Abstract
Lecanoromycetes is the class of Ascomycota with the largest number of lichen-forming fungi. Members of this class are important components of most terrestrial ecosystems and occur in various habitats and on different substrates, from tropical to polar regions. Morphological, anatomical, and chemical characters have traditionally been used to classify orders, families, and genera within Lecanoromycetes. In the last two decades, molecular phylogenies have shown that traditional classification systems were not always consistent with the evolutionary history of this fungal class, resulting in changes in the delimitation of orders and families. Here, we revisit the taxonomic value of the main characters traditionally used for classification in light of current molecular phylogenies. The current delimitation of the 14 orders of Lecanoromycetes is also discussed, and recent changes in classification are highlighted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Lecanoromycetes (formally named by Eriksson and Winka in 1997) constitutes one of the largest classes of Ascomycota with 14,199 known species (Kirk et al. 2008). The class mainly includes ascomycetes characterized by apothecial ascomata, amyloid asci with a two-layered wall and an apical thickening, and a hamathecium (interascal hyphae) formed with paraphyses or pseudoparaphyses. This group comprises the largest number of lichen-forming ascomycetes, with approximately 90 % of all known lichen-forming fungi (Miadlikowska et al. 2006). However, not all members of this class are lichenized; several nonlichenized species are nested within this lineage (Lutzoni et al. 2001, 2004; Schoch et al. 2009). Lecanoromycetes are well known for the broad range of secondary compounds, such as depsidones, terpenoids, and xanthones (Huneck and Yoshimura 1996), that they exclusively produce, in some cases in large quantities out of proportion to the dry weight of their thalli.
In the past, ascoma structure or development and ascus types were used as the main characters in the classification of ascomycete fungi. Nannfeldt (1932) classified the euascomycetes according to their type of ascomatal development: Plectascales, Ascoloculares, and Ascohymeniales, where most Lecanoromycetes belonged. Luttrell (1951, 1955) in his classification system gave more importance to the ascus types and classified the euascomycetes as Bitunicatae (the Ascoloculares) and Unitunicatae (the Ascohymeniales and Plectascales). Unitunicatae were then subdivided according to their ascomata morphology: Plectomycetes, Pyrenomycetes, and Discomycetes, where most Lecanoromycetes belonged (Luttrell 1955). However, subsequent ultrastructural and ontogenetical studies showed that the complexity of these traits had been underestimated and that many taxa did not fit into these broad categories because of intermediate types (e.g., Bellemère and Letrouit-Galinou 1987; Henssen and Jahns 1974; Honegger 1982a). The reliability of these characters for higher-level classification was therefore doubted even before the beginning of the molecular era (e.g., Bellemère 1994; Poelt 1973; Reynolds 1989).
Molecular studies confirmed that classifications based on ascomatal characters often failed to capture monophyletic lineages within the ascomycetes (Berbee 1996; Lindemuth et al. 2001; Lumbsch and Huhndorf 2007a; Lutzoni et al. 2001; Liu and Hall 2004; Spatafora et al. 2006; Schoch et al. 2009). In particular, some groups within Lecanoromycetes (e.g., Ostropales, Caliciales) were shown to include a much larger diversity of ascoma and asci types than expected (Grube et al. 2004; Schmitt et al. 2005; Wedin and Tibell 1997; Wedin et al. 2000a). Molecular phylogenetic studies also cast doubts on the morphology-based delimitation of orders and families in Lecanoromycetes. As a result, many families and genera were segregated from the large order Lecanorales , while several additional families were included in Ostropales , the second largest order in Lecanoromycetes (Grube et al. 2004; Hofstetter et al. 2007; Kauff and Lutzoni 2002; Lumbsch et al. 2004; Miadlikowska and Lutzoni 2004; Miadlikowska et al. 2006; Wedin et al. 2005). Lecanoromycetes currently includes 14 orders and 3 subclasses: Acarosporomycetidae, Lecanoromycetidae, and Ostropomycetidae (Gaya et al. 2012; Hodkinson and Lendemer 2011; Lumbsch and Huhndorf 2010; Schmull et al. 2011).
II. Occurrence and Distribution
Lecanoromycetes have a broad distribution and can be found from the tropics to the poles. Their biomass is substantial in boreal to arctic climates, especially in the tundra, where species such as Cladonia rangiferina can constitute the main vegetation cover and are the main primary producer and food source for large herbivorous mammals (Brodo et al. 2001). In Antarctica, lichens (mostly from the class Lecanoromycetes) form the most species-diverse group within the vegetation cover, and some of them are found in the coldest and driest habitats of continental Antarctica (Green et al. 1999; Øvstedal and Lewis Smith 2001), where they are the basis of terrestrial life closest to a pole on Earth.
Lecanoromycetes are also very common and diverse in temperate regions, where they have been extensively studied (e.g., Brodo et al. 2001; Clauzade and Roux 1985; McCarthy 2003; Smith et al. 2009). In the tropics, they are thought to be as species diverse as in temperate regions, if not more so (Aptroot and Sipman 1997; Coppins and Wolseley 2002; Lücking 2008). A recent study in Papua New Guinea showed that a single Elaeocarpus tree could harbor up to 173 species of lichens, among which approximately 130 species were Lecanoromycetes (Aptroot 2001). Many tropical regions of the world are still mainly understudied, and many new species remain to be discovered (Sipman and Aptroot 2001).
Lecanoromycetes are found in a large number of natural terrestrial habitats, such as woodlands, heathlands, lowland and alpine grasslands, deserts, and arid shrublands. They are also commonly found in urban areas, wastelands, and other anthropogenous habitats (Gilbert 1990; Smith et al. 2010). Although most species occur in terrestrial environments, some species can grow on temporarily submerged substrates, in freshwater (Gilbert 1996; Gilbert and Giavarini 1997; Thüs and Schultz 2009), or in saltwater in the supralittoral zone (Brodo and Sloan 2004; Fletcher 1980).
III. Substrate Range and Ecology
A. Substrate Range
Lecanoromycetes grow predominantly on tree bark (corticolous species) and rocks (saxicolous species) but are also found on leaves (foliicolous species), soil (terricolous species), wood (lignicolous species), mosses (muscicolous species), and other lichens (lichenicolous species). Most species tend to be specific to a particular type of substrate, although some can colonize several (e.g., Parmelia sulcata). In addition to a natural substrate, some Lecanoromycetes can grow on human-made materials, such as brick, concrete, asphalt, metal, plastic, glass, and rubber (Brodo et al. 2001; Gilbert 1990).
A few species even occur on the bones or shells of land tortoises (Brodo et al. 2001) and indeed on any material with a stable surface exposed for long enough. A few others, mainly from the genera Aspicilia s.l. and Xanthoparmelia, do not attach to a substrate and occur as an erratic or vagrant (e.g., Pérez 1997; Rosentreter 1993).
B. Lifestyles
In Lecanoromycetes, most lichen-forming (mycobionts) fungi form mutualistic associations with one or two photosynthetic partners, either a green alga or a cyanobacterium (photobiont). Mycobionts obtain carbon from their photobiont, in the form of glucose when associated with cyanobacteria or in the form of polyhydric sugar alcohols (polyols) when associated with green algae (Palmqvist et al. 2008). Cyanobacteria found in lichens, such as Nostoc, can fix nitrogen and, therefore, can be a source of nitrogen for the mycobiont. The mycobiont protects the photobiont from UV light, temperature extremes, and, to some extent, dessication (Nash 2008). The great majority of lichenized fungi in Lecanoromycetes are obligate mutualists, but a few species of Stictis can occur either as lichen symbionts or as nonlichenized saprotrophs, depending on the substrate (Wedin et al. 2004). Some lichenized fungi can also be parasitic on other lichens, either throughout their life (Lawrey and Diederich 2003) or only in the first stage of their development (e.g., Diploschistes muscorum) (Friedl 1987). Finally, several lineages within Lecanoromycetes are always nonlichenized, most likely because of secondary losses of lichenization (Baloch et al. 2010; Gueidan et al. 2008; Lutzoni et al. 2001; Schoch et al. 2009). These nonlichenized lineages can have diverse lifestyles, ranging from saprophytism to parasitism (Lawrey and Diederich 2003; Sherwood 1977a, b; Sherwood-Pike 1987). They mostly occur on lichens (Lawrey and Diederich 2003) but are also found on diverse substrates such as bark or wood (e.g., Stictidaceae, Odontotremataceae).
C. Mycobiont–Photobiont Associations
Most Lecanoromycetes species associate with a single photobiont , a green alga (chlorobiont) or a cyanobacterium (cyanobiont). Some lichen-forming fungi are associated with both types of photobionts, forming tripartite thalli where the cyanobacterial photobiont is an accessory partner. The cyanobiont is then restricted to special organs of the thallus called cephalodia [although see exceptions in Henskens et al. (2012)], which can be internal or external outgrowths. Cyanobionts fix atmospheric nitrogen, transferring ammonia to the other partners. These tripartite associations are common in Lecanorales (e.g., Stereocaulon) and Peltigerales (e.g., Lobaria, Nephroma, Peltigera).
The majority of photobionts belong to Chlorophyta [approximately 90 % according to Tschermak-Woess (1988a); see also Ahmadjian (1967, 1993)]. The green algal genera Trebouxia, Asterochloris (Trebouxiophyceae), and Trentepohlia (Ulvophyceae) are the most common photobionts for Lecanoromycetes.
Other genera of photobionts associated with Lecanoromycetes include Chlorella (e.g., in Pseudocyphellaria) (Tschermak-Woess 1988b), Chlorosarcinopsis (in Lecidea) (Plessl 1963), Coccomyxa (e.g., in Icmadophila, Peltigera, Solorina) (Jaag 1933), Dictyochloropsis (e.g., in Lobaria, Pseudocyphellaria) (Tschermak-Woess 1984), Elliptochloris (e.g., in Baeomyces) (Tschermak-Woess 1985), Leptosira (in Vezdaea and Thrombium) (Tschermak-Woess 1953; Tschermak-Woess and Poelt 1976), Myrmecia (e.g., in Psora) (Geitler 1963), and Phycopeltis (e.g., in Porinaceae) (Santesson 1952).
Cyanobacterial photobionts are associated with a relatively small number of lichen-forming fungal species (10 %, Tschermak-Woess 1988a; approximately 1,700 species, Rikkinen 2002) and, in Lecanoromycetes, are restricted to Arctomiaceae, Stereocaulaceae, Trapeliaceae, and Peltigerales. The most common cyanobacterial (primary or accessory) lichen photobionts are Nostoc and Rhizonema (Lücking et al. 2009b; Rambold et al. 1998; Tschermak-Woess 1988a). The cyanobacterial genus Scytonema was also listed in the past as one of the most common lichen photobionts, but a recent molecular study showed that all lichenized strains of Scytonema studied (including those from the Lecanoromycetes genera Coccocarpia and Stereocaulon) belonged to Rhizonema, a new strictly lichenized cyanobacterial lineage genetically distinct from Scytonema (Lücking et al. 2009b). The current status of the genus Scytonema as a lichen photobiont is therefore in need of determination using molecular data. Stigonema and Gloeocapsa are also often found in lichens but more often as accessory photobionts (Rambold et al. 1998).
Other cyanobacteria occasionally found as photobionts are Anabaena (in Stereocaulon) (Duvigneaud 1955), Calothrix (in Coccotrema, Stereocaulon) (Brodo 1973; Lamb 1977), Dichothrix (in Placynthium nigrum) (Geitler 1934), Hyphomorpha (in Spilonema) (Henssen 1981), and Tolypothrix (in Hertella) (Henssen 1985).
Interestingly, patterns of mycobiont–photobiont associations have been observed at a high taxonomic level (Miadlikowska et al. 2006; Rambold et al. 1998). This is somewhat surprising for a symbiotic system that is believed to transmit its photobiont mostly horizonally across generations. However, the contribution of fungal sexual (horizontal transmission) versus asexual (vertical transmission) reproduction to the genetic composition of the mycobiont and photobiont populations has rarely been quantified. Dal Grande et al. (2012) estimated that more than 70 % of lichen thalli across worldwide populations of Lobaria pulmonaria were derived from asexual propagules. No recombination was detected for the photobiont, while it was detected in only 7.7 % of the mycobiont individuals. Therefore, vertical transmission of the photobiont through thallus fragments or differentiated asexual propagules might be more prominent than expected in shaping lichen populations and could explain in part the pattern of association observed at a high taxonomic level within Lecanoromycetes.
A population ecology study of the geographically widespread lichen Cetraria aculeata revealed that climate and codispersal are the most relevant factors shaping the genetic structure of the photobiont and that rare photobiont switches enabled the mycobiont to achieve a broad geographical distribution crossing bioclimatic zones (Fernandez-Mendoza et al. 2011).
In Lecanoromycetes, the following patterns of mycobiont–photobiont associations have been observed (Miadlikowska et al. 2006; Rambold et al. 1998). Certain lineages, such as Parmeliaceae and Teloschistales, are mainly associated with Trebouxia, whereas Cladoniaceae, Stereocaulaceae, and Icmadophilaceae are mostly associated with Asterochloris. In Peltigerales, Nostoc is the main photobiont, and in Ostropales, Trentepohlia is the most common photobiont. Although some photobiont associations could potentially be used to classify higher taxa within Lecanoromycetes, current knowledge on the identity of lichen photobionts is still too sparse. They are notoriously difficult to identify when lichenized, or even when cultured, based on morphology only (Friedl and Büdel 2008), and molecular data are currently only available for a limited number of taxa.
IV. Mycobiont–Photobiont Cellular Contacts
The type of cellular contacts between symbionts ranges from appressoria to haustoria in Lecanoromycetes (Honegger 2008), and their structure depends on various factors. The taxonomic affiliation of photobionts plays a large role, especially its morphology and cell wall composition (Honegger 2008). A correlation has been observed between thallus growth forms and types of cellular contact between symbionts, with simple crustose species tending to have less complex contact structures than do foliose or fruticose species (Honegger 2008). Environmental conditions can also be responsible for variation in the extent of penetration by the fungus (Ben-Shaul et al. 1969; Galun et al. 1970), as well as thallus age (Collins and Farrar 1978; Galun 1988; Geitler 1963). As a result, this character has not been used in the classification of lichen-forming fungi (Poelt 1973).
V. Morphological and Chemical Features
A. Thalli
The lichen thallus is a vegetative structure formed by the fungal hyphae and algal cells. In Lecanoromycetes, thallus structures range from simple to more complex organizations. The simplest thalli are found in leprose (powderlike, Fig. 4.1a) and byssoid (cottonlike) species, which lack differentiation into strata, and are formed of loosely intermingled fungal hyphae and algal cells (Ekman and Tønsberg 2002; Kantvilas 1996; Poelt 1987). The complex thalli (heteromerous) are differentiated into layers (Fig. 4.2): upper cortex, photobiont layer, medulla, and lower cortex.
Different types of thallus in Lecanoromycetes. (a) Powderlike leprose thallus of Lepraria membranacea. (b) Crustose areolate thallus of saxicolous species Rhizocarpon macrosporum. (c) Foliose thallus of Peltigera rufescens, with white rhizines visible on lower surface (white arrows). (d) Cordlike branches of fruticose thallus of Usnea subfloridana. Bars = 5 mm
Two main types of lichen thalli are recognized, mainly for convenience: microlichens and macrolichens. Microlichens can consist of powderlike or cottonlike thalli (leprose and byssoid thalli) or corticated granules (granulose thalli) or appear as crusts tightly appressed to the substrate and generally lacking rhizines and a lower cortex (crustose thalli) (Fig. 4.1b). Crustose thalli are quite diverse, ranging from continuous or slightly cracked crusts to crusts formed by areoles or small lobes often on a fungal hyphal mat (hypothallus). Crustose species generally grow above the substrate, but some have a thallus developing within the superficial layer of the substrate, either in rock (endolithic species such as Clauzadea immersa) or in bark (endophloeic species such as Caloplaca cerinella or Lecanora persimilis).
The largest thalli are generally found in squamulose, foliose, and fruticose lichens (macrolichens). Squamulose lichens are composed of scattered or imbricated scalelike lobes. Foliose lichens have dorsiventral thalli formed by lobes mostly with a lower cortex, which are often only loosely attached to the substrate, most often by attachment structures such as rhizines (e.g., Peltigera) (Fig. 4.1c). Foliose thalli attached to the substrate with a single holdfast are called umbilicate and can be formed either by a single lobe or by several lobes (e.g., Umbilicaria) (Hestmark 1997). Lichens with long striplike or cordlike branches that are hanging or standing upward from their substrates are called fruticose (e.g., Usnea, Ramalina) (Fig. 4.1d). Some fruticose lichens (e.g., Cladonia) have a mixed thallus formed by a basal squamulose primary thallus and trumpetlike or spikelike structures (podetia) growing upward from the primary thallus and bearing fruiting bodies (secondary thallus).
