Abstract
A limbic brain area, the amygdala plays a key role in emotional responses and affective states and disorders such as learned fear, anxiety, and depression. The amygdala has also emerged as an important brain center for the emotional–affective dimension of pain and for pain modulation. Hyperactivity in the laterocapsular division of the central nucleus of the amygdala (CeLC, also termed the “nociceptive amygdala”) accounts for pain-related emotional responses and anxiety-like behavior. Abnormally enhanced output from the CeLC is the consequence of an imbalance between excitatory and inhibitory mechanisms. Impaired inhibitory control mediated by a cluster of GABAergic interneurons in the intercalated cell masses (ITC) allows the development of glutamate- and neuropeptide-driven synaptic plasticity of excitatory inputs from the brainstem (parabrachial area) and from the lateral–basolateral amygdala network (LA-BLA, site of integration of polymodal sensory information). BLA hyperactivity also generates abnormally enhanced feedforward inhibition of principal cells in the medial prefrontal cortex (mPFC), a limbic cortical area that is strongly interconnected with the amygdala. Pain-related mPFC deactivation results in cognitive deficits and failure to engage cortically driven ITC-mediated inhibitory control of amygdala processing. Impaired cortical control allows the uncontrolled persistence of amygdala pain mechanisms.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Pain is a complex disorder with sensorimotor as well as emotional–affective and cognitive components (see Fig. 1). The amygdala, an almond-shaped brain area in the medial temporal lobe, plays an important role in the emotional–affective dimension of pain (Neugebauer et al. 2004, 2009) and, through interactions with cortical areas, also contributes to cognitive aspects such as pain-related decision-making deficits (Ji et al. 2010).
1 Pain-Related Amygdala Circuitry
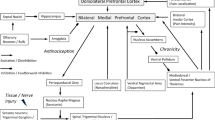
The amygdala is closely interconnected with numerous cortical, subcortical, and brainstem areas. Figure 2 shows key amygdala nuclei and their connections relevant to sensory and pain-related processing. The lateral–basolateral nuclei (LA-BLA) form the input region for sensory, including nociceptive, information from thalamus (posterior areas) and cortical areas such as insula, anterior cingulate cortex, and other medial prefrontal cortical areas (Orsini and Maren 2012; Pape and Pare 2010; Marek et al. 2013; Price 2003). BLA projections of the medial prefrontal cortex (mPFC) provide emotion- and value-based information to guide executive functions such as decision-making and behavior control (McGaugh 2004; Holland and Gallagher 2004; Laviolette and Grace 2006). The BLA contains neurons that respond preferentially to noxious stimuli (Ji et al. 2010).
Amygdala circuitry and interactions with cortical systems and brainstem. Input regions (lateral and basolateral amygdala, LA, BLA) process and transmit polymodal sensory and nociceptive information from thalamocortical systems to the amygdala output region (central nucleus, CeA) through direct excitatory projections or indirect feedforward inhibition involving interneurons in the intercalated cell mass (ITC). BLA forms close connections with mPFC that involve inhibitory interneurons in the cortex resulting in feedforward inhibition of mPFC principal cells. mPFC output neurons can engage ITC cells to control amygdala output. Dashed line indices area of the amygdala
Highly processed information generated in the LA-BLA network is transmitted to the central nucleus (CeA), which serves major amygdala output functions and projects to pain modulatory systems through forebrain and brainstem connections (Mason 2005; Neugebauer et al. 2004; Bourgeais et al. 2001; Price 2003) (Fig. 2). The laterocapsular division of the CeA (CeLC) receives nociceptive-specific information from the spinal cord and brainstem (external lateral parabrachial area) through the spino-parabrachio-amygdala pain pathway (Gauriau and Bernard 2002). The vast majority of CeLC neurons respond exclusively or predominantly to noxious stimuli and have large bilateral, mostly symmetrical receptive fields (Neugebauer et al. 2004, 2009). These CeLC neurons show non-accommodating spike firing properties characteristic of medium-size spine-laden peptidergic or GABAergic Type A projection neurons with targets in the brainstem, including PAG, and forebrain (Schiess et al. 1999; Jongen-Relo and Amaral 1998; Sun and Cassell 1993). Peptidergic (CRF or enkephalin containing) CeA projection neurons are innervated by calcitonin gene-related peptide (CGRP) containing terminals from the parabrachial area (Schwaber et al. 1988; Dobolyi et al. 2005; Harrigan et al. 1994), which is consistent with the peptidergic nature of the spino-parabrachio-amygdala pain pathway.
Interposed between LA-BLA and CeA is a cluster of inhibitory interneurons in the intercalated cell mass (ITC cells) which serve as a gate keeper to control amygdala output (Pape and Pare 2010; Likhtik et al. 2008; Marek et al. 2013; Jungling et al. 2008). ITC cells that inhibit CeA neurons are the target of excitatory projections from the infralimbic mPFC (McDonald 1998; Busti et al. 2011; Amir et al. 2011; Pinard et al. 2012) and are activated during behavioral extinction of negative emotional responses (Orsini and Maren 2012; Herry et al. 2010; Pape and Pare 2010; Sotres-Bayon and Quirk 2010). Pharmacological or mPFC-driven activation of ITC cells can also inhibit pain-related CeLC output and behaviors (Ren et al. 2013).
2 Pain-Related Amygdala Plasticity
Electrophysiological studies in anesthetized animals have consistently found increases in background and stimulus-evoked activity of individual CeLC neurons (Neugebauer and Li 2003; Li and Neugebauer 2004b, 2006; Ji and Neugebauer 2007, 2009; Ji et al. 2009) and BLA neurons (Ji et al. 2010) in a model of arthritic pain and in CeA neurons in a neuropathic pain model (Goncalves and Dickenson 2012) (Fig. 3). These “multireceptive” amygdala neurons are activated more strongly by noxious than innocuous stimuli and likely serve to integrate and evaluate sensory–affective information in the context of pain (Neugebauer et al. 2004). Biochemical and electrophysiological changes were observed only in the right amygdala in models of inflammatory pain (Ji and Neugebauer 2009; Carrasquillo and Gereau 2007, 2008). In neuropathic pain a transient activity increase was observed in the left amygdala but a persistent change in the right amygdala (Goncalves and Dickenson 2012). Mechanisms of pain-related lateralization remain to be determined.
Role of the amygdala in pain. Pain-producing events generate hyperactivity in the amygdala network of lateral, basolateral, and central nuclei (LA, BLA, CeA), which accounts for emotional–affective aspects of pain. Output from BLA deactivates medial prefrontal cortex through feedforward inhibition, resulting in cognitive deficits such as impaired decision-making. Decreased medial prefrontal cortical output to the amygdala allows the uncontrolled persistence of amygdala hyperactivity, hence persistence of pain
Neuronal activity changes in the amygdala are not simply a reflection of continued afferent input from spinal cord and other sources, but they result from an imbalance between excitatory and inhibitory synaptic mechanisms in the amygdala circuitry. Pain-related neuroplasticity in the amygdala has been established in electrophysiological and biochemical studies in brain slice preparations obtained from animals after the induction of different pain states, suggesting that brain changes persist at least in part independently of continued afferent input. Excitatory synaptic transmission to the CeLC is increased in acute models of arthritis (Neugebauer et al. 2003; Han et al. 2005; Bird et al. 2005; Fu and Neugebauer 2008; Fu et al. 2008), colitis (Han and Neugebauer 2004), and formalin-induced inflammation (Adedoyin et al. 2010) and in the spinal nerve ligation model of neuropathic pain (Ikeda et al. 2007; Nakao et al. 2012). Studies in the neuropathic pain model also reported right-hemispheric lateralization that depends on C-fiber-mediated inputs. Synaptic plasticity of parabrachial input to the CeLC is a consistent finding in various pain models, but there is also evidence for enhanced synaptic transmission at the LA-BLA (Ji et al. 2010) and BLA–CeLC synapses (Neugebauer et al. 2003; Fu and Neugebauer 2008; Ikeda et al. 2007; Ren and Neugebauer 2010; Ren et al. 2013).
