Abstract
In this study, the potential for heterologous protein expression and secretion via twin-arginine translocation (Tat) pathway was investigated in Escherichia coli DH5α host using enhanced green fluorescent protein (EGFP) as model protein reporter. To construct the shuttle vector pBT2-ET-5X-EGFP, 17 kinds of PCR-amplified 5x-egfp fragments were, respectively, cloned into plasmid pBT2-Peftu-Tat-EGFP and transformed into E. coli DH5α host. By SDS-PAGE, fluorescence microscope observation and flow cytometry analyzation, EGFP was expressed in an active form in the cells of E. coli DH5α, but failed to translocate to the culture medium.
B. Xu and Y. Cheng—Co-first authors.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- E. coli DH5α
- Enhanced green fluorescent protein (EGFP)
- Twin-arginine translocation pathway
- Plasmid pBT2
1 Introduction
Compared to the general protein secretion (Sec) system [1], the twin-arginine translocation (Tat) system can deliver the properly folded proteins to the extracellular environment [2]. Thus, the Tat system can be used in gene engineering to secrete exogenous protein which cannot utilize the Sec system [3].
Green fluorescent protein (GFP) can be expressed in Escherichia coli as an ideal reporter [4], but whether it is correctly folded and transported across the plasma membrane depends on the choice of the Tat signal peptide sequence [2]. The wild-type GFP is sensitive to temperature, sometimes less expressed and weakly fluorescent in some cells [5], thus researchers have optimized GFP through site-directed mutagenesis. The enhanced green fluorescent protein (EGFP) is now a widely used GFP mutant with two independent substitutions (F64L, S65T), which has improved the fluorescence intensity as well as stability to be detectable for 16–24 h after excitation [6]. In this study, N-terminal modified EGFP was used as model reporter to study the Tat-dependent secretion pathway in E. coli DH5α host.
Shuttle vector has two different replication origins of two microbial plasmids, selection marker gene and a multicloning site, which can be replicated in two different organisms, and is usually used for cloning the amplified cloned gene. In this study, pBT2-ET-5X-EGFP, a shuttle vector of E. coli and Staphylococcus derived from a shuttle vector pBT2 [7], was constructed to study the expression and secretion of EGFP via Tat-pathway.
2 Materials and Methods
2.1 Bacterial Strain, Plasmids, and Growth Medium
The bacterial strain and plasmids used in this study are listed in Table 19.1, and all the recombinant DNA manipulations were carried out in E. coli DH5α host. E. coli DH5α was cultured at 37 °C in LB broth (0.5 % yeast extract, 1 % tryptone, and 1 % NaCl) supplemented with 100 μg/mL ampicillin for plasmid selection.
2.2 Preparation of the 5x-egfp Fragment
Based on our previous experiments, a linker consisting of five identical amino acids was designed and inserted between the Tat signal peptide and the N-terminal of egfp gene to generate a 5x-egfp fusion gene fragment, where x here stands for a certain amino acid codon. Such 5x-egfp fragment is found to be helpful for secretion from the bacterial host (data not shown). Since 5N-, 5Q- and 5R-EGFP were previously expressed in E. coli DH5ɑ host, in order to construct the other 17 kinds of 5x-egfp fragment, the primers (Table 19.2) were correspondingly designed according to the sequence of egfp gene (No. AF302837).
The primers above were used to, respectively, amplificate the 5x-egfp fragment using plasmid pBT2-ET-EGFP as PCR template. The following reaction mixture was set up as: 17.5 μL ddH2O, 2.5 μL parent plasmid, 2.5 μL each primer, 25 μL 2× Taq PCR Master Mix. The cycling parameters for the PCR were as follows: 30 cycles of 95 °C for 5 min, 95 °C for 50 s, 70 °C for 30 s, 72 °C for 1 min followed by a single cycle of 72 °C for 10 min. The PCR products were purified by GeneJET Gel Extraction Kit (Thermo, Germany) after 1 % agarose gel electrophoresis.
