Abstract
With a major role in revealing epileptogenic lesions, magnetic resonance imaging (MRI) has also been very helpful in surgical planning and postoperative follow-up of drug-resistant focal epilepsies. In this article, in addition to discussing the most common epileptogenic lesions, advanced quantitative and functional MRI techniques in detecting abnormalities and revealing hemodynamic and microstructural changes are emphasized.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Among the modalities of neuroimaging, magnetic resonance imaging (MRI) has greatly impacted the management and outcome of patients with epilepsy. With a major role of revealing epileptogenic lesions, MRI has also been very helpful in the surgical planning and postoperative follow-up of drug-resistant focal epilepsies which constitute about 25 % of epilepsy cases. Although it is known that MRI in patients with idiopathic generalized epilepsy, benign rolandic epilepsy, and febrile seizures usually does not yield abnormalities but rather than ongoing epilepsy-related changes, the International League Against Epilepsy (ILAE) states that “Everybody with epilepsy should have, in the ideal situation, a high quality MRI!” Computed tomography (CT), once accepted as a first-line imaging modality, now is considered supplementary in the detection of calcification, as in cases of Sturge-Weber disease, tuberous sclerosis, or epileptogenic tumors.
Regardless of the underlying disease, patients in whom the lesions are visualized at preoperative MR imaging tend to have a better outcome after surgery for epilepsy than do patients without lesions [30, 45, 48].
In this article, in addition to discussing the most common lesions, advances in MRI and its potential in detecting abnormalities and revealing hemodynamic and microstuctural changes are emphasized.
Success of MRI in detecting abnormalities is determined by the scanner and applied techniques, the nature of the epileptogenic lesions, and the experience of the radiologist. One study found that the diagnostic yield of an MRI increases from 39 % with routine imaging interpreted by a general radiologist up to 90 % with an epilepsy-dedicated protocol interpreted by an experienced radiologist [68]. The radiologist should be experienced in epilepsy imaging and should assess the imaging with knowledge of the clinical semiology and electrophysiologic information (EEG).
Conventional MRI
An optimal MRI technique for detection of the epileptogenic lesion shows minor differences according to the patient’s age. Due to maturating white matter, it can be more difficult to detect lesions and interpret them during first 24 months of life. While only T2-weighted (W) imaging should be replaced with that of minimum slice thickness without losing signal-to-noise ratio (SNR) in routine cranial MR imaging (which includes sagittal T1W and transverse and coronal T1W and T2W imaging in the author’s institute) up to 8–10 months of age, addition of 3D T1 magnetization-prepared gradient-recalled echo (MPRAGE) or spoiled gradient echo (SPGR) with a 1–1.5 mm slice thickness is necessary from 8–10 to 24–30 months. These sequences provide a good gray-white matter contrast at this stage of myelination. Afterward, epilepsy-dedicated MRI protocol does not differ from that of adults: Adult protocol differs from pediatric cases older than 24–30 months in that it should include coronal high-resolution T2W and inversion recovery (IR) slices with a 2–3-mm thickness oriented perpendicular to the hippocampi, especially in cases of temporal lobe epilepsy (TLE) [3]. In patients with a history or suspicion of trauma or a vascular lesion, a more definitive diagnosis can be achieved by adding T2* gradient echo or susceptibility-weighted imaging (SWI) to the protocol, depending on the facility [55].
Gadolinium (Gd)-based contrast materials should be considered when the radiologist or technician comes across a tumor or tumor-like lesion to get a more specific differential diagnosis or to plan the surgery. Additionally, intravenous contrast materials can be used in dynamic-enhanced susceptibility-weighted perfusion imaging to supplement other techniques such as evaluation of peri-ictal hemodynamic changes or determination of the extent of the pial angiomas in Sturge-Weber syndrome [49, 56].
Neuroimaging of Common Epileptogenic Substrates
Although epileptogenic substrates are practically the same in children and adults, their proportion changes, i.e., malformation of cortical development and developmental tumors are more common in children and young adults while hippocampal sclerosis is more common in adults [17].
Epilepsy-Associated Tumors
Epilepsy-associated tumors are slow-growing, well-defined, non-necrotic lesions that develop from the cortex and do have a common association with malformation of cortical development. Clinical presentation in seizures and complete surgical removal of these lesions result in successful seizure control [50].
Images of these tumors have common features: a cortical well-defined lesion, no accompanying edema or necrosis, and a scalloped/remodeled adjacent bone due to the tumor’s long-term presence. Most critical radiological points of evaluation include its location, detection of associated focal cortical dysplasia (FCD), presence of calcification and cyst, and enhancement. Once an epilepsy-associated tumor is detected, one should also search for hippocampal sclerosis because it is common in double pathologies in epilepsy patients [18, 19].
Gangliogliomas and Gangliocytomas
Gangliomas are more frequent (approximately ten times) and bigger in macroscopic size in children than in adults [11, 60]. The usual location is the temporal lobe with a mild predilection for the mesial surface. Together with low-grade astrocytomas, they are the most common tumors in temporal lobectomy specimens [7]. Because they can present as a cyst, a solid mass, or a mixture of both, their density on CT varies, with about 30–50 % calcification. Usually they are hypointense, sometimes having an internal mild hyperintensity on T1-W series of MRI and hyperintensity on T2-W imaging unless they have a concretion, which gives a dark appearance. Approximately half of them enhance either in a nodular, solid, or peripheral pattern [46] (Fig. 1a, b).