Pain-related increased excitatory transmission and amygdala output can develop because inhibitory control mechanisms are impaired. Feedforward inhibition of CeLC neurons involves glutamatergic projections from BLA and mPFC to a cluster of GABAergic neurons in the intercalated cell masses (ITC cells). Decreased activation of this inhibitory gating mechanism in pain allows the development of glutamate- and neuropeptide-driven synaptic plasticity in the CeLC (Ren and Neugebauer 2010; Ren et al. 2013). A mechanism of impaired inhibition is the loss of cortical output (Ren et al. 2013) as the consequence of BLA hyperactivity that generates abnormally enhanced feedforward inhibition of principal cells in the mPFC (Ji et al. 2010; Ji and Neugebauer 2011). Failure to engage cortically driven ITC-mediated inhibitory control of amygdala processing may be a general mechanism of the abnormal persistence of emotional–affective states not just in pain (Apkarian et al. 2013; Ochsner and Gross 2005; Dalley et al. 2011).
Increased amygdala output as the result of neuroplasticity in the LA-BLA and CeLC has emerged as an important contributor to emotional–affective behaviors in animal pain models (Neugebauer et al. 2004, 2009). Decreasing amygdala activity with lesions or pharmacological interventions inhibits pain-related behaviors in different models (Han and Neugebauer 2005; Ren et al. 2013; Pedersen et al. 2007; Han et al. 2005; Manning 1998; Fu and Neugebauer 2008; Fu et al. 2008; Palazzo et al. 2008; Ji et al. 2010; Hebert et al. 1999). Importantly, increasing activity in the amygdala exogenously can exacerbate or generate pain responses under normal conditions in the absence of any tissue pathology (Han et al. 2010; Carrasquillo and Gereau 2007; Kolber et al. 2010; Qin et al. 2003; Myers et al. 2005; Myers and Greenwood-Van Meerveld 2010; Li et al. 2011; Ji et al. 2013). Increased amygdala activity is now also well documented in experimental and clinical pain conditions in humans (Liu et al. 2010; Baliki et al. 2008; Tillisch et al. 2010; Kulkarni et al. 2007; Simons et al. 2012).
3 Pharmacology of Pain-Related Processing in the Amygdala
The contributions of amino acid neurotransmitters (glutamate and GABA) and neuropeptides in the amygdala network relevant to pain-related processing are summarized in Fig. 4. A discussion of opioid function in the amygdala is beyond the scope of this article. There is strong evidence for a critical role of the amygdala, and the CeA in particular, in opioid-dependent pain modulation (Manning and Mayer 1995a, b; Zhang et al. 2013; Fields 2000; Manning 1998) and reward mechanisms (Bie et al. 2012; Cai et al. 2013), and μ-, κ-, and δ-opioid agonists can have direct effects on CeA amygdala neurons (Zhu and Pan 2004; Bie et al. 2009). However their role in pain-related amygdala processing and plasticity remains to be determined.
Pharmacology of pain-related amygdala changes. Glutamatergic/GABAergic and peptidergic mechanisms are depicted in separate diagrams for the sake of clarity. BLA basolateral amygdala, CeA central nucleus of the amygdala, ITC intercalated cells, mGluR metabotropic glutamate receptor, NR1/2B NMDA receptor subunits NR1 and NR2B, NPSR neuropeptide S receptor. Dashed blue line indicates intra-amygdalar CRF release from CeA neurons
3.1 Ionotropic Glutamate Receptors
The CeLC receives glutamatergic inputs from the LA-BLA network and from brainstem areas (parabrachial input has been studied most extensively with regard to amygdala pain mechanisms). Activation of NMDA and non-NMDA receptors is required for the generation of hyperactivity of CeLC neurons in the arthritis pain model, because administration of antagonists for NMDA (AP5) or non-NMDA (NBQX) receptors into the CeA 6 h postinduction inhibited the increased background activity and evoked responses of these neurons in anesthetized animals (Li and Neugebauer 2004a). In the same experimental in vivo preparation, an NR2B-selective antagonist (Ro-256981) showed a different profile, inhibiting evoked but not background activity (Ji et al. 2009). In brain slices obtained from arthritic rats 6 h postinduction (acute stage), NMDA receptor-mediated transmission at the PB-CeLC synapse is increased through PKA-dependent phosphorylation of the NR1 subunit, and AP5 blocks the synaptic potentiation (Bird et al. 2005). In the subacute stage of the formalin pain model (24 h postinduction), NMDA receptors contribute to enhanced transmission at the PB-CeLC synapse (the NMDA receptor antagonist CPP inhibited transmission), but the NMDA/AMPA ratio is not increased compared to controls (Adedoyin et al. 2010). In contrast, synaptic plasticity of PB inputs to CeLC neurons in the spinal nerve ligation model of neuropathic pain (6–7 days postinduction) largely depends on non-NMDA but not NMDA receptors (Ikeda et al. 2007).
While the electrophysiological studies may suggest that NMDA receptors are more important for the induction rather than maintenance phase in models of inflammatory but not neuropathic pain, behavioral data argue against pain type- or stage-specific contributions. Bilateral intra-CeA injection of an NMDA receptor antagonist (MK-801) inhibited nocifensive (hind limb withdrawal reflex) and affective (place avoidance test) behaviors in the spared nerve injury model of neuropathic pain (Ansah et al. 2010). Even in normal animals, vocalization afterdischarges evoked by a noxious footshock (a measure of pain affect) were inhibited by antagonists for NMDA (AP5) or non-NMDA (CNQX) receptors administered into the CeA, whereas spinal reflexes (tail flick and hind limb movements) were unaffected (Spuz and Borszcz 2012). In this study bilateral administration of AP5 or CNQX was more effective than unilateral administration into either hemisphere.
3.2 Metabotropic Glutamate Receptors
The family of G-protein-coupled metabotropic glutamate receptors (mGluRs) comprises eight subtypes with splice variants, which can be classified into three groups (I–III) based on their sequence homology, signal transduction mechanism, and pharmacological profile.
Group I consists of mGluR1 and mGluR5 subtypes that couple to Gq/11 proteins to activate phospholipase C, resulting in the formation of IP3 to stimulate intracellular calcium release, generation of diacylglycerol (DAG) activating PKC, and activation of MAP kinases such as ERK (Nicoletti et al. 2011; Ferraguti et al. 2008; Niswender and Conn 2010). Electrophysiological, biochemical, and behavior data support an important role of mGluR1 and mGluR5 in pain-related amygdala neuroplasticity and amygdala-dependent behaviors. Antagonists for mGluR1 (CPCCOEt) and mGluR5 (MPEP) inhibited increased evoked responses of CeLC neurons recorded in anesthetized rats in a model of arthritis pain (Li and Neugebauer 2004b). MPEP, but not CPCCOEt, also inhibited enhanced background activity in the arthritis model and had inhibitory effects under normal conditions (Li and Neugebauer 2004b). Underlying mechanisms involve presynaptic mGluR1-mediated increase in excitatory and decrease in inhibitory transmission and postsynaptic mGluR5-mediated effects based on electrophysiological analyses in brain slices from arthritic rats (Ren and Neugebauer 2010; Neugebauer et al. 2003). In these studies selective mGluR1 antagonists (CPCCOEt and LY367385) inhibited excitatory but facilitated inhibitory transmission only in arthritis, whereas selective mGluR5 antagonists (MPEP and MTEP) inhibited synaptic transmission both under normal conditions and in arthritis.
