Abstract
Development of the endoscopic techniques has had a major contribution to the diagnosis of early pathologic lesions. Now a day, the role of endoscopy is not only diagnostic tools but also treatment modality. In addition, as colonoscopy is enforced widely as a colorectal cancer screening test, the advanced polyps and early colorectal cancer is increased. It means that needs for the therapeutic endoscope is also increasing.
Laterally spreading tumors (LST) of the colon are best removed by endoscopic mucosal resection as they extend laterally rather than vertically. Since they sometimes invade deeply into the submucosal layer, it is important to assess the depth of invasion endoscopically before treatment. Recently, endoscopic submucosal dissection (ESD) has often been used for the treatment of colorectal LST. It is necessary to carefully select the treatment modality considering the risk of malignancy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
3.1 Laterally Spreading Tumor
Development of the endoscopic techniques has had a major contribution to the diagnosis of early pathologic lesions. Now a day, the role of endoscopy is not only diagnostic tools but also treatment modality. In addition, as colonoscopy is enforced widely as a colorectal cancer screening test, the advanced polyps and early colorectal cancer is increased. It means that needs for the therapeutic endoscope is also increasing.
Most colorectal polyps are protruded or pedunculated type. It can be removed by snare polypectomy easily. About 7–36% of colorectal tumors are flat or depressed lesions which are known to have a high possibility of submucosal invasion comparing to pedunculated polyps [1,2,3]. These sessile or non-polypoid colorectal polyps are still challenging to remove endoscopically.
The laterally spreading tumor (LST) is a colorectal neoplasm, larger than 10 mm in diameter, characterized by a horizontally extending growth pattern with a relatively low vertical axis. LSTs are classified into two types, the granular (LST-G ) and non-granular (LST-NG ) types depending on the presence or absence of a surface nodularity. The granular type consists of collecting nodules that form uneven granular or nodular surfaces, whereas the non-granular type exhibits a flat, smooth surface. The detection of the LST-NGs is also not easy. To avoid missing these lesions, we should take note of pale redness, little deformation of wrinkles, loss of blood vessels.
The LST-G is subclassified into homogeneous (G-H) and nodular mixed (G-NM) types and the LST-NG into flat elevated (NG-FE) and pseudo-depressed (NG-PD) types. LSTs have been regarded as less invasive than other polypoid tumors of similar size. However it is important to observe the lesion carefully before deciding the treatment, because LSTs have different malignant potentials depending on its type. Nodular mixed tumors were associated more frequently with a villous adenoma component, and the giant nodules or concavities present in nodular mixed tumors were related to malignant potential. The LST-NGs, especially pseudo-depressed types, have a higher malignant potential than LST-Gs [4] and thus are considered a good indication for ESD to avoid unintended piecemeal resections (Fig. 3.1).
The size and type of LSTs are good predictors of invasive cancer, and the proportion of submucosal carcinoma increases with increasing size of flat depressed types. Non-granular LSTs larger than 30 mm were submucosal invasive carcinoma in 60% of cases. In contrast, homogeneous tumors are not associated with submucosal invasive carcinoma, even when they are larger than 30 mm. Generally, large size, depressed phenotype, and large nodules (≥10 mm) are known to be predictive markers of invasive carcinoma in LSTs [5].
In treating LSTs, conventional endoscopic mucosal resection (EMR) or endoscopic piecemeal resection (EPMR) technique can be suitable for homogeneous tumors. It is also recommended that flat elevated or pseudo-depressed or nodular mixed tumors larger than 20 mm, should be managed using endoscopic submucosal dissection (ESD) with en-bloc resection by the experienced endoscopists.
