Abstract
Dental caries uniquely is prevalent worldwide and the annual oral health costs in Europe are estimated at €79 billion [1]. Essentially, the tooth’s outer covering, dental enamel and subsequently the sub-adjacent dentine are attacked and eventually destroyed by bacterially produced acid. If untreated this often results in infection of the alveolar bone of the jaw leading in turn to systemic infection including endocarditis. If the tooth is lost, supporting bone of the tooth socket is also resorbed exposing roots of previously healthy adjacent teeth to further attack by oral bacteria and toothbrush wear. Therapeutic treatment is only possible before the enamel surface is breached, after which restoration materials are inserted following drilling out of porous carious tissue. Since enamel is acellular, enamel caries occurs without the participation of host cells and is essentially a chemical process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction to Dental Caries
Dental caries uniquely is prevalent worldwide and the annual oral health costs in Europe are estimated at €79 billion [1]. Essentially, the tooth’s outer covering, dental enamel and subsequently the sub-adjacent dentine are attacked and eventually destroyed by bacterially produced acid. If untreated this often results in infection of the alveolar bone of the jaw leading in turn to systemic infection including endocarditis. If the tooth is lost, supporting bone of the tooth socket is also resorbed exposing roots of previously healthy adjacent teeth to further attack by oral bacteria and toothbrush wear. Therapeutic treatment is only possible before the enamel surface is breached, after which restoration materials are inserted following drilling out of porous carious tissue. Since enamel is acellular, enamel caries occurs without the participation of host cells and is essentially a chemical process.
Dental enamel comprises ~97 % inorganic crystals of a calcium hydroxyapatite mineral, similar to crystals in dentine, cementum and bone (see chapter “Overview” under part “Teeth and bones”). Enamel crystals are, however, much larger and better formed being 30–50 nm in thickness and width and up to 500 nm in length [2]. Several thousand crystals are packed parallel to each other into 4–5 μm diameter bundles, the enamel prisms, extending from dentine towards the enamel surface [3].
Apatite crystals exhibit many ion substitutions (Fig. 1) [4–6]. Important from a therapeutic viewpoint, fluoride ion substitution for hydroxyl ions dramatically reduces acid solubility and facilitates precipitation while carbonate and magnesium have an opposite effect [7]. Fluoride ion stabilises the crystal by reducing lattice energy and improving crystallinity, while carbonate and magnesium distort the regular arrangement of ions leading to instability.
Chemical composition of hydroxyapatite. (a) View of a regular, non-distorted hexagonal unit cell of hydroxyapatite in the tooth down the long c-axis. One layer of a unit crystal is shown. The positions of inner (Ca 2+ I) and outer (Ca 2+ II) calcium ions, as well as phosphate ions (P i ), are shown. Please note that outer Ca2+ ions also “belong” to two neighbouring apatite crystals (shown with dotted lines). A hydroxyl ion (OH −) is shown in the centre. For a complete hydroxyapatite unit cell, a second layer must be added, rotated by 60°, so that Ca2+ will be placed atop Pi, resulting in the chemical formula of Ca2+ 10(PO4 3−)6)(OH−)2. (b) View of a substituted, distorted hydroxyapatite hexagonal unit cell down the long c-axis showing possible substitutes. A fluoride (F −) ion is shown in the centre position. Close fit and high electronegativity of fluoride ions confer stability on the crystal with regard to acid. Distortions due to carbonate (CO 3 2−) and magnesium (Mg 2+) ions are indicated resulting in less well-ordered crystals and greater acid solubility. Locations of other substituent ions are shown, e.g. Sr2+, Pb2+, Zn2+. HPO4 2− and HCO3 − can also be present. Charge balance is maintained by loss of calcium or hydroxyl ions. (c) View of hydroxyapatite crystal showing the long c-axis with a fluoride substituting for one hydroxyl ion. The orientation of hydroxyl ions is altered such that the hydroxyl protons are oriented towards the fluoride ion. This increases stability of the crystals towards acid [13]
Pathophysiology of Dental Caries and Metabolic Alterations
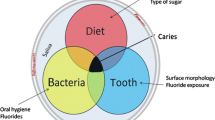
Several hundred species of bacteria are resident in the oral cavity [8]. Of these 30–50 are regarded as cariogenic. The mutans streptococci have received most attention in this respect. Cariogenic bacteria form a biofilm, dental plaque, in the deeper fissures of molar teeth, around contact areas between adjacent teeth (interproximal sites) and at the gum margin. Oral bacteria colonise a protein layer on enamel surfaces, many proteins of which are unique to saliva [9]. This produces a biofilm with channels often extending from saliva to the enamel surface and mushroom-shaped biomass, structures typical of nutrient rich biofilms [10]. Caries is mediated by acid products of plaque biofilm metabolism [11].