Behavioral studies support an important role of amygdalar group I mGluRs in models of formalin-induced, arthritis, visceral, and neuropathic pain. Blockade of mGluR1 in the CeA with CPCCOEt inhibited stimulus-evoked audible and ultrasonic vocalizations and spinal reflexes that were increased in the kaolin/carrageenan arthritis pain model, whereas blockade of mGluR5 in the CeA with MPEP inhibited vocalizations but not spinal hind limb withdrawal reflexes. Vocalizations organized in limbic forebrain areas and particularly in the amygdala (measured as vocalization afterdischarges) were inhibited by both CPCCOEt and MPEP. Vocalizations during stimulation, which are organized at the brainstem level, were inhibited by CPCCOEt but not MPEP (Han and Neugebauer 2005). The results may suggest that mGluR1 and mGluR5 contribute to affective behaviors generated in the amygdala, whereas mGluR1, but not mGluR5, also regulate amygdala-dependent modulation of “sensory” nocifensive behaviors by descending brainstem systems. A similar pattern of differential contributions of mGluR1 and mGluR5 to amygdala functions was found in the spared nerve injury model of neuropathic pain. Application of antagonists for mGluR1 (CPCCOEt) or mGluR5 (MPEP) into the CeA inhibited aversive behaviors in the place avoidance test. CPCCOEt, but not MPEP, also inhibited hind limb withdrawal reflexes (Ansah et al. 2010). These studies were done in rats. Disruption of mGluR5 function in the CeLC of mice pharmacologically or with a conditional knockout approach inhibited paw withdrawal reflexes in the formalin pain model (Kolber et al. 2010) and visceromotor reflexes in the bladder distension pain model (Crock et al. 2012).
A possible conclusion emerging from these findings would be that mGluR1 contribute in a broader way to any type of pain behaviors generated or modulated by the amygdala, whereas mGluR5 function switches over time (in arthritis and neuropathic pain models) towards a preferential contribution to affective forebrain-mediated pain mechanisms. The electrophysiological data support this scenario because the presynaptic function of mGluR1 could affect the drive onto various amygdala output neurons, whereas the postsynaptic action of mGluR5 allows for the discrete activation of subsets of CeA neurons.
Under normal conditions, mGluR5 appears to mediate the facilitatory effects of group I mGluR activation in the CeA on vocalizations and spinal reflexes in the awake animal, on neuronal activity in the anesthetized preparation, and on synaptic transmission in amygdala brain slices (Neugebauer et al. 2003; Li et al. 2011; Ji and Neugebauer 2010; Li and Neugebauer 2004b). In these studies, the effects of an mGluR1/5 agonist (DHPG) were mimicked by a presumed mGluR5-selective agonist (CHPG) and/or blocked by a selective mGluR5 antagonist (MTEP). A selective mGluR1 antagonist (LY367385) inhibited the facilitatory effects of DHPG only on neuronal responses to visceral stimuli (colorectal distention) (Ji and Neugebauer 2010). The facilitatory effects of mGluR5 involved IP3-mediated calcium release to increase mitochondrial ROS production resulting in the activation of ERK1/2 and PKA, but not PKC. Inhibition of ERK (U0126) and PKA (KT5720) was necessary to block completely the excitatory effects of a ROS donor (tBOOH); a PKC inhibitor (GF109203X) had no effect (Li et al. 2011). ERK activation downstream of mGluR5 is also supported by studies in mice. DHPG administered into the CeA induced mechanical (decreased paw withdrawal thresholds) and visceral (response to bladder distension) hypersensitivity under normal conditions. The facilitatory effect was reduced by an mGluR5 antagonist (MPEP) or mGluR5 knockdown and was accompanied by ERK1/2 activation in the CeA (Kolber et al. 2010) and in the spinal cord (Crock et al. 2012).
Group II comprises mGluR2 and mGluR3 subtypes that couple negatively to adenylyl cyclase, cAMP, and PKA activation via Gi proteins (Nicoletti et al. 2011; Niswender and Conn 2010). Typically localized extrasynaptically they serve as presynaptic auto- or hetero-receptors (Niswender and Conn 2010). Pharmacological activation of group II mGluRs in the CeA with mGluR2/3 agonists (LCCG1 or LY354740) decreased the responses of CeLC neurons under normal conditions and in the kaolin-/carrageenan-induced knee joint arthritis pain model (Li and Neugebauer 2006). There was an increase in potency for inhibition of responses to noxious stimulation of the arthritic knee in the pain model but not for inhibition of responses to innocuous stimulation of the knee or of the intact ankle or background activity, suggesting perhaps an input- and activity-dependent effect, because noxious stimulation of the arthritic knee would likely generate the strongest input to the amygdala. A group II mGluR antagonist (EGLU) had no effect under normal conditions but increased the responses to stimulation of the knee in the arthritis pain state. This would be consistent with activity-dependent endogenous activation and gain of function of group II mGluRs in pain.
Electrophysiological analysis in brain slices showed that group II mGluRs act presynaptically to modulate synaptic plasticity in the amygdala in a model of arthritic pain (Han et al. 2006). A selective group II mGluR agonist (LY354740) inhibited excitatory transmission at the PB-CeLC synapse in slices from arthritic rats more potently than in controls without affecting neuronal excitability. A group II mGluR antagonist (EGLU) but not a GABAA receptor antagonist (bicuculline) reversed the inhibitory effect of LY354740. EGLU had no effect on its own, suggesting that the endogenous activation of group II mGluRs in the in vivo condition (see previous paragraph) may be due to inputs not preserved or active in the brain slice preparation. The pain-related function of group II mGluRs at the BLA–CeLC synapse remains to be determined, but group II mGluR agonists (LCCG1 and LY354740) inhibited excitatory transmission from BLA to CeLC under normal conditions, and the inhibitory effect persisted in the presence of antagonists for mGluR1 (CPCCOEt), mGluR5 (SIB-1893), GABAA (picrotoxin), and GABAB (CGP 55845) (Neugebauer et al. 2000).
The relative contribution of mGluR2 and 3 was examined in amygdala brain slices using an inhibitor (ZJ43) of the peptide neurotransmitter N-acetylaspartylglutamate (NAAG) that activates preferentially mGluR3 (Adedoyin et al. 2010). ZJ43 inhibited excitatory transmission at the PB-CeLC synapse more strongly than a group II mGluR agonist that does not distinguish mGluR2 and 3 (SLx-3095-1), suggesting a role for endogenous NAAG and perhaps a predominant contribution of mGluR3 under normal conditions. Both effects were blocked by a group II mGluR antagonist (LY341495). In the formalin pain model (24 h postinduction), ZJ43 was much less effective than SLx-3095-1 in reducing excitatory synaptic transmission, which would be consistent with a loss of function of NAAG or an increase in the contribution of mGluR2 relative to mGluR3. Electrophysiological effects correlated with behavior because systemic injection of ZJ43 blocked formalin pain-related synaptic plasticity in the CeLC as well as mechanical allodynia (Adedoyin et al. 2010).
Group III consists of mGluR4, 6, 7, and 8 subtypes. With the exception of mGluR6, which is found only in retinal ON-bipolar cells, they couple negatively to adenylyl cyclase, cAMP and PKA activation via Gi proteins like group II mGluRs (Nicoletti et al. 2011; Niswender and Conn 2010). Predominantly presynaptic receptors, mGluR4 and mGluR8 have high affinity for glutamate and are localized extrasynaptically on glutamatergic terminals, whereas low affinity mGluR7 is found in or near the active zone of the synapse on GABAergic terminals (Niswender and Conn 2010).
Evidence from electrophysiological studies using the prototypical broad spectrum agonist (LAP4) suggests that group III mGluRs can inhibit pain-related amygdala neuroplasticity. LAP4 inhibited evoked responses of CeLC neurons recorded in anesthetized rats more potently in the arthritis pain state than under normal conditions, whereas the inhibitory effect on background activity did not change (Li and Neugebauer 2006). A group III mGluR antagonist (UBP1112) had no effect under normal conditions but facilitated the evoked responses in arthritic rats. Electrophysiological analysis in brain slices showed that LAP4 decreased excitatory transmission at the PB-CeLC synapse more potently in the arthritis pain model than in control slices, and the inhibitory effects involved a presynaptic site of action (Han et al. 2004). UBP1112 reversed the inhibitory effect of LAP4 but had no effect on its own, which is different from the in vivo situation where the facilitatory effects of the antagonist suggest the possibility of endogenous receptor activation in the pain model. A likely explanation would be the requirement of amygdala inputs not present or active in the slice preparation. A group III agonist (LAP4) also inhibited transmission at the BLA–CeLC synapse under normal conditions, but pain-related function of group III mGluRs at this synapse remains to be determined.
