Abstract
Overall survival from childhood malignancies has dramatically improved, with survival rates now reaching over 70 %. Although improvements in radiotherapy and surgery have reduced the late sequelae of curative therapy, chemotherapy still remains the mainstay of treatment for most childhood cancers. Nevertheless, some types of childhood cancer remain a difficult challenge, and for those who survive the burden of late effects can be considerable. The current paradigm for new cancer therapies is to increase our knowledge of the molecular basis of carcinogenesis, followed by the development of cancer-cell specific therapies. During the past 10 years, initiatives have been undertaken by paediatric oncologists to further promote the clinical evaluation of new anti cancer compounds in children within national academic paediatric groups. Through proper evaluation in collaborative clinical trials we will learn how best to use these new therapeutic approaches and improve the survival rates and reduce toxicity for children with cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Solid tumours
- Children
- Staging
- Treatment
- Chemotherapy
- Cytotoxic drugs
- Surgery
- Resistance
- Toxicity
- Targeted treatment
Introduction
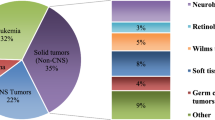
Children’s cancers are rare and account for 1 % of all malignancies. Within Europe this represents some 12,000 new cases each year, with approximately 1,600 per year in the United Kingdom. In the UK, 1 in every 600 children under 15 years of age develop cancer. Although rare, childhood cancer is the second commonest cause of death in children between 1 and 14 years of age. These cancers are quite different from cancers affecting adults. Most adult tumours are carcinomas and are usually classified by their site of origin, whereas paediatric tumours occur in different parts of the body, look different under the microscope and are classified by histological subtypes. Tumour types that are common to both adults and children, such as lymphomas and leukaemia, differ in their biology, behaviour and prognosis and hence demand different treatment. They also respond differently to treatment. Some embryonal tumours presenting in infancy undergo spontaneous remission or maturation (e.g., Stage IVS neuroblastoma).
Survival rates for childhood cancer have improved dramatically over the last 20 years, such that approximately 70 % of children can expect to become long-term survivors [1, 2]. This is reflected by the fact that today, 1 in 750 of the young adult population is now a survivor of childhood cancer. Treatments used to achieve this success are surgery, chemotherapy and/or radiotherapy. Factors contributing to these improved survival rates are: the development of dedicated paediatric oncology centres, advances in surgical techniques, novel chemotherapy agents and regimens, targeted radiotherapy and improvements in supportive care (early treatment of febrile neutropenia, better intensive care, improved transfusion services).
Surgery was the mainstay of treatment of solid tumours in children before the advent of effective chemotherapy. Cure could be obtained by surgery alone in the proportion of children with localised disease, and good palliation obtained in many others, and the surgeon was often the key clinician in the management of paediatric solid tumours. However, very few tumours present as a purely localised surgical problem. The surgeon becomes part of a larger team, needing to integrate surgical procedures with chemotherapy and/or radiotherapy. Although improvements in radiotherapy and surgery have reduced the late sequelae of curative therapy, chemotherapy now remains the mainstay of treatment for most childhood cancers. This chapter aims to discuss the factors which affect the way the paediatric surgeon interacts with a multidisciplinary team of experts, including the paediatric oncologist, radiologist, pathologist and radiotherapist. The best outcome will be achieved by collaboration of interested specialists clearly understanding the efficacies and limitations of various forms of treatment.
Although complete tumour resection is of paramount importance for cure, most paediatric cancers are advanced at presentation (e.g., 55–60 % sarcomas are High Risk at diagnosis, 25 % of Bone tumours are metastatic at diagnosis, 90 % of Neuroblastomas occurring after infancy are stage IV) and require systemic treatment. The prognosis for malignant solid tumours has improved since the introduction of effective chemotherapy capable of reducing the tumour volume and making previously unresectable tumours resectable. The operation also becomes safer and easier after pre-operative chemotherapy. Furthermore, there is no delay in treating metastatic disease, which is detectable at diagnosis in a significant proportion of patients.
Some diseases, such as osteosarcoma, cannot be cured except with surgery to remove the local tumour, whereas in others such as lymphoma, biopsy followed by chemotherapy is all that is needed. In others, such as Ewing’s sarcoma and rhabdomyosarcoma, the best treatment results may be obtained with systemic chemotherapy and a combination of surgery and/or radiotherapy for local control. In Europe, since the early 1990s, the concept of pre-operative chemotherapy and delayed surgery for solid tumours of childhood became standard clinical practice due to successful Wilms’ tumour trials of the SIOP (International Society of Paediatric Oncology) Group [3–5].
Children presenting with malignant diseases other than leukaemia often present with palpable masses and are usually seen first by a surgeon. Except in emergencies, a thorough consideration of the possible differential diagnosis should be made before any surgical procedures are undertaken. This should ideally be done in discussion with the paediatric oncology team. Any necessary pre-surgical staging or investigations can then be planned, depending on the nature of the suspected lesion and the facilities available. Biopsy should ideally be performed in the regional specialist centres, where the necessary support services are available (e.g., molecular biology services) and once radiological examination of the lesion is complete. If appropriate, a number of interventions (such as bone marrow aspiration/trephine for staging) can be carried out while the child is anaesthetised for biopsy/surgery.