3.2 EPMR vs. ESD
EMR can replace the surgery in the treatment of early colon cancer without lymph node metastasis. Most colorectal polyps can be treated using simple snaring or EMR, although large sessile colorectal tumors exceeding 20 mm can’t be removed by traditional EMR procedure. For these tumors, piecemeal resection is recommended [6]. The merit of piecemeal resection is that it is safe for resecting large sessile polyp . LSTs with granular homogenous type can be safely treated by EPMR, even it is larger than 30 mm. However, the fragmentation of specimens during conventional polypectomy or EMR prevents the evaluation of the resection margin involvement or the depth of tumor invasion, making it difficult to plan further treatment. In addition, any tumor cells remaining after piecemeal polypectomy can grow on the polypectomy scar and invade the submucosal layer more rapidly. Thus care should be used to remove suspicious malignant tumor including pseudo-depressed type LSTs by EPMR [6,7,8]. ESD introduced to overcome these limitations is now widely used for excising various gastrointestinal tumors, including colorectal tumors. ESD results in higher rates of en-bloc tumor resection, reducing local recurrences and providing more accurate pathologic information for planning further treatment. However, the procedure time is much longer and the complication rate is much higher for ESD than for EMR, limiting the use of ESD in the removal of colorectal tumors [9, 10].
According to several studies reported the outcomes of ESD, en-bloc resection rate is 84.9% (95% CI, 77.8–90.8), curative resection rate is 75.4% (95% CI, 66.7–82.2). A study on the long term outcomes of colorectal ESD found that the local recurrence rate was 2%, and the 3 and 5 year disease free survival rates were 97% and 95%, respectively. Safety outcomes of colorectal ESD are also important, in as much as perforation associated with this procedure was reported in 3.3–20.4%, with tumor size and the presence of fibrosis being independent risk factors for perforation [11,12,13,14]. Although many cases showing perforation have been improved with conservative treatment without surgery, using endoscopic clipping, it make to prolonged hospitalization and to need additional treatments.
In summary, both EPMR and ESD techniques can be used for large sessile polyps or LSTs. EPMR technique has merits of safety, ease and demerits of the difficulty of pathologic evaluation, high rates of local recurrences. ESD technique has merits of en-bloc resection, low rate of recurrence and demerits of long operation time, high rates of complications. Thus, ESD should be recommended for LSTs with suspected malignancy or lesions are technically difficult to treat with conventional EMR, and EPMR should be recommended for adenomatous lesions, LSTs with granular homogenous type [12, 15,16,17,18].
3.3 Indication of ESD
Basically, all endoscopic treatment is recommended only for lesions diagnosed as non-invasive tumors with a low metastatic potential. The risk factors for lymph node metastasis are poorly differentiated, signet-ring cell, and mucinous adenocarcinoma, massive submucosal invasion, lymphovascular invasion and tumor budding.
Current indications for colorectal ESD include (1) early colorectal cancer, (2) laterally spreading tumors ≥2 cm in diameter, (3) submucosal tumors, and (4) colorectal polyps with fibrosis. In detail, ESD study group in Japan announced the indication of colorectal ESD include LSTs with non-granular pseudo-depressed type, mucosal lesions with fibrosis caused by inflammation or scar change after biopsy, the tumor with underlying ulcerative colitis, recurred tumors after EMR resection, etc. They excluded the size criteria, more than 20 mm, in ESD indication, because depressed-type tumors with less than 20 mm in size can invade submucosal layer. And they also commented the submucosal infiltration of tumors should be shallow (Table 3.1).
3.4 Preoperative Diagnosis
Before procedure, malignant potential and margins should be clearly identified. Tumor morphology including color, unevenness, depression, fold convergence also carefully evaluated. Malignant tumors have the loss of the surface pattern of pits or the structure of micro-vessels. Dye spraying, magnifying endoscopy, narrow band image can be helpful to identifying it. For predicting the malignant polyps, the accuracy of conventional endoscopy is about 80%, and that of chromo-magnifying endoscopy is up to 96–98%. Endoscopic ultrasonography (EUS) is also helpful to diagnose the submucosal invasive cancer, however it is still not popular because the additional equipment is required.
