Abstract
In this work, γ-aminobutyric acid (GABA) was prepared by the decarboxylation of l-glutamate via l-glutamate decarboxylase in the resting cells of Lactobacillus brevis CGMCC No. 3414. The influence of cell concentration, cell age, buffer system, reaction time, and substrate concentration were investigated. The optimal composition of bioconversion system was composed of 50 g/L resting cells, cell age at 48 h fermentation, 0.2 M disodium hydrogen phosphate–citric acid buffer, and 25 mM monosodium glutamate. When the bioconversion system was performed at pH 4.6, 30°C, and 180 r/min shaking for 4 h, GABA production in biotransformation solution was 23.29 mM and the molar yield rate of bioconversion reached 93.15 %.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- γ-Aminobutyric acid (GABA)
- Lactobacillus brevis
- Resting cells
- l-Glutamate decarboxylase
- Bioconversion
1 Introduction
γ-Aminobutyric acid (GABA), an ubiquitous natural nonprotein amino acid, is widely distributed in plants and animals [1]. GABA, which acts as an important type of inhibitory neurotransmitter in mammal brain and spinal cord, could play an important role in hormone and nutritional factors in non-nerve tissues [2]; so GABA has an important regulating effect on the normal physiological functions of an organism [3]. GABA has several well-known physiological functions, such as antihypotension effects, improving cerebral function, delaying senility, tranquilizer effects, activating renal and liver functions, and promoting the secretion of growth hormone [4–9]. GABA, therefore, has the potential as a bioactive component in foods and pharmaceuticals due to its bioactive effects. Glutamate decarboxylase (GAD, EC 4.1.1.15), which is widely distributed in prokaryotic and eukaryotic cells [10, 11], is the unique enzyme to catalyze the conversion of l-glutamate or its salts to GABA and CO2 via the single-step α-decarboxylation [12–14].
With the increasing commercial demand for GABA, various chemical and biological synthesis methods for GABA have been studied [15–17]. Chemical synthesis of GABA has a high cost and a low production ratio. Furthermore, it cannot be used in the food industry due to dangerous or poisonous solvents used in the synthesis. Microbiological fermentation method is characterized by easy operation, moderate reaction conditions, safety, and low cost, whereas the downstream process and production cycle is long. Bioconversion method of GABA may be a much more promising method due to simple reaction procedure, high catalytic efficiency, saving production cost, shortening production cycle, less by-product, less pollution, and environmental compatibility [18]. In the current study, the process of enzymatic conversion for γ-aminobutyric acid by Lactobacillus brevis CGMCC No. 3414 resting cells and the influence of several factors on GABA production were investigated and the optimal composition of bioconversion system was determined in order to provide reference for using GAD of GRAS lactic acid bacterium to catalyze the conversion of l-glutamate or its salts to GABA.
2 Materials and Methods
2.1 Materials and Equipment
The L. brevis strain used in the present work was isolated from naturally pickled Chinese vegetables in our previous work, and it is stored at the China General Microbiological Culture Collection Center as L. brevis CGMCC No. 3414. The experimental equipments are high performance liquid chromatography (Agilent 1,260 infinity, Agilent Technologies, USA) with an Agilent ZORBAX StableBond C18 column (4.6 × 250 mm), Hitachi CR22GIII high-speed refrigerated centrifuge (Hitachi Koki Co., Ltd., Japan), and desk high speed centrifuge (Hunan Xiang Yi Laboratory Instrument Development Co., Ltd., China).
The glucose-yeast extract-peptone (GYP) medium for seed preparation contained (g/L): glucose, 10; yeast extract, 10; peptone, 5; sodium acetate, 2; MgSO4·7H2O, 0.02; MnSO4·4H2O, 0.001; FeSO4·7H2O, 0.001; NaCl, 0.001; and pH 6.8.
The fermentation medium consisted of the following (g/L): glucose, 30; yeast extract, 30; peptone, 5; sodium acetate, 3; FeSO4·7H2O, 0.001; MgSO4·7H2O, 0.03; NaCl, 0.001; MnSO4·4H2O, 0.019; l-sodium glutamate (L-MSG), 10; pH 6.8.