2.3 Transformation of E. coli DH5α
The E. coli DH5α competent cells were thawed in ice for 5 min, then a 10-μL ligation product was added to the cells, and the mixture was incubated in ice for 30 min, heat-shocked for 90 s in a 42 °C water bath, immediately transferred to an ice bath for 1–2 min, followed by the addition of 1 mL sterile LB broth and constant shaking at 37 °C and 200 r/min for 1.5 h; a 100-μL culture broth was spread on LB agar plates containing 100 μg/mL chloramphenicol, and incubated at 37 °C for 12 h [9].
2.4 Preparation of Plasmid pBT2-ET-5X-EGFP
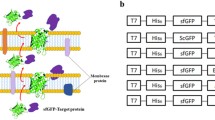
The resulting PCR products were, respectively, cloned into pMD19-T Simple Vector after overnight ligation at 16 °C and transformed into E. coli DH5α host. Plasmid pMD19-T-5X-EGFP prepared from E. coli was purified using the TIANprep Mini Plasmid Kit (TIANGEN, Beijing, China). The constructed 5x-egfp fragment was first digested by double digestion of HindIII and NheI (Fermentas, Lietuvos) from plasmid pMD19-T-5X-EGFP, then inserted into the synonymous sites in pBT2-Peftu-Tat-EGFP, a shuttle vector of E. coli and Staphylococcus, by T4 ligase (Fermentas, Lietuvos). After overnight ligation at 16 °C, the linked products were transformed into E. coli DH5α to create the recombinant plasmid pBT2-ET-5X-EGFP (Fig. 19.1). All plasmid constructions were verified by double restriction enzyme digestion and DNA sequence analysis.
Diagram of plasmid pBT2-ET-5X-EGFP. 5X-EGFP, EGFP with a 5 identical amino acids linker at its N-terminal (X represents for an amino acid of A, C, D, F, G, H, I, K, L, M, N, P, S, T, V, W and Y, respectively); Tat a twin-arginine translocation signal peptide of efeB gene in S. carnosus TM300; Peftu a strong promoter of tufA gene in S. carnosus TM300; bla ampicillin resistance gene; cat chloramphenicol resistance gene; E.c.ori origin of replication in E. coli; ss.ori origin of replication in Staphylococcus
2.5 Extraction of EGFP
Single colonies of E. coli DH5α/pBT2-ET-5X-EGFP and E. coli DH5α were, respectively, grown in 50 mL LB broth at 37 °C and 180 r/min for 16 h. The cell pellets were harvested by centrifugation at 12,000 r/min and 4 °C for 15 min. Proteins present in the culture medium were treated with 10 % trichloroacetic acid (TCA) for 12 h at 4 °C. The cells were resuspended in 2 mL PBS buffer, disrupted by sonication, and centrifuged to separate the supernatant from cell fragment. The supernatant was carefully transferred to a new vial and then precipitated by adding 10 % TCA for 12 h at 4 °C. The precipitated proteins were washed successively in 100 and 80 % acetone and dried at room temperature.
2.6 Detection of EGFP
After fermentation culture, the expression of the modified 5X-EGFP in E. coli DH5α host was observed by fluorescence microscopy (Olympus (China) Co., Ltd., China), the number of fluorescent cells per 10,000 was counted by flow cytometry (Accuri C6, BD, America), and the expression of EGFP in cells and culture medium of E. coli DH5α/pBT2-ET-5X-EGFP was also analyzed by SDS-PAGE.
3 Result
3.1 Construction of 5x-egfp Fragment
The 17 kinds of 5x-egfp fragment obtained from PCR were examined by agarose gel electrophoresis (Fig. 19.2a, b) and purified with GeneJET Gel Extraction Kit (Fig. 19.2c). Double restriction digestion and sequence alignment of plasmid pMD19-T-5X-EGFP (Fig. 19.2d) showed the same result with the target gene.