Different from gangliogliomas, which consist of neuronal and glial cells, gangliocytomas histologically consist of only neurons and are rarely seen. These lesions usually have solid and cystic components and show enhancement on post-Gd T1-W series.
Dysembryoplastic Neuroepithelial Tumors (DNET)
These are wedge-shaped tumors with a temporal and frontal lobe predominance and frequently coexist with FCDs DNET are commonly missed or invisible on MRI. They are characterized by a “tail” toward the ventricles, “bubbly” appearance due to a well-marginated multilobulated configuration, and very high T2 and low T1 signal intensities [16, 31] (Fig. 2a, b). Fluid-attenuated inversion-recovery (FLAIR) sequence gives invaluable information about their differential diagnosis from other cortical tumors. First, DNETs are proven to be “pseudocystic” because they have a high signal on FLAIR, which shows their solid nature, in contrast to their “cystic” signals on T1- and T2-W imaging. Second, a thin peripheral bright rim on FLAIR imaging has been reported to be pathognomonic of DNETs [59]. Although a classic DNET does not enhance, a faint, punctate, or rim enhancement can be seen in some cases. A tail extending to the ventricles can also be observed in cortical tubers and FCD with balloon cells (Fig. 2c). DNETs usually remain stable in size; however, seizures may continue despite multiple antiepileptic drug regimens. An MRI for unsuccessful seizure control following surgery should be performed, with the protocol dedicated to epilepsy (as described in the previous section). The radiologist should look for an incomplete resection of the tumor and/or a previously missed associated cortical dysplasia [61] (Fig. 3a, b). The latter is more common when preoperative MRI is performed routinely or with imaging sequences having poorer resolution.
(a) A DNET in the left parietal lobe appears as a hyperintense, rounded, well-defined cortical mass without peripheral edema on an axial FLAIR image. (b) Another DNET is seen in the left temporal lobe on a coronal T2W image. Note the prominent hyperintensity and remodeled thin adjacent bone as a result of the tumor’s longstanding presence. (c) A tail toward the ventricle (arrow) can be seen
(a) Coronal T2W image obtained immediately after resection of a DNET (not shown, scanned in another institution) from the left temporal lobe in a 12-year-old boy shows acute hemorrhage in the operation bed as profound hypointensity. As a result of uncontrolled seizures following surgery, another MRI was obtained 2 years later. (b) High-resolution T2-W TSE coronal image shows increased cortical thickness and blurred white-gray-matter junction suggestive of cortical dysplasia
Pleomorphic Xanthoastrocytomas
As common features with other epilepsy-associated tumors, pleomorphic xanthoastrocytomas (PXAs) affect predominantly the supratentorial compartment, and most commonly the temporal lobe followed by the frontal lobe cortex. These tumors also show solid and deeper cystic portions and, thus, solid and deeper signal intensities on MRI. Peripheral cortical location, solid enhancing nodule, and continuity with enhancing dura (tail) are well known features of PXAs. Usually no calcification is seen on CT and no perilesional edema is observed [32, 41]. They share common radiologic features with desmoplastic infantile ganglioglioma; however, PXAs usually present in the second decade or around late childhood. A close association with FCD in adjacent cortex should be considered during evaluation [47].
Low-Grade Astrocytomas
MRI is more successful in detecting these cortical, infiltrative, ill-defined masses. Low-grade astrocytomas (LGAs) do not enhance. When present, a more aggressive/anaplastic form should be suspected. Expansion of the cortex, which is commonly the frontal and temporal lobes, is usual but not the rule. Thus, sometimes it is necessary to perform a follow-up MRI or add diffusion-weighted imaging (DWI) or magnetic resonance spectroscopy (MRS) to differentiate these lesions from FCDs, infarcts, and encephalitis radiologically. Valuable information comes from the history and clinical findings of the patient and usually solves the problem.
Rarer epilepsy-associated tumors will not be mentioned here because of their nonspecific radiological findings.
Hippocampal Sclerosis
Mesial temporal sclerosis (MTS) or hippocampal sclerosis (HS) occurs from pyramidal and neuronal cell loss in the cornu ammonis and dentate of the hippocampus. It is not only the most frequent epilepsy substrate in patients undergoing surgery for epilepsy, but also the most frequent cause of complex partial seizures in adults [52]. Patients often have a history of complicated febrile seizures during childhood [26, 67]. A familial tendency of mesial temporal lobe epilepsy has been reported [25, 36]. Currently, the most preferred surgical procedure for treatment is the anterior temporal lobectomy [17, 35].
Three major findings that should suggest HS can easily be evaluated on coronal thin section (2–3 mm) T2W imaging acquired perpendicular to the hippocampi. These are atrophy, T2 signal increase, and loss of internal structure of the hippocampus (Fig. 4). Inversion recovery sequences help determine internal structure of a hippocampus [13, 14]. Supplementary MRI findings, such as loss of ipsilateral pes hippocampus digitations, decreased thickness of the collateral white matter, and dilatation of the ipsilateral temporal horn of the lateral ventricle, usually occur secondary to hippocampal involvement. Other MRI abnormalities result from degeneration through the components of the Papez circuit and include atrophy of the ipsilateral fornix and mammillary body (Fig. 4) [20]. Increased signal intensity of the ipsilateral anterior temporal lobe white matter and loss of the gray-white-matter boundary are often seen and can be due to degeneration and myelin loss or associated malformation of cortical development [54]. Involvement of the amygdala, which is usually indicated by a subtle increase in the T2 signal and a volume change, should be reported for prognostic importance because isolated HS has a better outcome after surgery [15].