GABA standard was purchased from Sigma-Aldrich. L-MSG (purity ≥ 99 %) was provided by Tianjin Red Rose Food Co., Ltd, China. Chromatography reagents were obtained from Tianjin Kemiou Chemical Reagent Co., Ltd, China. Other reagents were of analytical reagent grade and biochemical reagents.
2.2 GABA Biotransformation by L. brevis CGMCC No. 3414 Resting Cells
One loop of slant culture of L. brevis CGMCC No. 3414 was inoculated into a 50-mL seed medium in a 500-mL Erlenmeyer flask and incubated without agitation at 30 °C for 24 h. The seed culture was then inoculated at 10 % (v/v) into 200 mL fermentation medium in a 500-mL Erlenmeyer flask and cultivated without agitation at 30 °C. After 16–72 h cultivation, the cells were harvested by centrifugation at 5,000×g for 10 min at 4 °C, washed twice with sterile water, and then resuspended in 0.2 M disodium hydrogen phosphate–citric acid buffer (containing 25 mM L-MSG, pH 4.6) containing 50 g wet cells per liter. The bioconversion was performed at 30 °C and 180 r/min shaking for 4 h, and then the bioconversion broth was detected after centrifugal filtration.
2.3 Analytical Methods
The volume of 1 mL bioconversion broth was centrifuged at 12,000 r/min for 2 min, then a 10-μL supernatant was pipetted into a 1.5-mL centrifuge tube, added with 100 μL derived buffer, 100 μL derivative agent (2,4-dinitro-1-fluorobenzene), and 790 μL potassium dihydrogen phosphate buffer, uniformly mixed, water bath at 60 °C for 1 h. After that, the derivation mixture was filtered by a 0.22 μm cellulose acetate membrane filter, and the filtrate was stored at 4 °C for HPLC assay. Gradient elution conditions: Agilent ZORBAX StableBond C18 column, 30 °C column temperature, 360 nm ultraviolet detection wavelength, 0.8 mL/min flow velocity, 10 μL sample amount, mobile phase A: water, mobile phase B: acetonitrile, mobile phase C: methyl alcohol, and mobile phase C: 0.05 M sodium acetate solution [19].
3 Results and Discussion
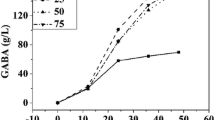
3.1 Effect of Cell Age on GABA Production by L. brevis CGMCC No. 3414 Resting Cells
The glutamate decarboxylase produced by lactic acid bacteria is an inducible enzyme [20]. The current study evaluated the resting cell of different cell age bioconversion of MSG to GABA. L. brevis CGMCC No. 3414 cells were harvested at culture time of 16, 24, 36, 48, 60, and 72 h, respectively, which is collected according to the above methods. The resting cells with the same concentration were resuspended in bioconversion system, and then the bioconversion broth was detected after a period of reaction time.
As shown in Fig. 63.1, the GABA yield was greatly improved with the increase of culture time. When the L. brevis CGMCC No. 3414 cells were harvested at culture time of 60 h, the cells had the strongest transformation ability, whereas at 72 h, the transformation ability of cells decreased. This is mainly because that a great amount of secondary metabolites produced by the cells began to accumulate after strains were developed to the stationary phase, especially the glutamate decarboxylase. When L. brevis CGMCC No. 3414 cells were harvested in this period for transformation, the yield of GABA could reach the highest. However, we conjecture that the content of GAD had little difference between the cells cultivated at 48 and 60 h. Therefore, the yield of GABA is of no significant difference between them. Considering the fermentation period and cost, culture time of 48 h was used for subsequent studies on GABA production by L. brevis CGMCC No. 3414 resting cells.
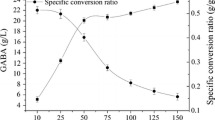
3.2 Effect of Cell Concentration on GABA Production by L. brevis CGMCC No. 3414 Resting Cells
Generally speaking, the yield of GABA will increase with the increased cell concentration. As shown in Fig. 63.2, the yield of GABA increased rapidly in the concentration range of 10–25 g/L. When the cell concentration increased further, the yield of GABA increased little. The specific conversion ratio was investigated in the current study. Figure 63.2 indicates that the specific conversion ratio decreases with the increased cell concentration, and the turning point appears at cell concentration of 50 g/L. Given the yield of GABA, training cost, and efficiency of the cell culture, 50 g/L cell concentration was used for subsequent studies on GABA production by L. brevis CGMCC No. 3414 resting cells.