In nearly all cases of malignancy, diagnosis must be confirmed by biopsy of the primary tumour. Traditionally, tumour material would be obtained by incisional or excisional biopsy at open operation, but advances in imaging techniques have led to much greater use of trucut biopsies obtained with ultrasound or computerised termography (CT) guidance. In a tumour with obvious heterogeneity on initial imaging, open biopsy may still be preferable, to ensure that a representative sample is obtained. Biopsy sites must be within potential radiation fields, as malignant cells may seed along the biopsy track. In rare instances, a combination of radiological and biochemical or molecular biological findings may enable a definitive diagnosis to be made without biopsy, e.g., a tumour in the characteristic site, such as the anterior mediastinum or pineal region, with high alphafetoprotein (AFP) levels in the blood can be confidently diagnosed as a germ cell tumour and a heterogeneous abdominal mass with calcification, raised urinary catecholamines and infiltration of the bone marrow, is a neuroblastoma. However, failure to obtain tissue makes it impossible to acquire important information regarding the biological and genetic characteristics of the tumour that often determine the risk factors affecting therapeutic decisions. Although the overall cure rate for childhood tumours is now around 70 %, it is only by increased understanding at the biological level that further progress will be made, particularly in an appropriate risk stratification of current intensive treatments and in the development of novel therapies. With increased survival rates for childhood cancer, philosophy of treatment has changed over the years from ‘Cure at any cost’ to ‘Cure at least possible cost’.
Staging
Once the diagnosis has been confirmed, the extent of the tumour (size, position, relationship to surrounding structures, appearance of lymph nodes) must be established. Unfortunately, there is no single uniform staging approach for childhood malignancies and the surgeon will need to be aware of the requirements for staging of each tumour type according to the current protocols (see Table 9.1).
-
The staging of disease directs the treatment given and should help to avoid excessive therapy: in easily curable conditions excessive therapy is known to put the child at increased risk of adverse late effects of treatment.
-
The stage of the disease also tends to reflect the prognosis and, consequently, aids counselling of the family.
-
Staging systems generally progress from localised disease (stage I) to widespread disease (stage IV) and are based on the results obtained from clinical examination, radiology and pathology.
More extensive tissue sampling and biopsy is usually only needed at the time of definitive operation. This information will determine what type of further treatment is required post-operatively. For example, in the current SIOP Wilms’ tumour trial, pathologists make precise evaluation of the stage of the disease post nephrectomy. Children are then risk stratefied and treated according to different therapy, depending on tumour histological subtype and stage of disease.
Increasingly, the chemotherapy response of the primary tumour in the post-surgical specimen is used in deciding post-operative treatment for a number of malignant solid tumour (e.g., in osteosarcoma and Ewing’s Sarcoma <90 % necrosis of the tumour is considered a poor response and these patients are now randomised to receive more intensive treatment to improve the chances of long-term survival.
Intra-operative photography or clear diagrams can be very helpful to the radiotherapist and, even in the era of three-dimensional imaging, a description of the tumour in relation to fixed anatomical points is also useful. The use of titanium clips is valuable to delineate tumour margins and does not affect subsequent imaging.
Although chemotherapy is needed for nearly all tumours in childhood and is often given before definitive surgery, primary surgical excision is still indicated for a number of malignancies. These include stage I testicular tumours, where no further treatment is needed if an associated raised AFP titre falls to normal with and expected half-life of 2.7 days post-operatively, stage I or II neuroblastoma (abdominal or thoracic), some adult-type soft tissue sarcomas, most brain tumours such as astrocytomas and medulloblastomas.
Debulking of tumours are rarely indicated as primary surgical procedures, except for some brain tumours. In particular, they confirm no advantage in the treatment of lymphoma, which may present with widespread intra-abdominal disease, although surgery may be necessary if chemotherapy results in a complication such as perforation or bleeding, or if the patient presents with intestinal obstruction. It is important that the surgeon is then as conservative as possible in his approach, since the chance of complete remission of disease following chemotherapy is high and surgery, performed at any stage in the disease does not lead to improved cure rates.
Emergency operations are unavoidable for intussusceptions, torsion of the tumour, perforation and some rapid enlargement due to intra-tumoural bleeding, cystic degeneration or necrosis.
Insertion of central venous catheter is probably the single most frequent operation that paediatric surgeons perform while caring for a child with malignancy. Centrally placed, long-term venous catheters are used for the administration of chemotherapy, antibiotics and for blood sampling. Central venous catheters make the care of the child easier, both for the child and for the medical team. Currently there are two main types of catheters used in clinical practice – tunnelled, external catheters (Hickman line, Broviac line, Groshong catheters) and totally implanted access devices such as a portocath. External, tunnel catheters are generally easier to access, are less expensive than portocaths, offer less risk of extravasation into subcutaneous tissue, allow more rapid infusions and can be removed easily at the end of treatment. However, the portocath offers an improved cosmetic result, less restriction in normal activities, less maintenance care and they are well protected, thus decreasing the chance of damage and are associated with a lower risk of infection. Numerous methods of catheter care, flushing, are practised in various paediatric oncology centres and none have proved superior when the literature is taken as a whole.
In addition to the insertion of central venous lines, diagnostic biopsies and resection of individual tumours, the surgeon has a role in facilitating treatment given by other members of the oncology team, i.e., insertion of a mesh to displace the bowel out of the future field of radiation or insertion of pain control devices and surgical exposure for brachietherapy.
Furthermore, a surgeon also has a role in providing enteral access in patients receiving intensive chemotherapy. Children with cancer often have associated cachexia, with significant weight loss and malnutrition. The intensity and type of primary therapy (chemotherapy, surgery and/or radiotherapy) is associated with decline in the nutritional status. Furthermore, patients receiving intensive chemotherapy have prolonged illnesses – mucositis, diarrhoea, sub-optimal dietary intake and decreased appetite – all are side effects of chemotherapy that contribute to further weight loss. Numerous studies have demonstrated that a nutritionally-repleted patient tolerates therapy better and with fewer complications [6–8]. In addition to providing nutritional requirements, gastrostomy tubes can perform other functions. Clinical experience has demonstrated that gastrostomy tubes are an effective way to delivery medications and to provide hydration to children experiencing excessive emesis. The quality of life of both the child and family also appears to improve, as eating is a frequent source of conflict between the child and parents. Providing nutrition through a gastrostomy tube alleviates the frustration associated with forced feeding of the child via the mouth. Maintenance of normal patient nutrition throughout cancer treatment allows normal growth and improves quality of life.