The recent availability of subtype selective agonists allowed a more detailed analysis that showed opposing functions of mGluR7 and mGluR8 in the amygdala related to pain processing and modulation (Palazzo et al. 2008; Ren et al. 2011). Activation of mGluR7 with AMN082 increased CeLC output (synaptically evoked spiking) in brain slices from control rats but not from arthritic rats (Ren et al. 2011). AMN082 acted presynaptically to inhibit glutamate-driven synaptic inhibition of CeLC neurons (feedforward inhibition from BLA), but not monosynaptic inhibitory transmission, implicating BLA-activated GABAergic interneurons such as intercalated cells (ITC; see Sect. 4.2). In support of this interpretation, the effect of AMN082 was occluded in the presence of bicuculline to block GABAA receptors. The mGluR7-mediated disinhibition facilitated the excitatory drive of CeLC neurons. Thus, mGluR7 would act as a gatekeeper to regulate information flow to the CeLC by releasing GABAergic control, hence permitting excitatory inputs from the LA-BLA network to reach the CeLC under normal conditions. In contrast, a selective mGluR8 agonist (DCPG) inhibited excitatory transmission at the BLA–CeLC synapse and output of CeLC neurons more potently in brain slices from arthritic rats than under normal conditions. The mechanism was presynaptic on glutamatergic terminals and did not involve GABAergic modulation; DCPG had no effect on inhibitory transmission (Ren et al. 2011). The direct inhibitory effect of mGluR8 and the disinhibition by mGluR7 could be blocked with a group III antagonist (MAP4).
Behavioral data are consistent with these electrophysiological findings (Palazzo et al. 2008). Activation of mGluR7 in the CeA of normal rats with AMN082 facilitated spinal reflexes (withdrawal thresholds) and supraspinally organized affective responses (vocalizations); AMN082 also increased anxiety-like behavior (decreased open-arm preference in the elevated plus maze). The facilitatory effects of AMN082 were not detected in arthritic rats showing increased pain behaviors. Intra-CeA administration of an mGluR8 agonist (DCPG) had no effect in normal animals but inhibited the increased spinal reflexes and vocalizations of arthritic rats and had anxiolytic-like effects in these animals tested in the elevated plus maze (Palazzo et al. 2008). DCPG also decreased thermal hypersensitivity in a carrageenan-induced hindpaw inflammation model and the antinociceptive effect was blocked with a group III mGluR antagonist (MSOP) (Palazzo et al. 2011). Furthermore, an increase in mGluR8 gene, protein, and staining, the latter being associated with vesicular GABA transporter-positive profiles, has been found in the CeA after carrageenan-induced inflammatory pain. These results show that stimulation of mGluR8, which was overexpressed within the CeA in inflammatory pain conditions, inhibits nociceptive behavior. Such an effect is associated with an increase in 5-HT and Glu release, a decrease in GABA, and the inhibition of ON- and the stimulation of OFF-cell activities within RVM.
The results suggest that under normal conditions mGluR7, but not mGluR8, facilitates pain responses and has anxiogenic-like properties. In pain models, however, mGluR8, but not mGluR7, has inhibitory behavioral effects (Palazzo et al. 2008, 2011), and this functional change may involve upregulation of mGluR8 gene and protein expression (Palazzo et al. 2011).
3.3 GABA
The amygdala is rich in GABAergic neurons and GABA receptors, allowing the control of amygdala output through direct inhibition, feedforward inhibition, and disinhibition. Recent work has focused on a cluster of inhibitory interneurons in the intercalated cell mass (ITC cells) positioned between BLA and CeLC (Ren et al. 2013).
Evidence from electrophysiological studies in brain slices suggests that GABAergic transmission can control excitatory inputs and synaptically evoked outputs of CeLC neurons (Fu and Neugebauer 2008; Ren et al. 2011, 2013; Ren and Neugebauer 2010). However, GABAA receptor-mediated synaptic inhibition is lost or impaired in a model of arthritic pain. Decreased monosynaptic (Ren et al. 2011) and glutamate-driven disynaptic inhibition (feedforward inhibition sensitive to NBQX) (Ren and Neugebauer 2010; Ren et al. 2013) of CeLC neurons was found in brain slices from arthritis rats compared to controls. Feedforward inhibition can be evoked by stimulation of BLA output (Ren and Neugebauer 2010) or of medial prefrontal cortical fibers in the external capsule (Ren et al. 2013). ITC cells have been implicated in feedforward inhibition of CeLC neurons because pharmacological activation of ITC cells with neuropeptide S (NPS) (see Sect. 5) or high-frequency stimulation of excitatory external capsule input to ITC cells inhibited synaptic activation of CeLC neurons (Ren et al. 2013). These inhibitory inputs exert a tonic GABAergic tone on CeLC neurons under normal conditions but not in the arthritis pain model. Blockade of GABAA receptors with bicuculline in the CeLC under normal conditions facilitated excitatory transmission at the BLA–CeLC synapse through an indirect action in the network (Ren and Neugebauer 2010) and also increased synaptically evoked action potential firing, a measure of neuronal output (Ren et al. 2011). In brain slices from arthritic rats, however, bicuculline had no significant effect on excitatory synaptic responses of CeLC neurons, suggesting a loss of GABAergic inhibitory control that may contribute to the pain-related increase of excitatory transmission (Ren and Neugebauer 2010).
Behavioral data somewhat agree with the electrophysiological findings. In a model of neuropathic pain chronic constriction injury, bicuculline administered into the CeA had no effect on mechanical allodynia and hyperalgesia, but attenuated, rather than facilitated, affective pain behaviors (escape/avoidance test). The data would be consistent with a loss, and possibly even reversal, of tonic GABAergic inhibitory control (Pedersen et al. 2007). Exogenous activation of GABAA receptors in the CeA with muscimol, however, had antinociceptive effects and attenuated escape/avoidance behaviors, which would indicate the presence of functional GABAA receptors. Neurochemical studies found no evidence for significant changes of extracellular GABA levels in the BLA in the formalin pain model (Rea et al. 2009) and in the CeA in a model of carrageenan-induced hindpaw inflammation (Palazzo et al. 2011). In these studies, baseline rather than evoked release was studied. Electrophysiological data implicated mGluR1 in the reduced or lost GABAergic inhibition (see Sect. 4.1 Group I). The mechanisms of impaired GABAergic control of amygdala function remain to be determined.
3.4 Neuropeptide CGRP
CGRP is a 37-amino-acid peptide that binds to G-protein-coupled receptors, including CGRP1, which couple positively to adenylyl cyclase, cyclic AMP formation, and protein kinase A (PKA) activation (Wimalawansa 1996; Poyner et al. 2002; Van Rossum et al. 1997). Functional CGRP1 receptors are formed by a heterodimeric complex of the calcitonin receptor-like receptor (CRLR) and receptor activity-modifying protein 1 (RAMP1) (Robinson et al. 2009; McLatchie et al. 1998). Particularly high levels of CGRP (de Lacalle and Saper 2000; Schwaber et al. 1988; Kruger et al. 1988; Dobolyi et al. 2005), CGRP binding sites (Wimalawansa 1996; Van Rossum et al. 1997), and proteins (CRLR and RAMP1) required for functional CGRP1 receptors (Ma et al. 2003; Oliver et al. 2001) have been described in the amygdala (CeA). CGRP can interact with other receptors (Robinson et al. 2009; Hay 2007; Poyner et al. 2002) such as a putative CGRP2 receptor that may include RAMP2 or RAMP3 rather than RAMP1 (Hay 2007). Whereas RAMP1 dominates in the CeA, the BLA contains relatively more RAMP2 than RAMP1 (Oliver et al. 2001). The exclusive source of CGRP in the amygdala is the lateral parabrachial area, and CGRP-immunoreactive parabrachial fibers essentially delineate the CeLC (Schwaber et al. 1988; de Lacalle and Saper 2000; Kruger et al. 1988; Dobolyi et al. 2005), making CGRP a marker of parabrachial inputs to the “nociceptive amygdala.”