3.3 Effect of Bioconversion Time on GABA Production by L. brevis CGMCC No. 3414 Resting Cells
When GABA is produced by bioconversion, glutamate reacts with GAD after penetrating the cell membrane and getting into the cell cytoplasm. The reaction ratios of GABA bioconversion were the highest at (0–3) h, slowed down at (4–5) h, and then became almost constant after 5 h (Fig. 63.3). This is mainly because that substrate consumption and product accumulation reach relative balance, and the decrease of substrate has an effect on reaction ratio. Thus, considering product accumulation and reaction ratio, a conversion time of 4 h was used for subsequent studies on GABA production by L. brevis CGMCC No. 3414 resting cells.
3.4 Effect of pH on GABA Production by L. brevis CGMCC No. 3414 Resting Cells
The reaction pH of GAD from different sources has a great difference. GADs of plant origin show the highest activity at pH 5.5–6.6 [21]. Nomura et al. [22] reported that the optimum pH of GAD produced by Lactococcus lactis subsp. lactis was 4.7. Huang and Mei [23] reported that the optimum pH of GAD produced by L. brevis CGMCC 1306 was 4.4. Figure 63.4 exhibits that the effect of pH has influence on bioconversion. The result showed that the suitable pH range of GAD was 4.6–5.0. The study proves that L. brevis GAD displays good pH stability in the pH 4.0–5.0 and the optimum pH is about 4.5. The optimum pH of bioconversion in the current study is 4.6 which is similar with the references. Thus, pH 4.6 was used for subsequent experiments.
3.5 Effect of MSG Substrate Concentration on GABA Production by L. brevis CGMCC No. 3414 Resting Cells
Substrate concentration has great influence on reaction ratio in enzymatic reaction. Substrate usually has double effects of accelerating the reaction speed and inhibiting enzyme activity. The influence of initial MSG concentration on GABA production and the ratio of bioconversion by L. brevis CGMCC No. 3414 resting cells were studied in current research. In Fig. 63.5, the yield of GABA by resting cell transformation at lower ranges of MSG substrate concentration (10–25 mM) gradually increased with the increase of MSG concentration by 4-h biotransformation. The final GABA yield was almost constant while the substrate concentration increased to over 25 mM; however, the conversion ratio decreased significantly. This result indicates that low concentration of substrate has little effect on enzymatic reaction and the product inhibition is not found during this bioconversion. Thus, 25 mM MSG was used for GABA bioconversion by L. brevis CGMCC No. 3414 resting cells.
4 Discussion
Lactobacillus brevis, which has full GAD activity and is the preferable species to catalyze the conversion of l-glutamate or its salts to GABA, is a GRAS microorganism. Using bioconversion method for GABA production not only has advantages like high yield and short production cycle, but also obtains high purity product and overcomes the limitation of microbiological fermentation method. Since each influencing factor of GABA production by L. brevis CGMCC No. 3414 resting cells was analyzed and discussed, the optimal composition of bioconversion system contained 50 g/L resting cells, cell age at 48 h fermentation, 0.2 M disodium hydrogen phosphate–citric acid buffer, and 25 mM MSG. When the bioconversion system was performed at pH 4.6, 30 °C, and 180 r/min shaking for 4 h, GABA production in biotransformation mixture was 23.29 mM, and the molar yield rate of bioconversion reached 93.15 %. Overall, this study laid a foundation for catalyzing the conversion of l-glutamate or its salts to GABA by L. brevis resting cells.