An experienced radiologist can recognize HS with a sensitivity of 80–90 %. However, in bilateral cases (10–20 %) [38] or in cases without a visible radiologic abnormality, quantitative assessment such as MR volumetry and T2 relaxation measurements or use of more advanced techniques such as magnetization-transfer contrast (MTC), MRS, and diffusion-tensor imaging (DTI) may be necessary. A quantitative approach, however, requires extra energy, trained personnel, software, and time, all of which are especially precious to busy radiology departments. Volume loss has been found to correlate with the duration of epileptic disorder, frequency of childhood febrile seizures, memory function, and cell loss on pathological examinations [21, 24]. A literature review by Keller and Roberts [44] summarized brain changes that occur in temporal lobe epilepsy (TLE) patients revealed by a total of 18 voxel-based morphometry (VBM) studies. In this fully automated quantitative technique, gray matter concentration and volume are measured via voxel-wise statistical analyses. Following a series of preprocessing steps, i.e., spatial normalization, tissue segmentation, and spatial smoothing in standard fashion, morphologic differences are detected in two groups of people [2]. Bilateral asymmetric widespread abnormalities occur preferentially ipsilateral to the side of seizure focus. These structures include hippocampus, amygdala, parahippocampal gyrus, entorhinal and perirhinal cortexes, fusiform gyrus, temporal pole, superior, middle, and inferior temporal gyri, fornix, orbital frontal lobe, frontal pole, insula, parietal lobe, and cingulated gyrus. White matter (WM) reductions also occur in predominantly ipsilateral temporal and extratemporal lobes, although VBM is not the technique of choice for evaluating WM due to insufficient WM tracts for effective spatial normalization. Additionally, some studies found an increase in gray matter (GM) concentration mostly in the temporal lobe. However, a parallel increase in volume of GM was not observed. Although this remains speculative, the frequent coexistence of malformation of cortical development (MCD), observed as blurred gray-to-white-matter [53] transition on MRI, can manifest as increased GM concentration or displacement of the parahippocampal gyrus due to severe atrophy [43].
Malformation of Cortical Development
Malformation of cortical development lesions and their imaging should be discussed in a separate article on migration disorders. However, two lesions that undergo surgical resection or other types of surgical procedures are discussed here: FCDs and tuberous sclerosis.
Focal Cortical Dysplasia
Since its initial description by Taylor et al. [65], FCDs have been extensively studied and classified and refer to a wide range of cortical formation abnormalities. Classification of FCDs based on the neuropathological findings by Palmini et al. in 2004 has been widely accepted and used [58]. However, in 2011, the ILAE task force developed a new classification system to distinguish isolated forms (FCD types I and II) from those associated with another principal lesion (FCD type III) [12]. While mild FCD usually shows no visible radiologic abnormality, as suggested by Palmini et al., a subtle increase in the T2 signal and hypoplasia of mostly the temporal lobe with type 1A and 1B can be seen. The usual findings of a FCD include thick cortex, blurring of the gray-to-white-matter transition, abnormally increased signal in the subjacent white matter, and deep and asymmetric abnormal sulci (Fig. 5a, b) [51]. Broadening of the gyri with increased T2 signal and accompanying “tail” tapering toward the ventricle [23, 66] have been accepted characteristics of type 2B (FCD with balloon cells) (Fig. 6). Keeping mild FCD types 1 and 2 suggested by Palmini et al., this new classification by the ILAE task force took those FCDs plus adjacent HS (a), glial/glioneural tumor (b), vascular malformation (c), or lesions acquired early in life such as trauma, ischemic sequela, encephalitis (d) into consideration (Fig. 7) [12]. However, a rare association between FCD type 2 and these entities within different locations of the brain parenchyma is not considered FCD type 3. FCD type 3a, which indicates FCD in an adjacent parenchyma and HS, should be differentiated from “double” and “dual pathology” terms. In cases without HS, the presence of two epileptogenic lesions is defined as “double pathology,” while HS plus an extratemporal lesion is defined as “dual pathology” (Fig. 8a, b). Since dual pathology is observed in approximately 15 % of the patients with HS, one should always search for abnormalities in addition to HS [18].
Tuberous Sclerosis
Multiple cortical and subcortical tubers and subependymal nodules along the lateral ventricles are characteristic features of tuberous sclerosis (TSC). Tubers expand the gyri they originate and cause an increased T2 signal in the cortex and subjacent white matter. When there is calcification, the signal can differ, i.e., a lower T2 signal and a higher T1 signal. Subependymal nodules usually are calcified and seen as T1 hyper- and T2 hypointense nodules (Fig. 9a). They can be observed along the ventricles on 3D MPRAGE/SPGR images, even when they are in the submillimeter size range. The similar radiologic appearance of a cortical tuber and that of a FCD with balloon cells or a DNET leads one to consider TSC as a “syndromic variant” of FCD or that they all have a common precursor cell (Fig. 9b) [37]. From a radiologic point of view, multiplicity of the tubers and the presence of subependymal nodules help in the diagnosis of TSC. Early in life, due to the lack of myelin, recognition of a tuber may be difficult. Thus, T1W imaging is a most valuable sequence with hyperintensity of these lesions (Fig. 10a, b) [4].