Thallus growth forms were of prime importance in shaping the first classifications of lichens (Zahlbruckner 1926). With improved microscopical techniques, anatomical and ultrastructural characters became available, and ascomatal characters replaced thallus morphology as the main characters used in classification (e.g., Hafellner 1984; Henssen and Jahns 1974; Luttrell 1951, 1955; Nannfeldt 1932; Poelt 1973). Molecular data confirmed that thallus morphology could not be strictly used for classification purposes because most growth forms were shown to have evolved several times independently in Lecanoromycetes and in lichens in general [e.g., Grube and Arup 2001; Schmitt et al. 2001; Stenroos and DePriest 1998; see also the review in Grube and Hawksworth (2007)].
B. Ascomata
1. Ascoma Morphology
Ascomata (Fig. 4.3) are structures producing spore-bearing cells called asci. They generally consist of a hymenium enveloped by a protective structure called an excipulum. The hymenium comprises asci and sterile interascal hyphae. There are two main types of ascomata in Lecanoromycetes. The most common type is the disc-shaped apothecium , where the upper portion of the hymenium is mostly exposed (Fig. 4.3a). When laterally elongated they are referred to as lirellate apothecia (Fig. 4.3b). In Lecanoromycetes, lirellate apothecia are restricted to Ostropales but can also be found in Arthoniomycetes, a distinct class within Leotiomyceta (Pezizomycotina), which also includes many lichen-forming fungi. The second type of fruiting body in Lecanoromycetes is the flask-shaped perithecium (Fig. 4.3c), where the hymenium is exposed only through an ostiole. The term perithecium was restricted in the past to species with an ascohymenial development (ascostromatic flask-shaped ascomata were called pseudothecia), but it is now used in a broad sense for all flask-shaped ascomata, regardless of their development type (Kirk et al. 2008). Perithecia are only found in a few lineages in Lecanoromycetes (e.g., Porinaceae, Protothelenellaceae, and Thelenellaceae). Some species have apothecia that can be confused with perithecia because they are immersed in the thallus and the hymenium is exposed only through a small aperture. Called perithecioid apothecia (Fig. 4.3d), these ascomata are frequently found in the subclass Ostropomycetidae.
Examples of fruiting bodies in Lecanoromycetes. (a) Disc-shaped fruiting bodies (apothecia) in Xanthoparmelia tinctina. (b) Elongated fruiting bodies (lirellate apothecia) in Graphis scripta. (c) Flask-shaped fruiting bodies (perithecia) in Porina guentheri. (d) Enclosed disc-shaped fruiting bodies (perithecioid apothecia) in Thelotrema lepadinum. Bars = 2.5 mm
In early classifications, ascoma morphology was used together with ascus characters to classify ascomycetes (e.g., Luttrell 1955; Nannfeldt 1932). However, molecular phylogenies demonstrated that some types of ascomata evolved several times independently in Lecanoromycetes [e.g., Grube et al. 2004; Schmitt et al. 2005, 2009; Wedin and Tibell 1997; see also the review in Grube and Hawksworth (2007)]. In particular, some groups with perithecioid ascomata (e.g., Porinaceae, Protothelenellaceae, and Thelenellaceae) were shown to be nested within the predominantly apothecial subclass Ostropomycetidae (Grube et al. 2004; Schmitt et al. 2005, 2009). In Lecanoromycetes, the convergent evolution of ascoma types also prevents the use of this character for defining orders and families, although some broad trends can be observed (e.g., predominantly lirellate apothecia in Graphidaceae).
In Ostropomycetidae, a correlation between ascoma type and ascoma development (Schmitt et al. 2009) suggested that angiocarpous development was a prerequisite adaptation in lineages in which perithecia have evolved. These perithecioid fruiting ascomata may therefore have a neotenic origin (Grube et al. 2004; Schmitt et al. 2009).
2. Ascoma Development
Types of ascoma development have been used in the past to define higher divisions in filamentous ascomycetes. Originally, two main types of development were distinguished, ascolocular and ascohymenial (Nannfeldt 1932). In ascolocular ascomata, the asci develop in the cavity in a preformed stroma, whereas in ascohymenial ascomata, the asci develop in a hymenium not located in a preformed stroma (Kirk et al. 2008). In Lecanoromycetes, ontogenetic studies have helped describe in more detail the development of ascomata (e.g., Henssen 1976; Henssen and Jahns 1974; Letrouit-Galinou and Bellemère 1989). Henssen and Jahns (1974) recognized two main types of development in ascohymenial lichen-forming fungi, depending on the origin of the structure enveloping the ascogonia (or primordium), as well as other more specific types. These development types can lead to apothecia (angiocarps), perithecia (gymnocarps), or perithecioid apothecia (hemiangiocarps).
Letrouit-Galinou and Bellemère (1989) recognized two types of development depending on the differentiation of the excipulum (or parathecium, proper margin). Among the types without any differentiated excipulum are the Pertusaria and Thelotrema types for perithecioid apothecia and the Graphis and Baeomyces types for apothecia or lirellate apothecia. Among the types with a differentiated excipulum are the Aspicilia and Gyalecta types (excipulum reduced to a crown), the Peltigera, Diploicia, and Xanthoria types (typical excipulum), and Cladonia and Parmelia types (atypical excipulum).
Molecular studies showed that the classification in ascolocular and ascohymenial fungi did not reflect monophyletic groupings (e.g., Berbee 1996; Lindemuth et al. 2001; Lumbsch and Huhndorf 2007a; Lutzoni et al. 2004). As a result, these characters are now rarely mentioned in classification work (e.g., Hibbett et al. 2007). Although the use of ascoma developmental types is inadequate for delimiting higher taxa of Ascomycota, their use at the ordinal and family levels may have some value since they can be characteristic of some groups of Lecanoromycetes (e.g., Parmeliaceae and Agyriaceae) (Henssen et al. 1981; Lumbsch et al. 2001a).
C. Asci
1. Ascus Walls
The first studies of asci with light microscopy (e.g., Chadefaud 1942; Luttrell 1951; Nannfeldt 1932) and electron microscopy resulted in the use of ascus characters in combination with ascomatal characters to establish higher-rank classification systems among filamentous ascomycetes (Barr 1983; Eriksson 1982; Luttrell 1951, 1955; Nannfeldt 1932). Thus, the structure of the ascus wall was one of the main ascus features used in higher classification. Currently, ascus walls are classified in three main types: a thin ascus wall formed of a single layer ( prototunicate ascus), an ascus wall formed of two layers functioning as a single layer ( unitunicate ascus), and an ascus wall formed of two layers functioning as two layers ( bitunicate ascus). Most members of Lecanoromycetes have unitunicate asci (Honegger 1982a; Letrouit-Galinou 1973a).
Among unitunicate ascus types are the Lecanora, Pertusaria, and Teloschistes types from Honegger (1982a, b). Bellemère and Letrouit-Galinou (1987) grouped these three ascus types within the “archeacés” and distinguished the further Lecidella, Catillaria, Psora, Lecidea, Cladonia, and Collema types. Unitunicate asci are also found in Anzina (Scheidegger 1985), Baeomyces (Bellemère 1977; Honegger 1983), Dactylospora (Bellemère and Hafellner 1982), Gyalectaceae (Kauff and Büdel 2005), and Trapeliopsis (Bellemère and Letrouit-Galinou 1987).
Functionally bitunicate asci are less common in the Lecanoromycetes. They are found in Collemataceae, Peltigeraceae, and Rhizocarpaceae (Bellemère and Letrouit-Galinou 1987; Honegger 1982a). Prototunicate asci are rather rare in this fungal class and are only found in the families Caliciaceae and Sphaerophoraceae (Wedin and Tibell 1997; Wedin et al. 2000b), in which the released spores form a loose mass.
2. Ascus Apical Structure
With improving microscopy technologies, the apical structure of asci was shown to be particularly variable within Lecanoromycetes (e.g., Chadefaud 1973; Chadefaud et al. 1963; Honegger 1978, 1980; Letrouit-Galinou 1973a). Among the variations were the presence or absence of differentiated apical structures (apical thickening, ocular chamber, apical ring, apical nasse, subapical bourrelet) and their reaction to various stains such as iodine (e.g., Chadefaud 1973; Hafellner 1984; Letrouit-Galinou 1973a). Hafellner (1984) was the first to use these characters to systematically revise the classification within Lecanorales s.l. at the family and genus levels. He recircumscribed some species-rich families (e.g., Lecanoraceae and Lecideacae) and described new families (e.g., Catillariaceae and Dactylosporaceae) based on the ascus apical structure (Bellemère and Hafellner 1982; Hafellner 1984). His classification system was broadly accepted (e.g., Eriksson and Hawksworth 1993; Hafellner 1994; Rambold and Triebel 1992), until molecular data showed that these characters were not as conserved as initially thought and that similar apical structures have evolved several times independently in unrelated lineages [Ekman and Wedin 2000; Ekman et al. 2008; Lumbsch et al. 2001b, 2007c; Wedin et al. 2005, 2009; see also the review in Grube and Hawksworth (2007) and Printzen (2010)].
3. Dehiscence Mechanisms
The type of dehiscence (or the process leading to the liberation of ascospores from mature asci) varies greatly in Lecanoromycetes (Bellemère and Letrouit-Galinou 1987; Honegger 1982a). In most members of this class, dehiscence occurs by apical rupture of the ascus, combined with the ejection of the internal part of the ascus wall. When the ejection is not coupled with a sliding between the two layers of the ascus wall, this type of dehiscence is called a rostrum type or Lecanora type (Bellemère and Letrouit-Galinou 1987; Honegger 1978). It is the most common type of dehiscence in Lecanoromycetes (e.g., Lecanoraceae, Physciaceae, Parmeliaceae), and it has been suggested that it might be the ancestral dehiscence type in this class based on both anatomical data (“type archaeascé”) (Chadefaud et al. 1967) and molecular data (Miadlikowska et al. 2006; Wedin et al. 2005).
In Rhizocarpon, the internal layer of the ascus wall slides slightly along the external layer (Rhizocarpon type or hemifissitunicate type) (Bellemère 1994; Honegger 1980). In Peltigera, the sliding of the internal layer along the external layer is more important (Peltigera type, fissitunicate type or “Jack in the box”) (Bellemère and Letrouit-Galinou 1987; Honegger 1978).
Dehiscence can also occur by apical rupture of the ascus without ejection of the internal part of the ascus wall (e.g., Trapelia and Coccocarpia) (Bellemère 1994). In Teloschistes, the apical pore forms after predehiscent elongation of the ascus wall (Teloschistes type or chimney type of dehiscence) (Bellemère and Letrouit-Galinou 1987; Honegger 1978). In Pertusaria, a predehiscent elongation also occurs, but the ascospores are released after bursting or splitting of the ascus tip (Honegger 1982b). Finally, in Caliciaceae and Sphaerophoraceae, ascospores are passively released after deliquescence of prototunicate asci (evanescent type of dehiscence) (Wedin and Tibell 1997). Although ascus dehiscence has been used in high-rank classifications (Luttrell 1955), the systematic value of this character has been questioned because dehiscence types more likely correspond to adaptations to various environmental conditions (Bellemère 1994).
D. Ascospores
Ascospore characters, such as septation, pigmentation, size, shape, and number per ascus, were originally used to delimit families or genera in many groups of Lecanoromycetes [e.g., in Graphidaceae (Müller 1880, 1882) or in Acarosporaceae (Zahlbruckner 1907)]. However, many authors have recognized subsequently that classification based on these characters might not reflect evolutionary history because of convergent evolution in ascospores of lichenized and nonlichenized fungi (e.g., Poelt 1973; Vainio 1890). Morphologically similar ascospores are indeed often found in distantly related groups, as confirmed by molecular data.
Molecular phylogenetic results have further thrown doubts on the use of ascospores as a main source of characters for classification at the generic level. Several Lecanoromycetes genera that were primarily circumscribed on ascospore characters were shown to be polyphyletic, and characters such as ascospore septation and color were frequently shown to be homoplasious (e.g., Ihlen and Ekman 2002; Rivas Plata and Lumbsch 2011; Staiger et al. 2006). Despite this conclusion, when combined with other features, ascospore characters provide important taxonomic information for most groups of lichenized fungi at the species and generic levels (e.g., within Thelotremataceae ) (Frisch et al. 2006). Ascospore characters can also, in some cases, be useful at higher taxonomic levels since some broad trends can be observed in some groups. For example, polarilocular ascopores are characteristic of the order Teloschistales and ascospores with lens-shaped lumina, of the family Graphidaceae s.s. (Poelt 1973).
E. Interascal Filaments
Sterile filaments are usually present in fruiting bodies alongside asci. These interascal filaments constitute the hamathecium and are thought to protect the asci or promote their function (Poelt 1973). Historically, two broad categories of interascal filaments were described in ascomycetes depending on their origin in the development of the ascoma. Ascohymenial development leads to the formation of paraphyses , whereas ascolocular development forms paraphysoids or pseudoparaphyses. These two types of filament may look very similar in mature fruiting bodies and can be confused, so they have been applied only in more recent classification systems of Ascomycota (e.g., Barr 1983). At lower taxonomic levels, hamathecial characters (septation, anastomoses and branching, color, chemistry, and shape of the upper cell) have been used together with other characters to delimitate genera or families in Lecanoromycetes (e.g., Kärnefelt and Thell 1992; Staiger and Kalb 1999; Timdal 1992). The importance of some of these characters has been discussed in light of molecular data (e.g., Rivas Plata and Lumbsch 2011; Staiger et al. 2006). However, a comprehensive reevaluation of the evolution of hamathecial characters and their taxonomic value is still needed.
F. Pycnidia
In lichenized fungi, pycnidia (= conidiomata) are minute flask-shaped structures located on the surface of, or embedded within, lichen thalli and producing small asexual spores called pycnidiospores (also called conidiospores or conidia ). The role of pycnidia has been insufficiently studied in lichens. Culture studies first suggested that conidiospores might act as asexual dispersal units (Möller 1888), but this possibility was questioned because more recent studies showed that, in Lecanoromycetes, conidiospores germinate only rarely (Ahmadjian 1969; Bailey 1976; Vobis 1977). Microscopical observations revealed that conidiospores most probably act as spermatia since they have been found attached to trichogynes in several species (e.g., Honegger 1984a, b; Jahns 1970).
The systematic value of pycnidial characters was recognized early on (Choisy 1954; Steiner 1901; Zahlbruckner 1903–1907), but their use in systematic studies remains rather limited, mostly because they are difficult to observe. In the 1970s and 1980s, cytological and ontogenetical studies triggered a renewed interest in pycnidial characters, and several types of pycnidia and conidiophores were described in the Lecanoromycetes (e.g., Honegger 1984b; Janex-Favre 1977, 1982; Letrouit-Galinou 1972, 1973b; Letrouit-Galinou and Lallement 1977; Vobis 1980). However, data available on pycnidia remain sparse (Roux et al. 1986), and only relatively few studies have tried to assess their use for classification within Lecanoromycetes (e.g., Krog 1982; Matsumoto and Deguchi 1999; Thell et al. 2002).
G. Asexual Propagules
Lecanoromycetes can disperse asexually by thallus fragmentation aided by undifferentiated or specialized structures. The two most common types of specialized dispersal structures are isidia (corticated and more or less cylindrical thallus outgrowths) (Fig. 4.4a) and soredia (ecorticated thallus granules produced via openings in the cortex called soralia) (Fig. 4.4b). Such propagules facilitate successful codispersal of both lichen partners, in contrast to fungal dispersal through sexual ascospores, which requires finding appropriate photobionts to reestablish lichen thalli de novo. The taxonomic value of asexual propagules was first recognized by Du Rietz (1924). He described and classified them and introduced the concept of species pairs for species that differ only by their mode of reproduction, either primarily sexual or primarily vegetative. Poelt (1970, 1972) later developed this concept, and since then, it has been debated whether they correspond to conspecific individuals or separate sister species (e.g., Mattsson and Lumbsch 1989; Tehler 1982). Molecular studies on several species pairs have so far not confirmed them as separate species (e.g., Buschbom and Mueller 2006; Kroken and Taylor 2001; Myllys et al. 2001). The contribution of asexual reproduction to the ecology and evolution of lichens has rarely been addressed (e.g., Buschbom and Barker 2006; Dal Grande et al. 2012; Fedrowitz et al. 2011; Hestmark et al. 2011).