Agarose gel electrophoresis of the PCR product of 5x-egfp fragment. M DNA Marker; a1–a12 PCR product of 5x-egfp (x = codon for A, C, D, F, G, H, I, K, L, M, N or P); b1–b5 PCR product of 5x-egfp (x = codon for S, T, V, W and Y); c1–c17 purified product of 5x-egfp (x = codon for A, C, D, F, G, H, I, K, L, M, N, P, S, T, V, W or Y); d1–d17 pMD19-T-5X-EGFP digested by HindIII and NheI (X = A, C, D, F, G, H, I, K, L, M, N, P, R, S, T, V, W or Y)
3.2 Verification of pBT2-ET-5X-EGFP
Recombinant plasmid pBT2-ET-5X-EGFP extracted from recombinant strain was examined by 1 % agarose gel electrophoresis (Fig. 19.3). Their molecular weights were expected as 8,000 bp for pBT2-ET-5X-EGFP and 800 bp for 5X-EGFP.
Agarose gel electrophoresis of pBT2-Peftu-Tat-5X-EGFP. M DNA Marker. a1–a17 pBT2-ET-5X-EGFP (X = A, C, D, F, G, H, I, K, L, M, N, P, S, T, V, W or Y); b1–b4 pBT2-ET-5X-EGFP digested by HindIII and NheI (X = A, C, D or F); c1–c13 pBT2-ET-5X-EGFP restricted with HindIII and NheI (X = F, G, H, I, K, L, M, N, P, R, S, T, V, W or Y)
3.3 Florescence Detection of EGFP in E. coli DH5α
A 10 μL culture broth of E. coli DH5α/pBT2-ET-5X-EGFP was used for florescence microscope observation. The E. coli DH5α host strain bearing pBT2-ET-5X-EGFP exhibited strong fluorescence, while the control strain showed no fluorescence (Fig. 19.4).
3.4 Detection of EGFP in E. coli DH5α by SDS-PAGE
Dried precipitated proteins in the culture medium and the supernatant were resuspended in 50 μL PBS. After adding 12.5 μL loading buffer, the 30 μL samples were applied for SDS-PAGE analysis. The molecular weights of the 17 homologous target proteins were expected at about 35 kDa for 5X-EGFP. The results proved that 5X-EGFP were properly expressed in a soluble form in E. coli, but could not be translocated outside the cell wall (Fig. 19.5).
Expression of 5X-EGFP in E. coli DH5α validated by SDS-PAGE. M Protein Marker (Fermentas, Lietuvos); a1–a9 5X-EGFP extracted from the cytoplasm of E. coli DH5α/pBT2-ET-5X-EGFP (X = A, C, D, F, G, H, I, K or L); b1–b8 5X-EGFP extracted from the cytoplasm of E. coli DH5α/pBT2-ET-5X-EGFP (X = M, N, P, S, T, V, W or Y); c1–c9 5X-EGFP extracted from the culture medium of E. coli DH5α/pBT2-ET-5X-EGFP (X = A, C, D, F, G, H, I, K or L); d1–d8 5X-EGFP extracted from the culture medium of E. coli DH5α/pBT2-ET-5X-EGFP (X = M, N, P, R, S, T, V, W or Y)
3.5 Expression of EGFP in E. coli DH5α Detected by Flow Cytometry
A 1-mL suspension of E. coli DH5α/pBT2-ET-5X-EGFP was analyzed by flow cytometry. An average of fluorescent cell number indicated that more than half of the cells expressed active 5X-EGFP in E. coli DH5α cells (Fig. 19.6).
4 Discussion
Tat pathway first found in E. coli was now a focus of protein transportation research around the world due to its feature to help translocate fully folded proteins across the bacterial plasma membrane. Bolhuis et al. [10] found that two integral cytoplasmic membrane proteins TatB and TatC make up a structural and functional unit of the twin-arginine translocases in E. coli. Tat-dependent heterologous protein secretion was analyzed in the three different Gram-positive bacteria Staphylococcus carnosus, Bacillus subtilis, and Corynebacterium glutamicum using GFP as reporter [11]. Differences about the final localization and the folding status of the exported GFP proved that the choice of potential bacteria host and suitable microorganism is essential in GFP secretion via Tat pathway.