In many cases of solid tumours, surgical excision of primary tumour is the preferred local treatment since radiotherapy has a much greater risk of long-term sequelae. The general principles of underlined choice of local treatment are that surgical excision is the treatment of choice where: (1) complete excision is possible and results in improved survival and cure; (2) it will give functional and cosmetic results better than those obtained by other treatment.
Surgeons may also be consulted to deal with complications related to other forms of treatment: extravasation of chemotherapy agents causing tissue necrosis, typhilits (neutropenic enterocolitis), intestinal perforation, strictures or avascular necrosis or other damage due to late effects of radiotherapy.
Surgical decisions, as well as those concerning chemotherapy, radiotherapy and overall treatment strategies are best made after joint discussion, which is facilitated by a formal system of consultations such as regular multi-disciplinary oncology team meetings (Tumour Board), as well as maintaining communication between the key team members during the treatment.
In the United Kingdom, more than 80 % of children with malignant disease are registered with the United Kingdom Children’s Cancer Leukaemia Group (UKCCLG) (Table 9.2) [9] and are treated according to agreed tumour protocols. Although there are approximately 1,600 cases of childhood cancer diagnosed in the UK annually, when broken down into individual tumour types, the numbers even for the commonest childhood tumours, are often too small to ensure that clinical trials can be completed satisfactorily at a national level. It is for this reason that the majority of the Phase III clinical trials in childhood cancer are now increasingly conducted at an international or collaborative basis (see Table 9.3). The power of such collaboration is the ability to conduct large trials with rapid accrual, which would allow the investigation of new agents to be undertaken quickly and effectively and thus be able to answer more rapidly some still unanswered questions regarding the treatment of children with malignant tumours. Active participation of all the interested clinicians treating childhood cancer in a group such as the UKCCLG or SIOP is therefore essential to keep up to date with the various protocols/clinical trials, which in turn will continue to improve the outcome of childhood cancer.
Chemotherapy
The effective use of cancer chemotherapy requires a thorough understanding of principles of neoblastic cell growth kinetics, basic pharmacologic mechanisms of drug action and pharmaco-kinetic and pharmaco-dynamic variability. Development of selective, highly effective therapy for cancer has been hindered by lack of understanding of the molecular mechanisms, malignant transformation and denovo or acquired drug resistance. In spite of scientific advances in the field of molecular oncology, information remains incomplete, therefore therapy continues to be largely empiric.
The Cell Cycle and Tumour Growth Kinetics
The growth pattern of individual neoplastic cells may greatly affect the overall biological behaviour of human tumours and their responses to specific types of cancer therapy. Tumour cells can be subdivided into three general populations: (1) cells that are not dividing and are terminally differentiated; (2) cells that continue to proliferate; and (3) nondividing cells that are currently quiescent but may be recruited into the cell cycle. The kinetic behaviour of dividing cells is best described by the concept of the cell cycle.
The cell cycle is composed of four distinct phases during which the cell prepares for and undergoes mitosis. The G1 phase consists of cells that have recently completed division and are committed to continued proliferation. After a variable period of time, these cells begin to synthesise DNA, marking the beginning of the S phase. After DNA synthesis is complete, the end of the S phase is followed by the premitotic rest interval called the G2 phase. Finally, chromosome condensation occurs and the cells divide during the mitotic M phase. Resting diploid cells that are not actively dividing are described as being in the G0 phase. The transition between cell cycle phases is strictly regulated by specific signalling proteins; however, these cell cycle checkpoints may become aberrant in some tumour types.
The most common anti-cancer drugs are cytotoxic agents which are cell poisons that act indiscriminately on most cells, either causing direct damage to DNA or inhibiting cell replication. The mechanism of action of most current anti-cancer drugs are non-selective and target vital micro-molecules (e.g., nucleic acid) or metabolic pathways that are critical to malignant and normal cells. The molecular basis of cytotoxic-induced cell death is the subject of considerable interest, and it is becoming clear that one of the important common pathway is that of programmed cell death or apoptosis [10].
Cancer chemotherapy relies on exploiting the therapeutic index – the ratio of cell killing in the malignant cell population compared with killing of normal cells. Mechanisms for recovery from damage are generally more efficient in normal cells than in their malignant counterparts and, if time is allowed between courses of treatment for this recovery to occur, malignant cells can be differentially killed by repeated courses of chemotherapy.
In the clinical development of anti-cancer drugs, the initial dose finding trials (phase I), and subsequent studies to define the spectrum of activity of a new agent (phase II) employ an empirical methodology. Phase I trials can be seen as toxicity-screening studies where a new drug is administered for the first time to humans in order to determine the maximum tolerated dose. There are usually two aims of the phase I trial – to establish the optimal dose to be used in the phase II trial for drug efficacy, and to determine the type and degree of toxicity (adverse effects) associated with the drug. In phase II trials, the response is evaluated in patients with different forms of cancer to determine which tumours the drug may have activity against. The end points of such trials are the response rate and toxicity. After a drug is found to have some activity in phase II trials, the next step is to determine its relative efficacy in a larger phase III trial, where the drug is compared – either alone or in combination – with other drugs, i.e., to a control group, usually the best available treatment, or a historical control. Most UKCCLG trials are phase III, comparing patients on a new treatment versus standard treatment, to try and establish whether new treatment is better than standard treatment. The dose and schedule of the anti-cancer drugs are empirically based. All patients receive the same fixed dose of drugs, adjusted for body weight or surface area, with subsequent dose or schedule modifications based only on ensuing toxicities, rather than on achieving a therapeutic plasma drug concentration. Commonly used cytotoxic agents and their metabolism use and side effects are listed in Table 9.4. Despite various limitations, several principles of cancer chemotherapy have evolved from clinical experience, including the use of multi-drug combination chemotherapy regimens, the administration of chemotherapy before the development of clinically evident metastatic disease (adjuvant chemotherapy) and administration of drugs in maximally tolerated doses (dose intensity).
