Abstract
Kaposi’s sarcoma (KS) is a multifocal tumor of endothelial cell origin associated with human herpesvirus 8 (HHV-8) infection. Four epidemiological and clinical variants are described: (1) classic KS, (2) endemic African KS, (3) KS in therapeutically immunosuppressed patients, and (4) acquired immunodeficiency syndrome (AIDS)-associated KS. The diagnosis of KS is based on the clinical picture and laboratory investigations (histopathology, serological immune tests). Treatment for KS with localized skin lesions includes surgical excision, local destruction (liquid nitrogen, laser, photodynamic therapy, etc.), and radiotherapy. For patients with aggressive disease, the recommendations include liposomal anthracyclines or alternatively different types of mono- or combination chemotherapies including vinblastine or doxorubicin, bleomycin, vincristine, etoposide, and dacarbazine. In AIDS-associated KS in addition, highly active antiretroviral therapies (HAART) need to be initiated. In advanced, nonresponsive KS, the HAART regimen is supplemented with paclitaxel. In organ transplant patients, the immunosuppressive therapy needs reevaluation – a switch to rapamycin has been shown to result in regression of KS. Despite the implication of HHV-8 in KS pathogenesis, anti-herpetic drugs have no effect in KS treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Kaposi’s sarcoma
- HHV-8 infection
- Surgical excision
- Local destruction
- Highly active antiretroviral therapies (HAART)
- Liposomal anthracyclines
- Rapamycin
- Paclitaxel
-
Kaposi’s sarcoma (KS) is a multifocal tumor of endothelial cell origin which occurs in four clinical variants: (1) classic KS, (2) endemic African KS, (3) KS in therapeutically immunosuppressed patients, and (4) AIDS-associated KS.
-
The diagnosis is established by the clinical aspect, histopathology, and demonstration of HHV-8 in tumor lesions.
-
The therapeutic approach is dependent on the clinical variant and on the extent of the lesions and comprises local destructive measures or systemic chemotherapies.
-
Localized cutaneous KS treatment includes surgical excision or lesion destruction with radiotherapy, liquid nitrogen, laser, photodynamic therapy, and topical therapy with 9-cis-retinoic acid.
-
AIDS-associated KS responds to liposomal anthracyclines: liposomal doxorubicin or liposomal daunorubicin. KS in organ transplant patients benefits from replacement of calcineurin inhibitors with rapamycin in their immunosuppressive regimen.
-
Classic KS with widespread skin lesions responds to chemotherapeutic drugs (doxorubicin, bleomycin, vincristine, etoposide, dacarbazine) as well as to liposomal anthracyclines.
-
In AIDS patients, it is pivotal to combine KS therapy with antiretroviral therapies.
Definition and Epidemiology
In 1872, the Austro-Hungarian dermatologist Moritz Kaposi described five patients with multicentric cutaneous and extracutaneous neoplasms that primarily affected older individuals. Kaposi’s sarcoma (KS) was originally described as “idiopathic multiple pigmented sarcoma” and was later named after its first describer. Based on distinct epidemiological features and clinical evolution, there are four clinical variants of KS. All of those variants have comparable histopathology, and in all of them the human herpesvirus type 8 (HHV-8), also known as Kaposi’s sarcoma–associated herpesvirus, plays a causative role.
Clinical Presentation
Classic KS
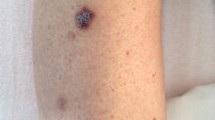
Classic KS preferentially occurs in Caucasians over 60 years of age with a Mediterranean or Jewish background. The male to female ratio is 3:1–5:1. Classic KS lesions manifest on the skin as unilateral or bilateral violaceous (hematoma-like) macules and plaques with acral disposition, frequently on the distal portions of the lower extremities (Fig. 46.1). These lesions progress slowly into coalescent firm plaques and nodules surrounded by pitting edema (Fig. 46.2) and, subsequently, into nodules, while additional lesions start to arise in the surroundings or at distant sites. This clinical variant is usually slowly progressive, and patients may live with slowly progressive disease for many years. However, also in classic KS, ultimately dissemination to other body sites such as lymph nodes, mucous membranes, and inner organs, in particular the gastrointestinal tract, may occur. Since classic KS mostly affects elderly patients, death from other causes may precede its full spread.
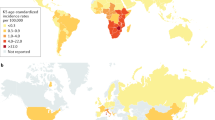
The higher incidence of KS in countries surrounding the Mediterranean Sea has led some authors to speak of a Mediterranean KS variant as a separate entity. Since the clinical course and the epidemiology are comparable to the classic KS, the authors prefer to not separate classic and Mediterranean KS.