Electrophysiological and behavioral data show an important role of CGRP and CGRP1 receptors in the CeA in pain-related neuroplasticity and behaviors. Administration of selective CGRP1 receptor antagonists (CGRP8-37 and BIBN4096BS) into the CeA inhibited increased responses of CeLC neurons to mechanical stimulation of the arthritic knee and non-injured ankle in anesthetized rats with a kaolin-/carrageenan-induced knee joint arthritis (Han et al. 2005). The antagonists were more efficacious in the pain model than under normal conditions. In amygdala brain slices from arthritic rats, CGRP1 receptor antagonists inhibited synaptic plasticity of parabrachial inputs to the CeLC but had no significant effect under normal conditions (Han et al. 2005). Detailed electrophysiological analyses showed that CGRP1 receptors contribute to pain-related plasticity through a protein kinase A (PKA)-dependent postsynaptic mechanism that involves NMDA, but not AMPA, receptors. Pharmacological blockade of CGRP1 receptors in the CeA with CGRP8-37 inhibited spinal hind limb withdrawal reflexes and supraspinally organized pain behaviors (audible and ultrasonic vocalizations) of awake arthritic rats (Han et al. 2005). CGRP8-37 had no effect on these behaviors in normal animals without arthritis.
Under normal conditions CGRP in the CeA can facilitate synaptic transmission and generate nocifensive and affective pain behaviors (Han et al. 2010). In brain slices from normal rats, CGRP increased excitatory transmission of parabrachial inputs to CeLC neurons through a postsynaptic mechanism and also increased neuronal excitability. CGRP-induced synaptic facilitation was reversed by an NMDA receptor antagonist (AP5) or a PKA inhibitor (KT5720), but not by a PKC inhibitor (GF109203X). Stereotaxic administration of CGRP into the CeLC of awake rats increased audible and ultrasonic vocalizations and decreased hind limb withdrawal thresholds (Han et al. 2010). Behavioral effects of CGRP were largely blocked by KT5720 but not GF109203X. Electrophysiological and behavioral effects of CGRP were blocked by a CGRP1 receptor antagonist (CGRP8-37). The data suggest that CGRP in the amygdala exacerbates pain behaviors under normal conditions through a PKA and NMDA receptor-dependent direct action on CeLC neurons.
In contrast, antinociceptive effects of CGRP have been reported in the BLA of normal animals (Li et al. 2008). Hindpaw withdrawal latencies to noxious thermal and mechanical stimulations increased significantly after intra-BLA administration of CGRP, and the antinociceptive effect was blocked by CGRP8-37. CGRP receptor composition in different nuclei of the amygdala may explain the differential effects of CGRP. CGRP could also activate inhibitory projections from the BLA to the CeLC (see Sect. 4.2). Neuronal effects of CGRP in the BLA remain to be determined.
3.5 Neuropeptide CRF
Corticotropin-releasing factor (CRF) is not only a “stress hormone” but also a neuromodulator outside the hypothalamic-pituitary-adrenocortical (HPA) axis, and the amygdala is a major site of extrahypothalamic expression of CRF and its receptors (Charney 2003; Gray 1993; Koob 2010; Asan et al. 2005; Sanchez et al. 1999; Takahashi 2001; Tache and Bonaz 2007; Bale and Vale 2004; Reul and Holsboer 2002; Hauger et al. 2009). CRF receptors can couple to a number of G-proteins to activate a variety of intracellular signaling pathways, and PKA and PKC appear to play particular important roles (Blank et al. 2003). Sources of CRF in the amygdala are CeA neurons as well as afferents from the lateral hypothalamic area and dorsal raphe nucleus (Uryu et al. 1992; Commons et al. 2003). CRF containing neurons in the CeA are innervated by CGRP containing terminals from the parabrachial area (Schwaber et al. 1988; Dobolyi et al. 2005; Harrigan et al. 1994) and project to widespread regions of the basal forebrain and brain stem (Gray 1993).
Electrophysiological studies showed an important contribution of endogenously activated CRF1 receptors to neuroplasticity in the CeA and BLA in a model of arthritic pain (Ji and Neugebauer 2007, 2008; Fu and Neugebauer 2008). In anesthetized rats, a selective CRF1 receptor antagonist (NBI27914) administered into the CeA inhibited the evoked responses and background activity of CeLC neurons in an arthritis pain model (Ji and Neugebauer 2007). Administration of a NBI27914 into the BLA, but not CeA, also inhibited the increased background and evoked activity of BLA neurons in the arthritis model (Ji et al. 2010). In contrast, a selective CRF2 receptor antagonist (astressin-2B) had facilitatory effects on CeLC neurons under normal conditions but not in the pain model (Ji and Neugebauer 2007).
Patch-clamp analysis in brain slices found differential pre- and postsynaptic mechanisms of CRF1 and CRF2 receptor-mediated effects in the amygdala. In brain slices from arthritic rats, NBI27914 inhibited enhanced synaptic inputs to CeLC neurons from the parabrachial area and the BLA (Fu and Neugebauer 2008) as well as synaptic plasticity in BLA neurons (Ji et al. 2010). NBI27914 had no significant effect on CeLC or BLA neurons under normal conditions. The synaptic effects involved a postsynaptic mechanism and PKA-dependent inhibition of an NMDA receptor-mediated synaptic component. NBI27914 also decreased neuronal excitability by inhibiting ion channels important for action potential repolarization such as Kv3-type potassium channels. In contrast, a CRF2 receptor antagonist (astressin-2B) had no effect on neuronal excitability but facilitated excitatory transmission at the parabrachial–CeLC and BLA–CeLC synapses through presynaptic inhibition of GABAergic transmission (disinhibition) (Fu and Neugebauer 2008). Astressin-2B inhibited GABAA receptor-mediated inhibitory monosynaptic transmission from BLA to CeLC, whereas NBI27914 had no effect on inhibitory transmission. In brain slices from normal rats neither antagonist had no effects on basal synaptic transmission (Fu and Neugebauer 2008).
In conclusion, endogenous CRF1 receptor activation in the amygdala contributes to pain-related synaptic plasticity and hyperactivity through a postsynaptic mechanism, whereas presynaptic CRF2 receptor-mediated inhibitory function is lost in the arthritis pain model. Studies in whole animals (Ji and Neugebauer 2007) and in brain slice preparations (Fu and Neugebauer 2008) are largely in agreement. Discrepancies regarding CRF2 receptor function under normal conditions may be due to differences in the availability of extra-amygdaloid inputs providing endogenous ligands and tonic inhibition required for CRF2 receptor blockade to have a detectable effect.
Behavioral evidence suggests that pain-related endogenous activation of CRF1 receptors in the amygdala contributes to pain modulation and pain affect. Blockade of CRF1 receptors in the CeA with NBI27914 inhibited pain-related behaviors (audible and ultrasonic vocalizations and hind limb withdrawal reflexes) and anxiety-like behaviors in a model of arthritic pain but had no effect in normal animals (Ji et al. 2007; Fu and Neugebauer 2008). CRF2 receptor-mediated inhibition does not reach behavioral significance since astressin-2B had no significant effect on pain behaviors (Fu and Neugebauer 2008). In a neuropathic pain model (spared nerve injury) a nonselective CRF receptor antagonist (CRF9-41) had no effect on emotional–affective (aversive place-conditioning test) and nocifensive (hind limb withdrawal thresholds) pain behaviors (Bourbia et al. 2010). Increasing endogenous CRF in the CeA with a CRF-binding protein inhibitor (CRF6-33) had mixed effects, facilitating nocifensive responses while attenuating emotional–affective behaviors (Bourbia et al. 2010). Microinjections of a CRF receptor antagonist (CRF9-41) into the CeA reduced hyperalgesia (tail flick test) associated with morphine withdrawal but had no effect in normal controls (McNally and Akil 2002).
Pain-related changes of CRF function in the amygdala are supported by biochemical data. CRF and CRF mRNA are increased in CeA neurons in the chronic constriction injury model of neuropathic pain independently of HPA axis activation (Rouwette et al. 2011; Ulrich-Lai et al. 2006). Increased CRF mRNA expression in the CeA was also found in a colitis model of visceral pain (Greenwood-Van Meerveld et al. 2006).