Asexual propagules in Lecanoromycetes. (a) Upper surface of thallus of Parmelina tiliacea covered with small and easily detached, corticated outgrowths called isidia. In this species, isidia are simple to branched and brown at the tip (inset: detail of a long branched isidium). (b) Round openings (soralia) in the upper cortex of Pertusaria flavicans releasing ecorticated granules called soredia. Bars = 0.5 mm
H. Secondary Compounds
Secondary compounds are insoluble metabolites that are often deposited extracellularly by the fungal partner on the surface of hyphae. These compounds are very diverse and numerous, with more than 700 described so far from lichens (Elix and Stocker-Wörgötter 2008). Most of these compounds are found exclusively in lichens. They are derived from three main biosynthetic pathways (acetyl-polymalonyl, shikimic, and mevalonic acid pathways) and belong to different chemical groups such as anthraquinones, depsidones, triterpenes, pulvinic acids, and xanthones (Asahina and Shibata 1954; Culberson and Elix 1989; Huneck 2001). Mostly present in the cortex and the medulla, they can help repel herbivores and microorganisms (Lawrey 1986, 1989) but in the cortex also act as light and UV screens (Armaleo et al. 2008; Millot et al. 2012; Solhaug et al. 2003). Armaleo et al. (2011) were the first to identify a gene cluster involved in the biosynthesis of a depside and a depsidone.
Approximately 5,000 lichen species have been investigated for their secondary compounds (Elix and Stocker-Wörgötter 2008). Methods of detection vary from simple spot tests to analysis of extracts with thin-layer chromatography and high-performance liquid chromatography (Huneck and Yoshimura 1996). The presence of secondary compounds has been used at all taxonomic levels, for both classification and species identification. Most secondary compounds are found widely in Lecanoromycetes and only rarely form synapomorphies for single lineages. However, the presence of some secondary compounds was shown to be informative at the genus and family levels (Lumbsch 1998a; Schmitt and Lumbsch 2004). The use of chemical characters for species delimitation has been controversial (Lumbsch 1998b). Recent molecular studies show that, depending on the group studied, chemotypes may correspond to separate species (e.g., Tehler and Källersjö 2001) or can represent infraspecific variability (e.g., Leavitt et al. 2011a, b; Nelsen and Gargas 2009; Velmala et al. 2009).
VI. Origin and Diversification
Dating the divergence time of fungi is not an easy task because of the relatively poor fossil record for these organisms and the variable rates of nucleotide substitution across this kingdom (Lumbsch et al. 2008a; Lutzoni and Pagel 1997; Woolfit and Bromham 2003; Zoller and Lutzoni 2003). As a result, estimates of divergence times in fungi once varied considerably depending on the methods and calibrations used (Berbee and Taylor 1993, 2001; Heckman et al. 2001; Padovan et al. 2005; Taylor and Berbee 2006). With the reinterpretation of fossil data and the development of new phylogenetic methods allowing for rates to vary across lineages, divergence estimates for the main fungal lineages have now reached a consensus (Lücking et al. 2009a; Taylor and Berbee 2010), and divergences of more recent lineages have started to be investigated (Amo de Paz et al. 2011; Gueidan et al. 2011).
Soon after the divergence of Pezizomycotina, Leotiomyceta underwent a radiation during which the Lecanoromycetes lineage originated (Gazis et al. 2012; Schoch et al. 2009; Spatafora et al. 2006). So far, all ancestral state reconstruction studies agree that lichenization in Lecanoromycetes evolved at or prior to the onset of the evolution of this fungal class. This acquisition of lichenization has been placed at the base of a lineage including Lecanoromycetes, Eurotiomycetes, and Lichinomycetes (James et al. 2006; Lutzoni et al. 2001), at the base of a lineage including Lecanoromycetes and Lichinomycetes (Gueidan et al. 2008), or at the base of Lecanoromycetes (Schoch et al. 2009). According to Gueidan et al. (2011), Lecanoromycetes diverged from Eurotiomycetes during the late Devonian, around 371 million years ago (mya) (between 322 and 424 mya), and the diversification of extant Lecanoromycetes species (crown group) originated during the Carboniferous, approximately 322 mya (between 269 and 380 mya).
Within Lecanoromycetes, several well-interpreted fossils from amber are available to calibrate the molecular clock. A species of Anzia was described from European amber (35–40 mya) (Mägdefrau 1957), two species of Parmelia from Dominican amber (15–45 mya) (Poinar et al. 2000), and a species of Alectoria from Baltic amber (35–40 mya) (Rikkinen and Poinar 2002). A more recent discovery by Honegger et al. (2013) of exceptionally well-preserved lichen thalli fragments in siltstone of the lower Devonian (415 mya) provided the oldest record of modern lichens.
One of the Parmelia fossils and the Alectoria fossil were used in a recent study to investigate the origin of Parmeliaceae, one of the largest families within Lecanoromycetes (Amo de Paz et al. 2011). Results show that this family radiated around the Cretaceous–Tertiary boundary (±65 mya), just before a climatic period characterized by temperature and atmospheric CO2 maxima. Most major parmelioid genera originated during the Eocene and early Oligocene and diversified during the cooler periods of late Oligocene to early Pliocene.
VII. Orders and Classification
A. Acarosporales
Acarosporales (Acarosporomycetidae) (Fig. 4.5) is a small order with a single family, Acarosporaceae , 11 genera, and 183 species (Kirk et al. 2008). They are mostly crustose species with apothecial ascomata, poorly to moderately branched and anastomosed paraphyses, unitunicate polysporous asci, and small hyaline simple ascospores (Hibbett et al. 2007). They occur worldwide and mostly colonize rocks. Traditionally, Acarosporaceae had been placed in the order Lecanorales based on ascus characters (Kirk et al. 2001). However, molecular studies showed that they did not belong to this order but represent instead the earliest diverging lineage in Lecanoromycetes (Lumbsch et al. 2007b; Miadlikowska et al. 2006; Reeb et al. 2004; but see Hofstetter et al. 2007). The order Acarosporales was therefore formally described for this family (Hibbett et al. 2007).
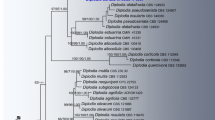
Schematic representation of phylogeny and classification of Lecanoromycetes based on selected published sources (Andersen and Ekman 2005; Arup et al. 2007; Baloch et al. 2010; Bylin et al. 2007; Ekman et al. 2008; Gaya et al. 2012; Hodkinson and Lendemer 2011; Hofstetter et al. 2007; Kirk et al. 2008; Lumbsch and Huhndorf 2010; Lumbsch et al. 2004, 2007b, 2008b; Lutzoni et al. 2004; Miadlikowska et al. 2006; Muggia et al. 2011; Reeb et al. 2004; Rivas Plata et al. 2012; Schmitt et al. 2005, 2012; Schoch et al. 2009; Wedin et al. 2009; Widhelm and Lumbsch 2011; Zhou and Wei 2007). Phylogenetic relationships among taxa (families, orders, and subclasses) were compiled using a “super tree” approach and are shown as resolved if reported with posterior probability ≥95 % or maximum likelihood bootstrap ≥70 % in multiple studies (in most cases) and are not in conflict at the same level of support. The number of recognized families in each order is provided in parentheses after the order name. Shaded boxes represent subclasses and suborders. Oblique bars across branches indicate families with unknown placement in the Lecanoromycetidae or Ostropomycetidae. Stars indicate families for which no DNA sequence is currently available in GenBank. Question mark indicates that Dactylosporaceae may be placed outside of Lecanoromycetes (in Eurotiomycetes) (Schoch et al. 2009)
Although polyspory is not uncommon in nonlichenized and lichenized ascomycetes, the family Acarosporaceae was originally characterized by its true polyspory (polyspory resulting from a meiosis followed by several mitoses generating more than 100 ascospores, as opposed to polyspory resulting from budding or fragmenting ascospores). However, in Lecanoromycetes, true polyspory is not restricted to the Acarosporaceae. As a result, the circumscription of this family has been variable, with many polysporous genera (e.g., Maronea, Pleopsidium, and Thelocarpon) being successively excluded from, or included in, the family depending on the system adopted for their classification [Golubkova 1988; Hafellner 1995; Magnusson 1936; Zahlbruckner 1907; see the detailed review in Reeb et al. (2004)].
Molecular data have helped resolve the circumscription of Acarosporaceae (Reeb et al. 2004; Wedin et al. 2005), and the genera Acarospora, Glypholecia, Pleopsidium, Polysporina, Sarcogyne, Thelocarpella, and Timdalia were confirmed as part of this family, whereas the genera Biatoridium, Maronea, Sporastatia, Strangospora, and Thelocarpon were excluded. Molecular studies also confirmed the multiple independent origins of true polyspory in Lecanoromycetes (Reeb et al. 2004), i.e., Acarosporaceae (with a loss in the two species Acarospora macrospora and Glypholecia scabra, which have only 30–100 ascospores), Maronea, Sporastatia, Strangospora, and a lineage including Thelocarpon and Biatoridium.
B. Baeomycetales
The order Baeomycetales (Ostropomycetidae) (Fig. 4.5) currently includes two families, Baeomycetaceae and the monotypic Anamylopsoraceae (Hodkinson and Lendemer 2011; Lumbsch et al. 1995). The family Baeomycetaceae comprises the three genera Ainoa, Baeomyces, and Phyllobaeis (Hibbett et al. 2007; Lumbsch and Huhndorf 2010) and a total of approximately 15 species with crustose to squamulose or foliose thalli, sessile to stipitate apothecia, branched paraphyses, nonamyloid asci or asci with a slightly amyloid apex, and simple to transversally septate hyaline ascospores (Hibbett et al. 2007; Johnston 2001). The species grow on soils, rocks, and bryophytes in moist areas and often are primary colonizers of disturbed substrates (Johnston 2001). First placed in Lecanorales close to Cladoniaceae (Henssen and Jahns 1974; Poelt 1973), this family (including at the time the genera Baeomyces and Icmadophila) was then transferred to the predominantly nonlichenized order Helotiales (now Leotiales) based on its Leotia-type ascus (Chadefaud 1960, 1973; Hafellner 1988; Honegger 1983; Rambold et al. 1993; Tehler 1996).
Early molecular studies suggested that Baeomyces might not be related to Leotiales (Platt and Spatafora 1999; Stenroos and DePriest 1998). Later, this genus was shown to form a sister group to the Ostropales s.l., and the ordinal name Baeomycetales was then suggested for this lineage (Kauff and Lutzoni 2002). Additional molecular data confirmed its phylogenetic placement within Ostropomycetidae (Lumbsch et al. 2007b; Miadlikowska et al. 2006), and the order Baeomycetales was therefore formally erected for the family Baeomycetaceae (Hibbett et al. 2007).
The delimitation of Baeomycetaceae also underwent some important changes during the last two decades. Some genera previously placed in this family (e.g., Dibaeis, Icmadophila, Siphulella) were segregated into the new family Icmadophilaceae based on morphological evidence (Rambold et al. 1993), and subsequent molecular studies confirmed that these genera are not related to Baeomycetaceae (Platt and Spatafora 1999; Stenroos and DePriest 1998). A new genus (Phyllobaeis) was also described for Baeomyces species with squamulose primary thalli and brown apothecia (Gierl and Kalb 1993). Finally, molecular data showed that the genus Ainoa also belongs to Baeomycetales (Lumbsch et al. 2007b, c). This genus had previously been described to accommodate two morphologically different species of Trapelia that did not cluster with Trapelia s.s. in a molecular phylogenetic study (Lumbsch et al. 2001b).
The circumscription of the order Baeomycetales is likely to undergo more changes in the future. A morphological study of the family Anamylopsoraceae shows that this monotypic family shares several characters with Baeomycetaceae, such as the ascocarp ontogeny, stipitate ascogonia, annular exciple, and conidiophore type (Lumbsch et al. 1995). However, these two families also differ in other characters, such as the ascus type. Anamylopsoraceae had been placed tentatively in Agyriineae and Agyriales (Lumbsch et al. 1995, 2001a, respectively), but a transfer to Baeomycetales was recently suggested (Hodkinson and Lendemer 2011; Lumbsch et al. 2007b). A comprehensive phylogenetic analysis is needed to determine whether this family belongs to Baeomycetales.
C. Caliciales
The order Caliciales (Lecanoromycetidae) (Fig. 4.5) was in the past recognized as a phenotypically well-delimited group that included species with mazaedium-forming ascomata and passive ascospore dispersal (e.g., Zahlbruckner 1903–1907; see detailed review in Tibell 1984) and was erected as an order by Bessey (1907). However, this order underwent drastic changes in circumscription after a first extensive revision by Tibell (1984). Based on a careful morphological study, Tibell excluded several families and genera from Caliciales s.s. (e.g., Sphaerophoraceae and Chaenotheca) and restricted the order to the three families Caliciaceae, Mycocaliciaceae, and Sphinctrinaceae (Tibell 1984, 1996). More recently, a study using molecular data demonstrated that Mycocaliciaceae and Sphinctrinaceae belong in fact to Eurotiomycetes and that only the family Caliciaceae was strongly supported as part of the order Lecanorales (Wedin and Tibell 1997).
Subsequent studies with broader taxon samplings showed that Caliciaceae were in fact closely related to Physciaceae , in the order Lecanorales (Wedin et al. 2000a, 2005). Both Physciaceae and Caliciaceae were later tentatively placed in Teloschistales as part of the suborder Physciineae (Miadlikowska et al. 2006). In a more recent molecular study focusing on Teloschistales s.l., Gaya et al. (2012) demonstrated phylogenetic instability for relationships among Physciineae, Teloschistineae, and Lecanorales, where the two suborders did not always form a monophyletic group. To ensure the classification was resilient to the various resolution of these three clades, Gaya et al. (2012) elevated Physciineae to the ordinal level by resurrecting the order Caliciales and restricted Teloschistales to Brigantiaeaceae, Letrouitiaceae, Megalosporaceae, and Teloschistaceae. In this new phylogenetic context, Caliciales includes two families: Caliciaceae (with two subfamilies Calicioidea and Buellioidea) and Physciaceae.
D. Candelariales
The order Candelariales (Candelariomycetidae?) (Fig. 4.5) includes a single family, Candelariaceae , and four genera: Candelaria, Candelariella, Candelina, and Placomaronea (Lumbsch and Huhndorf 2010; Miadlikowska et al. 2006; Westberg et al. 2009). It is a small group with approximately 66 lichenized species (Kirk et al. 2008) characterized by yellow to orange thalli (secondary chemistry based on pulvinic acid and derivatives), disciform apothecia, unitunicate asci (often polysporous), mostly unbranched paraphyses, and hyaline mostly simple ascospores (Hibbett et al. 2007; Westberg et al. 2007, 2009). Formerly, the family Candelariaceae had been placed in Lecanorales s.l. (Henssen and Jahns 1974; Poelt 1973). Molecular data showed that this family did not cluster within Lecanorales as currently delimited (Hofstetter et al. 2007; Miadlikowska et al. 2006; Wedin et al. 2005), and so the order Candelariales was erected for this family (Hibbett et al. 2007). Candelariales was considered to be a sister order to Acarosporales (Wedin et al. 2005) or possibly formed the second lineage to diverge within Lecanoromycetes after Acarosporales (Miadlikowska et al. 2006) or perhaps the first diverging lineage in Lecanoromycetes (Hofstetter et al. 2007). Because of this phylogenetic uncertainty, this order was only tentatively recognized as the subclass Candelariomycetidae by Miadlikowska et al. (2006) and Hofstetter et al. (2007). A morphological and molecular revision of the family showed that the current generic delimitation does not reflect monophyletic groups, and further work will be needed to clarify the generic boundaries (Westberg et al. 2007, 2009).