Patients with TSC frequently are not candidates for epilepsy surgery because of multiplicity of the lesions and types of seizures. However, when electrophysiology and imaging with single photon emission computed tomography (SPECT), along with the seizures that the patient experiences, indicate a specific focus, then surgery is considered.
Other
Cavernomas are best depicted on T2*GRE, SWI, but their internal structure, with its multiple phases of blood products and dark hemosiderin periphery, is usually seen on T1- and T2W series (Fig. 11a, b). These cortical and juxtacortical lesions are highly epileptogenic and are not difficult to diagnose radiologically unless they are huge and complicated with recent hemorrhage.
Arteriovenous malformations (AVMs) with their tangle of vessels, large feeding and draining vasculature, and accompanying aneurysm usually pose no diagnostic challenge.
‘Similarly, with characteristic CT and MRI findings of Sturge-Weber Syndrome such as calcification of subcortical parenchyma which are affected by overlying pial angiomas, asymmetric atrophy of the parenchyma most frequently parietal and occipital lobes, enlarged ipsilateral choroid plexus and linear superficial enhancement with Gd, diagnosis of this syndrome is straight forward (Fig. 12a, b). Determining the regions of the affected brain may be more challenging than diagnosis. SPECT, PET, and perfusion-weighted MR imaging can delineate hemodynamically and metabolically abnormal areas in candidates for surgery, especially because involvement of other hemisphere becomes important.
Status Epilepticus
During the peri-ictal phase of status epilepticus (SE), regional cortical changes and remote lesions, including cerebellar diaschisis, ipsilateral thalamic lesions, and basal ganglia lesions, have been reported. These lesions are observed as tissue swelling, hyperintensity most prominent on FLAIR imaging, and restricted diffusion (Fig. 13a, b) [39]. Peri-ictal radiologic findings are usually reversible, and these abnormalities may reflect the real extent of epileptogenic activity. Later sequelae, including focal brain atrophy, cortical laminar necrosis, and mesial temporal sclerosis, can occur. Thalamic DWI hyperintense lesions, occurring after prolonged partial SE, have received attention recently and probably represent participation of the thalamus in the propagation of partial seizures in SE because of reciprocal connections with the involved cortex (Fig. 13b) [39, 42]. Gd enhancement may occur in the hippocampi, which may be followed by sclerosis (Fig.14a, b). Diffusion restriction as shown by reduced ADC in the hippocampi, the pulvinar region of the thalamus, and cortical regions may give correlates of hyperperfusion assessed by SPECT or perfusion-weighted MR imaging (PWI) [63].
Cryptogenic Epilepsy
Although prolonged EEG recordings with intracranial electrodes have increased the success in resecting the epileptogenic cortex, because of complications of this procedure and the possibility of removing the electrodes without identifying the seizure focus, surgery in the absence of a visible MR lesion continues to be a challenge. As imaging techniques and their diagnostic yield improve, previously accepted “cryptogenic” epilepsies may turn out to be “lesional” [9]. The most common histopathological finding in cryptogenic epilepsy is focal cortical dysplasia. High magnetic field systems and phase-array coils will enable detection and delineation of malformed areas and, in turn, increase the number of patients who will benefit from surgery. T1W volumetric acquisition with isotropic 1-mm3 voxels not only increases the visibility of the lesions, it also provides multiplanar reformats. Curvilinear reformatting in particular decreases artifactual cortical thickening and increases the detection of these abnormalities [6, 40].
Voxel-based morphometry (VBM) is a technique that enables identification of regional differences in the whole brain through a multiple-step process. However, it is time consuming to perform, and subtle increased cortical thickness due to cortical reorganization from personal abilities and specifications and prominent signal changes of the lesions may lead to confusion and reduced sensitivity [22]. Histopathological correlation of findings obtained from VBM is needed. Besides VBM and T2-relaxivity measurements, DTI, by quantifying diffusivity of water, adds information about the microstructure of brain tissue [33, 51]. DTI revealed abnormalities in white matter adjacent to and often beyond the cortical dysplasia [28, 69]. TLE patients, as discussed in the relevant section of this chapter, show widespread diffusion abnormality in white matter [33].
Since blood oxygen level-dependent (BOLD) imaging can detect local changes in oxy- and deoxyhemoglobin concentrations due to neuronal activity related to epileptic discharge, combined EEG and fMRI studies, coregistered with anatomical data, can reveal an epileptic zone [34].
In addition to dynamic susceptibility-weighted perfusion imaging, another technique recently available in the market, “Arterial Spin Labeling” (ASL), gives information comparable to that of single photon emission computed tomography (SPECT). An advantage of ASL is that it quantifies cerebral blood flow. If by chance the patient is not having a seizure during the MR examination, ASL will disclose interictal abnormalities. Adding ASL may provide additional information about the lateralization of the focus [51, 71]. 18-Fluorodeoxyglucose (FDG) positron emission tomography (PET) is especially helpful in lateralization of the focus by showing interictal hypometabolism. The focus may actually be at the site of margin [51]. Coregistration of PET data with volumetric T1W imaging overcomes the poor resolution of this technique.