In this study, the constructed 5X-EGFP was used as a model protein reporter to study the possibility of Tat-dependent heterologous protein secretion in E. coli DH5α. By means of SDS-PAGE, fluorescence microscope observation and flow analyzation, all the 17 kinds of 5X-EGFP were successfully expressed in a soluble and active form in E. coli DH5α cytoplasm, but failed to be translocated out of the cell wall. One possible explanation is the lack of a series of cofactor-binding-proficient proteins which plays an important role in protein transportation [12], the existence of Gram-negative E. coli outer membrane is another reason for this failure.
This research constructed an E. coli-Staphylococcus shuttle vector to study the function of Tat-pathway upon exogenous protein secretion. In the future research, the recombinant plasmid pBT2-ET-5X-EGFP is to be transformed into Staphylococcus carnosus TM300, a valuable genetic engineering strain in food industry [13] with low extracellular proteolytic activity and no by-products like toxin, hemolysin, coagulase [14], and thereby lays the preliminary experimental basis for further insight into heterologous protein secretion via twin-arginine translocation pathway.
References
Driessen AJM, Fekkes P, van der Wolk JPW (1998) The Sec system[J]. Curr Opin Microbiol 1(2):216–222
Tjalsma H, Bolhuis A, Jongbloed JDH et al (2000) Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev 64:515–547
Wu LF, Bérengère I, Angélique C et al (2000) Bacterial twin-Arginine signal peptide-dependent protein translocation pathway: evolution and Mechanism. J Mol Microbiol Biotechnol 2(2):179–189
Chalife M, Tu Y, Euskirchen G et al (1994) Green fluorescent protein as a maker for gene expression. Science 263(5148):802–805
Pédelacq JD, Cabantous S, Tran T et al (2006) Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24(9):79–88
Cormack BP, Valdivia RH, Falkow S (1996) FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173(1):33–38
Brückner R (1997) Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol Lett 151(1):1–8
Gao Q, Wang MX, Yu CY et al (2012) Optimization of the electroporation conditions for DNA transformation of Staphylococcus carnosus. In: Zhang TC, Ouyang PK, Kaplan S et al (eds) Proceedings of the 2012 international conference on applied biotechnology. Lecture Notes in Electrical Engineering, vol 251. Springer, Heidelberg, pp 1699–1707
Yu C, Zheng X, Zhu Y et al (2011) Construction and expression of tat-gfp fusion vector. Biotechnol Bull 08:203–207
Bolhuis A, Mathers JE, Thomas JD et al (2001) TatB and TatC form a functional and structural unit of the twin-arginine translocase from Escherichia coli. J Biol Chem 276(23):20213–20219
Meissner D, Vollstedt A, van Dijl JM et al (2007) Comparative analysis of twin-arginine (Tat)-dependent protein secretion of a heterologous model protein (GFP) in three different Gram-positive bacteria. Appl Genet Mol Biotechnol 76:633–642
Blaudeck N, Springer GA, Freudl R, Wiegert T (2001) Specificity of signal peptide recognition in Tat-dependent bacterial protein translocation. J Bacteriol 183:604–610
Papamanoli E, Kotzekidou P (2002) Characterization of Micrococcaceae isolated from dry fermented sausage. Food Microbiol 19:441–449
Götz F (1990) Staphylococcus carnosus: a new host organism for gene cloning and protein production. Soc Appl Microbiol Symp Ser 19:49–53
Acknowledgments
We are very grateful to Prof. Dr. Friedrich Goetz of University of Tuebingen, Germany, for kindly providing plasmid pBT2, we also thank Ms. Lin Huang and Mr. Hao Zhou for excellent technical assistance and instrument support. This work was sponsored by the Natural Science Foundation of China (31370075 & 31471725), the National 973 Program of China (2013CB734004), and the National 863 Program of China (2012AA021302).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this paper
Cite this paper
Xu, By. et al. (2015). Construction of Eschericha coli-Staphylococcus Shuttle Vector for EGFP Expression and Potential Secretion via Tat Pathway. In: Zhang, TC., Nakajima, M. (eds) Advances in Applied Biotechnology. Lecture Notes in Electrical Engineering, vol 333. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46318-5_19
Download citation
DOI: https://doi.org/10.1007/978-3-662-46318-5_19
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46317-8
Online ISBN: 978-3-662-46318-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)