African Endemic Kaposi’s Sarcoma
An increased frequency of Kaposi’s sarcoma in black Africans in Central Africa was reported already in the 1950s. This clinical form is more prevalent in male patients, with ratios ranging from 3:1 in children up to 18:1 in adults. In some Equatorial African countries, endemic KS comprises up to 10 % of all malignancies in men. The age at onset is lower than in classic KS, with a peak at 25–39 years in women and 35–39 years in men. There are four clinical variants of the African endemic KS: nodular, florid, infiltrative, and lymphadenopathic. The florid and infiltrative variants are showing an aggressive behavior, and the lesions may extend deeply into the muscle and bone. The lymphadenopathic variant is mainly seen in children and young adults and may rapidly progress.
Kaposi’s Sarcoma in Patients Receiving Immunosuppressive Therapies
After organ transplantation had become a routine procedure in the 1970s, a high incidence of KS was reported for organ-transplant recipients undergoing immunosuppressive therapy. The occurrence of KS has been reported, less frequently, in patients who receive therapeutic immunosuppression for other reasons such as the treatment of autoimmune diseases. Transplantation-associated KS is observed predominantly in kidney allograft recipients, while in recipients of other solid organs and bone marrow allografts, it is rarely seen. An important risk factor for KS development is the dose and type of the immunosuppressive drug. The risk in association with cyclosporin A is higher than with glucocorticoids and azathioprine, and the disease onset is earlier. Discontinuation of immunosuppressive therapy was followed in some cases by the regression of KS lesions.
The clinical course of KS in therapeutically immunosuppressed patients may be slowly progressing like in the classic variant or rapidly disseminating like in AIDS-associated KS. Skin lesions are present in more than 85 % of the patients with transplantation-associated KS, while the remaining 15 % of patients have visceral disease without skin involvement.
AIDS-Associated KS
Rapidly progressing, multifocal KS occurring in young homosexual men was one of the diseases which alerted health authorities in the USA on the advent of a new disease, i.e., the acquired immunodeficiency syndrome (AIDS). KS is considered an AIDS-defining illness. At the beginning of the AIDS pandemic, KS was seen in more than 20 % of HIV-1–infected patients. In recent years, it is in a strong decline due to efficient anti-HIV drugs. AIDS-associated KS is more frequent in homosexual or bisexual men and less common in injection drug users or transfusion recipients. In Europe and the USA, the AIDS-associated KS is seen mostly in males, whereas in Africa, it is encountered in high numbers in HIV-1–infected women and children.
AIDS-associated KS evolves with multifocal dissemination and undergoes a more rapid course than classic KS. The KS lesions in AIDS are asymptomatic, are elliptical, and occur along the skin tension lines, frequently on the face (nose, eyelids, ears) and on the trunk (Fig. 46.3). Over time, KS lesions may unite to form large plaques that can interfere with the normal movements of the limbs. Mucosal lesions occur early in the disease. Hard palate (Fig. 46.4), gingival, and pharyngeal lesions may easily ulcerate and result in difficulties of eating, speaking, and breathing.
AIDS-associated KS involves rapidly all visceral sites. Tumors are frequently found in lymph nodes, gastrointestinal tract, lungs, liver, pancreas, heart, bone marrow, etc. Involvement of the gastrointestinal tract may cause severe clinical symptoms including extensive bleeding, ileus, malabsorption, nausea, vomiting, and abdominal pain. Pulmonary involvement can be manifest with coughing, chest pain, hemoptysis, bronchospasm, and progressive respiratory insufficiency.
Diagnosis of KS
The diagnosis of KS is based on the clinical aspect and confirmed by the histological examination. To assess the dissemination of KS and the coexistence of other diseases, laboratory and imaging studies are needed.
Histopathology is similar for the different clinical forms and depends on the stage of KS lesions. Early patch lesions show a discrete increase in dermal vessels, which may appear slightly irregular and may form slits and clefts in the papillary dermis. The dermis also shows hemosiderin deposits and extravasated erythrocytes. In the plaque stage, extensive vascular proliferation can be seen at all levels of the dermis with dilated and angulated vascular spaces dissecting the collagen and leaving a spongy network. At this stage, solid cords and fascicles of spindle cells arranged between the vascular channels may be already present. On further progression to the nodular stage, bundles of spindle cells delineating irregular slit-like vascular spaces without endothelial linings become predominant. In these advanced lesions, pronounced pleomorphism, nuclear atypia, and mitotic figures are frequently present. In all stages of the KS lesions, a moderate inflammatory infiltrate consisting of lymphocytes, histiocytes, plasma cells, and, sporadically, neutrophils is regularly present. On immunophenotyping KS tumor cells are vWF+/−, PAL-E−, CD31+, CD34+, andVEGFR3+ which characterizes them as part of the lymphatic endothelial differentiation lineage.