While there is little evidence for endogenous activation of the CRF system in the amygdala under normal conditions (Ji and Neugebauer 2007; Fu and Neugebauer 2008), exogenously administered CRF can have CRF1 receptor-mediated facilitatory and CRF2 receptor-mediated inhibitory effects in the CeA (Ji and Neugebauer 2008; Ji et al. 2013). In anesthetized normal rats CRF increased background and evoked activity of CeLC neurons but decreased neuronal activity at higher concentrations (Ji and Neugebauer 2008). Facilitatory effects of CRF were blocked by a selective CRF1 receptor antagonist (NBI27914) but not a CRF2 receptor antagonist (astressin-2B) and by a PKA, but not PKC, inhibitor. Inhibitory effects of CRF were reversed by astressin-2B. In brain slices from normal rats without injury, CRF increased excitatory transmission at the parabrachial–CeLC synapse and also neuronal output (synaptically evoked spiking) through a postsynaptic PKA-dependent action (Ji et al. 2013). The CRF effects were blocked by NBI27914, but not astressin-2B, and by an inhibitor of PKA (KT5720), but not PKC (GF109203x). CRF increased a latent NMDA receptor-mediated synaptic component through CRF1 receptor-mediated PKA activation. Thus CRF can induce changes resembling pain-related plasticity of CeLC neurons that involves PKA-dependent NR1 subunit phosphorylation and increased NMDA receptor-mediated synaptic transmission (Bird et al. 2005; Han et al. 2005).
Behavioral consequences of non-pain-related activation of CRF1 receptors in the amygdala (CeA) are increased audible and ultrasonic vocalizations and decreased hind limb withdrawal thresholds (Ji et al. 2013). In agreement with the electrophysiological findings, behavioral effects of CRF were blocked by NBI27914 and KT5720 but not GF109203x. Importantly, CRF effects persisted when HPA axis function was suppressed by pretreatment with dexamethasone (subcutaneously). It is conceivable that conditions of increased amygdala CRF levels can contribute to pain in the absence of tissue pathology or disease state.
3.6 Neuropeptide S
Recently discovered NPS selectively enhances ITC-dependent feedforward inhibition of CeA neurons to produce powerful anxiolytic effects (Jungling et al. 2008). NPS acts on a Gq-/Gs-coupled receptor (NPSR) to increase intracellular calcium and cAMP-PKA signaling (Reinscheid 2008; Guerrini et al. 2010). The amygdala is one of the brain areas with the strongest expression of NPSR (Leonard and Ring 2011). In the rat amygdala, the highest level of NPSR mRNA is found in and around ITC cells but not in other elements of the pain-related amygdala circuitry (Xu et al. 2007).
Electrophysiological and behavioral data suggest an important role for NPS in the control of pain-related amygdala output and affective behaviors through a direct action on inhibitory ITC cells (Ren et al. 2013). In brain slices from arthritic rats, NPS inhibited the enhanced excitatory drive of CeLC neurons from BLA. The inhibitory effect of NPS was not due to a direct postsynaptic action on CeLC neurons but involved a presynaptic, action potential-dependent network mechanism. In fact, NPS increased feedforward inhibition of excitatory drive and output of CeLC neurons by activating GABAergic ITC neurons. The cellular mechanisms by which feedforward inhibition controls CeLC output remain to be determined. Feedforward inhibition was generated by cortical (mPFC) fiber activation in the external capsule. NPS increased excitatory drive and synaptically evoked output of ITC cells through a PKA-dependent facilitatory postsynaptic NPSR-mediated action. A selective NPSR antagonist ([D-Cys(tBu)5]NPS) blocked the electrophysiological effects of NPS but had no effect on its own (Ren et al. 2013).
As a consequence of synaptic inhibition of amygdala output NPS inhibited pain behaviors in an arthritis pain model (Ren et al. 2013). Administration of NPS into the ITC, but not CeLC, inhibited vocalizations and anxiety-like behavior in arthritic rats. A selective NPS receptor antagonist ([D-Cys(tBu)5]NPS) blocked the behavioral effects of NPS but had no effect on its own. These findings are in line with previous reports that intracerebroventricular administration of NPS had anxiolytic (Ruzza et al. 2012; Xu et al. 2004; Jungling et al. 2008) and antinociceptive (Li et al. 2009; Peng et al. 2010) effects. Intracerebroventricular NPS attenuated nociceptive behaviors (paw licking) in the mouse formalin pain model (Peng et al. 2010) but also had antinociceptive effects on the tail withdrawal and hot-plate tests in normal mice (Li et al. 2009). NPSR antagonists ([D-Val5]NPS and [D-Cys(tBu)5]NPS) blocked the effects of NPS but had no effect on their own. In conclusion, the amygdala is an important site for pain-inhibiting effects of NPS through a mechanism that involves activation of feedforward inhibition of CeA neurons through a PKA-dependent postsynaptic action on inhibitory ITC cells.
Abbreviations
- BLA:
-
Basolateral amygdala
- CB1:
-
Cannabinoid receptor 1
- CeA:
-
Central nucleus of the amygdala
- CeLC:
-
Laterocapsular division of the central nucleus of the amygdala
- ITC:
-
Intercalated cell mass
- LA:
-
Lateral amygdala
- mGluR:
-
Metabotropic glutamate receptor
- mPFC:
-
Medial prefrontal cortex
- NPS:
-
Neuropeptide S
- PB:
-
Parabrachial area
References
Adedoyin MO, Vicini S, Neale JH (2010) Endogenous N-acetylaspartylglutamate (NAAG) inhibits synaptic plasticity/transmission in the amygdala in a mouse inflammatory pain model. Mol Pain 6:60–77
Amir A, Amano T, Pare D (2011) Physiological identification and infralimbic responsiveness of rat intercalated amygdala neurons. J Neurophysiol 105:3054–3066
Ansah OB, Bourbia N, Goncalves L, Almeida A, Pertovaara A (2010) Influence of amygdaloid glutamatergic receptors on sensory and emotional pain-related behavior in the neuropathic rat. Behav Brain Res 209:174–178
Apkarian AV, Neugebauer V, Koob G, Edwards S, Levine JD, Ferrari L, Egli M, Regunathan S (2013) Neural mechanisms of pain and alcohol dependence. Pharmacol Biochem Behav 112C:34–41
Asan E, Yilmazer-Hanke DM, Eliava M, Hantsch M, Lesch KP, Schmitt A (2005) The corticotropin-releasing factor (CRF)-system and monoaminergic afferents in the central amygdala: investigations in different mouse strains and comparison with the rat. Neuroscience 131:953–967
Bale TL, Vale WW (2004) CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol 44:525–557
Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV (2008) A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain 4:47
Bie B, Zhu W, Pan ZZ (2009) Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther 329:290–296
Bie B, Wang Y, Cai YQ, Zhang Z, Hou YY, Pan ZZ (2012) Upregulation of nerve growth factor in central amygdala increases sensitivity to opioid reward. Neuropsychopharmacology 37:2780–2788
Bird GC, Lash LL, Han JS, Zou X, Willis WD, Neugebauer V (2005) Protein kinase A-dependent enhanced NMDA receptor function in pain-related synaptic plasticity in rat amygdala neurones. J Physiol 564:907–921
Blank T, Nijholt I, Grammatopoulos DK, Randeva HS, Hillhouse EW, Spiess J (2003) Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci 23:700–707
Bourbia N, Ansah OB, Pertovaara A (2010) Corticotropin-releasing factor in the rat amygdala differentially influences sensory-discriminative and emotional-like pain response in peripheral neuropathy. J Pain 11:1461–1471
Bourgeais L, Gauriau C, Bernard J-F (2001) Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci 14:229–255
Busti D, Geracitano R, Whittle N, Dalezios Y, Manko M, Kaufmann W, Satzler K, Singewald N, Capogna M, Ferraguti F (2011) Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci 31:5131–5144
Cai YQ, Wang W, Hou YY, Zhang Z, Xie J, Pan ZZ (2013) Central amygdala GluA1 facilitates associative learning of opioid reward. J Neurosci 33:1577–1588
Carrasquillo Y, Gereau RW (2007) Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci 27:1543–1551
Carrasquillo Y, Gereau RW (2008) Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain 4:24
Charney DS (2003) Neuroanatomical circuits modulating fear and anxiety behaviors. Acta Psychiatr Scand Suppl (417):38–50
Commons KG, Connolley KR, Valentino RJ (2003) A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28:206–215