Combination Chemotherapy
Multi-agent therapy has three important theoretical advantages over single-agent therapy. Firstly, it maximises the cell kill, while minimising host toxicities by using agents with non overlapping dose-limiting toxicities. Secondly, it may increase the range of drug activity against tumour cells with endogenous resistance to specific types of therapy. Finally, it may also prevent or slow the development of newly resistant tumour cells. Specific principles for selecting agents for use in combination chemotherapy regimens are listed in Table 9.5 [11].
Adjuvant Chemotherapy
The aim of adjuvant chemotherapy is to prevent metastatic recurrence by eliminating micro-metastatic tumour deposits in the lungs, bone, bone marrow or other sites at the time of diagnosis. Adjuvant chemotherapy has been demonstrated to be efficacious for most of the common paediatric cancers, including Wilms’ tumour, Ewing’s sarcoma, osteosarcoma and rhabdomyosarcoma. Adjuvant chemotherapy should be given as soon as possible after definitive local therapy. A delay to allow for recovery from surgery or radiation therapy may compromise the chance of curing the patient.
Increasingly, chemotherapy is now used in a neo-adjuvant setting (before the definitive treatment) in paediatric solid tumours as chemotherapy shrinks the tumour and the operation becomes safer and easier. Neo-adjuvant chemotherapy also provides earlier set treatment for micro-metastases.
Dose Intensity
Most anti-cancer drugs have a steep dose response curve, and a small increment in the dose can significantly enhance the therapeutic effect of the drug. The maximum tolerated dose of the drug combination should be given as frequently as possible to achieve optimal cell kill at a time when the size of the drug-resistant population is limited. Methods for maximising dose intensity include: greater physician and patient willingness to tolerate drug toxicities, more aggressive supportive care, selective rescue of the patient from toxicity such as with peripheral stem cell transplantation or the administration of colony-stimulating factors such as G-CSF, use of regional chemotherapy (intra-arterial, intrathecal delivery) to achieve high drug concentrations at local tumour sites and the development of new treatment schedules such as long-term continuous infusions that may allow more drugs to be administered over a given period.
Whatever the final pathway of cell death, there remains a correlation between sensitivity to anti-cancer drugs and the stage of the cell cycle at the time of drug exposure. During the S-phase most agents are effective, in contrast to the G0-resting phase, during which most tumour cells will be chemo-resistant. Anti-cancer drugs can be classified on the basis of the cell cycle phase during which they are more effective. For example, Nitrogen mustard, alkylating agents and gamma radiation are non-cycle-specific, being effective at most phases of the cell cycle and in some cases including the G0 population. Phase specific agents include Vinblastine and Vincristine, which are active during the mitotic phase; Etoposide and Tenoposide effective during the G2 pre-mitotic phase; and Methotrexate, 6-Mercaptopurine and Cytosine Arabinoside effective during the S-phase. Agents that are cycle but not phase specific include 5-Fluorouracil, Actinomycin D and Doxorubicin (Table 9.6, Fig. 9.1).
All cytotoxic drugs produce DNA damage but by different mechanisms. Alkylating agents induce arrest of DNA transcription regulation. Antimetabolites produce DNA injury by inhibiting thymidine synthetase, or blocking purine synthesis or DNA repair. Anthracyclines produce intercalation (cross-linking) between strands of DNA, may generate free radicals or interact with DNA-modifying enzymes. The final common pathway of cytotoxic induced cell death is apoptosis but, by exploiting these different mechanisms of damage, greater tumour cell kill can be achieved. One of the main reasons why chemotherapy may fail to kill all tumour cells is that clones of ‘resistant’ cells may develop. Tumour resistance is related to a genetic event in the cell – a mutation, gene amplification, deletion or chromosome translocation which may affect drug transport, intracellular drug activation or efflux from the cell.
Three genes have so far been implicated in multiple drug resistance (MDR) – IP glycoprotein, which acts as a transmembrane pump to reduce the intracellular drug concentration, multiple drug resistance-associated protein gene (MRP) which is related to non p-glycoprotein mediated resistance, and DNA topoisomerase II mutations which affect DNA conformation [12, 13].
Irrespective of the precise mechanism of drug resistance, it has been suggested by Goldie – Coldman hypothesis [14] that there is a high likelihood of drug resistant mutants at the time of initial diagnosis and that two important considerations must therefore be taken into account in protocol design. Firstly, the earliest use of non-cross resistant drugs (combination chemotherapy), and secondly the maximum tolerated dose of the drug combinations should be given as frequently as possible to achieve optimum cell kill, at a time when the size of the drug resistant population and number of mechanisms are limited.
High-dose chemotherapy offers a strategy for overcoming multiple drug resistance and is feasible as long as bone marrow suppression, which can be overcome by bone marrow or peripheral stem cell rescue, is the only dose limiting toxicity.
In adults, local perfusion of cytotoxic agents has been attempted in a number of situations. Isolated limb perfusion has achieved some success as a treatment for melanoma, but this tumour is extremely uncommon in children. Hepatic artery infusion has been used to treat liver metastases, particularly from colonic cancers in adults. Again the usefulness of this approach is limited in children where systemic therapy is needed for most tumours because of the pattern of tumour spread. It may have some place in the treatment of hepatoblastoma. Randomised trials comparing regional perfusion with systemic therapy have not shown a specific advantage for localized therapy.
For solid tumours, chemotherapy has two main goals – to eliminate overt metastases or microscopic spread, and to destroy or reduce the primary tumour mass so that, with or without further local treatment, complete response (CR) can be obtained. Without complete response, cure will never be possible. For the purposes of comparison in trials, different categories of Response criteria are used to assess the effectiveness of systemic treatment (Table 9.7).
Management of Side Effects of Chemotherapy
Acute Complications
Early complications include metabolic disorders (tumour lysis syndrome), bone marrow suppression, immunosuppression, nausea and vomiting.