Laboratory studies include a complete blood count (CBC) and blood chemistry tests together with screening for sexually transmitted infections including HIV tests. In HIV-positive patients, CD4 T-lymphocyte count and plasma HIV viral-load studies should be performed.
Imaging Studies
-
Conventional radiology of the chest is nonspecific in case of pulmonary KS showing diffuse reticulonodular infiltrates, lymphadenopathy, pulmonary nodules, and pleural effusion.
-
Computerized tomography (CT) of the thorax can also identify nonspecific lesions in pulmonary KS: nodules (poorly defined), bronchovascular infiltrates, lymphadenopathy, or pleural effusions. Contrast CT scan can detect liver KS nodules.
-
Magnetic resonance imaging (MRI) can identify KS lesions with better contrast resolution than CT. MRI findings can be suggestive for pulmonary KS and for cardiac or bone involvement.
-
99Tc scintigraphy (planer or single photon emission computerized tomography (SPECT)) can detect KS lesions and can be used in combination with ultrasound to indentify KS lymph node involvement or with 67Ga scintigraphy to differentiate lung infection.
Medical procedures can be used to identify KS lesions in internal organs and take tissue samples for histopathologic examination: bronchoscopy, upper endoscopy, or colonoscopy.
KS and Human Herpesvirus Type 8 (HHV-8)
Epidemiological data and electron microscopic studies had suggested the involvement of an infectious agent in the etiology of KS for a long time. Finally, in 1994 Chang and colleague set the stage for a viral cause of KS when they discovered and characterized a novel human herpesvirus: HHV-8. This virus is present in all KS variants. HHV-8 is a member of the γ-Herpesviridae subfamily, genus Rhadinovirus. Several of the HHV-8 regulatory genes are homologous to human genes involved in regulation of apoptosis, cell proliferation, and angiogenesis, and some of them have been shown to transform cells. It is therefore generally accepted that HHV-8 is a transforming herpesvirus and plays a direct role in the development of KS. In addition to KS, HHV-8 has been implicated in the pathogenesis of pleural effusion lymphoma, a rare B cell neoplasm seen primarily in AIDS patients, and in multicentric Castleman’s disease. The routes of HHV-8 transmission are not yet entirely known. Epidemiological studies have confirmed that sexual transmission occurs during oral-genital and oral-anal contacts. In areas where the virus is highly prevalent, such as in Mediterranean countries and Africa, nonsexual transmission is possible as the HHV-8 can be shed in saliva.
The prevalence of HHV-8 is largely overlapping with that of KS. In regions of low KS incidence such as the USA and Northern Europe, HHV-8 prevalence is below 0.1 %, whereas in regions with high KS incidence such as in Southern Italy and Central Africa, HHV-8 prevalence reaches 20 and 70 %, respectively. In HIV-1–infected patients in the USA and Northern Europe, HHV-8 prevalence is highest in homosexual and bisexual men and reflects the incidence of KS in this risk group.
Differential Diagnosis
The violaceous lesions of KS can be sometimes misdiagnosed, mainly in patients with few skin or mucosal lesions:
-
Bacillary angiomatosis
-
Pseudo-Kaposi’s sarcoma (acral angiodermatitis)
-
Blue rubber bleb nevus syndrome
-
Pyogenic granuloma
-
Hematoma
-
Tufted angioma
-
Melanocytic nevi
-
Melanoma
-
Angiokeratoma
-
Stewart-Treves syndrome
-
Nodal angiomatosis
-
Glomus tumor
-
Lymphangioma
-
Arteriovenous malformations (pseudo-KS)
-
Severe stasis dermatitis (pseudo-KS)
-
Skin metastasis of other tumors (e.g., renal cell carcinoma)
-
Erythema elevatum et diutinum
-
Insect bites
General Principles of Treatment
The treatment of KS depends on the clinical variant, on the extent of the skin lesions, and on the internal organ involvement (Table 46.1). Despite the presence of the oncogenic herpesvirus HHV-8, the treatment with modern anti-herpetic drugs did not prove to be effective.
Localized Cutaneous Disease
Regardless of the clinical variant, if a patient presents with limited disease confined to the skin, local therapies aimed for the destruction of individual lesions are recommended as first-line treatment. These include surgical excision or local destruction with liquid nitrogen, laser or photodynamic therapy, and topical therapy with 9-cis-retinoic acid. Radiotherapy can be used at body sites difficult to reach by other local therapies such as on the nose and in the oral mucosa. Radiation therapy may be given as low-voltage (100 kV) photons or electron-beam radiotherapy. For localized skin lesions, electron beam therapy can be used once weekly in 4 Gy fractions. In patients with widespread skin involvement, extended-field electron beam radiation therapy was reported to be effective. The treatment is maintained for 6–8 weeks/cure.