3.5 ESD Instruments
3.5.1 Knifes
Some kinds of knifes are used for ESD treatment of colon tumors. The Dual knife (Olympus Optical Co., Tokyo, Japan) is most commonly used, and also the Flush knife BT (Fujifilm Medical, Tokyo, Japan) and the Jet B-knife (ZeonMedical, Tokyo, Japan) which are capable of injecting the submucosa solution, are used for colorectal ESD. A notable characteristic of the Jet B-knife is the use of the bipolar current system—it can minimize the damage to the muscle layer and reduce the risk of perforation. The Insulated tip knife-nano (IT-nano, Olympus Optical Co.) has also been developed and have been utilized in colorectal ESD, which has a merit of bringing out the relatively fast treatment. The Hook knife (Olympus Optical Co.) can be used to lift and cut the tissues in cases of the LSTs with fibrosis or the difficult-to-reach tumors. And recently, the clutch cutter (Fujifilm Corp., Tokyo, Japan) of grasping-type scissor forceps and the SB knife Jr. (Sumitomo Bakelite Co., Tokyo, Japan) has been developed and used (Fig. 3.2).
3.5.2 Hemostatic Forceps
The Hemostat-Y (H-S2518; Pentax) of a bipolar-type hemostatic Forceps and the Coagrasper (FD-410LR; Olympus Medical Systems Co., Tokyo, Japan) of a monopolar-type forceps have been currently used in ESD procedures.
3.5.3 Distal Attachments
Various distal attachments such as a standard transparent cap or the ST hood short-type (DH-28GR and 29CR; Fujifilm Medical Co., Tokyo, Japan) are useful for colorectal ESD. A transparent cap is usually attached at the distal end of the endoscope, which make it easy to dissect the submucosal layer with lifting up the lesions. This also can be used as an auxiliary tool for compressing the tissue during bleeding (Fig. 3.3).
3.5.4 Submucosal Injection Solutions
The maintenance of the sufficient submucosal elevation using injecting hypertonic solutions is essential for the success of the ESD. An ideal submucosal injection solution should be inexpensive, readily available, non-toxic, easy to prepare and inject, and should provide a long-lasting submucosal cushion. The normal saline solution is the most commonly used as the injection solution for conventional EMR. Saline-epinephrine injection has been shown to be an effective method for the complete endoscopic polypectomy, especially in flat or sessile lesions. However, other substances such as sodium hyaluronate, hydroxypropyl methylcellulose and glycerol, have been preferred for ESD procedures because of their ability to create a longer lasting submucosal cushion as a result of their viscous properties. A small amount of Indigo Carmine is also mixed to the submucosal-injecting solution to enhance the lesion and margins. Recently the ready-to-use sodium hyaluronate also commercially available—MucoUp® (Seikagaku Co., Tokyo, Japan) and Endo-ease (Unimed Co., Seoul, Korea).
3.5.5 CO2 Insufflation Systems
The use of carbon dioxide (CO2) gas is usually recommended in colorectal ESD. The CO2 insufflation into the colonic lumen has been proven effective to let the patient stand the long ESD procedure and to reduce the risk of pneumoperitoneum in cases of perforation and other complications. Operators should pay always attention to abdominal distension due to over-insufflation of the gas during ESD procedures.
3.5.6 Electrosurgical Generators
For ESD procedures, the multi-functioning electrosurgical generators are usually used, such as the VIO300D (ERBE, Tübingen, Germany) or the ESG100 (Olympus Medical Co.). The ERBE generator was set to the Endo-Cut mode (Effect 3, 60–80 W) for incision of the mucosa, and to the Endo-Cut mode (Effect 3, 60–80 W) or forced coagulation mode (40–50 W) for incision of the submucosa. Bleeding was controlled using hemostatic forceps, such as the Coagrasper (Olympus Optical) in the soft coagulation mode (50–80 W).
3.6 ESD Procedures
3.6.1 Incision of Mucosa
Because the boundary of colon lesions is usually clear, mucosal marking is non-essential for colorectal ESD. Only marking is needed for selective cases with a blurred margin. Generally, mucosal incision was made around margin with at least 5 mm after submucosal injection. For lifting flap easily, an initial mucosal incision was recommended in more than 1 cm apart from margin. And it is not recommended that the 360° surrounding incision around a tumor have been made without enough submucosal dissection because the leak of the injection solutions from the submucosal layer leads the loss of fields of views in submucosal layers and make it difficult to complete dissection. In order to achieve the complete dissection, it is necessary to formulate the strategy including the repeated sequences of submucosal injection, mucosal incision and submucosal dissection (Fig. 3.4).