E. Lecanorales
Lecanorales (Lecanoromycetidae) (Fig. 4.5) is the largest order in Lecanoromycetes with 19 families and approximately 250 genera (Lumbsch and Huhndorf 2010). The number of species in this order was estimated to be 5,695 (Kirk et al. 2008). It includes mostly lichenized species with varied thallus types, predominantly apothecial ascomata, usually unbranched paraphyses, mostly thick-walled unitunicate asci (often amyloid), and varied ascospores (Kirk et al. 2008). Traditionally, this apparently heterogenous order included most lichenized apothecial ascomycetes, with the exception of those included in Ostropales (e.g., Henssen and Jahns 1974; Poelt 1973; Rambold and Triebel 1992). A new circumscription of this order based on the ascus apical structure led to the exclusion of several taxa from Lecanorales (e.g., Peltigerales and Teloschistales) (Hafellner 1988). This circumscription was further narrowed after molecular data were used to test the relationships among the main Lecanorales taxa. This circumscription was confirmed, and the trend continued with the advent of molecular phylogenetics, resulting in the following orders currently being recognized outside the Lecanorales: Acarosporales, Caliciales (with the Physciaceae), Candelariellales, Lecideales, Peltigerales, Pertusariales, Teloschistales, and Umbilicariales (e.g., Gaya et al. 2012; Miadlikowska and Lutzoni 2004; Miadlikowska et al. 2006; Reeb et al. 2004; Schmull et al. 2011; Wedin et al. 2005). Among the remaining 19 families in Lecanorales are the broadly distributed and well-known species-rich Cladoniaceae, Lecanoraceae, Parmeliaceae, and Ramalinaceae (Lumbsch and Huhndorf 2010).
Parmeliaceae is the largest family within Lecanorales, with approximately 2,500 species and 88 genera (Kirk et al. 2008). Earlier, many groups within Parmeliaceae had been tentatively segregated from this family based on morphological, anatomical, and chemical characters (e.g., Alectoriaceae, Anziaceae, Hypogymniaceae, and Usneaceae). But most of these segregate families were not confirmed by molecular phylogenies (e.g., Arup et al. 2007; Mattsson and Wedin 1999; Wedin et al. 1999), and a wider circumscription of Parmeliaceae is currently accepted (Eriksson 2006; Kirk et al. 2008; Lumbsch and Huhndorf 2010). This family comprises a large variety of growth forms (e.g., parmeliod, usneoid, or cetrarioid species), which do not define large monophyletic groups within Parmeliaceae but nevertheless were found to be useful in characterizing and identifying smaller clades within the family (Crespo et al. 2007).
The generic concept is a strongly debated area in lichen research (e.g., Elix 1993; Hale 1984a; Nimis 1998). In Parmeliaceae, large genera (e.g., Parmelia s.l.) have been split into multiple smaller genera (e.g., Culberson and Culberson 1981; Elix and Hale 1987; Elix et al. 1986; Hale 1974, 1984b). Molecular phylogenetic studies have shown that many of these newly segregated genera are not monophyletic (e.g., Blanco et al. 2004, 2005; Crespo et al. 2010; Nelsen et al. 2011), and the taxonomic importance of some characters traditionally used to classify parmelioid lichens (e.g., chemistry of the cortex, presence or absence of pores and pseudocyphellae) may have been overestimated (Blanco et al. 2006).
Similar problems were found in other families of Lecanorales (e.g, Lecanoraceae , Ramalinaceae ). In the predominantly crustose groups of Lecanorales, which were mainly classified based on ascus characters (Hafellner 1984), molecular phylogenetic studies reported that many of these crustose groups (e.g., Bacidiaceae, Lecanoraceae, Micareaceae) were not monophyletic and that the evolution of the ascus was more complex than had been anticipated and of limited value for classification at this taxonomic level (Andersen and Ekman 2004; Ekman 2001; Ekman and Wedin 2000; Ekman et al. 2008). Because of a similar composite thallus, Cladoniaceae was placed together with Stereocaulaceae and two other families within the suborder Cladoniineae (Lecanorales s.l.) (Poelt 1973). Molecular phylogenies showed that, although the composite growth form evolved several times in Lecanoromycetes (Stenroos and DePriest 1998), Cladoniaceae and Stereocaulaceae do form a sister group to which the suborder Cladoniineae is now restricted (Miadlikowska et al. 2006; Myllys et al. 2005; Wedin et al. 2000b).
F. Lecideales
Lecideales (Lecanoromycetidae) (Fig. 4.5) is an order recently resurrected for a single family, Lecideaceae , now restricted to the genus Lecidea s.s. (sensu Hertel) and some species of Porpidia (Schmull et al. 2011). In Zahlbruckner’s classification system (1903–1907), Lecideaceae was a large artificial family within the order Lecanorales that included a heterogeneous assemblage of crustose taxa with lecideine or biatorine apothecia (e.g., Bacidia, Catillaria, Toninia), among which Lecidea was one of the largest lichen genera. The delimitation of this poorly studied family was questioned in later taxonomic works and classification systems (Henssen and Jahns 1974; Hertel and Rambold 1985; Poelt 1973; Santesson 1952; Timdal 1987). In his classification of Lecanorales, Hafellner (1984) was the first to attempt to recircumscribe the two families Lecideaceae and Lecanoraceae. Based on ascus characters, he segregated several new families from the Lecideaceae, among which was Porpidiaceae . His system was broadly accepted (e.g., Eriksson and Hawksworth 1993; Hafellner 1994; Rambold and Triebel 1992), although also sometimes criticized (e.g., Timdal 1992).
Molecular phylogenetic studies confirmed the heterogeneity of early circumscriptions of Lecideaceae and Lecanoraceae (Andersen and Ekman 2004, 2005; Buschbom and Mueller 2004; Ekman 2001; Ekman et al. 2008; Schmull et al. 2011). For example, the genus Bacidia, included in Lecideaceae in early classifications (Henssen and Jahns 1974; Poelt 1973; Zahlbruckner 1903–1907), was shown to belong to Ramalinaceae, a family classified within Lecanorales (Ekman 2001). Molecular phylogenies also shed a light on Hafellner’s classification system (1984). Characters of the ascus tip used by this author to redelimitate genera and families within Lecanorales do not seem to characterize monophyletic entities in Lecideaceae and related taxa (e.g., Porpidiaceae) (Buschbom and Mueller 2004). Some taxa previously attributed to Lecideaceae were shown to belong to different lineages within Lecanoromycetes, and genera within this family were shown to be poorly delimited (Schmull et al. 2011). The phylogenetic positions of most members of Lecideaceae are still unknown or unsettled (Miadlikowska et al. 2006; Schmull et al. 2011). However, they were found to form five distinct groups within Lecanoromycetidae, one of which included only saxicolous species belonging to the genera Lecidea and Porpidia, including the type species Lecidea fuscoatra, which led to the resurrection of the order Lecideales s.s. (Schmull et al. 2011). Additional molecular data are greatly needed to further investigate this species-rich and broadly defined lichen group.
G. Ostropales
Ostropales (Ostropomycetidae) (Fig. 4.5) is a large order of mostly crustose lichenized and nonlichenized species, with high species diversity in the tropics. It includes approximately 2,750 species (Kirk et al. 2008) currently classified in ten families: Coenogoniaceae , Graphidaceae (including Gomphillaceae and Thelotremataceae), Gyalectaceae , Myeloconidaceae , Odontotremataceae , Phaneromycetaceae , Phlyctidaceae , Porinaceae , Sagiolechiaceae, and Stictidaceae (Baloch et al. 2010; Lumbsch and Huhndorf 2010; Rivas Plata et al. 2012). This order is characterized by ascomata ranging from perithecial to apothecial, with unbranched or anastomosate paraphyses, unitunicate nonamyloid asci, and morphologically variable ascospores (Kirk et al. 2008; Lücking et al. 2004; Lumbsch et al. 2007b).
The circumscription of the order Ostropales has undergone many changes in the past. It was originally described to accommodate the nonlichenized family Ostropaceae (Nannfeldt 1932), now known as Stictidaceae. Gilenstam (1969) was the first to include lichenized taxa within Ostropales. He recognized the close relationship between the lichenized genus Conotrema and the nonlichenized genus Stictis and attributed Conotrema to Ostropales. He also suggested that the lichenized genera Diploschistes, Graphis, and Thelotrema should be transferred to Ostropales because of their close relationship with Conotrema (Gilenstam 1969). Henssen and Jahns (1974) considered these genera and further lichenized groups (then included in Asterothyriaceae, Graphidaceae, and Thelotremataceae) as part of Ostropales. Subsequently, in a morphological revision of Ostropalean fungi, Sherwood (1977a, b) restricted Ostropales to Odontotrema, Ramonia, most current genera of Stictidaceae, and other genera now excluded from Lecanoromycetes. In this classification, many lichenized taxa (e.g., Graphidaceae and Thelotremataceae) were excluded from Ostropales based on differences in ascospore type (Sherwood 1977a, b).
Early molecular phylogenetic studies confirmed the close relationship between Stictis and Conotrema, and between Stictidaceae and both Graphidaceae and Thelotremataceae (Winka et al. 1998). The two families Coenogoniaceae and Gyalectaceae (Gyalectales) were then shown to be related to Graphidaceae and Thelotremataceae based on molecular data, and a broad delimitation was adopted for Ostropales (Kauff and Lutzoni 2002): Ostropales s.l. with Coenogoniaceae, Graphidaceae [including Thelotremataceae, as shown by Mangold et al. (2008)], Gyalectaceae, Stictidaceae, and Trapeliaceae. Other families were subsequently attributed to Ostropales s.l. based on additional molecular data: Asterothyriaceae and Gomphillaceae (Lücking et al. 2004), Phlyctidaceae and Solorinellaceae (Miadlikowska et al. 2006), the reinstated family Sagiolechiaceae (Baloch et al. 2010), and, more surprisingly, Porinaceae, a family of lichenized perithecioid ascomycetes (Grube et al. 2004). Trapeliaceae (as Agyriaceae) is now excluded from Ostropales s.l. (Grube et al. 2004; Miadlikowska et al. 2006). Although still recently largely debated (Grube et al. 2004; Lücking et al. 2004; Lumbsch et al. 2004; Miadlikowska et al. 2006), the broader delimitation of Ostropales (but without Trapeliaceae) has been accepted in current classification systems (Hibbett et al. 2007; Lumbsch and Huhndorf 2010).
The phylogenetic placement of Ostropales within Lecanoromycetes has also long been unclear due to an unstable backbone of the Lecanoromycetes phylogeny (Lumbsch et al. 2007b). Ostropales has been found to be sister to all other Lecanoromycetes (Grube et al. 2004; Lücking et al. 2004; Lumbsch et al. 2004), sister to Trapeliales and Hymeneliaceae (Kauff and Lutzoni 2002; Miadlikowska and Lutzoni 2004) or to a lineage including Trapeliales and Baeomycetales (Miadlikowska et al. 2006), sister to Fuscideaceae, a family incertae sedis in Lecanoromycetes (Reeb et al. 2004), and sister to a lineage including Anzina and Arthroraphis (Wedin et al. 2005), although none of these relationships were strongly supported. Schmitt et al. (2005) reported the Thelenellaceae as sister to the Ostropales s.l. with a high posterior probability, supporting the resolution shown in Fig. 4.5. Nevertheless, more loci and broader taxon samplings are needed to establish the sister taxa of Ostropales with high phylogenetic confidence (Lumbsch et al. 2007b).
H. Peltigerales
Peltigerales (Lecanoromycetidae) (Fig. 4.5) is an order of mainly foliose species, with rounded apothecia, unbranched paraphyses, bitunicate asci with fissitunicate dehiscence, and multiseptate ascospores (Honegger 1978; Kirk et al. 2008). They have a worldwide distribution and colonize diverse substrates, mostly in humid habitats. Most species in this order are associated with cyanobacteria, either as primary or secondary photobionts. All Lecanoromycetes with cyanobacteria as their primary photobiont belong to this order [with the only exception being Arctomiaceae, which are classified in Ostropomycetidae (Lumbsch et al. 2005)]. When cyanobacteria occur only as secondary photobionts, the primary photobionts are then green algae from the genera Coccomyxa, Dictyochloropsis, or Myrmecia (Tschermak-Woess 1988a), and the cyanobacterial secondary photobionts are restricted to gall-like structures called cephalodia. Peltigeralean species associated only with a green alga are rare. The most recent common ancestor of Peltigerales was inferred to be associated with a cyanobacterium as its primary photobiont, which means that the green algal photobionts were most likely acquired secondarily in this order (Miadlikowska and Lutzoni 2004). Moreover, the anatomically nonlayered gelatinous thalli mostly found in some genera of Collematineae (e.g., Collema and Leptogium) seem to have evolved from more complex and anatomically layered thalli (Wedin et al. 2009). The phylogenetic relationships, an overview of phenotypic characters, and the major types of ascus structures within Peltigerales are reported in Spribille and Muggia (2013).
Peltigerales currently includes two suborders, Collematineae and Peltigerineae (Miadlikowska and Lutzoni 2004), and ten families (Spribille and Muggia 2013). Collematineae includes four families: Coccocarpiaceae , Collemataceae , Pannariaceae, and Placynthiaceae . Peltigerinae includes six families: Koerberiaceae , Lobariaceae , Massalongiaceae , Nephromataceae , Peltigeraceae, and Vahliellaceae (Miadlikowska and Lutzoni 2004; Muggia et al. 2011; Spribille and Muggia 2013; Wedin et al. 2007, 2011). Previously, peltigeralean lichens had been recognized at either the ordinal level (Peltigerales) (Hafellner 1988; Kirk et al. 2001) or the subordinal level within the order Lecanorales (Peltigerinae) (Eriksson et al. 2003; Henssen and Jahns 1974; Poelt 1973; Rambold and Triebel 1992; Tehler 1996). Despite this ranking inconsistency, all large-scale molecular phylogenetic studies confirmed the placement of this lineage within Lecanoromycetes (e.g., Kauff and Lutzoni 2002; Lutzoni et al. 2001, 2004; Miadlikowska et al. 2006; Wedin and Wiklund 2004). The current recognition of this clade at the ordinal level and the establishment of the two suborders Peltigerineae and Collematineae were proposed by Miadlikowska and Lutzoni (2004) and are now largely adopted (Hibbett et al. 2007; Lumbsch and Huhndorf 2007b, 2010).
The number of families within Peltigerales changed greatly over time [see details in Miadlikowska and Lutzoni (2004)]. The two families Lobariaceae and Peltigeraceae have always been included in Peltigerales (Hafellner 1988; Poelt 1973), but Coccocarpiaceae, Collemataceae, and Pannariaceae have sometimes been excluded and transferred to the Lecanorales s.l. or classified as incertae sedis within Lecanorales s.l. (Eriksson et al. 2003; Hafellner 1988; Henssen and Jahns 1974; Kirk et al. 2001; Poelt 1973). Moreover, Nephrotomataceae and Solorinaceae were recognized as separate families from Peltigeraceae by certain authors (Hafellner 1988; Poelt 1973). Molecular phylogenetic studies have confirmed the placement of Coccocarpiaceae, Collemataceae, Pannariaceae, and Placynthiaceae within the Collematineae, and Lobariaceae, Nephromataceae, and Peltigeraceae within the Peltigerineae (Miadlikowska and Lutzoni 2004; Miadlikowska et al. 2006; Wedin and Wiklund 2004; Wedin et al. 2009). The families Massalongiaceae, Vahliellaceae, and Koerberiaceae were more recently described and attributed to Peltigerineae (Spribille and Muggia 2013; Wedin et al. 2007, 2011).
I. Pertusariales
The order Pertusariales (Ostropomycetidae) (Fig. 4.5) mostly comprises crustose species with disciform to poriform apothecia, thick-walled asci, branched paraphysoids, and generally large ascospores (Lumbsch et al. 1994; Schmitt et al. 2006). They have a worldwide distribution and colonize a broad range of habitats and substrates. Earlier, these species were classified in the suborder Pertusarineae within the order Lecanorales s.l. (Henssen and Jahns 1974; Poelt 1973) and later as the order Pertusariales (Hawksworth and Eriksson 1986). Molecular phylogenies revealed that Pertusariales belongs to Ostropomycetidae (Lutzoni et al. 2004; Miadlikowska et al. 2006; Reeb et al. 2004), and this order was accepted in all recent classifications of Ascomycota (Hibbett et al. 2007; Lumbsch and Huhndorf 2010).
A recent molecular study showed that the type species of Agyrium did not cluster with other Agyriaceae but nested within Pertusariales (Schmitt et al. 2010). As a result, these authors reduced the order Pertusariales to synonymy with Agyriales based on the priority principle. The name Agyriales would then be used for a group including a large majority of species traditionally classified in Pertusariales and only a few mostly nonlichenized and poorly known species of Agyrium. Hodkinson and Lendemer (2011) proposed that the name Pertusariales should be retained over Agyriales because the principle of priority is not mandatory for taxa above the family rank, and because the name Agyriales was most recently misapplied to a monophyletic group, including the family Trapeliaceae, now recognized as Trapeliales .
