Abstract
Oxygen isotope ratios of organisms are closely linked to the hydrological environment in which they grow up. This is especially the case for mammals. Mammal-bone phosphate is formed at a constant body temperature and, thus, relatively insensitive temperature-dependent isotope fractionation. The applicability of oxygen isotopes from mammal-bone phosphate for environmental reconstructions is tested here using bones of deer, domestic pig, and domestic cattle from 16 archaeological sites situated along a North-South transect crossing the Alps. Bones of 118 specimens in total were analyzed, which covered an age span from the Late Neolithic to the Roman period. The main control on oxygen-isotope ratios was site altitude. Significant negative correlations between phosphate-isotope values and altitude were registered especially for cattle and pig. Possibly nonlocal pig bones from one site were deviating from this altitudinal pattern and were excluded from further correlations. Modern equations for translating oxygen-isotope ratios of the three species to those of source water were applied. The reconstructed source water isotopic composition showed a similar altitudinal relation as modern Alpine precipitation. As a consequence, our study confirms the utility of oxygen-isotope ratios of mammal-bone phosphate for palaeoaltitudinal reconstructions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Prehistoric ritual immolation site s in the Alpine area have been investigated for the past 40 years. Research on ritual immolation sites in the Alps started in 1966 by a paper by Werner Krämer. He describes: … where masses of calcinated bones allow the interpretation of ritual immolation…. …as well as the occurrence of large masses of ceramic fragments which can also be interpreted as sacrificial offerings…. For the first time these sites were considered from an archaeological standpoint as a group of their own. Fire is considered to have cleansing properties. The so “cleansed” sacrifice is transferred to the gods via smoke. Weiss (1997) showed that due to the lack of surface characteristics, ritual burning sites are very hard to identify and indeed their identification is mainly accidental. Weiss (1997) mentions about 120 ritual immolation sites from an area extending between the Alps and the Danube, which probably represents a lower limiting number of the actual sites. A more recent study by Gleirscher et al. (2002) extended the number of ritual immolation sites to 201 in the Alps. In the last few years, investigations concerning alpine ritual immolation sites intensified by the extensive studies of Steiner (2010) on the ritual immolation site of St. Walburg/Ulten in South-Tyrol and a proceedings volume (Stadler et al. 2013) following a conference on Alpine ritual immolation sites in Nenzing 2012. The study by Steiner represents a comprehensive investigation involving not only archaeological but also archaeobotanical and archaeozoological aspects.
Based on literature consensus, ritual immolation of offerings started roughly in the Early Bronze Age (ca. 1800 bc) and occasionally lasted until the Roman period. It was mostly carried out by farming populations asking the gods for good harvests and herds. In the mountains the sacrifices were goats and sheep and in the valleys cows, pigs , deer, etc. Usually skulls and extremities of the animals were sacrificed. Besides animals the offerings also contained grains, tools, pottery, jewelry, and coins. In the Bronze Age, these activities took place in isolated sites whereas in the Iron Age they took place in the vicinity of dwellings. Increasing ritual immolation activities from the Middle Bronze Age onward coincide with increasing populations and hence increasing farming.
What are the characteristics of alpine ritual immolation site s ? According to Steiner (2010) a unique definition of what clearly defines a ritual immolation site is not an easy task. The most important criterion is the presence of calcinated bones. It has been shown that the following features are also typical for a ritual immolation site: exposed position, ash layers, the presence of stone altars, ceramic fragments, and other sacrificed libations, the occurrence of pyrometamorphic slag s , and the presence of adjacent bone deposits. Therefore, most sites were identified based on the presence of pottery sherds, metal artifacts, and bone fragments. However, until 2004 none of the sites had ever been investigated from a mineralogical point of view, since characteristic P-bearing minerals can form due to the interaction between bone material and rocks in the course of the immolation process (Tropper et al. 2004, 2006; Spielmann 2013). Two prehistoric immolation sites in Tyrol associated with slags were chosen for mineralogical/petrological investigations, the Goldbichl in Igls near Innsbruck (Schneider et al. 2013) and a site outside of Oetz in the Ötz Valley approximately 50 km W of Innsbruck (Tropper et al. 2004). In both areas, the site is always on top of a hill. Ritual sites have been known in Tyrol for a long time, but Von Chlingensperg (1904) was the first to interpret these sites as localities where ritual immolations took place, based in part on the abundant presence of bone fragments of domestic animals such as cows, sheep, goats, and pigs (Weiss 1997; Tomedi and Nicolussi Castellan 2000). Tropper et al. (2004) investigated slags from the ritual immolation site near Oetz and concluded, based on experiments using natural animal bones (Tropper et al. 2006), that mineralogical observations such as the assemblage phosphorus -rich olivine + whitlockite can indeed provide possible evidence for the burning of bone material and thus be very helpful in the identification of prehistoric immolation sites in cases where clear archaeological evidence is lacking. Schneider et al. (2013) investigated the Goldbichl immolation site and found the highly unusual mineral assemblage P-bearing olivine + stanfieldite . They concluded that the formation of phosphoran olivine and stanfieldite is not due to the interaction between bone material and rocks but can form locally due to the pyrometamorphic breakdown of a P-rich accessory precursor phase such as detrital apatite .
Scope of This Contribution
As stated previously, although prehistoric sacrificial burning sites have been studied in the Alpine region for the past 40 years, these investigations only focused on the pottery, metal, and bone fragments and only two of these sites have ever been investigated from a mineralogical point of view, namely the Goldbichl site (Schneider et al. 2013) and the site in Oetz (Tropper et al. 2004). The scope of this contribution is a summary of the mineralogical and petrological characterization of slags from the two ritual immolation places: Oetz and Goldbichl. These petrographic observations are then compared to experimental investigations concerning the T-fO2 conditions of the pyrometamorphic overprint. The experiments presented in this article were done using either whole-rock samples tempered at different temperatures with or without the presence of bones or mineral separates of sheet silicates (chlorite ). These were investigated separately with several methods (high-temperature diffractometry under oxidizing conditions, HT-XRD; differential thermal analyses and thermogravimetry under reducing conditions, DTA-TG). Finally the archaeological implications of the petrological and experimental results will be discussed.
Archaeological Setting
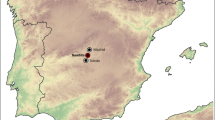
Goldbichl/Igls: This ritual immolation site is on top of the Goldbichl, a small hill a few kilometers to the south of Innsbruck near the village of Igls (Fig. 1). The Goldbichl has been used as a prehistoric cult site since the Neolithic Age (Tomedi and Nicolussi Castellan 2000). In the Early Bronze Age (1900–1650 bc) it gained importance as a ritual immolation place. In the process of these ritual immolations, cattle , goats, and sheep were sacrificed on a stepped altar made of loam and local rocks (quartzphyllites ). Due to the presence of a natural air draft, huge fires were made so that the flames could be easily seen from far away (Tomedi and Nicolussi Castellan 2000). The immolation fires were set on a circular place on the loamy ground. It was only at a later point in time that stone altars were built. Archaeological excavations yielded fragments of ceramic vessels that contained libations representing offerings of liquids and vessels sacrificed during these rituals. After every ritual immolation the place was cleaned and the precious sacrificial offerings were carried to a secret depot. According to Tomedi and Nicolussi Castellan (2000) immolation activity started again at this site during the Iron Age (ca. 450–15 bc) following a long period of neglect. At the end of this period the site was ritually “closed down” with a huge fire that completely destroyed the site leaving behind large amounts of slags .