Plaque acid initially dissolves the calcium hydroxyapatite crystals at prism and crystal surfaces, which are rich in carbonate [2]. While the lesion front advances relatively quickly, the lesion surface appears to remain intact due to reprecipitation of dissolved crystals facilitated by high surface levels of fluoride [12–14].
Histological studies of caries lesions reveal pores, which progressively increase in size until mechanical breakdown occurs. These are visible as a series of zones (Fig. 2a) [12]. Initial dissolution of crystal surfaces produces the translucent zone, with ~1 % mineral loss (Fig. 2b). This is succeeded by the dark zone [15] with larger pores and ~5 % mineral loss. The dark appearance is due to the additional generation of much smaller pores, which do not admit media such as quinoline, which has the same refractive index as apatite crystals. Importantly this indicates some closing up of existing pores by regrowth of existing crystals and new crystal precipitation, i.e. remineralisation. Some accumulation of organic material has also been reported. This remineralisation, via calcium and phosphate from saliva, is facilitated by the massive loss of carbonate and magnesium, which are crystal growth inhibitors because of their destabilising effect on apatite crystals and an increase in the crystal growth promoter fluoride. The subsequently formed body of the lesion with even larger pores (20–30 % mineral loss) represents continued dissolution of even the reprecipitated material as more acid penetrates from the plaque [13].
Pathophysiology of dental caries. (a) Overview image of an affected tooth indicating the section shown in (b). (b) Section through an interproximal (white spot) caries lesion of enamel showing histological zones of the lesion. (c) Schematic drawing of pore structure and changes in crystal appearance, due to dissolution, at each stage with the earliest stage to the right: translucent zone (1 % mineral loss) through the dark zone (5 % mineral loss) to the body of the lesion (20–50 % mineral loss). Pore size increases at each stage with additional small pores indicating crystal regrowth/remineralisation in the dark zone indicated by darker areas, later dissolved in the lesion body. (d) Graphical illustration of chemical changes to enamel crystals (hexagons) at each stage of lesion development (from right to left). Intact prisms and crystals are present in healthy teeth (sound enamel). Large, selective loss of carbonate and magnesium ions and increase in fluoride occurs during the first stage (translucent zone), where dissolution of prism boundaries and crystal surfaces starts. Subsequent reprecipitation of dissolved mineral occurs in the dark zone (dark areas in c). Dissolution continues even of this stable reprecipitated mineral (lesion body) as pH falls towards the plaque biofilm on the enamel surface. The surface zone contains a range of poorly defined/bulky calcium phosphate materials. (e) Ionic alterations in different zones of a caries lesion showing relative changes of important ions and conditions (arbitrary units). Whereas the fluoride content is relatively low in sound enamel, it continuously rises along the lesion progression, with a steep rise during the body and highest concentration on the enamel surface. Calcium and phosphate are also high at the surface and in sound enamel. Their minimal concentration can be found in the body of the lesion. Carbonate and magnesium ions are maximal in sound enamel and drop continuously towards the surface. Similarly, pH is highest in sound enamel and continuously drops towards the surface [13]
Demineralisation and remineralisation can thus occur at the same time in the same lesion depending on concentration gradients of fluoride, carbonate, magnesium and pH [13].
Treatment and Implications for Patients
Earliest carious changes involve chemical alterations, which are not normally visible on the tooth surface. As the disease progresses, caries is most often seen as a white spot on the tooth surface resulting from porosity generating light scatter. This may progress to greater mineral loss which can be detected radiographically.