Pertusariales currently includes seven families: Agyriaceae (currently only represented by its generic type Agyrium rufum), Coccotremataceae , Icmadophilaceae , Megasporaceae , Miltideaceae , Ochrolechiaceae, and Pertusariaceae (Hodkinson and Lendemer 2011; Lumbsch and Huhndorf 2010; Schmitt et al. 2010; Widhelm and Lumbsch 2011), but its circumscription has been problematic. Only species from the families Coccotremataceae, Pertusariaceae, and Ochrolechiaceae had traditionally been included in Pertusariineae/Pertusariales (Eriksson and Hawksworth 1986; Henssen 1976; Henssen and Jahns 1974; Poelt 1973). Species from the Coccotremataceae family were segregated from Pertusariaceae based on differences in ascomata structure and ontogeny (David and Hawksworth 1991; Henssen 1976). Because species of Coccotremataceae also differ from those of Pertusariaceae in other aspects (e.g., the ascus structure, the presence of cephalodia), members of Coccotremataceae had previously been excluded from Pertusariales (Lumbsch et al. 1994), but molecular phylogenetic analyses confirmed their placement within this order (Lumbsch et al. 2002). The segregation of Ochrolechia from Pertusariaceae was first suggested by Harris (1990). Schmitt et al. (2006) then formally described and redelimited this family to also include the genus Varicellaria. Icmadophilaceae had earlier been classified within Baeomycetaceae (Henssen and Jahns 1974; Poelt 1973), but molecular phylogenies supported the placement of this family within Pertusariales (Miadlikowska et al. 2006; Reeb et al. 2004). The family Megasporaceae was erected for Megaspora verrucosa, a species previously classified as part of the genus Aspicilia (Clauzade and Roux 1984) and placed in Pertusariales (Lumbsch et al. 1994). This species was later shown based on molecular data to be sister to Aspicilia and part of Pertusariales (Schmitt et al. 2006). The genera Aspicilia and Lobothallia were therefore transferred to Megasporaceae, and Aspiciliaceae ined. was regarded as a synonym of this family (Lumbsch and Huhndorf 2010; Schmitt et al. 2006).
J. Rhizocarpales
The order Rhizocarpales (Lecanoromycetidae?) (Fig. 4.5) includes two families, Rhizocarpaceae and part of the Catillariaceae (the genus Sporastatia) , and approximately 489 species (Kirk et al. 2008). They are characterized by crustose areolate thalli, immersed to sessile apothecia, branched and often anastomosed paraphyses, asci with an amyloid apex, and simple to muriform ascospores (Hafellner 1984). The sister relationship between Sporastatia (Catillariaceae) and Rhizocarpon (Rhizocarpaceae) was first demonstrated in the study by Reeb et al. (2004). Further studies confirmed this result (Buschbom and Mueller 2004; Lutzoni et al. 2004), and the order Rhizocarpales was proposed by Miadlikowska et al. (2006) to accommodate selected taxa from these two families (many members were never subjected to phylogenetic studies). The phylogenetic position of Rhizocarpales as the first split within Lecanoromycetes has rarely been supported. If confirmed, this order should be considered as a member of Lecanoromycetidae.
K. Sarrameanales
The order Sarrameanales (Ostropomycetidae) (Fig. 4.5) includes a single family, Sarrameanaceae, and the two genera Loxospora and Sarrameana . It is a small family of approximately ten species occurring mostly in cool temperate regions of both Northern and Southern Hemispheres (Kantvilas 2004). They are crustose species with dark lecideine to lecanorine apothecia, simple to sparingly branched paraphyses, asci with an amyloid domelike tholus lacking an ocular chamber, and simple to transversally septate hyaline ascospores. The genus Loxospora was segregated from Haematommataceae and placed in Loxosporaceae (Staiger and Kalb 1995), which was later synonymized with Sarrameanaceae (Kantvilas 2004). Because of a unique combination of morphological characters, the systematic position of the genus Sarrameana has always been problematic, possibly as related to Fuscideaceae (Eriksson and Hawksworth 1986), Haematommataceae (Hafellner 1984; Vězda and Kantvilas 1988), Lecideaceae (Vězda and James 1973), and Ophioparmaceae (Kantvilas and Vězda 1996). Recent molecular phylogenetic analyses that included several species of Loxospora showed that Sarrameanaceae is not related to any of these families (Lumbsch et al. 2007a, b, 2008b). In several studies, the genus Loxospora forms the earliest diverging lineage within the subclass Ostropomycetidae (Lumbsch et al. 2007b; Miadlikowska et al. 2006; Schoch et al. 2009). As a result, Hodkinson and Lendemer (2011) erected the new order Sarrameanales for Sarrameanaceae.
L. Teloschistales
Teloschistales (Lecanoromycetidae) (Fig. 4.5), as recently recircumscribed by Gaya et al. (2012), comprises four families classified in the two suborders Teloschistineae (Megalosporaceae and Teloschistaceae ) and Letrouitineae (Brigantiaeaceae and Letrouitiaceae ). It includes mostly lichenized species with crustose to foliose or fruticose thalli with a yellow to orange color (anthraquinone pigments), apothecial ascomata, unbranched paraphyses, unitunicate asci with an apical thickening, and mostly hyaline polarilocular ascospores (Kärnefelt 1989; Kirk et al. 2008). They are found worldwide and often favor nutrient-rich substrates.
First recognized as a suborder within Lecanorales s.l. (Teloschistineae; Henssen and Jahns 1974) or placed within the suborder Buelliineae (Poelt 1973), the families Letrouitiaceae, Teloschistaceae, and, tentatively, Fuscideaceae were grouped by Eriksson and Hawksworth (1986) in the order Teloschistales, which they formally described. With the advent of molecular phylogenetics, several taxa were added to Teloschistales, namely, Megalosporaceae (Helms et al. 2003; Lutzoni et al. 2004) and both Caliciaceae and Physciaceae (Miadlikowska et al. 2006), which were shown to form a monophyletic group (Helms et al. 2003; Wedin et al. 2000a). As a result, two suborders were recognized within Teloschistales (Miadlikowska et al. 2006): Physciineae (Physciaceae, including Caliciaceae) and Teloschistineae (Letrouitiaceae, Megalosporaceae, and Teloschistaceae). However, the relationship between Physciineae and Teloschistineae never obtained strong support (Miadlikowska et al. 2006).
In a more recent molecular study, Gaya et al. (2012) detected two competing hypotheses for the relationships among the three clades Lecanorales, Physciineae, and Teloschistineae: either Lecanorales is sister to a lineage including Physciineae and Teloschistineae, or Physciineae is sister to a lineage including Lecanorales and Teloschistineae. To avoid this phylogenetic uncertainty contributing to taxonomic instability, Gaya et al. (2012) proposed to restrict the name Teloschistales to the Teloschistiineae and resurrect the order Caciliales for the Physciineae. This study also showed that Brigantiaeaceae, a family classified as incertae sedis in Lecanoromycetidae (Lumbsch and Huhndorf 2010) or as part of Lecanorales (Kirk et al. 2008), belongs to Teloschistales and is sister to Letrouitiaceae (Gaya et al. 2012).
M. Trapeliales
Trapeliales (Ostropomycetidae) (Fig. 4.5) currently includes a single family, Trapeliaceae (Hodkinson and Lendemer 2011). This family had been described for the four genera Orceolina, Placopsis, Trapelia, and Trapeliopsis (Hertel 1970). Originally, Trapeliaceae was placed in the Agyriineae, a suborder of Lecanorales with a similar ascus structure (Hafellner 1994). In a comprehensive morphological revision of Agyriineae, the placement of this suborder within Lecanorales was questioned (Lumbsch 1997). Early molecular studies showed that Agyriineae were indeed not related to Lecanorales, and Agyriales was resurrected for this group (Lumbsch et al. 2001a). More recent molecular studies showed that the type species of Agyrium (A. rufum) belongs to Pertusariales (Lumbsch et al. 2007c; Schmitt et al. 2010). Because no ordinal name was then available for the lineage including all genera of Trapeliaceae and other genera previously placed in Agyriaceae, Hodkinson and Lendemer ( 2011 ) proposed to erect the new order Trapeliales for them. Previous molecular data had confirmed the placement within Trapeliaceae of the four genera originally included in this family (Lumbsch et al. 2007b, c; Miadlikowska et al. 2006; Poulsen et al. 2001; Schmitt et al. 2003).
In addition, the genera Aspiciliopsis, Placynthiella, Ptychographa, Rimularia, and Xylographa had also been shown to belong to this family based on molecular data (Lumbsch et al. 2001b; Schmitt et al. 2003). The genera Amylora, Coppinsia, Lignoscripta, and Sarea have also been suggested as belonging to Trapeliaceae (Hodkinson and Lendemer 2011), but their phylogenetic placements still need confirmation from molecular phylogenetic studies.
N. Umbilicariales
The order Umbilicariales (Lecanoromycetes incertae sedis) (Fig. 4.5) is an order of approximately 191 species classified in four families: Elixiaceae , Fuscideaceae , Ophioparmaceae [including Rhizoplacopsidaceae, as it is now considered a synonym of Ophioparmaceae (Lumbsch and Huhndorf 2010)], and Umbilicariaceae (Kirk et al. 2008). The delimitation of the order Umbilicariales has changed greatly over the last decade. Diverse lichen families have recently been shown to belong to this order: Elixiaceae (Lumbsch et al. 2007a), Ophioparmaceae and Fuscideaceae (Bylin et al. 2007; Miadlikowska et al. 2006), Rhizoplacopsidaceae (Zhou and Wei 2006), and Ropalosporaceae, although without support for this last family (Bylin et al. 2007). Umbilicariales was formally described in two different publications during the same year (Hibbett et al. 2007; Zhou and Wei 2007), but the authorship was attributed to the earliest one (Zhou and Wei 2007).
Umbilicariaceae, the largest family within this order, includes lichenized foliose umbilicate species, with disciform apothecia, mostly unbranched paraphyses, unitunicate asci with a small amyloid cap, and simple to muriform ascospores (Hibbett et al. 2007; Louwhoff 2009). They mostly grow on rocks at high altitudes or high latitudes (Davydov 2007). They were traditionally placed in Lecanorales s.l. (Henssen and Jahns 1974), sometimes in a separate suborder, Umbilicariineae (Poelt 1973). Early molecular studies showed that Umbilicariaceae was not related to Lecanorales (Kauff and Lutzoni 2002; Lutzoni et al. 2001; Stenroos and DePriest 1998). They form a well-supported monophyletic group, and although their placement within Lecanoromycetes is not resolved, they do not seem to belong to any of the three currently recognized Lecanoromycetes subclasses Acarosporomycetidae, Lecanoromycetidae, and Ostropomycetidae (Hofstetter et al. 2007; Lumbsch et al. 2004; Miadlikowska et al. 2006; Reeb et al. 2004; Wedin et al. 2005). Miadlikowska et al. (2006) suggested that this group might be recognized as a separate subclass in the future.
VIII. Conclusion
Morphological, anatomical, and chemical characters have traditionally been used to classify orders, families, and genera within Lecanoromycetes, the class of Ascomycota with the largest number of lichen-forming fungi. In the last two decades, molecular phylogenies showed that traditional classification systems were not always consistent with the evolutionary history of this fungal class, resulting in changes in the delimitation of orders and families. For example, many families were segregated from the large and heterogenous order Lecanorales and raised to the ordinal level. As a result, the classification system of the Lecanoromycetes is now more on par with the overall supraordinal classification of the Fungi (Hibbett et al. 2007) and comprises 14 orders and 3 subclasses. Additional changes are expected in the future when better taxon and gene sampling will be available to resolve phylogenetic relationships within Lecanoromycetes.
References
Ahmadjian V (1967) A guide to the algae occurring as lichen symbionts: isolation, culture, cultural physiology, and identification. Phycologia 6:127–160
Ahmadjian V (1969) Lichen synthesis. Österr Bot Z 116:306–311
Ahmadjian V (1993) The lichen photobiont—what can it tell us about lichen systematics? Bryologist 96:310–313
Amo de Paz G, Cubas P, Divakar PK, Lumbsch HT, Crespo A (2011) Origin and diversification of major clades in parmelioid lichens (Parmeliaceae, Ascomycota) during the Paleogene inferred by Bayesian analysis. PLoS One 6:e28161
Andersen HL, Ekman S (2004) Phylogeny of the Micareaceae inferred from nrSSU DNA sequences. Lichenologist 36:27–35
Andersen HL, Ekman S (2005) Disintegration of the Micareaceae (lichenized Ascomycota): a molecular phylogeny based on mitochondrial rDNA sequences. Mycol Res 109:21–30
Aptroot A (2001) Lichenized and saprobic fungal biodiversity of a single Elaeocarpus tree in Papua New Guinea, with the report of 200 species of ascomycetes associated with one tree. Fungal Divers 6:1–11
Aptroot A, Sipman HJM (1997) Diversity of lichenized fungi in the tropics. In: Hyde KD (ed) Biodiversity of tropical microfungi. University Press, Hong Kong, pp 93–106
Armaleo D, Zhang Y, Cheung S (2008) Light might regulate divergently depside and depsidone accumulation in the lichen Parmotrema hypotropum by affecting thallus temperature and water potential. Mycologia 100:565–576
Armaleo D, Sun XM, Culberson C (2011) Insights from the first putative biosynthetic gene cluster for a lichen depside and depsidone. Mycologia 103:741–754
Arup U, Ekman S, Grube M, Mattsson JE, Wedin M (2007) The sister group relation of Parmeliaceae (Lecanorales, Ascomycota). Mycologia 99:42–49
Asahina Y, Shibata S (1954) Chemistry of lichen substances. Japan Society for the Promotion of Science, Ueno, Tokyo, Japan
Bailey RH (1976) Ecological aspects of dispersal and establishment in lichens. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. Academic, London, pp 215–247
Baloch E, Lücking R, Lumbsch HT, Wedin M (2010) Major clades and phylogenetic relationships between lichenized and non-lichenized lineages in Ostropales (Ascomycota: Lecanoromycetes). Taxon 59:1483–1494
Barr ME (1983) The ascomycete connection. Mycologia 75:1–13
Bellemère A (1977) L'appareil apical de l'asque chez quelques Discomycètes: étude ultrastructurale comparative. Rev Mycol 40:3–19
Bellemère A (1994) Asci and ascospores in ascomycete systematics. In: Hawksworth DL (ed) Ascomycete systematics. Problems and perspectives in the nineties, NATO advanced science institutes series. Plenum Press, New York, pp 111–126
Bellemère A, Hafellner J (1982) L'ultrastructure des asques du genre Dactylospora (Discomycètes) et son intérêt taxonomique. Cryptogamie Mycol 3:71–93
Bellemère A, Letrouit-Galinou MA (1987) Differentiation of lichen asci including dehiscence and sporogenesis: an ultrastructural survey. In: Peveling E (ed) Progress and problems in lichenology in the eighties, vol 25, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 137–161