Geological overview of the lower Inn Valley from Tropper et al. (2004). The locations of the Goldbichl/Igls (GB) and Oetz are shown. The Goldbichl site is geologically located in the Innsbruck quartzphyllite complex and the site in Oetz is in the Ötztal Complex (ÖC)
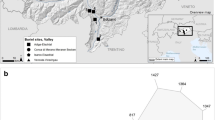
Oetz: The presumably La-Tène (450–15 bc) age ritual immolation site is situated south of Oetz at the entrance to the Ötz Valley (Fig. 1) and is located on the back of a small ridge composed of biotite –plagioclase gneisses. Archaeological evidence for a prehistoric ritual immolation site is provided by the presence of bone fragments, broken pieces of clay vessels, and the occurrence of foamy patches of dark glassy material at the surface of gneiss boulders (Tropper et al. 2004).
Geological Setting
Goldbichl/Igls: The stones for the altar are local and stem from the area around the Goldbichl. Geologically this site is situated in the westernmost part of the Innsbruck Quartzphyllite complex, which is part of the Austroalpine basement nappes north of the Tauern Window (Fig. 1). In the vicinity of Innsbruck the polymetamorphic Austroalpine basement consists of lower Ordovician porphyroid gneisses (Kellerjochgneiss or Schwazer Augengneiss), micaschists (Patscherkofel and Glungezer Crystalline Complex), and Palaeozoic schists (Innsbruck Quartzphyllite complex and Wildschönau Schists) with intercalated carbonates (Piber 2005).
Oetz: According to Hoinkes et al. (1997), the gneisses are part of the polymetamorphic Ötztal–Stubai Complex (ÖSC) and were metamorphosed during the Variscan metamorphic event under amphibolite-facies conditions (500–600 °C, 5–7 kbar).
Petrography and Textural Relations
Goldbichl/Igls: The westernmost part of the Innsbruck Quartzphyllite complex consists of metapsammites and metapelites. The mineral assemblage of the protolith quartzphyllites consists of muscovite + chlorite + plagioclase + quartz ± apatite ± biotite ± garnet ± clinozoisite ± ilmenite ± rutile ± titanite (Schneider et al. 2013). Titanite is the most abundant Ti-mineral and quartz and feldspar contents are highly variable and chlorite, muscovite, and if present biotite form the penetrative foliation. The rocks of the immolation place show signs of a strong thermal overprint, which often differs on a centimeter scale. The samples show foamy textures as well as thin (<0.5 cm) layers of glass. The foamy patches show a diameter of <3 cm and contain many vesicles which clearly indicate a high-temperature overprint while other less reactive domains still show the primary foliation (Fig. 2). Most of the slags show bloated structures. The minerals in the foamy patches were exposed to the highest temperatures , which resulted in the formation of high-T minerals (olivine, spinel ) and melt (Fig. 3a). The pyrometamorphic rocks mostly contain the mineral assemblage olivine + orthopyroxene + plagioclase + spinel + glass. During the investigation an apatite-rich domain was found in which P-rich phases occur. Elongated crystals of plagioclase dominate the texture of the microdomain and between these laths the highly unusual mineral assemblage stanfieldite [Ca4(Mg, Fe2+, Mn2+)5(PO4)6] + phosphoran olivine occurs (Fig. 3b). Relict detrital apatite grains still occur in this domain.
(a) BSE (backscattered electron) image of a chlorite -rich domain in a slag sample of the Goldbichl. The high-temperature assemblage is orthopyroxene (Opx) + spinel (Sp) + anorthite (An) + glass (L). (b) BSE close-up of the phosphorous domain in the slag. Stanfieldite (St) and phosphoran olivine (Ol) crystallized as interstitial phases between An-rich plagioclase (An) laths. In the right corner relict detrital apatite (Ap) still can be seen
Oetz: The rock samples still retain their gneissose texture showing an alternation of light and dark bands (Fig. 4). Unmelted gneiss samples show the assemblage biotite + plagioclase + quartz + accessories (apatite + zircon). During partial melting, foamy patches of dark glassy material formed at the surface of the rocks and also as layers within the rocks (Fig. 4). In this glassy crust the assemblage clinopyroxene + plagioclase + quartz + anorthite + glass was observed (Fig. 5a). Plagioclase mostly forms lath-shaped crystals overgrowing the mineral assemblage. Clinopyroxene tends to form dendritic crystals, whereas olivine always occurs as angular grains. In a few small areas, melt is still preserved and quenched to glass, whereas in the majority of cases, melt recrystallized to form very small dendritic crystals which could not be analyzed properly. Toward the contact between the dark glassy layers and the protolith, rock whitlockite [Ca9(Mg,Fe)(PO4)6(PO3OH)] occurs in the assemblage olivine + clinopyroxene + plagioclase + whitlockite + glass (Fig. 5b).
Backscatter electron (BSE) images from the vicinity of the contact between rock and glass layer. (a) Overview over a clinopyroxene (Cpx)- and olivine (Ol)-rich domain. Interstitial glass pockets (L) and Ti-bearing magnetite also occur. Abundant plagioclase (An) laths grow within the glass and the minerals. (b) The assemblage olivine (Ol) + whitlockite (Whit) + plagioclase (An) + interstitial glass (L)
Analytical Methods
Electron Microprobe Analysis (EMPA)
A JEOL 8100 SUPERPROBE electron microprobe was used at the Institute of Mineralogy and Petrography at the University of Innsbruck. For a preliminary identification of minerals the energy-dispersive spectrometer (Thermo Noran EDS system) was applied. The measurements were made using five wavelength-dispersive spectrometers (TAB, PETJ, PETH, LIF, and LIFH). Measurement conditions were 15 kV acceleration voltage and a sample current of 10 nA. The measurement time was 20 s for the peak and 10 s for each side of the background positions. The beam size was 1 μm and for corrections the Phi-Rho-Z method was used. Standardization was done using natural and synthetic standards. In order to minimize the loss of volatile elements such as K and Na, a defocused beam with a diameter of 5 μm was used for mica and feldspar analysis.