Although these advanced imaging techniques can provide valuable information about the epileptogenic zone, currently no single technique is capable of delineating the exact extent of cortical lesions. Further studies with correlation between disciplines, including histopathology and immunohistochemistry, are necessary to determine the clinical significance of these imaging findings.
Functional MR (fMR) Imaging
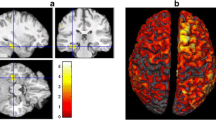
Among advanced MR imaging modalities, fMR imaging merits special attention. This technique relies on a signal that arises from the oxygenation status of hemoglobin, the so-called BOLD signal. Close correlation of neural activity and changes in tissue oxygen level enable visualization of the BOLD signal. A block (alternating active and baseline cycles) or event-related (discrete unequal events) paradigm is present; however, under clinical circumstances a block-design paradigm provides a higher signal-to-noise ratio and decreases the scan time necessary for robust fMR imaging activation. fMR studies are performed in epilepsy patients for two reasons: to evaluate the relationship of a lesion with eloquent cortex and to assess hemispheric dominance (Fig. 15). A variety of tasks may be used; however, this poses a problem in standardization of the technique and the results obtained from studies. For expressive language, frequently silent word generation from letters or words is used.
Because of the increased frequency of right-sided or bilateral language, lateralization or intrahemispheric reorganization can occur in patients with left hemisphere lesions [1, 17, 27], and an intracarotid amobarbital test (Wada test) or, increasingly, fMRI is performed to assess the language/speech deficit risks of the surgery [5, 64]. Up to 90 % of cases studied found agreement between these techniques [8, 29, 70] and even found fMR imaging to be a better predictor of postoperative cognitive outcome [10, 62]. It has been emphasized that at least three different language tasks of sufficient length and the combination of electrocortical stimulation (ECS) and fMR imaging should be obtained. fMR imaging should be performed by a technician/radiologist with expertise and be interpreted with special attention paid to the individual’s task performance and the extent of cluster size of the BOLD signal on postprocessing. fMR imaging has advantages over ECS: atypical locations of eloquent cortex can be detected by imaging. Although fMR imaging cannot replace ECS or WADA completely, it decreases the need for these invasive tests and helps in planning surgical strategy and the targeting of sites for ECS.
Fiber tractography showing 3D orientation of white matter tracts in combination with fMR imaging enables the surgeon to assess the relationship between the lesion and the WM bundle and eloquent cortex simultaneously (Fig. 16a–c).
Fiber tractography of the corticospinal tractus (CST) coregistered with FLAIR imaging (a) shows posterior displacement of the left CST by the tumor. BOLD activation from right-hand (b) and right-foot (c) motor tasks shows posterolateral (b) and posteromedial (c) deviation of the activation by the mass
Postoperative Imaging
Evaluation of a postoperative MR study necessitates knowledge of the surgery performed, its potential complications, and previous epileptogenic lesion characteristics if visible on MR imaging. Local postsurgical changes include hemorrhage and cytotoxic tissue edema displayed b`y DWI, and sometimes infarcts in arterial territory similar to those in other cranial operations [57]. MR imaging protocol should be planned with knowledge of the preoperative imaging findings, i.e., epilepsy protocol in FCD, contrast-enhanced routine imaging in neoplastic lesions, and contrast-enhanced imaging, including thin-section T2W imaging perpendicular to the temporal lobe developmental tumors.
In patients who were operated on but not seizure-free, a repeat MR imaging procedure should be performed if a higher magnetic field system and newer imaging modalities with higher resolution become available.
Conclusion
Patients with drug-resistant epilepsy benefit from advances in imaging for the localization and lateralization of epileptogenic substrate and the planning of surgery in cryptogenic and lesional epilepsy. MR imaging provides information about hemodynamic and microstructural tissue properties as well. Once overlooked subtle cortical dysplasias will probably be captured via developing quantitative and functional imaging techniques in the near future.