Therapeutic treatment falls into two areas: first and primarily reconstituting tooth material (by supplementing mineral ions) and second acid reduction (by antibacterial treatment to reduce the pH gradient). Acid production can be inhibited by broad-spectrum antibacterial agents in toothpastes and mouthwashes. These include chlorhexidine [16], a bisbiguanide, which disrupts cell membranes and triclosan [17], a polychlorophenoxyphenol, which inhibits fatty acid synthesis. This approach is effective, but repeated application to continuously renewing plaque raises issues of bacterial resistance and the possibility of long-term effects on oral soft tissues. Inclusion of buffers in toothpastes and mouthwashes to correct pH has a limited effect due to their relatively short half-life in the mouth.
Mineral ions can be supplemented to the lesion site to promote remineralisation [18]. Fluoride in particular is remarkably effective, reducing the disease by 50–70 %. Originally thought to increase enamel resistance via incorporation into crystals during development, later emphasis shifted towards a topical remineralising effect on the lesion itself [19]. Fluoride can be supplied to the tooth surface via the diet, drops or tablets entering the saliva directly or via the bloodstream. Topical application via toothpastes and mouthwashes is also effective [20, 21]. Why continuous supply of fluoride is necessary is not perfectly understood but is likely related to maintenance of a concentration gradient into the lesion through the plaque biofilm [22]. Fluoride inhibition of plaque acid production may also contribute. More generally, removal of biofilm by flossing and toothbrushing twice daily for about 2 min is also effective by removing acid generating bacteria and lowering the overall bacterial load.
Influence of Treatment on Metabolism
The antibacterials described above are not retained in the mouth except in small amounts and usually for limited periods. Significant effects on general metabolism have so far not been reported. Treatment with fluoride results in increased acid resistance of the enamel by substitution of fluoride for hydroxyl ions in the enamel apatite crystals. Fluoride also stimulates regrowth of acid damaged crystals and redeposition of new crystals enriched in fluoride and depleted in carbonate and magnesium [13].
Continuous availability of topical fluoride at the levels currently used has not resulted in discernible side effects on oral or indeed any other tissue. However, ingested fluoride in excessive amounts during tooth development can result in tissue changes [23]. Up to ~1 ppm in the water supply produces few observable effects. Above this level, crystal development is impaired and porosity in enamel results, which increases in severity with fluoride concentration. In extreme cases bone can be affected. Several mechanisms may operate here, including increased apatite-protein binding to crystal growth sites limiting crystal growth [23] and more recently alterations in cytoskeletal behaviour in the enamel forming ameloblasts [24]. This may inhibit removal of proteins and/or access of calcium phosphate to the growing crystals. Several guidelines have been published with regard to optimal dietary fluoride levels [25].
Perspectives
The most effective current anticaries therapy is fluoride. Administration via the drinking water is extremely effective and is relatively inexpensive. However, interpreted as mass medication and with the risk of fluorotic changes, this route is still somewhat controversial. Topical oral application remains the most effective and acceptable treatment but depends on individual compliance. More recently, attempts have been made to encourage remineralisation by supplying additional calcium to tooth surfaces using a calcium-protein complex and via administration of peptides, which nucleate the deposition of new hydroxyapatite crystals. New approaches to diagnosing very early chemical changes in the enamel surface, driving fluoride deep into the lesion more effectively together with disruption of plaque and specific targeting of bacterial acid production seem the most promising way forward.