High-T Powder XRD (HT-XRD)
The HT-XRD measurements were done using an AXS-Bruker D-8 powder X-ray diffractometer. The radiation used is CuKa1,2 with a wavelength of 0.15406 nm. The measurements occur under parallel beam optics, and an energy-dispersive counter was used. The detector and the beam optic were theta/theta coupled and the standard operating conditions were 40 kV and 40 mA with a continuous scan ranging from 2 to 70° in theta/2theta configuration. The measurement was done with a step size of 0.01 steps and a counting time of 2 s at room conditions. Experiments were done by heating up the chlorite mineral separate with a heating rate of 0.3 °C/s. At every 20 °C step a diffractogram was measured. A 2-theta range of 5–70° was scanned with a step size of 0.02°. Each step was measured for 4 s. The measurement started at 300 °C and ended at 1200 °C. All measurements were conducted under oxidizing conditions and took place over the course of several days. The advantage of this method is the in situ observation of mineral reactions as a function of temperature .
Differential Thermal Analysis–Thermogravimetry (DTA–TG)
The principle of the differential thermo analyses/thermogravimetry is that during heating, temperature changes and changes in the mass of a sample are recorded. Endothermal or exothermal effects in a sample can be observed as well as effects of dihydroxylation. As such, mineral reactions associated with these energetic effects can be monitored as a function of temperature. DTA-TG was used to investigate the breakdown of chlorite under reducing helium atmosphere . An empty crucible of corundum was used as a standard and was held at T = 25 °C. The heating rate was 3 °C/s, and the temperature range was from 25 to 1200 °C. The device was the Setsys Evolution 2400 by Setaram and corund crucibles with 100 μl were used. Helium was the flushing gas with a flow rate of 20 ml/min.
Mineral Chemistry of the Slags
Goldbichl/Igls
Olivines show X Fe [Fe/(Fe + Mg)] contents ranging from 0.44 to 0.84. Olivines also contain an extraordinary high amount of P of up to 23 wt% P2O5. The P content strongly varies but reaches a maximum of 0.536 apfu (atoms per formula unit). Figure 6 shows that not only the Si content decreases with increasing P contents (Fig. 6a), but also the sum of cations on the M1,2 positions decreases down to 1.655 apfu (Fig. 6b). The formula of olivine illustrates the strong compositional variations: Mg0.584–0.978Fe0.768–1.116Mn0.011–0.022Ca0.004–0.014P0.289–0.536Si0.480–0.777O4. No chemical zoning was observed in olivine. Boesenberg and Hewins (2010) postulated the following charge balancing scheme for P incorporation in olivine:
(a) Linear correlation between Si and P (apfu) in the phosphoran olivines of the samples from the Goldbichl immolation place. The dashed line represents the 1:1 P substitution for Si according to the charge balance scheme 2Si4+ + VIM2+ ↔ 2P5+ + VI□. (b) Correlation between P and the sum of M1,2 cations in the phosphoran olivines. The dashed line represents the correlation between the vacancies in M1,2 and the P content according to the charge balance scheme 2Si4+ + VIM2+ ↔ 2P5+ + VI□
Rearrangement of their charge-balancing scheme leads to the more simplified charge balance scheme according to Tropper et al. (2004):
In the course of this investigation, a tri-calcium phosphate phase (TCP) was found. Chemically, its composition fits several Ca-phosphates , namely graftonite (Ca, Mg, Fe2+, Mn2+)3(PO4)2, beusite (Mn2+, Fe2+, Ca, Mg)3(PO4)2, and stanfieldite Ca4(Mg, Fe2+, Mn2+)5(PO4)6. Stanfieldite has been described from stony-iron meteorites (Fuchs 1967), as well as pallasites (Buseck and Holdsworth 1977). The TCP from the P-rich domain has a high content of Fe, Ca, and especially Mg, which is in good agreement with the chemical composition of stanfieldite as reported by Fuchs (1967). The Ca contents of 3.41–3.83 apfu are lower than in the ideal stanfieldite formula by Fuchs (1967) but similar to the analysis by Buseck and Holdsworth (1977) who report Ca contents down to 3.69 apfu. Therefore based on the observed chemical composition the TCP is most likely stanfieldite. Spinel is a solid solution between spinel –magnetite –hercynite–magnesioferrite and ulvöspinel. The feldspar laths are plagioclase and also contain up to 0.6 wt% P2O5. Melt compositions outside the P-rich domains are peraluminous and also vary strongly in composition due to its formation in different microdomains. Minor orthopyroxene can be found in the slags as well as in the P-rich domain. The P-bearing orthopyroxenes vary strongly in their composition and all show a high Al component of 3–6 wt% Al2O3. Al is incorporated on the tetrahedral position due to the Tschermaks substitution (2Al3+↔Si4++M2+). The P2O5 content is around 0.5 wt% except for one orthopyroxene which contains 5.0 wt% P2O5 which represents 0.16 apfu P. This amount of P can only be accommodated by the substitution of P and Al instead of Si on the tetrahedral site according to the following vector (Boesenberg and Hewins 2010):
Oetz
Olivine is characterized by highly variable X Fe ranging from 0.26 to 0.52 and P2O5 contents from below the detection limit up to 8.8 wt% P2O5, respectively. Again all analyzed olivines show a linear negative correlation between P and Si with a slope close to −1, which is consistent with data from the literature (Goodrich 1984; Buseck and Clark 1984; Agrell et al. 1998). Moreover, a significant decrease in total cation sums with increasing P contents can be observed with ∑cat as low as 2.90 for the highest P content of 0.20 apfu. Clinopyroxenes are mostly augitic in composition with limited Ca-Tschermak (AlIV = 0.02–0.10 apfu) component. Clinopyroxenes may contain significant enstatite and ferrosilite solid solution with (Mg + Fe) contents on the M(2) site, ranging from 0.08 to 0.43 apfu. Plagioclase laths coexisting with olivine and clinopyroxene show anorthite contents in the range 40–65 mol%. In contrast to the chemical composition of apatite [Ca5(PO4)3(OH,F,Cl)], whitlockite [Ca9(Mg,Fe)(PO4)6(PO3OH)] contains significant amounts of MgO (3.29–3.69 wt%), FeO (0.70–1.33 wt%), and Na2O (1.46–1.85 wt%). All interstitial glasses are quartz -normative and show a granitic composition with SiO2 ranging from 62.52 to 69.06 wt%. FeO contents of the glass are highly variable and range from 4.97 to 16.11 wt%. The P2O5 contents are very low and are <0.99 wt%.
The chemical variability of the phases formed in the slags during the ritual immolation process and the occurrence of extensive P-substitution in olivine both strongly indicate disequilibrium growth in relatively SiO2-rich domains due to rapid quenching. This is consistent with the implications associated with phosphoran olivine growth in natural occurrences (Buseck and Clark 1984; Agrell et al. 1998; Goodrich 1984; Brunet and Chazot 2000) and in a ritual immolation site (Tropper et al. 2004), as well as in experimental investigations (Bousenberg et al. 2004; Boesenberg and Hewins 2010; Tropper et al. 2006).