Abbreviations
- ASL:
-
Arterial spin labeling
- AVM:
-
Arteriovenous malformation
- BOLD:
-
Blood oxygen level-dependent
- CT:
-
Computed tomography
- DNET:
-
Dysembryoplastic neuroepithelial tumor
- DTI:
-
Diffusion-tensor imaging
- DWI:
-
Diffusion-weighted imaging
- ECS:
-
Electrocortical stimulation
- EEG:
-
Electroencephalogram
- FCD:
-
Focal cortical dysplasia
- FLAIR:
-
Fluid-attenuated inversion recovery
- fMRI:
-
Functional magnetic resonance imaging
- Gd:
-
Gadolinium
- GM:
-
Gray matter
- HS:
-
Hippocampal sclerosis
- IR:
-
Inversion recovery
- LGA:
-
Low-grade astrocytoma
- MCD:
-
Malformation of cortical development
- MPRAGE:
-
Magnetization-prepared gradient-recalled echo
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- MTC:
-
Magnetization-transfer contrast
- MTS:
-
Mesial temporal sclerosis
- PET:
-
Fluorodeoxyglucose (FDG) positron emission tomography
- PWI:
-
Perfusion-weighted MR imaging
- PXA:
-
Pleomorphic xanthoastrocytoma
- SE:
-
Status epilepticus
- SNR:
-
Signal-to-noise ratio
- SPECT:
-
Single photon emission CT
- SPGR:
-
Spoiled gradient echo
- SWI:
-
Susceptibility-weighted imaging
- TLE:
-
Temporal lobe epilepsy
- TSC:
-
Tuberous sclerosis
- VBM:
-
Voxel-based morphometry
- WM:
-
White matter
References
Anderson DP, Harvey AS, Saling MM, Anderson V, Kean M, Abbott DF, Wellard RM, Jackson GD (2006) FMRI lateralization of expressive language in children with cerebral lesions. Epilepsia 47(6):998–1008
Ashburner J, Friston KJ (2000) Voxel-based morphometry – the methods. Neuroimage 11(6 Pt 1):805–821
Atlas SW (2009) Magnetic resonance imaging of the brain and spine, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 312–313
Baron Y, Barkovich AJ (1999) MR imaging of tuberous sclerosis in neonates and young infants. AJNR Am J Neuroradiol 20(5):907–916
Bargalló N (2008) Functional magnetic resonance: new applications in epilepsy. Eur J Radiol 67(3):401–408
Bastos AC, Comeau RM, Andermann F, Melanson D, Cendes F, Dubeau F, Fontaine S, Tampieri D, Olivier A (1999) Diagnosis of subtle focal dysplastic lesions: curvilinear reformatting from three-dimensional magnetic resonance imaging. Ann Neurol 46(1):88–94
Benifla M, Otsubo H, Ochi A, Weiss SK, Donner EJ, Shroff M, Chuang S, Hawkins C, Drake JM, Elliott I, Smith ML, Snead OC 3rd, Rutka JT (2006) Temporal lobe surgery for intractable epilepsy in children: an analysis of outcomes in 126 children. Neurosurgery 59(6): 1203–1213
Benke T, Köylü B, Visani P, Karner E, Brenneis C, Bartha L, Trinka E, Trieb T, Felber S, Bauer G, Chemelli A, Willmes K (2006) Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada Test. Epilepsia 47(8):1308–1319
Bernasconi A, Bernasconi N, Bernhardt BC, Schrader D (2011) Advances in MRI for ‘cryptogenic’ epilepsies. Nat Rev Neurol 7(2):99–108
Binder JR, Sabsevitz DS, Swanson SJ, Hammeke TA, Raghavan M, Mueller WM (2008) Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia 49(8):1377–1394
Blümcke I, Löbach M, Wolf HK, Wiestler OD (1999) Evidence for developmental precursor lesions in epilepsy-associated glioneuronal tumors. Microsc Res Tech 46(1):53–58
Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, Becker A, Cepeda C, Cendes F, Colombo N, Crino P, Cross JH, Delalande O, Dubeau F, Duncan J, Guerrini R, Kahane P, Mathern G, Najm I, Ozkara C, Raybaud C, Represa A, Roper SN, Salamon N, Schulze-Bonhage A, Tassi L, Vezzani A, Spreafico R (2011) The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 52(1):158–174
Bote RP, Blázquez-Llorca L, Fernández-Gil MA, Alonso-Nanclares L, Muñoz A, De Felipe J (2008) Hippocampal sclerosis: histopathology substrate and magnetic resonance imaging. Semin Ultrasound CT MR 29(1):2–14
Bronen RA, Cheung G, Charles JT, Kim JH, Spencer DD, Spencer SS, Sze G, McCarthy G (1991) Imaging findings in hippocampal sclerosis: correlation with pathology. AJNR Am J Neuroradiol 12(5):933–940
Bronen RA, Anderson AW, Spencer DD (1994) Quantitative MR for epilepsy: a clinical and research tool? AJNR Am J Neuroradiol 15(6):1157–1160
Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J, Urbach H (2009) Simple and complex dysembryoplastic neuroepithelial tumors (DNT) variants: clinical profile, MRI, and histopathology. Neuroradiology 51(7):433–443
Cataltepe O, Jallo GI (2010) Pediatric epilepsy surgery: preoperative assessment and surgical intervention, 1st edn. Thieme Medical Publishers, New York, pp 60–61
Cendes F, Cook MJ, Watson C, Andermann F, Fish DR, Shorvon SD, Bergin P, Free S, Dubeau F, Arnold DL (1995) Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology 45(11):2058–2064
Cendes F, Li LM, Andermann F, Watson C, Fish DR, Shorvon SD, Dubeau F, Arnold DL (1999) Dual pathology and its clinical relevance. Adv Neurol 81:153–164
Chan S, Erickson JK, Yoon SS (1997) Limbic system abnormalities associated with mesial temporal sclerosis: a model of chronic cerebral changes due to seizures. Radiographics 17(5):1095–1110