References
Oral health means more than just good teeth. www.oralhealthplatform.eu. As available on 1 Aug 2013
Johansen E (1965) Comparison of the ultrastructure and chemical composition of sound and carious enamel from human permanent tooth. In: Stack MV, Fearnhead RW (eds) Tooth enamel, its composition, properties, and fundamental structure. John Wright & Sons Ltd., Bristol, pp 177–181
Boyde A (1989) Enamel. In: Oksche A, Vollrath L (eds) Handbook of microscopic anatomy, vol V/6, Teeth. Springer, Berlin, p 309
Kay MI, Young RA, Posner AS (1964) Crystal structure of hydroxyapatite. Nature 204:1050–1052
Elliott JC, Holcomb DW, Young RA (1985) Infrared determination of the degree of substitution of hydroxyl by carbonate ions in human dental enamel. Calcif Tissue Int 37:372–375
Terpstra RA, Driessens FCM (1986) Magnesium in tooth enamel and synthetic apatites. Calcif Tissue Int 39:348–354
Moreno EC, Kresak M, Zahradnik RT (1974) Fluoridated hydroxyapatite, solubility and caries formation. Nature 247:64–65
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhurst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43:5721
Van Nieuw Amerongen A, Bolscher JG, Veerman EC (2004) Salivary proteins: protective and diagnostic value in cariology? Caries Res 38:247–253
Wood SR, Kirkham J, Marsh PD, Shore RC, Nattress B, Robinson C (2000) Architecture of intact natural human plaque biofilms studied by confocal laser scanning microscopy. J Dent Res 79:21–27
Kleinberg I (2002) A mixed – bacterial ecological approach to understanding the role of the oral bacteria in caries causation: an alternative to the Streptococcus mutans and specific plaque hypothesis. Crit Rev Oral Biol Med 13:108–125
Darling AI (1961) The selective attack of caries on the dental enamel. Ann R Coll Surg Engl 29:354–369
Robinson C, Kirkham J, Shore RC, Brookes SJ, Wood SR, Strafford SM (2000) The chemistry of enamel caries. Crit Rev Oral Biol Med 11:481–495
Weatherell JA, Deutsch D, Robinson C, Hallsworth AS (1977) Assimilation of fluoride by enamel through the life of the tooth. Caries Res 11(Suppl 1):85–115
Silverstone LM (1967) Observations on the dark zone in early enamel caries and in artificial caries-like lesions. Caries Res 1:261–274
Hope CK, Wilson M (2004) Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob Agents Chemother 48:1461–1468
van Loveren C, Buijs JF, Ten Cate JM (2000) The effect of triclosan toothpaste on enamel demineralisation in a bacterial demineralisation model. J Antimicrob Chemother 45:153–158
Pearce EI, Schamschula RG, Cooper MH (1983) Increases in fluoride, calcium and phosphate in dental plaque resulting from the use of a mineralising mouthwash containing urea and monofluorophosphate. J Dent Res 62:818–820
Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC (2010) New approaches to enhanced remineralisation of tooth enamel l. J Dent Res 89:1187–1197
Featherstone JD (1999) Prevention and reversal of dental caries: role of low level fluoride. Community Dent Oral Epidemiol 27:31–40
Marinho VC, Higgins JP, Sheiham A, Logan S (2003) Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev (1):CD002278
Watson PS, Pontefract HA, Devine DA, Shore RC, Nattress BR, Kirkham J, Robinson C (2005) Penetration of fluoride into natural plaque biofilms. J Dent Res 84:451–455
Robinson C, Connell S, Kirkham J, Brookes SJ, Shore RC, Smith AM (2004) The effect of fluoride on the developing tooth. Caries Res 38:268–276
Li Y, Decker S, Yuan ZA, DenBesten PK, Aragon MA, Jordan-Sciutto K, Abrams WR, Huh J, McDonald C, Chen E, MacDougall M, Gibson CW (2005) Effects of fluoride on the actin cytoskeleton of murine ameloblasts. Arch Oral Biol 50:681–688
Fawell J, Bailey K, Chikton E, Dahi E, Fewtrell L, Magara Y (2006) Fluoride in drinking water. WHO, IWA Publishing, London, Seattle
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Wien
About this chapter
Cite this chapter
Robinson, C. (2014). Dental Caries. In: Lammert, E., Zeeb, M. (eds) Metabolism of Human Diseases. Springer, Vienna. https://doi.org/10.1007/978-3-7091-0715-7_15
Download citation
DOI: https://doi.org/10.1007/978-3-7091-0715-7_15
Published:
Publisher Name: Springer, Vienna
Print ISBN: 978-3-7091-0714-0
Online ISBN: 978-3-7091-0715-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)