Experimental Investigations
Very few experimental investigations simulating pyrometamorphic processes are available and are mainly concerned with the formation and development of disequilibrium textures due to the breakdown of hydrous phases (e.g., Cultrone et al. 2001; Brearley and Rubie 1990). Tropper et al. (2006) performed firing experiments at 1 bar to investigate the formation of phosphorus olivines and thus compare the results with the observations of Tropper et al. (2004) from the ritual immolation site near Oetz in Tyrol . Schneider (2009) and Spielmann (2013) conducted additional experiments using natural quartzphyllite samples in order to place temperature constraints on the firing process in the samples from the Goldbichl. So far the experimental investigations can be placed in three groups: (1) whole-rock experiments without the presence of bones, (2) bone-rock experiments, and (3) high-T experiments (HT-XRD, DTA–TG) investigating the thermal evolution of a chlorite sample with intermediate Fe-content (X Fe = 0.46).
Whole-Rock Experiments
To place constraints on the temperature of formation of the Oetz slags melting experiments at 1 bar and 900–1300 °C were conducted in a box furnace (Tropper et al. 2006). To investigate the role of crucible material during firing, two experiments at 1000 °C were conducted: one in a graphite crucible and the other in a Pt crucible. Secondary electron (SE) images of the rock cubes from the experiment in the Pt-crucible at 1000 °C showed almost no melting textures on the surface (Fig. 7a) and therefore the experiments in the Pt crucible were not pursued any further. In contrast in the experiments with the graphite crucible visible melting took place at the surface of the rock cube (Fig. 7b) indicating that more reducing fO2 conditions facilitate a higher degree of partial melting. In the experiments without the addition of bones biotite is stable up to 900 °C and its breakdown yielded the mineral assemblage olivine + Ti-bearing magnetite + melt from 1000 °C on. In order to put preliminary temperature constraints on the pyrometamorphic formation of the slags from the Goldbichl site Schneider (2009) conducted an experiment using a quartzphyllite sample at 1100 °C and obtained the same mineral assemblage orthopyroxene + olivine + spinel + melt as observed in the slag samples from the Goldbichl site.
Secondary electron (SE) image of the surface of rock cubes from experiments at 1000 °C. (a) Experiment in a Pt crucible. The sharp edges of the biotites do not indicate a significant degree of melting. (b) Experiment in a graphite crucible. The rounded edges and open spaces indicate a considerable degree of melting
Bone–Whole-Rock Experiments
For this study Tropper et al. (2006) used samples of unmelted biotite -plagioclase -gneisses from the burning site near Oetz, which were cut into cubes of approximately 1 cm edge length. To be as close as possible to the observations simple experiments were designed where fO2 was only approximated to the CCO (graphite C/carbon monoxide CO) buffer but not fixed. Most experiments were performed with the rock cube placed on top of a layer of crushed chicken bones. These rock–bone aggregates were then subjected to temperatures between 900 and 1300 °C with run durations from 90 to 480 min. In addition, the presence of bone material to the rock cubes led to complete melting of the rock cubes at temperatures of 1300 °C as shown in Fig. 8a. Most experiments were quenched by quickly removing the crucible from the furnace. Although olivine occasionally formed, quenching and the presence of bone material on only one side of the rock cube did not lead to sufficient mineral reactions at the interface between the rock and the bone layer and thus experiments were conducted where bone material was sandwiched between rock cubes. Therefore, in order to allow a more intimate contact between bone and rock and thus to enable a stronger reaction, bone material was sandwiched between two rock slabs in two experiments (Fig. 8b). Instead of quenching, these experiments were cooled slowly from 1100 and 1200 °C down to 500 and 700 °C with cooling rates of 60 and 120 °C/h to allow slow crystallization from the melt. After the experiment the cubes were embedded in epoxy resin and polished for electron microprobe and scanning electron microscope analysis (Fig. 9a, b). The former bone domain now contains the assemblage whitlockite (Whit) + olivine (Ol) + melt (L). Olivines from the experiment at 1100 °C show a wide range in P2O5-concentrations from 0.18 to 1.19 wt% along with significant variations in their Fe/Mg-ratios (Fo30Fa70–Fo50Fa50). Compared to olivines from the immolation site in Oetz site the experimentally produced olivines extend to more Fe-rich compositions but do not contain as much P2O5. Similar to the olivines from the immolation site the experimentally grown olivines also show a negative correlation between P and Si apfu and also between P and total cation sums. Both correlations, however, are not as pronounced as those observed for olivines from the immolation site. The chemical analyses of whitlockites from the slowly cooled experiments at 1100 and 1200 °C are very similar to whitlockites reported by Tropper et al. (2004) and contain 3–4 wt% MgO and <1 wt% FeO. Na2O varies between 0.7 and 2.4 wt%.
(a) Comparison between two experiments at 1300 °C from an experiment in a graphite crucible with bone material added (left) and without bone material (right). The addition of bone material to the experiments leads to a strong increase in melting during the experiments. The diameter of the cube on the right is 1 cm
Backscatter electron (BSE) images of a slowly cooled bone-paragneiss experiment in a graphite crucible at 1100 °C from Tropper et al. (2006). (a) The layering of the former bone layer is still visible and contains the assemblage whitlockite (Whit) + olivine (Ol) + melt (L). Small injections of melt veins into the adjacent rock cubes are also visible. (b) Within the melt pockets the assemblage olivine (Ol) + magnetite (Mgt) + plagioclase (Pl) + melt (L) occurs
Spielmann (2013) carried out high-temperature investigations at 1200 °C by using typical rocks (paragneiss, quartzphyllite , granite , garnet-amphibolite) of the eastern Alps together with bone material. The paragneiss-bone experiment yielded the mineral assemblage whitlockite + clinopyroxene + anorthite + Fe-Ti-spinel + Fe metal + melt in the bone–rock contact area (Fig. 10a). The obtained textures and the mineral assemblage only lack olivine in the contact area between bone and rock when compared to the slags from Oetz. Olivine does form in this experiment slightly further away from the direct contact in former biotite domains. The granite-bone experiment yielded in the contact area the mineral assemblage whitlockite + wollastonite + anorthite + melt. The quartzphyllite-bone experiment yielded the mineral assemblage whitlockite + P-bearing olivine + spinel + Fe metal + melt (Fig. 10b). The most complex mineral assemblage was obtained in the garnet-amphibolite-bone experiment where the assemblage whitlockite + clinopyroxene + perovskite + Fe-Ti-spinel + anorthite + Fe metal + melt formed in the bone–rock contact zone. The chemical composition of whitlockite in the experiments strongly depends on the composition of the protolith rocks and shows MgO and FeO contents of 1–2 wt% and 0–3 wt%. In the quartzphyllite-bone experiments P-bearing olivine with P2O5 contents up to 4.5 wt% occurs. P-incorporation follows the similar coupled substitution as observed in the slags from the two immolation site s . In addition to olivine clinopyroxenes from the paragneiss-bone and garnet-amphibolite-bone experiments, contain significant P2O5 contents of up to 15 wt%. This amount of P can only be accommodated by the substitution of P and Al instead of Si on the tetrahedral site according to the following vector P5+ + Al3+ = 2Si4+. The occurrence of metallic Fe in the experiments indicates that fO2 conditions were too reducing when compared to the Goldbichl and Oetz slags where Fe occurs as Fe2+ in olivines and the QFM assemblage (quartz -olivine-spinel) is still stable.