Tumour Lysis Syndrome
Patients with large tumour burden may have substantial breakdown of tumour cells following the start of treatment and renal function may be impaired from uric acid nephropathy. This problem is seen most often in haematological malignancies, but can occur in solid tumours (Burkitt’s lymphoma, germ cell tumours, metastatic neuroblastoma). Before initiating treatment for these malignancies, renal function should be measured, adequate hydration should be ensured and Allopurinol (xanthine-oxidase inhibitor) should be given. In patients with very high risk of tumour lysis (bulky disease, high white cell count in acute leukaemia, high lactate dehydrogenase (LDH) and uric acid, those presenting with oliguria) Rasburicase (urate oxidase inhibitor) should be used to avoid tumour lysis syndrome. In the tumour lysis syndrome the phosphates and potassium are released into the circulation from cells that are lysed by chemotherapy, leading to hyperkalaemia, hyperphosphataemia, hypocalcaemia. It is prudent to inform the renal team as the treatment is initiated in these high risk cases. It is also important to remember that there other causes of renal failure (obstruction of urinary tract, sepsis, fluid shifts) apart from tumour lysis in these patients.
Bone Marrow Suppression
Tumours that invade the bone marrow can cause pancytopenia. The majority of chemotherapy drugs produce myelosuppression. Anaemia can be corrected by transfusions of packed red cells and thrombocytopenia by platelet transfusions. Neutropenia (ANC <0.5 × 109/l) poses a significant risk of life-threatening infection. Febrile neutropenia patients should be hospitalised and treated as an emergency with empirical broad spectrum intravenous antibiotics pending the results of appropriate cultures. Treatment should be continued until the fever resolves or neutrophil count rises. If there is no response to antibiotics, antifungal or antiviral drugs may be required. Fever may be related to sepsis from indwelling central venous line requiring its removal. Bone marrow recovery may be facilitated by the use of G-CSF (granulocyte colony stimulating factor).
Infection
Opportunistic infections with pneumocystis carinii can produce fatal interstitial pneumonitis and prophylaxis with Trimethoprim/Sulfa-methoxazole is recommended where severe immunosuppression is anticipated from chemotherapy.
Children receiving chemotherapy and exposed to chicken pox contact require zoster immunoglobulin and if clinical disease develops, they require hospitalisation and treatment with intravenous high dose Aciclovir.
Nausea and Vomiting
This is often the most troubling side-effect from the patient’s point of view and should be treated effectively from the first course of chemotherapy. Protocols containing Cisplatin, Actinomycin-D and Cyclophosphamide or Ifosfamide are associated with the highest incidence of vomiting, but sickness is also a problem with Procarbazine, Adriamycin® (Doxorubicin), Daunorubicin and Carboplatin. The new 5-hydroxytryptamine (5-HT3) antagonists such as Ondansetron and Granisetron act centrally in the chemo-receptor trigger zone in the brain and are effective in preventing vomiting with most agents. These drugs are given intravenously at the time of chemotherapy and orally for 5 days until the gastrointestinal side-effects resolve. Dexamethasone is often added, also for 5 days, and is effective, though its mechanism of action is uncertain. Additional sedation, and relative amnesia, can be obtained by including Benzodiazepine such as Lorazepam in the anti-emetic regimen. If emesis is less severe, Domperidone, Prochlorperazine, Chlorpromazine or Metoclopramide have been used but are less effective and may have troublesome side-effects.
Malnutrition/Mucositis
This is a particular risk in patients receiving intensive chemotherapy, radiotherapy to the abdomen or head and neck. As these treatments cause mucositis, careful oral hygiene is important during this phase. If oral or enteral intake is inadequate then patients may require intravenous fluid and electrolyte supplementation or total parenteral nutrition. Intravenous opiate analgesia may also be required at this time.
Late Effects
Successful treatment of childhood cancer with multi-agent chemotherapy in combination with surgery or radiotherapy causes significant morbidity in later life [15]. Successful surgical resection may require the loss of important functional structures. Radiotherapy can produce irreversible organ damage with symptoms and functional limitations depending on the organ involved and the severity of damage. Endocrine consultation regarding growth, sex maturation and thyroid function is necessary for any child who has received cranial or total body irradiation, or who has chemotherapy-induced ovarian or testicular damage.
Chemotherapy also carries the risk of severe organ damage. Of particular concerns are leucoencephalopathy after high dose Methotrexate therapy, myocardial damage from anthracyclines, pulmonary fibrosis after Bleomycin, sterility in patients treated with alkylating agents, hearing loss after Cisplatin chemotherapy and renal tubular damage from Ifosfamide. Patients must be closely monitored by obtaining baseline and sequential measurements during their treatment, wherever possible.
Psychosocial evaluation and educational support is often needed especially following treatment of brain tumours in children. Periods of physiological stress, for example pregnancy, may lead to overt expression of subclinical damage (e.g. heart failure after Adriamycin (doxorubicin) or foetal loss after uterine muscle irradiation). Long-term follow-up of all children treated for cancer is essential if we are to improve the cure rates and minimize harmful effects of treatment including the increased risk of second malignancy.
New Drugs for Children and Adolescents with Cancer
The overall cure rate for children diagnosed with cancer now approaches 80 % [16]. Although this means 1/5th of children will die of their disease and 40 % [17] of survivors are burdened by the late effects of therapy, this still represents one of the most remarkable improvements in outcome in modern medical history. Thirty years ago cure rates were <20 % and it is only 50 years ago that the outcome for children with cancer was so appalling that there were strong debates about the ethics of giving children chemotherapy at all.
As alluded to earlier in this chapter, part of this turnaround can be attributed to improvements in surgery, radiotherapy, and supportive care. As a consequence, it is now rare for children to die during their anticancer therapy. Much of the turnaround, however, has been as a direct consequence of a better use of standard chemotherapeutic drugs. It is unlikely that further improvements in cure rates will be achieved by modification of existing modalities of treatment. Novel compounds as well as novel approaches to treatment will be required to help children who are currently incurable.