Cheon JE, Chang KH, Kim HD, Han MH, Hong SH, Seong SO, Kim IO, Lee SG, Hwang YS, Kim HJ (1998) MR of hippocampal sclerosis: comparison of qualitative and quantitative assessments. AJNR Am J Neuroradiol 19(3):465–468
Colliot O, Bernasconi N, Khalili N, Antel SB, Naessens V, Bernasconi A (2006) Individual voxel-based analysis of gray matter in focal cortical dysplasia. Neuroimage 29(1):162–171
Colombo N, Tassi L, Galli C, Citterio A, Lo Russo G, Scialfa G, Spreafico R (2003) Focal cortical dysplasias: MR imaging, histopathologic, and clinical correlations in surgically treated patients with epilepsy. AJNR Am J Neuroradiol 24(4):724–733
Cook MJ (1994) Mesial temporal sclerosis and volumetric investigations. Acta Neurol Scand Suppl 152:109–114, discussion 115
Crompton DE, Scheffer IE, Taylor I, Cook MJ, McKelvie PA, Vears DF, Lawrence KM, McMahon JM, Grinton BE, McIntosh AM, Berkovic SF (2010) Familial mesial temporal lobe epilepsy: a benign epilepsy syndrome showing complex inheritance. Brain 133(11):3221–3231
Davies KG, Hermann BP, Dohan FC Jr, Foley KT, Bush AJ, Wyler AR (1996) Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res 24(2):119–126
Duchowny M, Jayakar P, Harvey AS, Resnick T, Alvarez L, Dean P, Levin B (1996) Language cortex representation: effects of developmental versus acquired pathology. Ann Neurol 40(1):31–38
Dumas de la Roque A, Oppenheim C, Chassoux F, Rodrigo S, Beuvon F, Daumas-Duport C, Devaux B, Meder JF (2005) Diffusion tensor imaging of partial intractable epilepsy. Eur Radiol 15(2):279–285
Dym RJ, Burns J, Freeman K, Lipton ML (2011) Is functional MR imaging assessment of hemispheric language dominance as good as the Wada test? A meta-analysis. Radiology 261:446–455
Fauser S, Bast T, Altenmüller DM, Schulte-Mönting J, Strobl K, Steinhoff BJ, Zentner J, Schulze-Bonhage A (2008) Factors influencing surgical outcome in patients with focal cortical dysplasia. J Neurol Neurosurg Psychiatry 79(1):103–105
Fernandez C, Girard N, Paz Paredes A, Bouvier-Labit C, Lena G, Figarella-Branger D (2003) The usefulness of MR imaging in the diagnosis of dysembryoplastic neuroepithelial tumor in children: a study of 14 cases. AJNR Am J Neuroradiol 24(5):829–834
Giannini C, Scheithauer BW, Burger PC, Brat DJ, Wollan PC, Lach B, O’Neill BP (1999) Pleomorphic xanthoastrocytoma: what do we really know about it? Cancer 85(9):2033–2045
Gross DW (2011) Diffusion tensor imaging in temporal lobe epilepsy. Epilepsia 52(Suppl 4): 32–34
Gotman J (2008) Epileptic networks studied with EEG-fMRI. Epilepsia 49(Suppl 3):42–51
Harkness W (2006) Temporal lobe resections. Childs Nerv Syst 22(8):936–944
Hedera P, Blair MA, Andermann E, Andermann F, D’Agostino D, Taylor KA, Chahine L, Pandolfo M, Bradford Y, Haines JL, Abou-Khalil B (2007) Familial mesial temporal lobe epilepsy maps to chromosome 4q13.2-q21.3. Neurology 68(24):2107–2112
Hirfanoglu T, Gupta A (2010) Tuberous sclerosis complex with a single brain lesion on MRI mimicking focal cortical dysplasia. Pediatr Neurol 42(5):343–347
Ho SS, Kuzniecky RI, Gilliam F, Faught E, Morawetz R (1998) Temporal lobe developmental malformations and epilepsy: dual pathology and bilateral hippocampal abnormalities. Neurology 50(3):748–754
Huang YC, Weng HH, Tsai YT, Huang YC, Hsiao MC, Wu CY, Lin YH, Hsu HL, Lee JD (2009) Periictal magnetic resonance imaging in status epilepticus. Epilepsy Res 86(1):72–81
Huppertz HJ, Kassubek J, Altenmüller DM, Breyer T, Fauser S (2008) Automatic curvilinear reformatting of three-dimensional MRI data of the cerebral cortex. Neuroimage 39(1):80–86
Im SH, Chung CK, Kim SK, Cho BK, Kim MK, Chi JG (2004) Pleomorphic xanthoastrocytoma: a developmental glioneuronal tumor with prominent glioproliferative changes. J Neurooncol 66(1–2):17–27
Katramados AM, Burdette D, Patel SC, Schultz LR, Gaddam S, Mitsias PD (2009) Periictal diffusion abnormalities of the thalamus in partial status epilepticus. Epilepsia 50(2):265–275
Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N (2002) Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage 16(1):23–31
Keller SS, Roberts N (2008) Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia 49(5):741–757
Kim DW, Lee SK, Chu K, Park KI, Lee SY, Lee CH, Chung CK, Choe G, Kim JY (2009) Predictors of surgical outcome and pathologic considerations in focal cortical dysplasia. Neurology 72(3):211–216
Koeller KK, Henry JM (2001) From the archives of the AFIP: superficial gliomas: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 21(6):1533–1556
Lach B, Duggal N, DaSilva VF, Benoit BG (1996) Association of pleomorphic xanthoastrocytoma with cortical dysplasia and neuronal tumors. A report of three cases. Cancer 78(12):2551–2563
Lerner JT, Salamon N, Hauptman JS, Velasco TR, Hemb M, Wu JY, Sankar R, Donald Shields W, Engel J Jr, Fried I, Cepeda C, Andre VM, Levine MS, Miyata H, Yong WH, Vinters HV, Mathern GW (2009) Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: a critical review and the UCLA experience. Epilepsia 50(6):1310–1335