Close-up backscatter electron (BSE) images of the contact zones in the bone-paragneiss (a) and bone-quartzphyllite (b) experiments in a graphite crucible at 1200 °C from Spielmann (2013). (a) In the contact zone between paragneiss and bone the assemblage whitlockite (Whit) + clinopyroxene (Cpx) + anorthite (An) + Fe metal (Fe) occurs. (b) In the contact zone between quartzphyllite and bone the assemblage whitlockite (Whit) + olivine (Ol) + spinel (Sp) + anorthite (An) + melt (L) occurs
High-T XRD Experiments of Chlorite Under Oxidizing Conditions
For this experiment a chlorite with an intermediate Fe content (X Fe = 0.46) similar to the composition from the protolith quartzphyllites from the Goldbichl site was used. With increasing temperature the chlorite lattice shrinks in c-direction (Schneider 2009). This is a consequence of the high water loss during heating. With rising temperature the (002)-peak disappears finally at 550 °C and the (001)-peak rises which was also reported by Villieras et al. (1994). This structure is known as the chlorite “modified structure” (Brindley and Chang 1974; Villieras et al. 1994; Guggenheim and Zhan 1999) and is stable until 760 °C. Above this temperature chlorite-like structure can no longer be observed. The shrinking of the lattice at higher temperatures due to dehydroxylation of the brucite-like layers to lower spacings for the (001)-peak is also reported by Villieras et al. (1994). At 800 °C the sample is completely decomposed and at 900 °C spinel forms. At 1120 °C sapphirine and at 1140 °C cristobalite appear.
DTA–TG Experiments of Chlorite Under Reducing Conditions
The X Fe = 0.46 chlorite shows a two-step dehydroxylation starting with a drastic weight loss at 507 °C. It is remarkable that no loss of adhesively bound water was observed, which usually occurs between a temperature range of 50–200 °C. Then at a temperature of 740 °C the second dehydroxylation step occurs until 812 °C where a mass gain starts again (Schneider 2009). This phenomenon of gaining mass is still unexplained and might be attributed due to oxidations due to the presence of small concentrations of oxygen in the flushing helium gas. XRD of the products of the DTA/TG measurement yielded the mineral assemblage spinel + orthopyroxene + cristoballite/β-quartz .
Discussion
Textural Evolution of the Slags from the Goldbichl Site
At atmospheric pressure the sheet silicates start changing their shape due to the rising temperatures with intensified bloating and microcracking due to dehydration of adhesive and structurally bound water (Grapes 2006). Until the α–β transition of quartz at 575 °C the dilation occurs moderately and continuously (Grapes 2006). Vitrification occurs at a temperature of ca. 900 °C while degasification channels and bloating structures are produced at temperatures ca. 1200 °C (Grapes 2006). During firing of the quartzphyllites the micas break down along a continuous process beginning with substantial water loss. The original textures of muscovite and chlorite are preserved only as relicts and at higher firing temperatures small cubic crystals of spinel form within the chlorite layers. Depending on the distance to the fire different temperatures and oxygen conditions affected the rocks . The highest temperature was at the contact area between the rock and the fire where the most obvious melting processes took place. In these areas high temperature led to the breakdown of the initial assemblage muscovite + chlorite + plagioclase + quartz ± biotite ± clinozoisite ± ilmenite to the formation of the new pyrometamorphic assemblage: plagioclase (an-rich) + olivine + spinel + melt ± orthopyroxene due to the following model reactions involving the breakdown of chlorite (Schneider et al. 2013):
Orthopyroxene can also form due to a reaction between olivine and quartz and occurs therefore in an advanced stage of pyrometamorphism (Grapes 2006). Adjacent to olivine, large blades of plagioclase crystallized.
Textural Evolution of the Slags from the Oetz Site
Unmelted gneiss samples from the immolation site show the mineral assemblage biotite + plagioclase + K-feldspar + quartz with feldspars showing strong retrograde alteration to clinozoisite + albite + muscovite (sericite). Based on the petrographic observation, dark bands within the partially molten rock samples are therefore interpreted as former layers rich in biotite where partial melting was initiated. The breakdown of biotite at high temperatures and very low pressures has been reported so far from partially fused metapelites and granites (Maury and Bizouard 1974; Le Maitre 1974; Grapes 1986; Brearley 1987). Textural observations revealed that olivine and Ti-bearing magnetite form within the former biotite domains according to the reaction (Tropper et al. 2004):
The presence of clinopyroxene in the partially molten rocks could be ascribed to a reaction involving clinozoisite, the latter being a product of plagioclase alteration:
which would lead to the formation of K-rich melt at high temperatures . The high enstatite and ferrosilite components in the clinopyroxene indicate the simultaneous proceeding of the biotite breakdown reaction:
Derived Chlorite Breakdown Reactions from the Experimental Results
DTA-TG experiments: The decomposition of chlorite is a complex process strongly dependent of its Fe content and fO2 conditions (Schneider 2009). Chlorite with a low Fe content (X Fe = 0.11) forms under vacuum (DTA-TG) as well as under air atmosphere (HT-XRD) the assemblage forsterite + spinel + enstatite + water. Under helium atmosphere chlorites with even a higher X Fe produce the assemblage spinel + enstatite (for X Fe = 0.46 and 0.62). Chlorite with X Fe = 0.62 formed cordierite in addition and chlorite with X Fe = 0.89 formed hercynite + melt. The derived mineral reactions that occur in helium atmosphere are:
High-T XRD experiments: Under strongly oxidizing conditions and with high X Fe, phases with Fe3+ appear. Chlorite with the medium Fe-content (X Fe = 0.46) decomposes to pleonaste (spinel soild solution), while the Fe-rich chlorite (X Fe = 0.89) forms hematite and mullite. Sapphirine occurs in both cases only in minor concentrations. The reactions that occur in oxidizing air atmosphere (Schneider 2009) are:
Comparison of these experimental results to the slags from the Goldbichl site clearly indicates that the firing process must have taken place under very reducing conditions (most likely QFM) since the mineral assemblage spinel + orthopyroxene + quartz from the X Fe = 0.46 DTA-TG experiment closely reproduced the observed mineral assemblage in the former chlorite domains of the Innsbruck quartzphyllite .