Crock LW, Kolber BJ, Morgan CD, Sadler KE, Vogt SK, Bruchas MR, Gereau RW (2012) Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci 32:14217–14226
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694
de Lacalle S, Saper CB (2000) Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience 100:115–130
Dobolyi A, Irwin S, Makara G, Usdin TB, Palkovits M (2005) Calcitonin gene-related peptide-containing pathways in the rat forebrain. J Comp Neurol 489:92–119
Ferraguti F, Crepaldi L, Nicoletti F (2008) Metabotropic glutamate 1 receptor: current concepts and perspectives. Pharmacol Rev 60:536–581
Fields HL (2000) Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 122:245–253
Fu Y, Neugebauer V (2008) Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci 28:3861–3876
Fu Y, Han J, Ishola T, Scerbo M, Adwanikar H, Ramsey C, Neugebauer V (2008) PKA and ERK, but not PKC, in the amygdala contribute to pain-related synaptic plasticity and behavior. Mol Pain 4:26–46
Gauriau C, Bernard J-F (2002) Pain pathways and parabrachial circuits in the rat. Exp Physiol 87(2):251–258
Goncalves L, Dickenson AH (2012) Asymmetric time-dependent activation of right central amygdala neurones in rats with peripheral neuropathy and pregabalin modulation. Eur J Neurosci 36:3204–3213
Gray TS (1993) Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci 697:53–60
Greenwood-Van Meerveld B, Johnson AC, Schulkin J, Myers DA (2006) Long-term expression of corticotropin-releasing factor (CRF) in the paraventricular nucleus of the hypothalamus in response to an acute colonic inflammation. Brain Res 1071:91–96
Guerrini R, Salvadori S, Rizzi A, Regoli D, Calo’ G (2010) Neurobiology, pharmacology, and medicinal chemistry of neuropeptide S and its receptor. Med Res Rev 30:751–777
Han JS, Neugebauer V (2004) Synaptic plasticity in the amygdala in a visceral pain model in rats. Neurosci Lett 361:254–257
Han JS, Neugebauer V (2005) mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain 113:211–222
Han JS, Bird GC, Neugebauer V (2004) Enhanced group III mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Neuropharmacology 46:918–926
Han JS, Li W, Neugebauer V (2005) Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J Neurosci 25:10717–10728
Han JS, Fu Y, Bird GC, Neugebauer V (2006) Enhanced group II mGluR-mediated inhibition of pain-related synaptic plasticity in the amygdala. Mol Pain 2:18–29
Han JS, Adwanikar H, Li Z, Ji G, Neugebauer V (2010) Facilitation of synaptic transmission and pain responses by CGRP in the amygdala of normal rats. Mol Pain 6:10–23
Harrigan EA, Magnuson DJ, Thunstedt GM, Gray TS (1994) Corticotropin releasing factor neurons are innervated by calcitonin gene-related peptide terminals in the rat central amygdaloid nucleus. Brain Res Bull 33:529–534
Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM (2009) Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci 1179:120–143
Hay DL (2007) What makes a CGRP2 receptor? Clin Exp Pharmacol Physiol 34:963–971
Hebert MA, Ardid D, Henrie JA, Tamashiro K, Blanchard DC, Blanchard RJ (1999) Amygdala lesions produce analgesia in a novel, ethologically relevant acute pain test. Physiol Behav 67(1):99–105
Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A (2010) Neuronal circuits of fear extinction. Eur J Neurosci 31:599–612
Holland PC, Gallagher M (2004) Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol 14:148–155
Ikeda R, Takahashi Y, Inoue K, Kato F (2007) NMDA receptor-independent synaptic plasticity in the central amygdala in the rat model of neuropathic pain. Pain 127:161–172
Ji G, Neugebauer V (2007) Differential effects of CRF1 and CRF2 receptor antagonists on pain-related sensitization of neurons in the central nucleus of the amygdala. J Neurophysiol 97:3893–3904
Ji G, Neugebauer V (2008) Pro- and anti-nociceptive effects of corticotropin-releasing factor (CRF) in central amygdala neurons are mediated through different receptors. J Neurophysiol 99:1201–1212
Ji G, Neugebauer V (2009) Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol 1102:2253–2264
Ji G, Neugebauer V (2010) Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons. J Neurophysiol 104:218–229
Ji G, Neugebauer V (2011) Pain-related deactivation of medial prefrontal cortical neurons involves mGluR1 and GABAA receptors. J Neurophysiol 106:2642–2652
Ji G, Fu Y, Ruppert KA, Neugebauer V (2007) Pain-related anxiety-like behavior requires CRF1 receptors in the amygdala. Mol Pain 3:13–17
Ji G, Horvath C, Neugebauer V (2009) NR2B receptor blockade inhibits pain-related sensitization of amygdala neurons. Mol Pain 5:21–26
Ji G, Sun H, Fu Y, Li Z, Pais-Vieira M, Galhardo V, Neugebauer V (2010) Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J Neurosci 30:5451–5464
Ji G, Fu Y, Adwanikar H, Neugebauer V (2013) Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Mol Pain 9:2
Jongen-Relo AL, Amaral DG (1998) Evidence for a GABAergic projection from the central nucleus of the amygdala to the brainstem of the macaque monkey: a combined retrograde tracing and in situ hybridization study. Eur J Neurosci 10:2924–2933
Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC (2008) Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron 59:298–310
Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ, Gereau RW (2010) Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci 30:8203–8213
Koob GF (2010) The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314:3–14
Kruger L, Sternini C, Brecha NC, Mantyh PW (1988) Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J Comp Neurol 273:149–162
Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El-Deredy W, Jones AK (2007) Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum 56:1345–1354
Laviolette SR, Grace AA (2006) Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26:6458–6468
Leonard SK, Ring RH (2011) Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience 172:153–163
Li W, Neugebauer V (2004a) Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain 110:112–122
Li W, Neugebauer V (2004b) Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol 91:13–24
Li W, Neugebauer V (2006) Differential changes of group II and group III mGluR function in central amygdala neurons in a model of arthritic pain. J Neurophysiol 96:1803–1815
Li N, Liang J, Fang CY, Han HR, Ma MS, Zhang GX (2008) Involvement of CGRP and CGRPl receptor in nociception in the basolateral nucleus of amygdala of rats. Neurosci Lett 443:184–187
Li W, Chang M, Peng YL, Gao YH, Zhang JN, Han RW, Wang R (2009) Neuropeptide S produces antinociceptive effects at the supraspinal level in mice. Regul Pept 156:90–95
Li Z, Ji G, Neugebauer V (2011) Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci 31:1114–1127
Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D (2008) Amygdala intercalated neurons are required for expression of fear extinction. Nature 454:642–645
Liu CC, Ohara S, Franaszczuk P, Zagzoog N, Gallagher M, Lenz FA (2010) Painful stimuli evoke potentials recorded from the medial temporal lobe in humans. Neuroscience 165:1402–1411
Ma W, Chabot J-G, Powell KJ, Jhamandas K, Dickerson IM, Quirion R (2003) Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 120:677–694
Manning BH (1998) A lateralized deficit in morphine antinociception after unilateral inactivation of the central amygdala. J Neurosci 18:9453–9470
Manning BH, Mayer DJ (1995a) The central nucleus of the amygdala contributes to the production of morphine antinociception in the formalin test. Pain 63:141–152
Manning BH, Mayer DJ (1995b) The central nucleus of the amygdala contributes to the production of morphine antinociception in the rat tail-flick test. J Neurosci 15(12):8199–8213
Marek R, Strobel C, Bredy TW, Sah P (2013) The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol 591:2381–2391
Mason P (2005) Deconstructing endogenous pain modulations. J Neurophysiol 94:1659–1663
McDonald AJ (1998) Cortical pathways to the mammalian amygdala. Prog Neurobiol 55:257–332
McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28
McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339
McNally GP, Akil H (2002) Role of corticotropin-releasing hormone in the amygdala and bed nucleus of the stria terminalis in the behavioral, pain modulatory, and endocrine consequences of opiate withdrawal. Neuroscience 112:605–617