ESD procedures. Endoscopic submucosal dissection for rectal neoplasia. (a) 50 mm sized middle rectal lesion which is a type 0-IIa, laterally spreading, intramucosal adenocarcinoma in adenoma. (b) Chromoendoscopic view with indigocarmine, showing demarcation of the margin of the lesion. (c) Cap applied. (d) Submucosal injection at the oral margin of the lesion, with the endoscope in a retroflexed position. (e) Initial mucosal incision at the anal margin of the lesion. (f) Extension of the incision in a circumferential manner around the lesion, with endoscope in a straight position. (g) Repetition of submucosal injections from the exposed submucosal layer. (h) Repetition of dissection of the submucosal connective tissue. (i) The artificial ulcer after removal. The vessels on the ulcer base are treated with hemostatic forceps to prevent bleeding. (j) Complete resection of the lesion in one piece
3.6.2 Submucosal Dissection
After making initial mucosal incision, firstly we should attempt the submucosal dissection up to identifying the muscle layer . A cap or a hood of the tip of endoscopy could be helpful to lift the mucosal flap. After identifying the muscle layer, then submucosal dissection would be kept continuously going at the level of lower 1/3 of submucosal layers. When the vessels are met, it can be controlled by coagulation using a knife or a coagrasper. Small-sized vessels were managed by slow-moving of a knife with a swift mode or a forced coagulation mode. Large-sized vessels are needed to a coagulation by the coagrasper with a soft coagulation mode with 50–80 W.
For dissecting the areas with submucosal fibrosis or infiltration by tumor, a careful approach should be needed with identifying an exact plane of dissection. The ESD is limited in these situations, because it makes it easier to occur the perforation (Fig. 3.4).
3.7 ESD for Special Situations
A lots of problems during ESD can be occurred in the cases of difficult locations of tumors, in which endoscopy is not reach to the tumor base, rather than in cases with large-sized tumors. In general, the difficulty of ESD is growing as tumor locations from the rectum up to cecum. To dissect it easier during submucosal dissection, the endoscopy should be placed in parallel to the muscle layer. However, it is difficult to make it in cecum, angulated areas of colon including hepatic flexure, splenic flexure, etc. Sometimes, the retroflexion of the scope would be helpful for reaching to the tumor. And also patients’ position change and air suction could be helpful (Fig. 3.5).
3.7.1 Rectal Lesions
ESD for rectal lesions is known to be easy, but ESD for the lesion closed to dentate line or anal canal is difficult because of the limited spaces. A perpendicular approach can be made by retroflextion of the scope, then the procedure from oral side to anal side is possible. The local anesthesia is also needed to reduce the pain during the dissection of tumors closed to anal canal. In this situation, the surgical local excision can be more preferred than ESD.
3.7.2 Sigmoid or Descending Colon
Sigmoid colon is severely bent and has the narrow interior space of the lumen. Because it is not attached to the retroperitoneum, it is freely movable and its shape can be altered during the procedure depending on the amount of air. Descending colon has also the narrow interior space of the lumen, even it is not much bent. It is difficult to perform ESD due to the limited movement of the scope. Because the endoscope is placed perpendicular to the lesion at the sigmoid-descending junction, it is hard to make a visualization of tumors. Endoscopists should not to try a retroflexion of the scope in sigmoid or descending colon. It is very a dangerous procedure because it is likely to damage a colon wall and to increase a risk of perforation.
3.7.3 Transverse Colon
The common problem of ESD at the transverse colon is a movement of colon due to an aortic pulsation and bowel peristalses. The patient’s aortic pulsation may be exaggerated in a supine position. The change of patient’s position such as the right decubitus or left decubitus can be helpful in this situation. However, the most important factor of deciding patients’ position is that tumors must be placed at the opposite site to the gravity. It is very important to make a visualization of submucosal dissection plane. It is necessary to find an optimal position of the patient in every ESD procedures.
3.7.4 Hepatic Flexure Colon
The endoscopic blind spot can be made due to the curved folds and acute angulation of the hepatic flexure colon . It is also very difficult to perform ESD in hepatic flexure colon. If necessary, a hook-knife and an IT-knife can be helpful to lift and dissect the lesions which is placed perpendicular to the scope.