Bone– rock experiments: The experimental investigations by Tropper et al. (2006) have shown that the interaction of bone material and metapelitic gneisses during partial melting led to the formation of P-rich olivines + whitlockite + plagioclase + K-rich glass. The experimental investigations indicate that the temperature of olivine formation due to the reaction biotite + quartz = olivine + Ti-bearing magnetite + K-rich melt must have exceeded 1000 °C at fO2 conditions near the CCO buffer and are in agreement with temperature estimates from pallasite meteorites (1143–1359 °C). The occurrence of phosphoran olivine and whitlockite in meteorites with compositions similar to those encountered in the experiments and the rocks at the firing site (Goodrich 1984; Buseck and Clark 1984; Agrell et al. 1998) further indicates a similarity with the experimental conditions. The chemical and experimental data strongly indicate olivine growth under disequilibrium conditions. Although phosphoran olivine did form in the experiments the extent of P-incorporation into olivine is much smaller compared to the olivines from the burning site at Oetz. Olivines with P2O5 contents similar to those found in the experiments do occur in Oetz but are restricted to microdomains more distant to the rock/bone interface. Clearly, local variations in firing temperature, oxygen fugacity, bulk phosphorus , and the geometry of the bone–rock aggregates must have controlled the P-incorporation in olivine. The experimental study of Spielmann (2013) extended the investigations of Tropper et al. (2006) by using four different rock types: paragneiss, quartzphyllite , granite , and garnet-amphibolite in the experiments. Whitlockite formed in all experiments and P-bearing olivine formed in some (quartzphyllite-bone) of the experiments. The occurrence of P-rich olivine and whitlockite in the quartzphyllite experiments is therefore also diagnostic for bone–rock interaction at high temperatures similar to the results from Tropper et al. (2006).
Temperature Constraints on Firing Temperatures During Pyrometamorphism
Temperature Estimates Based on Mineral Reactions in the Slags
Schneider (2009) has shown by using high-T XRD that the breakdown reactions of chlorites with different X Fe strongly differ. Fe-rich chlorite also dehdroxylates 140 °C earlier than clinochlore. DTA-TG experiments using a chlorite sample with intermediate Fe-contents yielded at 1100 °C the product assemblage of enstatite + spinell + quartz which forms from 740 to 810 °C according to the reaction chlorite (X Fe = 0.46) = spinel + enstatite + quartz + H2O. The slags from the immolation place contain the minerals: olivine + spinel + orthopyroxene + anorthite + glass. Thus, the occurrence of the assemblage spinel + orthopyroxene + olivine leads to the conclusion that temperatures of at least 800 °C were reached during the firing. The experimental investigations of Tropper et al. (2006) and Schneider (2009) using rock samples without the addition of bones also reproduced the mineral assemblages of the pyrometamorphic slag s olivine + spinel + melt (Oetz) and olivine + orthopyroxene + spinel + melt (Goldbichl) over a temperature range of 900–1100 °C.
Temperature Constraints Based on Coexisting Feldspars
Schneider (2009) analyzed coexisting feldspars in the pyrometamorph slags from the Goldbichl site. The texture of the feldspars indicates that they were the first crystals that crystallized from the melt. The feldspars are plagioclases with low K contents. The temperature dependence of the miscibility gap between plagioclase and K-feldspar can be used to estimate temperatures present during the immolation process (Fuhrmann and Lindsley 1988). Coexisting feldspar composition yielded temperatures ranging from 900 °C to 1200 °C. The spread in the data is probably due to the lack of equilibrium but consistent with the experimental results.
Temperature Constraints Based on Phase Diagrams
Phase equilibria in the system FeO–Al2O3–SiO2 by Osborn and Muan (1960 written comm., NIST 2004) show that fayalite and hercynite coexist along a cotectic line over a temperature range of 1100–1200 °C. These temperature constraints should only be viewed as lower constraints since the observed phase compositions deviate significantly from this end-member system due to the incorporation of a significant MgO (forsterite) component.
Temperature Constraints Based on XRD of Cremated Bones from Immolation Sites
Calcination of bones is a thermal process leading to thermal decomposition and hence removal of the volatile fraction of bone material. During this process organic matter (collagen ) burns off and minerals such as hydroxyl-apatite remain. During heating, the color of the bones changes from yellow to white. Due to heating shrinkage and extensive fragmentation of bones also occur. HAT-XRD studies of bone material show that at ca. 350 °C organic matter burns off and hydroxylapatite recrystallizes with increasing temperature (Haberko et al. 2006). This is indicated by XRD patterns which become sharper with increasing temperature due to better crystallization of apatite. At T > 700 °C CaO forms (Haberko et al. 2006). The size of the crystallites also strongly increases and Piga et al. (2008) conducted a calibration of apatite crystallite size as a function of temperature based on cremated human remains. It turns out that not only temperature is important but the duration of firing is important as well. Crystallite sizes of bone apatite from two ritual immolation site s namely the Scheibenstuhl near Nenzing in Vorarlberg and from the calcinated bone deposit of the ritual immolation site at Weer in the lower Inn Valley yielded apatite crystallite sizes of 300–380 nm which corresponds to temperatures of 650–750 °C according to Piga et al. (2008). It is interesting to note that the calcinated bones show much lower temperatures (>650 °C) than the slags (1000–1100 °C).
Mineralogical Implications
The investigated sites (Goldbichl/Igls and Oetz) contain pyrometamorphic slag s (Goldbichl shows massive amounts and Oetz only small amounts) and temperatures derived from these slags are >1000–1100 °C under highly reducing conditions (QFM). Tropper et al. (2004) pointed out the possible importance of P-bearing phases such as whitlockite and P-bearing olivines in pyrometamorphic slags as diagnostic phases for bone–rock interaction at very high temperatures. The subsequent experimental investigations by Tropper et al. (2006) and Spielmann (2013) confirmed this suggestion since whitlockite + P-bearing olivine indeed did form in the bone–rock experiments. The formation of whitlockite in a quartzphyllite precursor rock can be explained by using a model reaction such as:
On the other hand, Schneider et al. (2013) investigated the pyrometamorphic slag s from the Goldbichl site and found in a former apatite micro-domain P-rich olivine coexisting with stanfieldite instead of whitlockite . Therefore, Schneider (2009) concluded that the occurrence of phosphoran olivine + stanfieldite is restricted to extremely P-rich domains in the rocks and thus is only related to the breakdown of accessory detrital apatite in the slag . The formation of P-rich olivine from accessory apatite is thus in contrast to the investigations by Tropper et al. (2004) who proposed that bone–rock interactions alone are responsible for the formation of phosphoran olivines and phosphates . This fact might also be reflected by the presence of a different phosphate phase in the slag samples from the Goldbichl. In this investigation a TCP phase (stanfieldite) instead of whitlockite was found. Therefore, caution is advised concerning archaeological implications based on the occurrence of phosphoran phases in slags from ritual immolation site s since they can also form without the presence of bone material. Based on the mineralogical observations, P-rich minerals, which indicate the presence of a P-source in the fire, are as follows: whitlockite, P-rich olivine, P-rich clinopyroxene , and stanfieldite. The following mineral assemblage is experimentally shown to be associated with bone–rock interactions: P-rich olivine ± P-rich clinopyroxene, yet only when coexisting with whitlockite. P-rich olivine, P-rich clinopyroxene, and stanfieldite form by decomposition of detrital apatite in the slags of the Goldbichl and no bone material is involved in their formation. P-rich olivine and whitlockite have been found in the slags from Oetz and based upon the experimental results bone material might be involved in their formation.