Myers B, Greenwood-Van Meerveld B (2010) Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. Am J Physiol Gastrointest Liver Physiol 298:G295–G303
Myers DA, Gibson M, Schulkin J, Greenwood Van-Meerveld B (2005) Corticosterone implants to the amygdala and type 1 CRH receptor regulation: effects on behavior and colonic sensitivity. Behav Brain Res 161:39–44
Nakao A, Takahashi Y, Nagase M, Ikeda R, Kato F (2012) Role of capsaicin-sensitive C-fiber afferents in neuropathic pain-induced synaptic potentiation in the nociceptive amygdala. Mol Pain 8:51
Neugebauer V, Li W (2003) Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol 89:716–727
Neugebauer V, Zinebi F, Russell R, Gallagher JP, Shinnick-Gallagher P (2000) Cocaine and kindling alter the sensitivity of group II and III metabotropic glutamate receptors in the central amygdala. J Neurophysiol 84:759–770
Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW (2003) Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci 23:52–63
Neugebauer V, Li W, Bird GC, Han JS (2004) The amygdala and persistent pain. Neuroscientist 10:221–234
Neugebauer V, Galhardo V, Maione S, Mackey SC (2009) Forebrain pain mechanisms. Brain Res Rev 60:226–242
Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP (2011) Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60:1017–1041
Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50:295–322
Ochsner KN, Gross JJ (2005) The cognitive control of emotion. Trends Cogn Sci 9:242–249
Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS, Keyvan-Fouladi N, Heavens RP, Wainwright A, Jacobson M, Dickerson IM, Hill RG (2001) Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci 14:618–628
Orsini CA, Maren S (2012) Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36:1773–1802
Palazzo E, Fu Y, Ji G, Maione S, Neugebauer V (2008) Group III mGluR7 and mGluR8 in the amygdala differentially modulate nocifensive and affective pain behaviors. Neuropharmacology 55:537–545
Palazzo E, Marabese I, Soukupova M, Luongo L, Boccella S, Giordano C, de Novellis V, Rossi F, Maione S (2011) Metabotropic glutamate receptor subtype 8 in the amygdala modulates thermal threshold, neurotransmitter release, and rostral ventromedial medulla cell activity in inflammatory pain. J Neurosci 31:4687–4697
Pape HC, Pare D (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90:419–463
Pedersen LH, Scheel-Kruger J, Blackburn-Munro G (2007) Amygdala GABA-A receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 127:17–26
Peng YL, Zhang JN, Chang M, Li W, Han RW, Wang R (2010) Effects of central neuropeptide S in the mouse formalin test. Peptides 31:1878–1883
Pinard CR, Mascagni F, McDonald AJ (2012) Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 205:112–124
Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM (2002) International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev 54:233–246
Price JL (2003) Comparative aspects of amygdala connectivity. In: Shinnick-Gallagher P, Pitkanen A, Shekhar A, Cahill L (eds) The amygdala in brain function. Basic and clinical approaches, vol 985. The New York Academy of Sciences, New York, pp 50–58
Qin C, Greenwood-Van Meerveld B, Foreman RD (2003) Visceromotor and spinal neuronal responses to colorectal distension in rats with aldosterone onto the amygdala. J Neurophysiol 90:2–11
Rea K, Lang Y, Finn DP (2009) Alterations in extracellular levels of gamma-aminobutyric acid in the rat basolateral amygdala and periaqueductal gray during conditioned fear, persistent pain and fear-conditioned analgesia. J Pain 10:1088–1098
Reinscheid RK (2008) Neuropeptide S: anatomy, pharmacology, genetics and physiological functions. Results Probl Cell Differ 46:145–158
Ren W, Neugebauer V (2010) Pain-related increase of excitatory transmission and decrease of inhibitory transmission in the central nucleus of the amygdala are mediated by mGluR1. Mol Pain 6:93–106
Ren W, Palazzo E, Maione S, Neugebauer V (2011) Differential effects of mGluR7 and mGluR8 activation on pain-related synaptic activity in the amygdala. Neuropharmacology 61:1334–1344
Ren W, Kiritoshi T, Gregoire S, Ji G, Guerrini R, Calo G, Neugebauer V (2013) Neuropeptide S: a novel regulator of pain-related amygdala plasticity and behaviors. J Neurophysiol 110:1765–1781
Reul JM, Holsboer F (2002) Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol 2:23–33
Robinson SD, Aitken JF, Bailey RJ, Poyner DR, Hay DL (2009) Novel peptide antagonists of adrenomedullin and calcitonin gene-related peptide receptors: identification, pharmacological characterization, and interactions with position 74 in receptor activity-modifying protein 1/3. J Pharmacol Exp Ther 331:513–521
Rouwette T, Vanelderen P, Reus MD, Loohuis NO, Giele J, van Egmond J, Scheenen W, Scheffer GJ, Roubos E, Vissers K, Kozicz T (2011) Experimental neuropathy increases limbic forebrain CRF. Eur J Pain 16(1):61–71
Ruzza C, Pulga A, Rizzi A, Marzola G, Guerrini R, Calo’ G (2012) Behavioural phenotypic characterization of CD-1 mice lacking the neuropeptide S receptor. Neuropharmacology 62:1999–2009
Sanchez MM, Young LJ, Plotsky PM, Insel TR (1999) Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol 408(3):365–377
Schiess MC, Callahan PM, Zheng H (1999) Characterization of the electrophysiological and morphological properties of rat central amygdala neurons in vitro. J Neurosci Res 58:663–673
Schwaber JS, Sternini C, Brecha NC, Rogers WT, Card JP (1988) Neurons containing calcitonin gene-related peptide in the parabrachial nucleus project to the central nucleus of the amygdala. J Comp Neurol 270:416–426
Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D (2012) The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp 35(2):527–538. doi:10.1002/hbm.22199
Sotres-Bayon F, Quirk GJ (2010) Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20:231–235
Spuz CA, Borszcz GS (2012) NMDA or non-NMDA receptor antagonism within the amygdaloid central nucleus suppresses the affective dimension of pain in rats: evidence for hemispheric synergy. J Pain 13:328–337
Sun N, Cassell MD (1993) Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol 330:381–404
Tache Y, Bonaz B (2007) Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest 117:33–40
Takahashi LK (2001) Role of CRF(1) and CRF(2) receptors in fear and anxiety. Neurosci Biobehav Rev 25:627–636
Tillisch K, Mayer EA, Labus JS (2010) Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 140:91–100
Ulrich-Lai YM, Xie W, Meij JTA, Dolgas CM, Yu L, Herman JP (2006) Limbic and HPA axis function in an animal model of chronic neuropathic pain. Physiol Behav 88:67–76
Uryu K, Okumura T, Shibasaki T, Sakanaka M (1992) Fine structure and possible origins of nerve fibers with corticotropin-releasing factor-like immunoreactivity in the rat central amygdaloid nucleus. Brain Res 577:175–179
Van Rossum D, Hanish U-K, Quirion R (1997) Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev 21:649–678
Wimalawansa SJ (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17:533–585
Xu YL, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, De LL, Civelli O (2004) Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron 43:487–497
Xu YL, Gall CM, Jackson VR, Civelli O, Reinscheid RK (2007) Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J Comp Neurol 500:84–102
Zhang RX, Zhang M, Li A, Pan L, Berman BM, Ren K, Lao L (2013) DAMGO in the central amygdala alleviates the affective dimension of pain in a rat model of inflammatory hyperalgesia. Neuroscience 252:359–366
Zhu W, Pan ZZ (2004) Synaptic properties and postsynaptic opioid effects in rat central amygdala neurons. Neuroscience 127:871–879
Acknowledgements
Work in the author’s lab is supported by National Institute of Neurological Disorders and Stroke Grants NS-081121, NS-38261, and NS-11255.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Neugebauer, V. (2015). Amygdala Pain Mechanisms. In: Schaible, HG. (eds) Pain Control. Handbook of Experimental Pharmacology, vol 227. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46450-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-662-46450-2_13
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46449-6
Online ISBN: 978-3-662-46450-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)