Lin DD, Barker PB, Hatfield LA, Comi AM (2006) Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. J Magn Reson Imaging 24(2):274–281
Luyken C, Blümcke I, Fimmers R, Urbach H, Elger CE, Wiestler OD, Schramm J (2003) The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia 44(6):822–830
Madan N, Grant PE (2009) New directions in clinical imaging of cortical dysplasias. Epilepsia 50(9)
Margerison JH, Corsellis JA (1966) Epilepsy and the temporal lobes. A clinical, electroencephalographic, and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain 89:499–530
Meiners LC, van Gils A, Jansen GH, de Kort G, Witkamp TD, Ramos LM, Valk J, Debets RM, van Huffelen AC, van Veelen CW et al (1994) Temporal lobe epilepsy: the various MR appearances of histologically proven mesial temporal sclerosis. AJNR Am J Neuroradiol 15(8):1547–1555
Meiners LC, Witkamp TD, de Kort GA, van Huffelen AC, van der Graaf Y, Jansen GH, van der Grond J, van Veelen CW (1999) Relevance of temporal lobe white matter changes in hippocampal sclerosis. Magnetic resonance imaging and histology. Invest Radiol 34(1):38–45
Mittal S, Wu Z, Neelavalli J, Haacke EM (2009) Susceptibility-weighted imaging: technical aspects and clinical applications, part 2. AJNR Am J Neuroradiol 30(2):232–252
Oguz KK, Senturk S, Ozturk A, Anlar B, Topcu M, Cila A (2007) Impact of recent seizures on cerebral blood flow in patients with Sturge-Weber syndrome: study of 2 cases. J Child Neurol 22(5):617–620
Ozturk A, Oguz KK, Akalan N, Geyik PO, Cila A (2006) Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol 12(3):115–120
Palmini A, Najm I, Avanzini G, Babb T, Guerrini R, Foldvary-Schaefer N, Jackson G, Lüders HO, Prayson R, Spreafico R, Vinters HV (2004) Terminology and classification of the cortical dysplasias. Neurology 62(6 Suppl 3):S2–S8
Parmar HA, Hawkins C, Ozelame R, Chuang S, Rutka J, Blaser S (2007) Fluid-attenuated inversion recovery ring sign as a marker of dysembryoplastic neuroepithelial tumors. J Comput Assist Tomogr 31(3):348–353
Provenzale JM, Ali U, Barboriak DP, Kallmes DF, Delong DM, McLendon RE (2000) Comparison of patient age with MR imaging features of gangliogliomas. AJR Am J Roentgenol 174(3):859–862
Sakuta R, Otsubo H, Nolan MA, Weiss SK, Hawkins C, Rutka JT, Chuang NA, Chuang SH, Snead OC (2005) Recurrent intractable seizures in children with cortical dysplasia adjacent to dysembryoplastic neuroepithelial tumor. J Child Neurol 20(4):377–384
Szabo K, Poepel A, Pohlmann-Eden B, Hirsch J, Back T, Sedlaczek O, Hennerici M, Gass A (2003) Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology 60(11):1788–1792
Szabo K, Poepel A, Pohlmann-Eden B, Hirsch J, Back T, Sedlaczek O, Hennerici M, Gass A (2005) Diffusion-weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain 128(Pt 6):1369–1376
Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M (2008) Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav 12(1):74–83
Taylor DC, Falconer MA, Bruton CJ, Corsellis JA (1971) Focal dysplasia of the cerebral cortex in epilepsy. J Neurol Neurosurg Psychiatry 34(4):369–387
Urbach H, Scheffler B, Heinrichsmeier T, von Oertzen J, Kral T, Wellmer J, Schramm J, Wiestler OD, Blümcke I (2002) Focal cortical dysplasia of Taylor’s balloon cell type: a clinicopathological entity with characteristic neuroimaging and histopathological features, and favorable postsurgical outcome. Epilepsia 43(1):33–40
VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV (1998) Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol 43(4):413–426
Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, Elger CE (2002) Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry 73(6):643–647
Widjaja E, Zarei Mahmoodabadi S, Otsubo H, Snead OC, Holowka S, Bells S, Raybaud C (2009) Subcortical alterations in tissue microstructure adjacent to focal cortical dysplasia: detection at diffusion-tensor MR imaging by using magnetoencephalographic dipole cluster localization. Radiology 251(1):206–215
Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A (2003) Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology 61(5):699–701
Wolf RL, Alsop DC, Levy-Reis I, Meyer PT, Maldjian JA, Gonzalez-Atavales J, French JA, Alavi A, Detre JA (2001) Detection of mesial temporal lobe hypoperfusion in patients with temporal lobe epilepsy by use of arterial spin labeled perfusion MR imaging. AJNR Am J Neuroradiol 22(7):1334–1341
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer-Verlag Wien
About this chapter
Cite this chapter
Oguz, K.K. (2012). Magnetic Resonance Imaging in Epilepsy. In: Akalan, N., Di Rocco, C. (eds) Pediatric Epilepsy Surgery. Advances and Technical Standards in Neurosurgery, vol 39. Springer, Vienna. https://doi.org/10.1007/978-3-7091-1360-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-7091-1360-8_3
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-1359-2
Online ISBN: 978-3-7091-1360-8
eBook Packages: MedicineMedicine (R0)