Ben-Shaul Y, Paran N, Galun M (1969) The ultrastructure of the association between phycobiont and mycobiont in three ecotypes of the lichen Caloplaca aurantia var. aurantia. J Microsc 8:415–422
Berbee ML (1996) Loculoascomycete origins and evolution of filamentous ascomycete morphology based on 18S rRNA gene sequence data. Mol Biol Evol 13:462–470
Berbee ML, Taylor JW (1993) Dating the evolutionary radiations of the true fungi. Can J Bot 71:1114–1127
Berbee ML, Taylor JW (2001) Fungal molecular evolution: gene trees and geologic time. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The Mycota, vol VII, part B. Systematics and evolution. Springer, Berlin, Heidelberg, New York, pp 229–245
Bessey CE (1907) A synopsis of plant phyla. Univ Nebr Stud 7(4):275–373
Blanco O, Crespo A, Elix JA, Hawksworth DL, Lumbsch HT (2004) A molecular phylogeny and a new classification of parmelioid lichens containing Xanthoparmelia-type lichenan (Ascomycota: Lecanorales). Taxon 53:959–975
Blanco O, Crespo A, Divakar PK, Elix JA, Lumbsch HT (2005) Molecular phylogeny of parmotremoid lichens (Ascomycota, Parmeliaceae). Mycologia 97:150–159
Blanco O, Crespo A, Ree RH, Lumbsch HT (2006) Major clades of parmelioid lichens (Parmeliaceae, Ascomycota) and the evolution of their morphological and chemical diversity. Mol Phylogenet Evol 39:52–69
Brodo IM (1973) The lichen genus Coccotrema in North America. Bryologist 76:260–270
Brodo IM, Sloan NA (2004) Lichen zonation on coastal rocks in Gwaii Haanas National Park Reserve, Haida Gwaii (Queen Charlotte Islands), British Columbia. Can Field Nat 118:405–424
Brodo IM, Duran Sharnoff S, Sharnoff S (2001) Lichens of North America. Yale University Press, New Haven, London
Buschbom J, Barker D (2006) Evolutionary history of vegetative reproduction in Porpidia s.l. (lichen-forming Ascomcota). Syst Biol 55:471–484
Buschbom J, Mueller G (2004) Resolving evolutionary relationships in the lichen-forming genus Porpidia and related allies (Porpidiaceae, Ascomycota). Mol Phylogenet Evol 32:66–82
Buschbom J, Mueller GM (2006) Testing “species pair” hypotheses: evolutionary processes in the lichen-forming species complex Porpidia flavocoerulescens and Porpidia melinodes. Mol Biol Evol 23:574–586
Bylin A, Arnerup J, Högberg N, Thor G (2007) A phylogenetic study of Fuscideaceae using mtSSU rDNA. In: Frisch A, Lange U, Staiger B (eds) Lichenologische Nebenstunden. Contributions to lichen taxonomy and ecology in honour of Klaus Kalb, vol 96, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 49–60
Chadefaud M (1942) Étude d'asques: II. Structure et anatomie comparée de l'appareil apical chez divers Disco- et Pyrénomycètes. Rev Mycol 7:57–88
Chadefaud M (1960) Les végétaux non vasculaires (Cryptogamie). In: Chadefaud M, Emberger L (eds) Traité de botanique systématique. Masson, Paris, pp 524–529, 543–545 and 639–640
Chadefaud M (1973) Les asques et la systematique des Ascomycetes. Bull Soc Mycol France 89:127–170
Chadefaud M, Letrouit-Galinou MA, Favre MC (1963) Sur l'évolution des asques du type archaeascé chez les Discomycètes de l'ordre des Lécanorales. Compt Rend Acad Sci Paris 257:4003–4005
Chadefaud M, Letrouit-Galinou MA, Favre MC (1967) Sur l'origine phylogénétique et l'évolution des ascomycètes des lichens. Bull Soc Bot Fr Mém 115:79–111
Choisy M (1954) Catalogue des lichens de la region lyonnaise. Édition Paul Chevalier, Paris
Clauzade G, Roux C (1984) Les genres Aspicilia Massal. et Bellemerea Hafellner et Roux. Bull Soc Bot Centre-Ouest 15:127–141
Clauzade G, Roux C (1985) Likenoj de okcidenta Europo. Ilustrita determinlibro. Bull Soc Bot Centre-Ouest 7:1–893
Collins CR, Farrar JF (1978) Structural resistances to mass transfer in the lichen Xanthoria parietina. New Phytol 81:71–83
Coppins BJ, Wolseley P (2002) Lichens of tropical forests. In: Watling R, Frankland JC, Ainsworth AM, Isaac S, Robinson CH (eds) Tropical mycology, vol 2, Micromycetes. CABI Publishing, Wallingford, UK, pp 113–131
Crespo A, Lumbsch HT, Mattsson JE, Blanco O, Divakar PK, Articus K, Wiklund E, Bawingan PA, Wedin M (2007) Testing morphology-based hypotheses of phylogenetic relationships in Parmeliaceae (Ascomycota) using three ribosomal markers and the nuclear RPB1 gene. Mol Phylogenet Evol 44:812–824
Crespo A, Kauff F, Divakar PK, del Prado R, Pérez-Ortega S, de Paz GA, Ferencova Z, Blanco O, Roca-Valiente B, Núñez-Zapata J, Cubas P, Argüello A, Elix JA, Esslinger TL, Hawksworth DL, Millanes AM, Molina MC, Wedin M, Ahti T, Aptroot A, Barreno E, Bungartz F, Calvelo S, Candan M, Cole MJ, Ertz D, Goffinet B, Lindblom L, Lücking R, Lutzoni F, Mattsson JE, Messuti MI, Miadlikowska J, Piercey-Normore MD, Rico VJ, Sipman H, Schmitt I, Spribille T, Thell A, Thor G, Upreti DK, Lumbsch HT (2010) Phylogenetic generic classification of parmelioid lichens (Parmeliaceae, Ascomycota) based on molecular, morphological and chemical evidence. Taxon 59:1735–1753
Culberson WL, Culberson CF (1981) The genera Cetrariastrum and Concamerella (Parmeliaceae): a chemosynthetic synopsis. Bryologist 84:273–314
Culberson CF, Elix JA (1989) Lichen substances. In: Harborne JB (ed) Methods in plant biochemistry, vol 1, Plant phenolics. Academic, London, San Diego, pp 509–535
Dal Grande F, Widmer I, Wagner HH, Scheidegger C (2012) Vertical and horizontal photobiont transmission within populations of a lichen symbiosis. Mol Ecol 21:3159–3172
David JC, Hawksworth DL (1991) Validation of six family names of lichenized ascomycetes. Systema Ascomycetum 10:13–18
Davydov DA (2007) Approaches to a classification of the family Umbilicariaceae (lichenised Ascomycota) by anatomical and morphological characters. Turczaninowia 10:51–57
Du Rietz GE (1924) Die Soredien und Isidien der Flechten. Svensk Bot Tidskr 18:371–396
Duvigneaud P (1955) Les Stereocaulon des hautes montagnes du Kivu. Essai anatomo-systématique. Lejeunia Mem 14:1–144
Ekman S (2001) Molecular phylogeny of the Bacidiaceae (Lecanorales, lichenized Ascomycota). Mycol Res 105:783–797
Ekman S, Tønsberg T (2002) Most species of Lepraria and Leproloma form a monophyletic group closely related to Stereocaulon. Mycol Res 106:1262–1276
Ekman S, Wedin M (2000) The phylogeny of the families Lecanoraceae and Bacidiaceae (lichenized Ascomycota) inferred from nuclear SSU rDNA sequences. Plant Biol 2:350–360
Ekman S, Andersen HL, Wedin M (2008) The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota). Syst Biol 57:141–156
Elix JA (1993) Progress in the generic delimitation of Parmelia sensu lato lichens (Ascomycotina: Parmeliaceae) and a synoptic key to the Parmeliaceae. Bryologist 96:359–383
Elix JA, Hale ME (1987) Canomaculina, Myelochroa, Parmelinella, Parmelinopsis and Parmotremopsis, five new genera in the Parmeliaceae (lichenized Ascomycotina). Mycotaxon 29:233–244
Elix JA, Stocker-Wörgötter E (2008) Biochemistry and secondary metabolites. In: Nash TH III (ed) Lichen biology. Cambridge University Press, Cambridge, UK, pp 104–133
Elix JA, Johnston J, Verdon D (1986) Canoparmelia, Paraparmelia and Relicinopsis, three new genera in the Parmeliaceae (lichenized Ascomycotina). Mycotaxon 27:271–282
Eriksson OE (1982) The families of bitunicate Ascomycetes. Opera Bot 60:1–209
Eriksson OE (2006) Outline of Ascomycota—2006. Myconet 12:1–82
Eriksson OE, Hawksworth DL (1986) Outline of the ascomycetes—1986. Systema Ascomycetum 5:185–324
Eriksson OE, Hawksworth DL (1993) Outline of the ascomycetes—1993. Systema Ascomycetum 12:51–257
Eriksson OE, Winka K (1997) Supraordinal taxa of Ascomycota. Myconet 1:1–16
Eriksson OE, Baral HO, Currah RS, Hansen K, Kurtzman CP, Rambold G, Laessøe T (2003) Outline of Ascomycota—2003. Myconet 9:1–103
Fedrowitz K, Kaasalainen U, Rikkinen J (2011) Genotype variability of Nostoc symbionts associated with three epiphytic Nephroma species in a boreal forest landscape. Bryologist 114:220–230
Fernandez-Mendoza F, Domasche S, Garcia MA, Jordan P, Martin MP, Printzen C (2011) Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol Ecol 20:1208–1232
Fletcher A (1980) Marine and maritime lichens of rocky shores: their ecology, physiology and biological interactions. In: Price JH, Irvine DEG, Farnham WF (eds) The shore environment, vol 2, Ecosystems. Systematics Association special volume no. 17B. Academic, London, New York, pp 789–842
Friedl T (1987) Thallus development and phycobionts of the parasitic lichen Diploschistes muscorum. Lichenologist 19:183–191
Friedl T, Büdel B (2008) Photobionts. In: Nash TH III (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, UK, pp 7–26
Frisch A, Kalb K, Grube M (2006) Molecular phylogeny of the Thelotremataceae. A study based on Bayesian analysis of mitochondrial 16S rDNA gene data. In: Wirth V (ed) Contributions towards a new systematics of the lichen family Thelotremataceae, vol 92, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 517–539
Galun M (1988) The fungus-alga relation. In: Galun M (ed) CRC handbook of lichenology, vol I. CRC Press, Boca Raton, FL, pp 147–158
Galun M, Paran N, Ben-Shaul Y (1970) An ultrastructural study of the fungus alga association in Lecanora radiosa growing under different environmental conditions. J Microsc 8:801–806
Gaya E, Högnabba F, Holguin Á, Molnar K, Fernández-Brime S, Stenroos S, Arup U, Søchting U, van den Boom P, Lücking R, Sipman HJM, Lutzoni F (2012) Implementing a cumulative supermatrix approach for a comprehensive phylogenetic study of the Teloschistales (Pezizomycotina, Ascomycota). Mol Phylogenet Evol 63:374–387
Gazis R, Miadlikowska J, Lutzoni F, Arnold AE, Chaverri P (2012) Culture-based study of endophytes associated with rubber trees in Peru reveals a new class of Pezizomycotina: Xylonomycetes. Mol Phylogenet Evol 65:294–304
Geitler L (1934) Beiträge zur Kenntnis der Flechtensymbiose IV, V. Arch Protistenk 82:51–85
Geitler L (1963) Über Haustorien bei Flechten und über Myrmecia biatorellae in Psora globifera. Österr Bot Z 110:270–280
Gierl C, Kalb K (1993) Die Flechtengattung Dibaeis. Eine Übersicht über die rosafrüchtigen Arten von Baeomyces sens. lat. nebst Anmerkungen zu Phyllobaeis gen. nov. Herzogia 9:593–645
Gilbert OL (1990) The lichen flora of urban wasteland. Lichenologist 22:87–101
Gilbert OL (1996) The lichen vegetation of chalk and limestone streams in Britain. Lichenologist 28:145–159
Gilbert OL, Giavarini VJ (1997) The lichen vegetation of acid watercourses in England. Lichenologist 29:347–367
Gilenstam G (1969) Studies in the lichen genus Conotrema. Arch Bot 7:149–179
Golubkova NS (1988) The lichen family Acarosporaceae in the U.S.S.R. Komarov Botanical Institute, Academy of Sciences of the U.S.S.R., Leningrad
Green TGA, Schroeter B, Sancho LG (1999) Plant life in Antarctica. In: Pugnaire FI, Valladares F (eds) Handbook of functional plant ecology. Marcel Dekker, New York, Basel, pp 495–543
Grube M, Arup U (2001) Molecular and morphological evolution in the Physciaceae (Lecanorales, lichenized Ascomycotina), with special emphasis on the genus Rinodina. Lichenologist 33:63–72
Grube M, Hawksworth DL (2007) Trouble with lichen: the re-evaluation and re-interpretation of thallus form and fruit body types in the molecular era. Mycol Res 111:1116–1132
Grube M, Baloch E, Lumbsch HT (2004) The phylogeny of Porinaceae (Ostropomycetidae) suggests a neotenic origin of perithecia in Lecanoromycetes. Mycol Res 108:1111–1118
Gueidan C, Ruibal C, de Hoog GS, Gorbushina AA, Untereiner WA, Lutzoni F (2008) A rock-inhabiting ancestor for mutualistic and pathogen-rich fungal lineages. Stud Mycol 61:111–119
Gueidan C, Ruibal C, de Hoog GS, Schneider H (2011) Rock-inhabiting fungi originated during periods of dry climate in the late Devonian and middle Triassic. Fungal Biol 115:987–996
Hafellner J (1984) Studien in Richtung einer naturlicheren Gliederung der Sammelfamilien Lecanoraceae und Lecideaceae. In: Hertel H, Oberwinkler F (eds) Beitrage zur Lichenologie. Festscrift J. Poelt, vol 79, Beiheft zur Nova Hedwigia. J. Cramer, Vaduz, pp 241–371
Hafellner J (1988) Principles of classification and main taxonomic groups. In: Galun M (ed) CRC handbook of lichenology, vol III. CRC Press, Boca Raton, FL
Hafellner J (1994) Problems in Lecanorales systematics. In: Hawksworth DL (ed) Ascomycete systematics. Problems and perspectives in the nineties, NATO advanced science institutes series. Plenum, New York, pp 315–320
Hafellner J (1995) Towards a better circumscription of the Acarosporaceae (lichenized Ascomycotina, Lecanorales). Cryptog Bot 5:99–104
Hale ME (1974) Bulbothrix, Parmelina, Relicina, and Xanthoparmelia, four new genera in the Parmeliaceae. Phytologia 28:479–490
Hale ME (1984a) An historical review of the genus concept in lichenology. In: Hertel H, Oberwinkler F (eds) Beitrage zur Lichenologie. Festschrift J. Poelt, vol 79, Beiheft zur Nova Hedwigia. Vaduz, J. Cramer, pp 11–23
Hale ME (1984b) Flavopunctelia, a new genus in the Parmeliaceae (Ascomycotina). Mycotaxon 20:681–682
Hawksworth DL, Eriksson OE (1986) The names of accepted orders of ascomycetes. Systema Ascomycetum 5:175–184
Harris RC (1990) Some Florida lichens. Author, Bronx, NY
Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB (2001) Molecular evidence for the early colonization of land by fungi and plants. Science 293:1129–1133
Helms G, Friedl T, Rambold G (2003) Phylogenetic relationships of the Physciaceae inferred from rDNA sequence data and selected phenotypic characters. Mycologia 95:1078–1099
Henskens FL, Green TGA, Wilkins A (2012) Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Ann Bot 110:555–563
Henssen A (1976) Studies in the developmental morphology of lichenized Ascomycetes. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. Academic, London, pp 107–138
Henssen A (1981) Hyphomorpha als phycobiont in Flechten. Plant Syst Evol 137:139–143
Henssen A (1985) Hertella, a new lichen genus in the Peltigerales from the Southern Hemisphere. Mycotaxon 22:381–397
Henssen A, Jahns HM (1974) Lichenes. Georg Thieme, Stuttgart
Henssen A, Keuck G, Renner B, Vobis G (1981) The lecanoralean centrum. In: Reynolds DR (ed) Ascomycete systematics. The Luttrellian concept. Springer, New York, Heidelberg, Berlin, pp 138–234
Hertel H (1970) Trapeliaceae—eine neue Flechtenfamilie. Vortr Gesamtgeb Bot 4:171–185
Hertel H, Rambold G (1985) Lecidea sect. Armeniacae: lecideoide Arten der Flechtengattungen Lecanora und Tephromela (Lecanorales). Bot Jarb Syst 107:469–501
Hestmark G (1997) Growth from the centre in an umbilicate lichen. Lichenologist 29:379–383
Hestmark G, Miadlikowska J, Kauff F, Fraker E, Molnar K, Lutzoni F (2011) Single origin and subsequent diversification of central Andean endemic Umbilicaria species. Mycologia 103:45–56
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbsch HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the fungi. Mycol Res 111:509–547
Hodkinson BP, Lendemer JC (2011) The orders of Ostropomycetidae (Lecanoromycetes, Ascomycota): recognition of Sarrameanales and Trapeliales with a request to retain Pertusariales over Agyriales. Phytologia 93:407–412