The pyrometamorphic slag s as well as the experimental investigations show P-bearing olivines as a diagnostic phase. The occurrence of phosphoran olivine with significant phosphorus contents (>2 wt% P2O5) has been reported from only a few unusual places of the world (see Tropper et al. 2004). The terrestrial phosphoran olivines commonly occur together with farringtonite and/or stanfieldite (Agrell et al. 1998). In contrast, experimental investigations by Boesenberg and Hewins (2010) yielded phosphoran olivines with up to 27 wt% P2O5. Synthetic olivines with slightly elevated phosphorus contents (<1 wt% P2O5) are known from blast furnace slags from modern, prehistoric, and medieval smelting operations (Müller et al. 1988; Heimann et al. 1998) and phosphoran olivines with up to 10.5 wt% P2O5 were reported from wrought iron from the USS Monitor (Boesenberg 2006).
Because of the similarity of crystal chemical properties of P and Si (low cation radius, highly charged) both occupy preferentially the tetrahedral site. Previous descriptions of the occurrence of phosphoran olivines indicate that they grew under strongly disequilibrium conditions and P substitution for Si in olivine is not surprising since several phosphates with olivine-like structures exist (e.g., Langer et al. 2006, 2007). Therefore, with respect to the nature of the olivine–phosphate phase solid solution several possibilities for olivine-phosphate solid solutions exist. The structure of farringtonite Mg3(PO4)2 is closely related to the structure of forsterite, but shows monoclinic instead of orthorhombic symmetry. Similar to olivine, the three-dimensional framework of farringtonite is composed of PO4-tetrahedra that are linked together by metal-oxide polyhedra. There are also two sites M1 and M2, which are five and sixfold coordinated (MgO5, i.e., MgO6) according to Nord and Kierkegaard (1968). But even more closely related to the olivine structure are the phosphate minerals sarcopside (Fe3(PO4)2, Moore 1972) and its Mg-rich counterpart chopinite (Mg3(PO4)2, Grew et al. 2010) which also have an olivine-type structure with hexagonal closest oxygen package. Therefore, phosphoran olivines can form due to the breakdown of detrital apatite by model reactions such as:
Archaeological Implications
Contrary to the slags bone apatite crystallinity of calcinated bones yields much lower temperatures . This might be due to their position in “cooler” spots of the fire (e.g., at or very close to the surface). The high temperatures deduced from the slags (1000–1100 °C) are compatible with core temperatures of large bone fires with a possible wind-driven air circulation. Concerning the source of phosphorus in the slags one also has to bear in mind that bone material might not be the only source of P since meat and wood also contain P and if the samples were found in cultivated soil post-ritual immolation activities over the centuries such as fertilization of soil also alter the P contents of the samples. The archaeological implications for the Goldbichl site point toward the implication that the massive amounts of slags probably represent the last firing event of the immolation site most likely the ritual “closing” of the site by an enormous fire and hence they are most likely not associated with ritual immolation activities. The archaeological implications for the Oetz site imply that bone–rock interactions at very high temperatures might indeed have occurred although clear archaeological evidence (e.g., bone–slag sample) is lacking. High-temperature bone–rock interaction could occur when small bone fragments migrate into deeper and hence hotter portions of the fire since usually bones crack and defragment and no flesh is attached to the bones anymore.
The experiments have shown that the occurrence of the phosphate mineral whitlockite in pyrometamorphic slag s from ritual immolation site s could indeed be mineralogically diagnostic for bone–rock interactions but only when archaeological data are considered.
References
Agrell SO, Charnley NR, Chinner A (1998) Phosphoran olivine from Pine Canyon, Piute Co., Utah. Mineral Mag 62:265–269
Boesenberg JS (2006) Wrought iron from the USS monitor: mineralogy, petrology and metallography. Archaeometry 48:613–631
Boesenberg JS, Hewins RH (2010) An experimental investigation into the metastable formation of phosphoran olivine and pyroxene. Geochim Cosmochim Acta 74:1923–1941
Bousenberg JS, Ebel RH, Hewins RH (2004) An experimental study of phosphoran olivine and its significance in main group pallasites. In: Lunar and Planetary Science Conference XXXV, Lunar Planet Institute, Houston:#1368 (abstract)
Brearley AJ (1987) An experimental and kinetic study of the breakdown of aluminous biotite at 800 °C: reaction microstructures and mineral chemistry. Bull Mineral 110:513–532
Brearley AJ, Rubie DC (1990) Effects of H2O on the disequilibrium breakdown of muscovite + quartz. J Petrol 31:925–956
Brindley GW, Chang T (1974) Development of long basal spacings in chlorites by thermal treatment. Am Mineral 59:152–158
Brunet F, Chazot G (2000) Partitioning of phosphorus between olivine, clinopyroxene and silicate glass in a spinel lherzolite xenolith from Yemen. Chem Geol 176:51–72
Buseck PR, Clark J (1984) Zaisho – a pallasite containing pyroxene and phosphoran olivine. Mineral Mag 48:229–235
Buseck PR, Holdsworth E (1977) Phosphate minerals in pallasite meteorites. Mineral Mag 41:91–102
Chlingensperg MV (1904) Der Knochenhügel am Langacker und die vorgeschichtliche Herdstelle am Eisenbichl bei Reichenhall in Oberbayern. Mitt Anthrop Ges Wien 34:53–70
Cultrone G, Rodriguez-Navarro C, Sebastian E, Cazalla O, De La Torre MJ (2001) Carbonate and silicate phase reactions during ceramic firing. Eur J Mineral 13:621–634
Fuchs LH (1967) Stanfieldite: a new phosphate mineral from stony-iron meteorites. Science 158:910–911
Fuhrmann ML, Lindsley DL (1988) Ternary feldspar modelling and thermometry. Am Mineral 73:201–215