The explosion of molecular biological knowledge and techniques coupled with a better understanding of host/tumor interactions has spurred on whole new areas of drug development. However, host/tumor inter-actions have long been recognized: In the 1800s, Coley, demonstrated tumor regression following infection in some of his patients [18]. Donor Lymphocyte Infusions (DLI) following allogenic transplantation is now standard hematological practice (see below) The drive for all new therapeutic interventions is to devise compounds that maximally target the tumor and minimize or avoid systemic side effects. New risk based algorithms will be needed to better define an individual’s response to therapy and maximize each child’s chance of cure.
The era of personalized medicine has been ushered in by a revolution in the understanding of tumour and host biology. Hanahan and Weinberg’s model of cancer at a cellular level [19] provides a framework that neatly encapsulates how new drug development has evolved. Each of their 6 hallmarks have been targeted by novel compounds (See Fig. 9.2).
Hanahan and Weinberg’s model of cancer at a cellular level provides a framework that neatly encapsulates how new drug development has evolved. Each of their six hallmarks have been targeted by novel compounds [19]
They have recently further clarified this model adding two further emerging hallmarks and two enabling characteristics of tumours which will allow for further sophistication in drug selection and trial design.[20] (See Fig. 9.3).
Hanahan and Weinberg’s model further clarified by adding two further emerging hallmarks and two enabling characteristics of tumours which will allow for further sophistication in drug selection and trial design [20]
Targeted Therapy
Improved understanding of cellular biology, including surface markers and intra cellular pathways has led to an explosion in targeted agents. There are over 800 compounds currently in development. Some target specific paediatric diseases but many more share common targets with adult tumours (See table 9.8) [21].
Those drugs sharing a common target which may be beneficial to children should be identified and fast tracked for development.
Examples of successful targeting are illustrated by the Tyrosine Kinase Inhibitors: tyrosine kinases act on act on pathways and biological systems that are responsible for many aspects of cell survival. These pathways are important in cellular proliferation, differentiation, motility, and apoptosis. There are two main classes of tyrosine kinases: transmembrane proteins and those found within the cell (see Fig. 9.4). Both have enzymatic properties under strict regulation so that cells that are not rapidly dividing have very low levels of tyrosyl phosphorolated protein [22]. The first successful clinical use of a tyrosine kinase inhibitor was imatinib in chronic myeloid leukemia (CML) [23]. This dramatic response accelerated research into tyrosine kinase inhibitors for solid tumors. The most successful use so far has been in adults with gastrointestinal stromal tumors (GISTs) where Imatinib has been used to target mutations in c-KIT. Preclinical data show expression of c-KIT and platelet-derived growth factors (PDGF) in other solid tumors. Many of these affect children and include glioblastoma, sarcomas, and chondromas.
Mechanisms of activation of normal TKs. A typical receptor TK [platelet-derived growth factor receptor β (PDGFR β)] and nonreceptor TK (c-ABL) are depicted, with the ATP- binding (ATP) and catalyic (Cat) lobes of the kinase domains and the transmembrane (TM) region of PDGFRβ indicated. Panel (a) shows both kinases in their inactive states. Inactive PDGFRβ is monomeric and unphosphorylated, and the catalytic domain is inhibited by protrusion of a regulatory tyrosine (Tyr) in the activation loop into the substrate cleft and by an intramolecular interaction with the juxtamembrane (JM) domain. Inactive c-ABL is associated with the membrane through a covalent N-terminal myristate group (Myr) and is inhibited through intramolecular interaction of the Src homology-3 (SH3) domain with an adjacent proline (Pro) residue and by direct interaction of the catalytic domain with an inhibitory membrane lipid, phosphatidylinositol-4,5-bisphosphate (PIP2). In Panel (b), PDGFRβ is activated upon binding of the ligand (dimeric platelet-derived growth factor (PDGF), which induces oligomerization of the receptor and intermolecular phosphorylation (P, in yellow) of the activation on-loop tyrosine. This leads to a conformational change in the catalytic domain and increased enzymatic activity, while phosphorylation of other tyrosines within the intracellular domain of the receptor creates binding sites for SH2 domain-containing signaling proteins, including c-SRC (red oval) and phospholipase Cγ (PLCγ) (green oval). c- ABL is activated through the phosphorylation of two regulatory tyrosines, one in the activation loop and the other near the SH3 binding site, which can be phosphorylated by another TK, such as c-SRC. In addition, activated PLCγ can hydrolyze and destroy the lipid inhibitor PIP2 (Further detail is provided in the review by Krause and Van Etten [38])
Other targets that may be inhibited by small molecules include endothelial and vascular endothelial growth factors (EGF/VEGF) (See Fig. 9.3) and once again evidence of expression has been found in cell lines in many pediatric tumors. Drugs targeting these pathways are currently undergoing Phase I and II trials in the pediatric setting.
Similarly Vismodegib, a sonic hedgehog pathway inhibitor has improved outcomes for adult patients with basal cell carcinomas [24]. Crizotonib, which targets the ALK pathway is effective in non-small-cell lung cancer [25]. In some ways the commonality of pathways such as IGF-1R [26], mTOR [27] and PARP [28] overcomes the hurdle imposed by the epidemiological differences between paediatric and adult, teenage and young adult and adult tumor types (Fig. 9.5) [16].
However different alterations in the same gene have been noted in different diseases, for example ALK is translocated in anaplastic large cell lymphoma, lung cancer and inflammatory myofibroblastic tumours but amplified or mutated in neuroblastoma. This may result in differing outcomes in different diseases despite targeting the same pathway. Children could be exposed to inevitable side effects with no benefit, or even worse, the disease could be driven at a molecular level rather than being inhibited. Functional validation and evidence of anti tumour activity in pre clinical models is vital before “first time in child” clinical trials.