Hofstetter V, Miadlikowska J, Kauff F, Lutzoni F (2007) Phylogenetic comparison of protein-coding versus ribosomal RNA-coding sequence data: a case study of the Lecanoromycetes (Ascomycota). Mol Phylogenet Evol 44:412–426
Honegger R (1978) The ascus apex in lichenized fungi. I: The Lecanora-, Peltigera and Teloschistes-types. Lichenologist 10:47–67
Honegger R (1980) The ascus apex in lichenized fungi. II: The Rhizocarpon-type. Lichenologist 12:157–172
Honegger R (1982a) Ascus structure and function, ascospore delimitation, and phycobiont cell wall types associated with the Lecanorales (lichenized ascomycetes). J Hattori Bot Lab 52:417–429
Honegger R (1982b) The ascus apex in lichenized fungi. III: The Pertusaria-type. Lichenologist 14:205–217
Honegger R (1983) The ascus apex in lichenized fungi. IV: Baeomyces and Icmadophila in comparison with Cladonia (Lecanorales) and the non-lichenized Leotia (Helotiales). Lichenologist 15:57–71
Honegger R (1984a) Scanning electron microscopy of the contact site of conidia and trichogynes in Cladonia furcata. Lichenologist 16:11–19
Honegger R (1984b) Ultrastructural studies on conidiomata, conidiophores, and conidiogenous cells in six lichen-forming ascomycetes. Can J Bot 62:2081–2093
Honegger R (2008) Morphogenesis. In: Nash TH III (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, pp 69–93
Honegger R, Edwards D, Axe L (2013) The earliest records of internally stratified cyanobacterial and algal lichens from the Lower Devonian of the Welsh Borderland. New Phytol 197:164–275
Huneck S (2001) New results on the chemistry of lichen substances. In: Fortschritte der Chemie organischer Naturstoffe, vol 81. Springer, Vienna
Huneck S, Yoshimura I (1996) Identification of lichen substances. Springer, Berlin, Heidelberg
Ihlen PG, Ekman S (2002) Outline of phylogeny and character evolution in Rhizocarpon (Rhizocarpaceae, lichenized Ascomycota) based on nuclear ITS and mitochondrial SSU ribosomal DNA sequences. Biol J Linn Soc 77:535–546
Jaag O (1933) Coccomyxa Schmidle, Monographie einer Algengattung. Beitr Kryptogamenfl Schweiz 8:1–132
Jahns HM (1970) Untersuchungen zur Entwicklungsgeschichte der Cladoniaceen. Nova Hedwigia 20:1–177
James TY, Kauff F, Schoch C, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch T, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung GH, Johnson D, O’Rourke B, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüßler A, Longcore JE, O’Donnell K, Mozley-Standridge K, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann-Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KW, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Buck WR, Cole MS, Diederich P, Printzen C, Schmitt I, Schultz M, Yahr R, Zavarzin A, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R (2006) Reconstructing the early evolution of the fungi using a six-gene phylogeny. Nature 443:818–822
Janex-Favre MC (1977) Le developpement et la structure des pycnides de l'Umbilicaria cinereorufescens. Rev Bryol Lichenol 43:1–18
Janex-Favre MC (1982) Le developpement et la structure des pycnides du lichen Parmelia acetabulum. Cryptogamie Bryol L 3:337–349
Johnston J (2001) Baeomycetaceae. In: McCarthy PM (ed) Flora of Australia, vol 58A, Lichens 3. ABRS/CSIRO Australia, Melbourne, pp 14–16
Kantvilas G (1996) A new byssoid lichen genus from Tasmania. Lichenologist 28:229–237
Kantvilas G (2004) Sarrameanaceae. In: McCarthy PM, Mallett K (eds) Flora of Australia, vol 56A, Lichens 4. ABRS/CSIRO Australia, Melbourne, pp 74–77
Kantvilas G, Vězda A (1996) The lichen genus Sarrameana. Nord J Bot 16:325–333
Kärnefelt I (1989) Morphology and phylogeny in the Teloschistales. Cryptog Bot 1:147–203
Kärnefelt I, Thell A (1992) The evaluation of characters in lichenized families, exemplified with the alectorioid and some parmelioid genera. Plant Syst Evol 180:181–204
Kauff F, Büdel B (2005) Ascoma ontogeny and apothecial anatomy in the Gyalectaceae (Ostropales, Ascomycota) support the re-establishment of the Coenogoniaceae. Bryologist 108:272–281
Kauff F, Lutzoni F (2002) Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships. Mol Phylogenet Evol 25:138–156
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth & Bisby's dictionary of the fungi, 9th edn. CAB International, Wallingford, UK
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth & Bisby's dictionary of the fungi, 10th edn. CAB International, Wallingford, UK
Krog H (1982) Evolutionary trends in foliose and fruticose lichens of the Parmeliaceae. J Hattori Bot Lab 52:303–311
Kroken S, Taylor JW (2001) A gene genealogical approach to recognize phylogenetic species boundaries in the lichenized fungus Letharia. Mycologia 93:38–53
Lamb IM (1977) A conspectus of the lichen genus Stereocaulon (Schreb.) Hoffm. J Hattori Bot Lab 43:191–355
Lawrey JD (1986) Biological role of lichen substances. Bryologist 89:111–122
Lawrey JD (1989) Lichen secondary compounds: evidence for a correspondence between antiherbivore and antimicrobial function. Bryologist 92:326–328
Lawrey JD, Diederich P (2003) Lichenicolous fungi: interactions, evolution, and biodiversity. Bryologist 106:81–120
Leavitt SD, Johnson LA, Goward T, St Clair LL (2011a) Species delimitation in taxonomically difficult lichen-forming fungi: an example from morphologically and chemically diverse Xanthoparmelia (Parmeliaceae) in North America. Mol Phylogenet Evol 60:317–332
Leavitt SD, Johnson LA, St Clair LL (2011b) Species delimitation and evolution in morphologically and chemically diverse communities of the lichen-forming genus Xanthoparmelia (Parmeliaceae, Ascomycota) in western North America. Am J Bot 98:175–188
Letrouit-Galinou MA (1972) Études sur le Lobaria laetevirens. II: Le développement des pycnides. Bull Soc Bot Fr 119:477–485
Letrouit-Galinou MA (1973a) Les asques des lichens et le type archaeascé. Bryologist 76:30–47
Letrouit-Galinou MA (1973b) Les pycnospores et les pycnides du Gyalecta carneolutea (Turn.) Oliv. Bull Soc Bot Fr 120:373–384
Letrouit-Galinou MA, Bellemère A (1989) Ascomatal development in lichens: a review. Cryptogamie Bryol L 10:189–233
Letrouit-Galinou MA, Lallement R (1977) Le développement des pycnides du discolichen Buellia canescens (Dicks.) D.N. Ann Sci Nat Bot Biol 18:119–134
Lindemuth R, Wirtz N, Lumbsch HT (2001) Phylogenetic analysis of nuclear and mitochondrial rDNA sequences supports the view that loculoascomycetes (Ascomycota) are not monophyletic. Mycol Res 105:1176–1181
Liu YJ, Hall BD (2004) Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc Natl Acad Sci U S A 101:4507–4512
Louwhoff SHJJ (2009) Umbilicariaceae. In: McCarthy PM (ed) Flora of Australia, vol 57, Lichens 5. ABRS and CSIRO Publishing, Canberra, Melbourne, pp 553–562
Lücking R (2008) Foliicolous lichenized fungi, Flora Neotropica Monograph 103. Organization for Flora Neotropica and The New York Botanical Garden Press, Bronx, NY
Lücking R, Stuart BL, Lumbsch HT (2004) Phylogenetic relationships of Gomphillaceae and Asterothyriaceae: evidence from a combined Bayesian analysis of nuclear and mitochondrial sequences. Mycologia 96:283–294
Lücking R, Huhndorf S, Pfister DH, Rivas Plata E, Lumbsch HT (2009a) Fungi evolved right on track. Mycologia 101:810–822
Lücking R, Lawrey JD, Sikaroodi M, Gillevet PM, Chaves JL, Sipman HJM, Bungartz F (2009b) Do lichens domesticate photobionts like farmers domesticate crops? Evidence from a previously unrecognized lineage of filamentous cyanobacteria. Am J Bot 96:1409–1418
Lumbsch HT (1997) Systematic studies in the suborder Agyriineae (Lecanorales). J Hattori Bot Lab 83:1–73
Lumbsch HT (1998a) Taxonomic use of metabolic data in lichen-forming fungi. In: Frisvad JC, Bridge PD, Arora DK (eds) Chemical fungal taxonomy. Marcel Dekker, New York, pp 345–387
Lumbsch HT (1998b) The use of metabolic data in lichenology at the species and subspecific levels. Lichenologist 30:357–367
Lumbsch HT, Huhndorf SM (2007a) Whatever happened to the pyrenomycetes and loculoascomycetes? Mycol Res 111:1064–1074
Lumbsch HT, Huhndorf SM (2007b) Outline of Ascomycota 2007. Myconet 13:1–58
Lumbsch HT, Huhndorf SM (2010) Myconet, vol 14. Part one. Outline of Ascomycota—2009. Part two. Notes on ascomycete systematics. Nos. 4751–5113. Fieldiana Life Earth Sci 1:1–64
Lumbsch HT, Feige GB, Schmitz KE (1994) Systematic studies in the Pertusariales. I: Megasporaceae, a new family of lichenized Ascomycetes. J Hattori Bot Lab 75:295–304
Lumbsch HT, Lunke T, Feige GB, Huneck S (1995) Anamylopsoraceae—a new family of lichenized ascomycetes with stipitate apothecia (Lecanorales: Agyriineae). Plant Syst Evol 198:275–286
Lumbsch HT, Schmitt I, Döring H, Wedin M (2001a) Molecular systematics supports the recognition of an additional order of Ascomycota: the Agyriales (Ascomycota). Mycol Res 105:16–23
Lumbsch HT, Schmitt I, Döring H, Wedin M (2001b) ITS sequence data suggest variability of ascus types and support ontogenetic characters as phylogenetic discriminators in the Agyriales (Ascomycota). Mycol Res 105:265–274
Lumbsch HT, Wirtz N, Lindemuth R, Schmitt I (2002) Higher level phylogenetic relationships of euascomycetes (Pezizomycotina) inferred from a combined analysis of nuclear and mitochondrial sequence data. Mycol Prog 1:57–70
Lumbsch HT, Schmitt I, Palice Z, Wiklund E, Ekman S, Wedin M (2004) Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences. Mol Phylogenet Evol 31:822–832
Lumbsch HT, Del Prado R, Kantvilas G (2005) Gregorella, a new genus to accommodate Moelleropsis humida and a molecular phylogeny of Arctomiaceae. Lichenologist 37:291–302
Lumbsch HT, Archer AW, Elix JA (2007a) A new species of Loxospora (lichenized Ascomycota: Sarrameanaceae) from Australia. Lichenologist 39:509–517
Lumbsch HT, Schmitt I, Lücking R, Wiklund E, Wedin M (2007b) The phylogenetic placement of Ostropales within Lecanoromycetes (Ascomycota) revisited. Mycol Res 111:257–267
Lumbsch HT, Schmitt I, Mangold A, Wedin M (2007c) Ascus types are phylogenetically misleading in Trapeliaceae and Agyriaceae (Ostropomycetidae, Ascomycota). Mycol Res 111:1133–1141
Lumbsch HT, Hipp AL, Divakar PK, Blanco O, Crespo A (2008a) Accelerated evolutionary rates in tropical and oceanic parmelioid lichens (Ascomycota). BMC Evol Biol 8:257
Lumbsch HT, Nelsen MP, Lücking R (2008b) The phylogenetic position of Haematommataceae (Lecanorales, Ascomycota), with notes on secondary chemistry and species delimitation. Nova Hedwigia 86:104–114
Luttrell ES (1951) Taxonomy of pyrenomycetes. University of Missouri Studies 24, Curators of the University of Missouri, Columbia, MO, pp 1–120
Luttrell ES (1955) The ascostromatic Ascomycetes. Mycologia 47:511–532
Lutzoni F, Pagel M (1997) Accelerated evolution as a consequence of transitions to mutualism. Proc Natl Acad Sci U S A 94:11422–11427
Lutzoni F, Pagel M, Reeb V (2001) Major fungal lineages are derived from lichen symbiotic ancestors. Nature 411:937–940
Lutzoni F, Kauff F, Cox C, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O'Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R (2004) Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am J Bot 91:1446–1480
Mägdefrau K (1957) Flechten und Moose in baltischen Bernstein. Ber Deut Bot Ges 70:433–435
Magnusson AH (1936) Acarosporaceae und Thelocarpaceae. In: Zahlbruckner A (ed) Dr. L. Rabenhorst's Kryptogamen-Flora von Deutschland, Österreich und der Schweiz, band 9. Akademische Verlagsgesellschaft M.B.H., Leipzig, pp 1–318
Mangold A, Martín MP, Lücking R, Lumbsch HT (2008) Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 57:476–486
Matsumoto T, Deguchi H (1999) Pycnidial structures and genus concept in the Thelotremataceae. Bryologist 102:86–91
Mattsson JE, Lumbsch HT (1989) The use of the species pair concept in lichen taxonomy. Taxon 38:238–241
Mattsson JE, Wedin M (1999) A re-assessment of the family Alectoriaceae. Lichenologist 31:431–440
McCarthy PM (2003) Catalogue of Australian Lichens. Flora of Australia Supplementary Series, 19, Australian Biological Resources Study, Canberra
Miadlikowska J, Lutzoni F (2004) Phylogenetic classification of Peltigeralean fungi (Peltigerales, Ascomycota) based on ribosomal RNA small and large subunits. Am J Bot 91:449–464
Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lücking R, Hestmark G, Otalora MG, Rauhut A, Büdel B, Scheidegger C, Timdal E, Stenroos S, Brodo IM, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May PF, Schoch C, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98:1088–1103
Millot M, Di Meo F, Tomasi S, Boustie J, Trouillas P (2012) Photoprotective capacities of lichen metabolites: a joint theoretical and experimental study. J Photochem Photobiol B 111:17–26
Möller A (1888) Über die sogenannten Spermatien der Ascomyceten. Bot Zeitung 46:421–425
Muggia L, Nelson P, Wheeler T, Yakovchenko S, Tønsberg T, Spribille T (2011) Convergent evolution of a symbiotic duet: the case of the lichen genus Polychidium (Peltigerales, Ascomycota). Am J Bot 98:1647–1656
Müller J (1880) Lichenologische Beiträge, X. Flora 63:17–24, 40–45
Müller J (1882) Lichenologische Beiträge, XV. Flora 65:291–306, 313–322, 326–337, 381–386, 397–402
Myllys L, Lohtander K, Tehler A (2001) β-Tubulin, ITS and group I intron sequences challenge the species pair concept in Physcia aipolia and P. caesia. Mycologia 93:335–343
Myllys L, Hognabba F, Lohtander K, Thell A, Stenroos S, Hyvonen J (2005) Phylogenetic relationships of Stereocaulaceae based on simultaneous analysis of beta-tubulin, GAPDH and SSU rDNA sequences. Taxon 54:605–618
Nannfeldt JA (1932) Studien über die Morphologie und Systematik der nicht-lichenisierten inoperculaten Discomyceten. Nov Act Reg Soc Sci Nat Cherbourg 3:161–202
Nash TH III (2008) Introduction. In: Nash TH III (ed) Lichen biology. Cambridge University Press, Cambridge, UK, pp 1–8
Nelsen MP, Gargas A (2009) Assessing clonality and chemotype monophyly in Thamnolia (Icmadophilaceae). Bryologist 112:42–53
Nelsen MP, Chavez N, Sackett-Hermann E, Thell A, Randlane T, Divakar PK, Rico VJ, Lumbsch HT (2011) The cetrarioid core group revisited (Lecanorales: Parmeliaceae). Lichenologist 43:537–551
Nimis PL (1998) A critical appraisal of modern generic concepts in lichenology. Lichenologist 30:427–438
Øvstedal DO, Lewis Smith RI (2001) Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Studies in polar research. Cambridge University Press, Cambridge, UK
Padovan AC, Sanson GF, Brunstein A, Briones MR (2005) Fungi evolution revisited: application of the penalized likelihood method to a Bayesian fungal phylogeny provides a new perspective on phylogenetic relationships and divergence dates of Ascomycota groups. J Mol Evol 60:726–735
Palmqvist K, Dahlman L, Jonsson A, Nash TH III (2008) The carbon economy of lichens. In: Nash TH III (ed) Lichen biology, 2nd edn. Cambridge University Press, Cambridge, UK, pp 182–215
Pérez FL (1997) Geoecology of erratic lichens of Xanthoparmelia vagans in an equatorial Andean paramo. Plant Ecol 129:11–28
Platt JL, Spatafora JW (1999) A re-examination of generic concepts of baeomycetoid lichens based on phylogenetic analyses of nuclear SSU and LSU ribosomal DNA. Lichenologist 31:409–418