3.7.5 Cecum
It is very difficult to crossly approach to the dome-shape portion of the cecum . It is recommended to perform EMR or EPMR beside of ESD, if it looks like a benign lesion. However, if en-bloc resection is needed for a suspicious malignant lesion, various knives including a hook knife, a dual knife and an IT-nano knife would be used even time-consuming. The approaches from medial (ileocecal valve) to lateral (anti-mesenteric) would be helpful to complete ESD. Sometime it is needed to reduce the electrical power of the electro-surgical units for the safe dissection.
3.7.6 Appendiceal Orifice
It is better to enforce the surgical resection of cecum, if the tumor is in growing up into the appendiceal orifice . However, ESD can be tried when the tumor is located closely or focally involve to the appendiceal orifice. In this situation, the operator should explain to the patient in advance that acute appendicitis after EMR or ESD could be developed and that prophylactic antibiotics also could be needed.
3.7.7 Ileocecal Valve
Since the mucosal surface of the ileocecal valve is granular, it is easy to be overlooked even in the presence of adenomas. Sometimes the tumor located in the ileocecal valve can be grown up to the terminal ileum. Thus, it is not easy to perform ESD for tumors located at the ileocecal valve. However, the relatively-thickened wall of the ileocecal valve may reduce the chance of perforation during ESD. When the tumor is extended to the terminal ileum, it is better to dissect the ileal side firstly during ESD. The enough saline injection at the ileal submucosa can lead to the expulsion of the tumor from the ileum into the colon. Rapid dissection or excision of tumors can be performed at that time. Argon plasma coagulation is helpful for remove the remnant tumor at the terminal ileum. If the tumor involves the ileocecal valve circumferentially, it is very difficult to complete ESD. In this situation, the stricture can be occurred after complete removal of tumor by EPMR or ESD.
3.7.8 ESD in Submucosal Fibrosis
Submucosal fibrosis can be induced by cancer cell invasion, inflammation, etc. It is careful that the biopsy can make a submucosal fibrosis in flat tumors or the laterally spreading tumors. When there is severe submucosal fibrosis under the tumor, even a simple snaring is likely to make a perforation due to muscle injury. Thus, ESD is preferred to snaring or EMR in this situation. However, a non-lifting mucosal layer and a hard submucosal layer are not easy to make a visualization of a correct dissecting plane during ESD.
For setting the electrosurgical unit, a forced coagulation mode or a swift coagulation mode is commonly used during ESD. For the fibrotic lesions, it is helpful that the coagulation current is decreased and cutting current is increased.
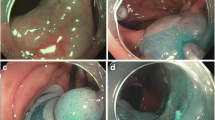
The knife should be carefully moved to submucosal plane crossly in fibrotic areas. Because severe fibrosis can lead to an incorrect plane and to increase a muscle injury, the dissection of severe fibrotic areas could be recommended to leave it to the last minute during ESD. Although the mucosal lifting after saline injection is not enough at the fibrotic areas, the frequent submucosal injection is needed to visualize clearly a correct plane, even a little (Fig. 3.6).
Submucosal fibrosis. Non-lifting mucosal layer and a hard submucosal layer are not easy to make a visualization of a correct dissecting plane during ESD. Upper three photos were fibrosis without submucosal invasion and lower three photos were massive submucosal invasion or proper muscle invasion cases
3.8 Histopathology
For a precise histological diagnosis, which determines the need for additional treatment after ESD, a proper handling of resected specimens by ESD is required. Microscopic tissue diagnosis is performed through the series of processes including fixing the specimen in the plate, a formalin fixation, a gross observation and a section of the specimen.
3.8.1 Specimen Fixation and Histologic Diagnosis
The resected specimen by ESD is firstly fixed in a rubber or cork-sheet plate using several pins. And then it has to be fixed into the 10–20% formalin solution after the saline-washing as soon as possible. The direction also should be marked in the specimen plate (Fig. 3.7).
Pathologic examination. (a) The specimen is fixed with formalin and sliced in 2- to 2.5-mm intervals. Red lines indicate the adenocarcinoma after mapping. (b) The adenocarcinoma is confined in the submucosa layer 500 μm sm1 depth with risk factor lymphovascular emboli (hematoxylin and eosin [H&E] stain, ×200). (c) Images arranged in line (H&E stain)
A gross observation including the size, color, shape and hardness of the tumor is needed before cutting the tumor. It is important to decide the cutting line and sections through the discussion of clinical information and gross observation between the endoscopist and the pathologist.