References
Kinnersley AM, Turano FJ (2000) Gamma aminobutyric acid (GABA) and plant responses to stress. Crit Rev Plant Sci 19(6):479–509
Manyam BV, Katz L, Hare TA et al (1981) Isoniazid-induced elevation of CSF GABA levels and effects on chorea in Huntington’s disease. Ann Neurol 10(1):35–37
Yang S, Lu Z, Lu F et al (2005) Research progress on microbial glutamate decarboxylase. Food Sci 26(9):546–551
Okada T, Sugishita T, Murakami T et al (2000) Effect of the defatted rice germ enriched with GABA for sleeplessness, depression, autonomic disorder by oral administration. J Jpn Soc Food Sci 47(8):596–603
Omori M, Yano T, Okamoto J et al (1987) Effect of anaerobically treated tea (Gabaron tea) on blood pressure of spontaneously hypertensive rats. J Agr Chem Sci Jpn 61:1449–1451
Jones EA (2002) Ammonia, the GABA neurotransmitter system, and hepatic encephalopathy. Metab Brain Dis 17(4):275–281
Hagiwara H, Seki T, Ariga T (2004) The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci Biotech Biochem (BBB) 68(2):444–447
DeFeudis FV (1983) γ-Aminobutyric acid and cardiovascular function. Experientia 39(8):845–849
Cavagnini F, Invitti C, Pinto M et al (1980) Effect of acute and repeated administration of gamma aminobutyric acid (GABA) on growth hormone and prolactin secretion in man. Endocrinol Acta 93(2):149–154
Zhao Y, Liang XL, Zhang H (2006) The Structure and function of the glutamate decarboxylase and its genes expression regulation in Escherichia coli. Food Ferment Ind 32(7):75–78
Peng C, Huang J, Hu S et al (2013) A two-stage pH and temperature control with substrate feeding strategy for production of gamma-aminobutyric acid by Lactobacillus brevis CGMCC 1306. Chinese J Chem Eng 21(10):1190–1194
Ueno H (2000) Enzymatic and structural aspects on glutamate decarboxylase. J Mol Catal B-Enzym 10(1):67–79
Battaglioli G, Liu H, Martin DL (2003) Kinetic differences between the isoforms of glutamate decarboxylase: implications for the regulation of GABA synthesis. J Neurochem 86(4):879–887
Gao Q, Duan Q, Wang D et al (2013) Separation and purification of γ-aminobutyric acid from fermentation broth by flocculation and chromatographic methodologies. J Agr Food Chem 61(8):1914–1919
Plokhov AY, Gusyatiner MM, Yampolskaya TA et al (2000) Preparation of γ-aminobutyric acid using E. coli cells with high activity of glutamate decarboxylase. Appl Biochem Biotech 88:257–265
Komatsuzaki N, Shima J, Kawamoto S et al (2005) Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol 22(6):497–504
Choi SI, Lee JW, Park SM et al (2006) Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J Microbiol Biotech 16:562–568
Zhang Y, Song L, Gao Q et al (2012) The two-step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Appl Microbiol Biotech 94(6):1619–1627
Chen HM, Gao Q, Su Z et al (2012) Screening, identification and flask fermentation optimization of a high-yield γ-aminobutyric acid Enterococcus raffinosus strain. Microbiol China 39(11):1642–1652
Fu YX, Zhang T, Jiang B et al (2008) Enzymatic conversion for γ-aminobutyric acid by Lactococcus lactis. Sci Tech Food Ind 09:166–169
Satyanarayan V, Nair PM (1985) Purification and characterization of glutamate decarboxylase from Solanum tuberosum. Eur J Biochem 150(1):53–60
Nomura M, Nakajima I, Fujita Y (1999) Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology 145(6):1375–1380
Huang J, Mei LH (2007) Purification and characterization of glutamate decarboxylase of Lactobacillus brevis CGMCC 1306 isolated from fresh milk. Chinese J Chem Eng 15(2):157–161
Acknowledgments
This research was supported by the National 863 Program of China (2012AA021302), the National 973 Program of China (2014CB734004), and the National Natural Science Foundation of China (31370075 & 31471725).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Shi, Xf., Zheng, B., Chang, Cy., Cao, P., Yang, Hj., Gao, Q. (2015). Enzymatic Bioconversion for γ-Aminobutyric Acid by Lactobacillus brevis CGMCC No. 3414 Resting Cells. In: Zhang, TC., Nakajima, M. (eds) Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, vol 333. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46318-5_63
Download citation
DOI: https://doi.org/10.1007/978-3-662-46318-5_63
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46317-8
Online ISBN: 978-3-662-46318-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)