Gleirscher P, Nothdurfter H, Schubert E (2002) Das Rungger Egg. Röm-German Forsch, Verlag Philipp von Zabern, Mainz, 61, 264 pp
Goodrich CA (1984) Phosphoran pyroxene and olivine in silicate inclusions in natural iron-carbon alloy, Disko Island, Greenland. Geochim Cosmochim Acta 48:2769–2771
Grapes RH (1986) Melting and thermal reconstitution of pelitic xenoliths, Wehr Volcano, East Eifel, West Germany. J Petrol 27:343–396
Grapes RH (2006) Pyrometamorphism. Springer, New York, 275 pp
Grew ES, Yates MG, Beane RJ, Floss C, Gerbi C (2010) Chopinite-sarcopside solid solution, [(Mg, Fe)3□](PO4)2, in GRA95209, a transitional acapulcoite: implications for phosphate genesis in meteorites. Am Mineral 95:260–272
Guggenheim S, Zhan WD (1999) Crystal structures of two partially dehydrated chlorites: the modified chlorite structure. Am Mineral 84:1415–1421
Haberko K, Bućko MM, Brzezińska-Miecznik J, Haberko M, Mozgawa W, Panz T, Pyda A, Zarębski J (2006) Natural hydroxyapatite—its behaviour during heat treatment. J Eur Ceram Soc 26:537–542
Heimann R, Kreher U, Oexele J, Hirsekom V, Ullrich O, Janke D, Ullrich B, Lindner H, Wagenbreth B (1998) Archaeometallurgical investigations into the iron production technology in Upper Lusatia, Saxony, from the early Iron Age (Billendorf period) to the 12th century A.D. Eur J Mineral 10:1015–1035
Hoinkes G, Thöni M, Bernhard F, Kaindl R, Lichem C, Schweigl J, Tropper P, Cosca M (1997) Metagranitoids and associated metasediments as indicators for the pre-Alpine magmatic and metamorphic evolution of the Western Austroalpine Ötztal Basement (Kaunertal, Tirol). Schweiz Mineral Petrogr Mitt 77:299–314
Krämer W (1966) Prähistorische Brandopferplätze. Helv Antiqua Festschr Emil Vogt 111–122
Langer K, Taran MN, Fransolet AM (2006) Electronic absorption spectra of phosphate minerals with olivine-type structures: I. Members of the triphylite-lithiophilite series, M1[6]LiM2[6](Fex2+Mn1-x 2+)[PO4]. Eur J Mineral 18:337–344
Langer K, Taran MN, Fransolet AM (2007) Electronic absorption spectra of phosphate minerals with olivine-type structures: II. The oxidized minerals ferrisicklerite, M1[6](□1−xLix)M2[6](Fe3+ 1−xMn2+ x)[PO4], and heterosite, M1[6](□ 1.00)M2[6](Fe3+ 1−xMn3+ x)[PO4], with x ≤ 0.5. Eur J Mineral 19:589–592
Le Maitre RW (1974) Partially fused granite blocks from Mt. Elephant, Victoria, Australia. J Petrol 15:403–412
Maury RC, Bizouard H (1974) Melting of acid xenoliths into a basanite: an approach to the possible mechanisms of crustal contamination. Contrib Mineral Petrol 48:275–286
Moore PB (1972) Sarcopside: its atomic arrangement. Am Mineral 57:24–35
Müller G, Schuster AK, Zippert Y (1988) Spinifex textures and texture zoning in fayalite-rich slags of medieval iron-works near Schieder Village, NW-Germany. N Jb Mineral Monatsh 3:111–120
NIST (2004) Phase Equilibria Database Version 3.0
Nord AG, Kierkegaard P (1968) The crystal structure of Mg3(PO4)2. Acta Chem Scand 22:1466–1474
Piber A (2005) The metamorphic evolution of the Austro-Alpine nappes north of the Tauern window (Innsbruck Quartzphyllite Complex – Patscherkofel Complex – Kellerjochgneiss and Wildschönau Schist). Unpublished Ph.D. Thesis, University of Innsbruck, 261 pp
Piga G, Malgosa A, Thompson TJU, Enzo S (2008) A new calibration of the XRD technique for the study of archaeological burned human remains. J Archaeol Sci 35:2171–2178
Schneider P (2009) Mineralogisch-petrologische Untersuchungen der Pyrometamorphose in Brandopferplätzen. Institut für Mineralogie und Petrographie. Unpubl MSc Thesis, University of Innsbruck, 124 pp
Schneider P, Tropper P, Kaindl R, Tomedi G (2013) The formation of P-rich olivine and stanfieldite from the pyrometamorphic breakdown of apatite in slags of a prehistoric ritual immolation site (Goldbichl, Igls, Tyrol, Austria). Mineral Petrol 107:327–340
Spielmann M (2013) Experimentelle Untersuchungen zu Knochen-Gesteinswechselwirkungen in Brandopferplätzen. Unpublished Bsc Thesis. University of Innsbruck, 59 pp
Stadler H, Leib S, Gamon T (2013) Brandopferplätze in den Alpen. Der Scheibenstuhl in Nenzing. Prearchos 3, 136 pp
Steiner H (2010) Alpine Brandopferplätze: archäologische und naturwissenschaftliche Untersuchungen. Forsch Denkmal Südtirol V, 907 pp
Tomedi G, Nicolussi Castellan S (2000) Ein bronze- und eisenzeitlicher Brandopferplatz am Goldbichl bei Igls (Bez. Innsbruck-Stadt). ArchaeoTirol Kl Schrift 2:122–123
Tropper P, Recheis A, Konzett J (2004) Experimental investigations on the pyrometamorphic formation of phosphorous-bearing olivines in partially molten metapelitic gneisses. Eur J Mineral 16:631–640
Tropper P, Konzett J, Recheis A (2006) Experimental investigations on the pyrometamorphic formation of phosphorus-bearing olivines in partially molten metapelitic gneisses. Mitt Österr Mineral Ges 152:47–56
Villieras F, Yvon J, Cases JM, De Donato P, Lhote F, Baeza R (1994) Development of microporosity in clinochlore upon heating. Clays Clay Mineral 42:679–688
Weiss RM (1997) Prähistorische Brandopferplätze in Bayern. Intern Archäologie 35, 203 pp
Acknowledgments
Financial support through the FWF special research program HiMAT (F3110-G02 to P.T.) is gratefully acknowledged. Thanks to Philipp Schneider and Magdalena Spielmann for the data, Daniela Schmidmair for her help with high-T XRD, and Waltraud Wertl for her help with DTA-TG. Gerhard Tomedi is thanked for helpful discussions concerning the archaeology of ritual immolation site s . Harald Stadler, Gerhard Tomedi, and Ulrike Töchterle are thanked for providing calcinated bone samples from immolation sites.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tropper, P. (2016). Bones, Rocks, and Flames: Mineralogy and Petrology of Slags and Cremated Bones from Ritual Immolation Sites in Tyrol. In: Grupe, G., McGlynn, G. (eds) Isotopic Landscapes in Bioarchaeology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-48339-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-662-48339-8_3
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-48338-1
Online ISBN: 978-3-662-48339-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)