Histologic diagnosis should be included to the histologic type, the depth of invasion, the presence of lymphovascular invasion, the resection margin including vertical and lateral, tumor buddings, a pattern of invasion. These factors are used for determining the additional treatment such as a radical surgery.
It would be necessary to surgical resection, if histopathology findings are shown to have one of follows; (1) deep submucosal invasion (sm2 or more), (2) if differentiation is bad (poorly differentiation), (3) mucinous carcinoma or signet-ring cell carcinoma, (4) lymphatic or vascular invasion, (5) positive tumor budding, (6) positive resection margin. Therefore, endoscopists should keep it in mind and try not to resect completely the tumor with deep submucosal invasion. And they also have to try to get an enough resection margin without tissue fragmentation.
Conclusions
When you perform the endoscopic treatment for colorectal lesions, you have to consider the indications, tumor location, size, depth of invasion, the skill of an endoscopist and the hospital facility for emergency surgery. Colorectal ESD is the most advanced skill in the fields of therapeutic endoscopy. Currently many of reports showed that ESD results in higher rates of en-bloc tumor resection, reducing local recurrences and providing more accurate pathologic information for planning further treatment. However, the procedure time is much longer and the complication rate much higher for ESD than for EMR. For the safe ESD procedures, endoscopists should be trained well to advance steadily.
References
Tsuda S, Veress B, Toth E, Fork FT. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut. 2002;51:550–5.
Saitoh Y, Waxman I, West AB, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–65.
O’Brien MJ, Winawer SJ, Zauber AG, et al. Flat adenomas in the National Polyp Study: is there increased risk for high-grade dysplasia initially or during surveillance? Clin Gastroenter Hepatol. 2004;2:905–11.
Shin-ei K. Endoscopic treatment of neoplasms in colon and rectum. Tokyo: Igaku-Shoin Ltd.; 2000.
Tanaka S. Basic technique of endoscopic mucosal resection and endoscopic submucosal dissection for colorectal tumors – knack, pitfall, and conclusive evidence of adjustment. Japan: Medical View; 2006.
Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–79.
Pech O, May A, Gossner L, Rabenstein T, Ell C. Management of pre-malignant and malignant lesions by endoscopic resection. Best Pract Res Clin Gastroenterol. 2004;18:61–76.
Larghi A, Waxman I. State of the art on endoscopic mucosal resection and endoscopic submucosal dissection. Gastrointest Endosc Clin N Am. 2007;17:441–69.
Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–7.
Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100–7.
Tanaka S, Terasaki M, Hayashi N, Oka S, Chayama K. Warning for unprincipled colorectal endoscopic submucosal dissection: accurate diagnosis and reasonable treatment strategy. Dig Endosc. 2013;25:107–16.
Saito Y, Fukuzawa M, Matsuda T, et al. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343–52.
Tanaka S, Haruma K, Oka S, et al. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62–6.
Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220–30.
Puli SR, Kakugawa Y, Saito Y, Antillon D, Gotoda T, Antillon MR. Successful complete cure en-bloc resection of large nonpedunculted colonic polyps by endoscopic submucosal dissection: a meta-analysis and systematic review. Ann Surg Oncol. 2009;38:493–7.
Fujishito M, Yahagi N, Nakamura M, et al. Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy. 2006;38:493–7.
Hurlstone DP, Atkinson R, Sanders DS, Thomson M, Cross SS, Brown S. Achieving R0 resection in the colorectum using endoscopic submucosal dissection. Br J Surg. 2007;94:1536–42.
Tanaka S, Kashida H, Saito Y, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Youk, EG. (2018). Management of Large Sessile Polyps: EPMR vs. ESD. In: Sohn, D. (eds) Practice and Principles in Therapeutic Colonoscopy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46552-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-662-46552-3_3
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46551-6
Online ISBN: 978-3-662-46552-3
eBook Packages: MedicineMedicine (R0)