Yauch LE, Shresta S (2014) Dengue virus vaccine development. Adv Virus Res 88:315–372
Author Contributions
Review of the literature: CMAA and HLG. Information contribution: JHEA, MHA: Analysis and Discussion of information: CMAA, HLG, JHEA, MHA.
Conflict of Interest
CMAA serves on the Independent Data and Monitoring Committee for ongoing dengue vaccine trials being conducted by Sanofi-Pasteur.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Wien
About this chapter
Cite this chapter
Lopez-Gatell, H., Hernandez-Avila, M., Hernández Avila, J.E., Alpuche-Aranda, C.M. (2015). Dengue in Latin America: A Persistent and Growing Public Health Challenge. In: Franco-Paredes, C., Santos-Preciado, J. (eds) Neglected Tropical Diseases - Latin America and the Caribbean. Neglected Tropical Diseases. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1422-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1422-3_11
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1421-6
Online ISBN: 978-3-7091-1422-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)