Cell surface markers can also act as targets for drug therapy, however the 30 years since Kohler and Millstone’s landmark publication [29] describing, for the first time, a generation of humanized monoclonal antibodies from mice has been frustrating. This tantalizing paper opened the promise of “magic bullet” therapy. Unfortunately, this initial enthusiasm gave way to the harsh reality of drug development. For this type of biological agent to be effective many hurdles have to be overcome. Tumor antigens need to be expressed on cell surfaces, there needs to be a high binding affinity between these markers and the compound and the antigens themselves should be specific-specific. Significant problems with allergy and toxicity also have to be overcome. However, much has been learned during this time and that knowledge in itself has spurred further drug development.
It is also important to understand whether the target for monoclonal antibody therapy is present on the tumor mass alone or on the tumor stem cells (e.g., CD33 is present on committed AML blasts but absent from the leukemic stem cell). If the target is only expressed on mature tumor cells the monoclonal therapy should be seen as cytoreductive therapy. However, if the tumour stem cell expresses the antigen, monoclonal antibody therapy can be used in the setting of minimal residual disease where a successful result is more likely.
The era of monoclonal therapy has firmly arrived: Rituximab, a monoclonal antibody targeting cells expressing CD20 antigens, is licensed for use against follicular lymphoma and diffuse large B-cell non- Hodgkin’s lymphoma (NHL) [30]. Cetuximab is active against tumors expressing epidermal growth factor (EGFR). It has been used in adult practice against metastatic colon cancer and advanced squamous cell cancers in the head and neck [31].
The most impressive use of monoclonal antibody therapy in childhood solid tumours has been seen in neuroblastoma treatment. The chimeric anti GD2-antibody ch14.18 was used in combination with IL2 and GM-CSF and seen to improve 2 year overall and event free survival in children in a state of minimal residual disease, treated with a variety of induction regimens and following autologous stem cell transplant [32]. Further encouraging 10 year survival figures for German children exposed to the antibody alone raise the possibility of prevention of late relapse [33]. However fundamental questions about the role of each of the immunomodulatory agents and how they should be used in combination still remain.
Other Immunomodulators
Other biological agents act by immunostimulation or by driving differentiation: Muramyl tripeptide phosphatidylethanolamine (MTP-PE) induces phagocytosis and costimulation of cytokines. Some useful effect has been seen in osteosarcoma [34] and it is likely to form part of the next international Phase III trial in that disease.
All-Transretinoic acid (ATRA) drives differentiation of the promyelocytes in the APML variant of Acute Myeloid Leukemia. Cis-retinoic acid drives differentiation of primitive neuroblasts to mature ganglioneuronal cells. Both of these retinoids are now incorporated in the standard treatment of these diseases in children.
Cancer Vaccination and T-cell Therapy
Vaccination works by stimulating host T-cells to fight off disease. Anticancer vaccines have been worked on for many years and recent increased understanding of cellular biology has meant there have been crucial developments in producing useful anticancer vaccines. Vaccination strategy is not only dependent on optimizing antigen presentation but also the interaction of that presenting cell with disease-modulating T-cells. The most exciting results have been seen using patient-specific vaccines derived from autologous tumor cell lines. Melanoma, which increasingly affects teenagers and young adults, has shown the most susceptibility to a vaccination approach. A recent report of patient-specific dendritic cell vaccines in a cohort of heavily pretreated patients with metastatic disease, will hopefully prove to be a large step forward in the long search for a successful anticancer vaccine [35]. The United Kingdom has recently opened a phase I trial of dendritic vaccine therapy for children with relapsed or progressive high grade gliomas.
The infusion of donor lymphocytes following bone marrow transplant in patients with relapsed leukemia has become standard practice in pediatric patients. These T-cells are not specific-specific and are associated with the development of significant graft-versus-host-disease (GVHD). Indeed, it is believed that the mechanism for GVHD is closely related to the mechanism for the graft-versus-leukemia-effect (GVL) and clinicians view mild GVHD post DLI as a marker of effect. However, specific-specific T-cell populations have the advantage of destroying the disease with less systemic side effects. Manipulated cytotoxic T-cells have been successful in eradicating viral-induced cancers: EBV-driven lymphoproliferative disease, Hodgkin’s disease, and nasopharyngeal carcinomas have all responded to EBV-specific cytotoxic T-cell therapy [36]. Despite the excitement generated by novel therapies, there are of course challenges to their use. Although their toxicity should be less than a conventional chemotherapeutic agent, this does not mean they are without significant side effects. Allergic reactions and cytokine release syndromes are common following monoclonal therapy. Cytokine storms have resulted in life-threatening events [37].
Immune disregulation, cardiotoxicity, and skin problems have all been noted as side effects of targeted small molecules. Resistance to therapy is increasingly recognized and mono-therapy with targeted agents is as unlikely to be successful as it is with conventional agents.
Future Challenges
We are unlikely to see the large step change in cure rates that has characterized the last 30 years of anticancer therapy in children. As important as an increased understanding of molecular biology will be a regulatory and fiscal environment that encourages new drug development in rare tumors. There will also need to be improvements in trial design and analysis to be able to identify real but small improvements in outcome. There will need to be co-operation and partnerships between clinicians, scientists, statisticians regulators and the pharmaceutical industry. Long-term follow up will be crucial in identifying any, as yet, unrecognized late effects.
So what does the future hold? Genetic analysis at birth may be able to predict life time risks and allow tailored lifestyle advice. Chemo-prevention and prophylactic surgery are already established adult practice. Real time micro array is now being used to augment pathology in a large breast cancer trial. Disease biomarkers will need to be better understood and validated. It is likely that gross disease will continue to be debulked by traditional treatment modalities. This may be followed by establishing a patient-specific, tumor profile with microarray technology, host genetics may give a clear picture of innate drug handling, allowing a truly bespoke, targeted attack of disease residuum with a combination of small molecules, immunomodulation, or vaccination.
References
Wallace WHB. Growth and endocrine function following the treatment of childhood malignant disease. In: Pinkerton CR, Plowman PN, et al., editors. Paediatric oncology: clinical practice and controversies. 2nd ed. London: Chapman and Hall Medical; 1997. p. 706–31. 29.