Plessl A (1963) Über die Beziehungen von Haustorienstypus and Organisationshöhe bei Flechten. Österr Bot Z 110:194–269
Poelt J (1970) Das Konzept der Artenpaare bei den Flechten. Vortr Gesamtgeb Bot 4:187–198
Poelt J (1972) Die taxonomische Behandlung von Artenpaaren bei den Flechten. Bot Not 125:77–81
Poelt J (1973) Systematic evaluation of morphological characters. In: Ahmadjian V, Hale ME (eds) The lichens. Academic, New York, London, pp 91–115, and 599–632
Poelt J (1987) On reductions of morphological structures in lichens. In: Peveling E (ed) Progress and problems in lichenology in the eighties, vol 25, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 35–45
Poinar GO, Peterson EB Jr, Platt JL (2000) Fossil Parmelia in new world amber. Lichenologist 32:263–269
Poulsen RS, Schmitt I, Søchting U, Lumbsch HT (2001) Molecular and morphological studies on the subantarctic genus Orceolina (Agyriaceae). Lichenologist 33:323–329
Printzen C (2010) Lichen systematics: the role of morphological and molecular data to reconstruct phylogenetic relationships. Prog Bot 71:233–278
Rambold G, Triebel D (1992) The inter-lecanoralean associations, vol 48, Bibliotheca Lichenologica. J Cramer, Berlin
Rambold G, Triebel D, Hertel H (1993) Icmadophilaceae, a new family in the Leotiales. In: Feige GB, Lumbsch HT (eds) Phytochemistry and chemotaxonomy of lichenized Ascomycetes—a festschrift in honour of Siegfried Huneck, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 217–240
Rambold G, Friedl T, Beck A (1998) Photobionts in lichens: possible indicators of phylogenetic relationships? Bryologist 101:392–397
Reeb V, Lutzoni F, Roux C (2004) Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phylogenet Evol 32:1036–1060
Reynolds DR (1989) The bitunicate ascus paradigm. Bot Rev 55:1–52
Rikkinen J (2002) Cyanolichens: an evolutionary overview. In: Rain AN, Bergman B, Rasmussen U (eds) Cyanobacteria in symbiosis. Kluwer, Netherlands, pp 31–72
Rikkinen J, Poinar GO (2002) Fossilised Anzia (Lecanorales, lichen-forming Ascomycota) from European Tertiary amber. Mycol Res 106:984–990
Rivas Plata E, Lumbsch HT (2011) Parallel evolution and phenotypic disparity in lichenized fungi: a case study in the lichen-forming fungal family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales). Mol Phylogenet Evol 61:45–63
Rivas Plata E, Lücking R, Lumbsch HT (2012) A new classification for the family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales). Fungal Divers 52:107–121
Roux C, Bellemère A, Boissière JC, Esnault J, Janex-Favre MC, Letrouit MA, Wagner J (1986) Les bases de la systématique moderne des lichens. Bull Soc Bot France, Actual Bot 133:7–40
Rosentreter R (1993) Vagrant lichens in North America. Bryologist 96:333–338
Santesson R (1952) Foliicolous lichens. I: A revision of the taxonomy of the obligately foliicolous, lichenized fungi. Symb Bot Upsal 12:1–590
Scheidegger C (1985) Systematische Studien zur Krustenflechte Anzina carneonivea (Trapeliaceae, Lecanorales). Nova Hedwigia 41:191–218
Schmitt I, Lumbsch HT (2004) Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol Phylogenet Evol 33:43–55
Schmitt I, Messuti MI, Feige GB, Lumbsch HT (2001) Molecular data support rejection of the generic concept in the Coccotremataceae (Ascomycota). Lichenologist 33:315–321
Schmitt I, Lumbsch HT, Sochting U (2003) Phylogeny of the lichen genus Placopsis and its allies based on Bayesian analyses of nuclear and mitochondrial sequences. Mycologia 95:827–835
Schmitt I, Mueller G, Lumbsch HT (2005) Ascoma morphology is homoplaseous and phylogenetically misleading in some pyrenocarpous lichens. Mycologia 97:362–374
Schmitt I, Yamamoto Y, Lumbsch HT (2006) Phylogeny of Pertusariales (Ascomycotina): resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. J Hattori Bot Lab 100:753–764
Schmitt I, del Prado R, Grube M, Lumbsch HT (2009) Repeated evolution of closed fruiting bodies is linked to ascoma development in the largest group of lichenized fungi (Lecanoromycetes, Ascomycota). Mol Phylogenet Evol 52:34–44
Schmitt I, Frankhauser JD, Sweeney K, Spribille T, Kalb K, Lumbsch HT (2010) Gyalectoid Pertusaria species form a sister-clade to Coccotrema (Ostropomycetidae, Ascomycota) and comprise the new lichen genus Gyalectaria. Mycology 1:75–83
Schmitt I, Otte J, Parnmen S, Sadowska-Des AD, Lücking R, Lumbsch HT (2012) A new circumscription of the genus Varicellaria (Pertusariales, Ascomycota). MycoKeys 4:23–26
Schmull M, Miadlikowska J, Pelzer M, Stocker-Wörgötter E, Hofstetter V, Fraker E, Hodkinson BP, Reeb V, Kukwa M, Lumbsch HT, Kauff F, Lutzoni F (2011) Phylogenetic affiliations of members of the heterogeneous lichen-forming fungi of the genus Lecidea sensu Zahlbruckner (Lecanoromycetes, Ascomycota). Mycologia 103:983–1003
Schoch C, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny B, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, De Hoog GS, Crous PW, Hewitt D, Pfister D, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman A, Lumbsch HT, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O'Donnell K, Sipman H, Rogers JD, Shoemaker R, Sugiyama J, Summerbell RC, Untereiner WA, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Sherwood MA (1977a) The ostropalean fungi. Mycotaxon 5:1–277
Sherwood MA (1977b) The ostropalean fungi. II: Schizoxylon, with notes on Stictis, Acarosporina, Coccopeziza, and Carestiella. Mycotaxon 6:215–260
Sherwood-Pike MA (1987) The ostropalean fungi. III: The Odontotremataceae. Mycotaxon 28:137–177
Sipman HJM, Aptroot A (2001) Where are the missing lichens? Mycol Res 105:1433–1439
Smith CW, Aptroot A, Coppins BJ, Fletcher A, Gilbert OL, James PW, Wolseley PA (2009) The lichens of Great Britain and Ireland. British Lichen Society, The Natural History Museum, London
Smith RM, Thompson K, Warren PH, Gaston KJ (2010) Urban domestic gardens (XIII): composition of the bryophyte and lichen floras, and determinants of species richness. Biol Conserv 143:873–882
Solhaug KA, Gauslaa Y, Nybakken L, Bilger W (2003) UV-induction of sun-screening pigments in lichens. New Phytol 158:91–100
Spatafora J, Sung GH, Johnson D, Hesse C, O'Rourke B, Serdani M, Spotts R, Lutzoni F, Hofstetter V, Miadlikowska J, Reeb V, Gueidan C, Fraker E, Lumbsch T, Lücking R, Schmitt I, Hosaka K, Aptroot A, Roux C, Miller AN, Geiser DM, Hafellner J, Hestmark G, Arnold AE, Büdel B, Rauhut A, Hewitt D, Untereiner WA, Cole MS, Scheidegger C, Schultz M, Sipman H, Schoch CL (2006) A five-gene phylogeny of Pezizomycotina. Mycologia 98:1018–1028
Spribille T, Muggia L (2013) Expanded taxon sampling disentangles evolutionary relationships and reveals a new family in Peltigerales (Lecanoromycetidae, Ascomycota). Fungal Divers 58:171–184
Staiger B, Kalb K (1995) Haematomma-studien. I. Die Flechtengattung Haematomma, vol 59, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 1–198
Staiger B, Kalb K (1999) Acanthothecis and other graphidioid lichens with warty periphysoids or paraphysis-tips. Mycotaxon 73:69–134
Staiger B, Kalb K, Grube M (2006) Phylogeny and phenotypic variation in the lichen family Graphidaceae (Ostropomycetidae, Ascomycota). Mycol Res 110:765–772
Steiner J (1901) Über die Function und den systematischen Wert der Pycnoconidien der Flechten. In: Festschrift zur Feier des zweihunderjährigen Bestandes des K.K. Staatsgymnasiums im VIII. Bezirke Wiens, 1901. Kainz & Liebhart, Wien, pp 119–154
Stenroos SK, DePriest PT (1998) SSU rDNA phylogeny of cladoniiform lichens. Am J Bot 85:1548–1559
Taylor JW, Berbee ML (2006) Dating divergences in the Fungal Tree of Life: review and new analyses. Mycologia 98:838–849
Taylor JW, Berbee ML (2010) Dating the molecular clock in fungi—how close are we? Fungal Biol Rev 24:1–16
Tehler A (1982) The species pair concept in lichenology. Taxon 31:708–717
Tehler A (1996) Systematics, phylogeny and classification. In: Nash TH III (ed) Lichen biology. Cambridge University Press, Cambridge, UK, pp 217–239
Tehler A, Källersjö M (2001) Parmeliopsis ambigua and P. hyperopta (Parmeliaceae): species or chemotypes? Lichenologist 33:403–408
Thell A, Stenroos S, Feuerer T, Kärnefelt I, Myllys L, Hyvönen J (2002) Phylogeny of cetrarioid lichens (Parmeliaceae) inferred from ITS and b-tubulin sequences, morphology, anatomy and secondary chemistry. Myc Prog 1:335–354
Thüs H, Schultz M (2009) Fungi, part 1: Lichens. In: Büdel B, Gärtner G, Krienitz L, Preisig HR, Schagerl M (eds) Freshwater flora of Central Europe. Spektrum Akademischer Verlag, Heidelberg, Germany
Tibell L (1984) A reappraisal of the taxonomy of Caliciales. In: Hertel H, Oberwinkler F (eds) Beitrage zur Lichenologie. Festschrift J. Poelt, vol 79, Beiheft zur Nova Hedwigia. J. Cramer, Vaduz, pp 597–713
Tibell L (1996) Caliciales. In: Flora Neotropica, vol 69. New York Botanical Garden, Bronx, NY
Timdal E (1987) Problems of generic delimitation among squamiform members of the Lecideaceae. In: Peveling E (ed) Progress and problems in lichenology in the eighties, vol 25, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 243–247
Timdal E (1992) A monograph of the genus Toninia (Lecideaceae, Ascomycetes). Opera Bot 110:1–137
Tschermak-Woess E (1953) Über wenig bekannte und neue Flechtengonidien III. Die Entwicklungsgeschichte von Leptosira thrombii nov. spec., der Gonidie von Thrombium epigaeum. Österr Bot Z 100:203–216
Tschermak-Woess E (1984) Über die weite Verbreitung lichenisierter Sippen von Dictyochloropsis und die systematische Stellung von Myrmecia reticulata (Chlorophyta). Plant Syst Evol 147:299–322
Tschermak-Woess E (1985) Elliptochloris bilobata kein ganz seltner Phycobiont. Herzogia 7:105–116
Tschermak-Woess E (1988a) The algal partner. In: Galun M (ed) CRC handbook of lichenology, vol I. CRC Press, Boca Raton, FL, pp 39–92
Tschermak-Woess E (1988b) New and known taxa of Chlorella (Chlorophyceae): occurrence as lichen phycobionts and observations on living dictyosomes. Plant Syst Evol 159:123–139
Tschermak-Woess E, Poelt J (1976) Vezdaea, a peculiar lichen genus, and its phycobiont. In: Brown DH, Hawksworth DL, Bailey RH (eds) Lichenology: progress and problems. Academic, London, pp 89–105
Vainio EA (1890) Étude sur la classification naturelle et la morphologie des lichens du Brésil. Acta Societatis Pro Fauna et Flora Fennica, vol 7. Héritiers J. Simelius, Helsingfors
Velmala S, Myllys L, Halonen P, Goward T, Ahti T (2009) Molecular data show that Bryoria fremontii and B. tortuosa (Parmeliaceae) are conspecific. Lichenologist 41:231–242
Vězda A, James PW (1973) Sarrameana paradoxa A. Vězda et P. James gen. nov et sp. nova, eine bemerkenswerte Flechte aus Neu-Kaledonien. Preslia 45:305–310
Vězda A, Kantvilas G (1988) Sarrameana tasmanica, a new Tasmanian lichen. Lichenologist 20:179–182
Vobis G (1977) Studies on the germination of lichen conidia. Lichenologist 9:131–136
Vobis G (1980) Bau und Entwicklung der Flechten-Pycnidien und ihrer Conidien, vol 14, Bibliotheca Lichenologica. J. Cramer, Vaduz
Wedin M, Tibell L (1997) Phylogeny and evolution of Caliciaceae, Mycocaliciaceae, and Sphinctrinaceae (Ascomycota), with notes on the evolution of the prototunicate ascus. Can J Bot 75:1236–1242
Wedin M, Wiklund E (2004) The phylogenetic relationships of Lecanorales suborder Peltigerineae revisited. Symb Bot Upsal 34:469–475
Wedin M, Döring H, Mattsson JE (1999) A multi-gene study of the phylogenetic relationships of the Parmeliaceae. Mycol Res 103:1185–1192
Wedin M, Döring H, Nordin A, Tibell L (2000a) Small subunit rDNA phylogeny shows the lichen families Caliciaceae and Physciaceae (Lecanorales, Ascomycotina) to form a monophyletic group. Can J Bot 78:246–254
Wedin M, Döring H, Ekman S (2000b) Molecular phylogeny of the lichen families Cladoniaceae, Sphaerophoraceae, and Stereocaulaceae (Lecanorales, Ascomycotina). Lichenologist 32:171–187
Wedin M, Doring H, Gilenstam G (2004) Saprotrophy and lichenization as options for the same fungalspecies on different substrata: environmental plasticity and fungal lifestyles in the Stictis-Conotrema complex. New Phytol 164:459–465
Wedin M, Wiklund E, Crewe A, Döring H, Ekman S, Nyberg Å, Schmitt I, Lumbsch HT (2005) Phylogenetic relationships of Lecanoromycetes (Ascomycota) as revealed by analyses of mtSSU and nLSU rDNA sequence data. Myc Res 109:159–172
Wedin M, Jørgensen PM, Wiklund E (2007) Massalongiaceae fam. nov., an overlooked monophyletic group among the cyanobacterial lichens (Peltigerales, Lecanoromycetes, Ascomycota). Lichenologist 39:61–67
Wedin M, Wiklund E, Jørgensen PM, Ekman S (2009) Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Mol Phylogenet Evol 53:862–871
Wedin M, Jørgensen PM, Ekman S (2011) Vahliellaceae, a new family of cyanobacterial lichens (Peltigerales, Ascomycetes). Lichenologist 43:67–72
Westberg M, Arup U, Kärnefelt I (2007) Phylogenetic studies in the Candelariaceae (lichenized Ascomycota) based on nuclear ITS DNA sequence data. Myc Res 111:1277–1284
Westberg M, Frödén P, Wedin M (2009) A monograph of the genus Placomaronea (Ascomycota, Candelariales). Lichenologist 41:513–527
Widhelm T, Lumbsch HT (2011) The phylogenetic placement of Miltideaceae inferred from ribosomal DNA sequence data. In: Bates ST, Bungartz F, Lücking R, Herrera-Campos MA, Zambrano A (eds) Biomonitoring, ecology, and systematics of lichens. Festschrift Thomas H. Nash III, vol 106, Bibliotheca Lichenologica. J. Cramer, Berlin, Stuttgart, pp 365–373
Winka K, Ahlberg C, Eriksson OE (1998) Are there lichenized Ostropales? Lichenologist 30:455–462
Wooolfit M, Bromham L (2003) Inceased rates of sequence evolution in endosymbiotic bacteria and fungi with small effective population size. Mol Biol Evol 20:1545–1555
Zahlbruckner A (1903–1907) Lichenes. B. Spezieller Teil. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol I(1). Engelmann, Leipzig, pp 49–249
Zahlbruckner A (1926) Lichenes. B. Specieller Teil. In: Engler A (ed) Die Natürlichen Pflanzenfamilien, 8, 2nd edn. Engelmann, Leipzig, pp 61–270
Zhou QM, Wei JC (2006) A new genus and species Rhizoplacopsis weichingii in a new family Rhizoplacopsidaceae (Ascomycota). Mycosystema 25:376–385
Zhou QM, Wei JC (2007) A new order Umbilicariales J.C. Wei & Q.M. Zhou (Ascomycota). Mycosystema 26:40–45
Zoller S, Lutzoni F (2003) Slow algae, fast fungi: exceptionally high nucleotide substitution rate differences between lichenized fungi Omphalina and their symbiotic green algae Coccomyxa. Mol Phylogenet Evol 29:629–640
Acknowledgments
Thanks to Armando Mendez and Andrea Hart (Botany Library, Natural History Museum) for their help in locating and providing the required literature.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gueidan, C., Hill, D.J., Miadlikowska, J., Lutzoni, F. (2015). 4 Pezizomycotina: Lecanoromycetes. In: McLaughlin, D., Spatafora, J. (eds) Systematics and Evolution. The Mycota, vol 7B. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-46011-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-662-46011-5_4
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-46010-8
Online ISBN: 978-3-662-46011-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)