Bleyer WA. The impact of childhood cancer on the United States and the world. Cancer. 1990;40:355–67.
Lemerle J, Voûte PA, Tournade MF, et al. Effectiveness of preoperative chemotherapy in Wilms’ tumour: results of an international society of paediatric oncology (SIOP) clinical trial. J Clin Oncol. 1983;1(10):604–9.
Voûte PA, Tournade MF, Delemarre JFM, et al. Preoperative chemotherapy as first treatment in children with Wilms’ tumour. Results of SIOP nephroblastoma trials and studies. SIOP proceedings, Abstract 123, Jerusalem; 1987.
Tournade MF, Com-Nougue C, Voute PA, Lemerle J, De Kraker J, et al. Results of the Sixth International Society of Pediatric Oncology Wilms’ Tumour Trial and Study: a risk-adapted therapy approach in Wilms’ tumour. J Clin Oncol. 1993;11:1014–23.
Rickard KA, Loghman ES, Grosfeld JL, et al. Short and long-term effectiveness of enteral and parenteral nutrition in reversing or preventing protein energy malnutrition in advanced neuroblastoma; a prospective randomised study. Cancer. 1985;56:2881.
Rickard KA, Coates TD, Grosfeld JL, et al. The value of nutritional support in children with cancer. Cancer. 1986;48:1904.
Capra S, Ferguson M, Ried K. Cancer: impact of nutrition intervention outcome – nutrition issues for patients. Nutrition. 2001;9:769–72.
UKCCSG. Annual scientific report. United Kingdom Children’s Cancer Study Group; 2009.
Wyllie AH. Apoptosis. Br J Cancer. 1993;67:205–8.
Takimoto C, Page R. Principles of chemotherapy. In: Pazdur R, Coia LR, Hoskins WJ, Wagman LD, editors. Cancer management: a multidisciplinary approach, vol. 3. 5th ed. New York: Oncology Group; 1991. p. 21–38.
Lum BL, Fisher GA, Brophy NA, Yahanda AM, Alder KM, et al. Clinical trials of modulation of multidrug resistance. Pharmacokinetic and pharmacodynamic considerations. Cancer. 1993;72(Suppl):3502–14.
Pinkerton CR, Hardy JR. Cancer chemotherapy and mechanisms of resistance. In: Pinkerton CR, Plowman PN, et al., editors. Paediatric oncology: clinical practice and controversies, vol. 6. 2nd ed. London: Chapman and Hall Medical; 1997. p. 159–88.
Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumours to their spontaneous mutation rate. Cancer Treat Rep. 1979;63:1727.
Wallace WHB, Blacklay A, Eiser C, et al. Developing strategies for long term follow up of survivors of childhood cancer. Br Med J. 2001;323:271–4.
Pritchard-Jones K, Pieters R, Reaman GH, et al. Sustaining innovation and improvement in the treatment of childhood cancer: lessons from high-income countries. Lancet Oncol. 2013;14(3):e95–103.
National Cancer Institute SEER Program. Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. Bethesda: National Cancer Institute; 1999.
Coley W. The treatment of malignant tumours by repeated inoculation of Erysipelas: With a report of 10 original cases. Am J Med Sci. 1893;105:487–511.
Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–69.
Vassal G, Zwaan CM, Ashley D, Le Deley MC, Hargrave D, Blanc P, Adamson PC. New drugs for children and adolescents with cancer: the need for novel development pathways. Lancet Oncol. 2013;14:e117–24.
Arceci RJ, Cripe TP. Emerging cancer-targeted therapies. Pediatr Clin North Am. 2002;49:1339–68.
Buchdunger E, Zimmermann J, et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2- Phenylaminopyrimidine derivative. Cancer Res. 1996;56(1):100–4.
Stegmeier F, Warmuth M, Sellers WR, Dorsch M. Targeted cancer therapies in the twenty-first century: lessons from imatinib. Clin Pharmacol Ther. 2010;87:543–52.
Adamson PC. Committee on shortening the time line for new cancer treatments. In: Adamson PC, Weiner SL, Simone JV, Gelband H, editors. Making better drugs for children with cancer. Washington DC: National Academies Press; 2005.
Kim SY, Toretsky JA, Scher D, Helman LJ. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91.
Franz DN, Belousova E, Sparagana S, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2013;381:125–32.
Garnett MJ, Edelman EJ, Heidorn SJ, et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–5.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of pre-defined specificity. Nature. 1975;256:495–7.
Lynch DA, Yang XT. Therapeutic potential of ABX- EGA: a fully human anti-epidermal growth factor receptor monoclonal antibody for cancer treatment. Semin Oncol. 2002;29:47–50.
Waksal HW. Role of anti-epidermal growth factor receptor in treating cancer. Cancer Metastasis Rev. 1999;18(4):427–36.
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, for the Children’s Oncology Group. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–34.
Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Klingebiel T, Berthold F. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21.
Worth LL, Jeha SS, Kleinermann ES. Biologic response modifiers in pediatric cancer. Hematol Oncol Clin North Am. 2001;15:723–40.
Dillman R, Dselvan F, Schiltz D. Patient-specific dendritic cell vaccines for metastatic melanoma. N Engl J Med. 2006;355:1179–81.
Foster A, Rooney C. Improving T cell therapy for cancer. Expert Opin Biol Ther. 2006;6(3):215–29.
Suntharalingan G, Perry MR, Ward S, et al. Cytokineand storm in a Phase I trial of the anti-CD28 monoclonal antibody PDN1412. N Engl J Med. 2006;335:1018–28.
Krause D, Van Etten RA. Tyrosine Kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–87.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ronghe, M.D., Murphy, D. (2016). Chemotherapy and Novel Cancer Targeted Therapies. In: Carachi, R., Grosfeld, J. (eds) The Surgery of Childhood Tumors. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-48590-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-662-48590-3_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48588-0
Online ISBN: 978-3-662-48590-3
eBook Packages: MedicineMedicine (R0)