Although recurrence rates are high, local therapies might be satisfactory for years in slowly progressing classic KS. By contrast, in the other clinical variants, it is necessary that additional measures such as change of the immunosuppressive therapy in transplantation-associated KS and initiation of HAART in patients with AIDS-associated KS.
Progressive Cutaneous Diseases and Involvement of Other Organs
AIDS-Associated KS. The advent of highly active antiretroviral therapies (HAART) had led to a decrease of AIDS-associated KS incidence, and remission of early KS lesions occurred after initiating HAART. However, advanced KS cannot be controlled or reversed by HAART but requires chemotherapy. Among several chemotherapeutic regimens used in the past decades, liposomal anthracyclines emerged as more efficient and better tolerated than the combination therapies of bleomycin with vincristine or both combined with doxorubicin. Liposomal doxorubicin is an anthracycline antibiotic liposome encapsulated with polyethylene glycol coating that has a preferential concentration in the skin. Doxorubicin inhibits DNA and RNA synthesis by intercalating between DNA base pairs. Liposomal doxorubicin is recommended for the treatment of AIDS-related KS in IV administration: 20 mg/m2once every 2–3 weeks. The treatment continues as long as the patient is responding and tolerating it. The doses need adjustments in case of hepatic impairment. Bone marrow suppression may occur during the treatment, and CBC monitoring is recommended before each drug administration. Leukopenia is usually transient. Neutropenia may result in superinfection and hemorrhage may occur due to thrombocytopenia. Doxorubicin may cause cumulative, dose-related myocardial toxicity that may lead to congestive heart failure as the cumulative dose of liposomal doxorubicin approaches 550 mg/m2. Left ventricular ejection fraction is used to evaluate the cardiac function prior to treatment and periodically during treatment. Other adverse reactions include peripheral edema, fever, palmar-plantar erythrodysesthesia (hand-foot syndrome), transient alopecia, nausea, and stomatitis. Doxorubicin may potentiate the toxicity of cyclophosphamide and mercaptopurine, may diminish the therapeutic effect of inactivated vaccines, and may enhance the adverse effect of live vaccines. Liposomal doxorubicin may diminish the therapeutic effect and enhance the adverse effect of zidovudine. The adverse effects of liposomal doxorubicin may be enhanced by taxane derivatives or tacrolimus.
Daunorubicin is another anthracycline antibiotic with antineoplastic activity, first obtained from Streptomyces peucetius. Cell structure studies have demonstrated the drug rapid cell penetration and perinuclear chromatin binding, rapid inhibition of mitotic activity and nucleic acid synthesis, and induction of mutagenesis and chromosomal aberrations. Encapsulating daunorubicin in liposomes maximizes the drug selectivity for solid tumors. Liposomal daunorubicin is indicated as a first-line cytotoxic therapy for advanced HIV-associated KS at a dose of 40 mg/m2administered IV at 2 weeks’ interval. Treatment is maintained as long as KS lesions are not progressing (over 25 % new or evolving lesions) or HIV disease complications do not impose the discontinuation. Due to the liposomal daunorubicin myelosuppressive effect, CBC monitoring is performed before each administration, and therapy is postponed when the total neutrophil count is less than 750 cells/mm3. Other side effects include cardiac toxicity (cardiomyopathy), back pain, flushing, chest tightness, and temporary hair loss. Echocardiographic monitoring is performed at a total cumulative dose of liposomal daunorubicin of 320 mg/m2 and afterward at every 160 mg/m2.
For patients with anthracycline-refractory AIDS-associated KS, paclitaxel has been recently advocated as potential rescue therapy. It is important to keep in mind that HAART needs to be continued during chemotherapy of KS in HIV-infected patients. Interferon alpha which was the most frequently used drug for AIDS-associated KS treatment during the 1980s and early 1990s still holds some promise for AIDS patients with early disseminated KS who simultaneously receive highly active antiretroviral therapy.
KS in organ transplant patients may respond well to a reduction or change of the immunosuppressive therapy. Replacement of calcineurin inhibitors by rapamycin may lead to dramatic therapeutic effects with complete remission of advanced KS lesions. This effect seems to be not only due to the modulation of the immune response by the change of therapy but also to direct antitumor effects of rapamycin.
Classic KS. Chemotherapeutic drugs including doxorubicin, bleomycin, vincristine, etoposide, and dacarbazine alone or in combination had been administered also to patients with progressive or widespread classic KS in particular in those with involvement of inner organs. However, large prospective studies are not available. Data from the literature (Martin-Carbonero, 2004) as well as the authors’ experience shows that liposomal anthracyclines are efficient as in the AIDS-associated KS.
Flow Diagram for KS treatment (Table 46.2).
Further Reading
Aoki Y, Tosato G. Therapeutic options for human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus-related disorders. Expert Rev Anti-Infect Ther. 2004;2:213–25.
Brambilla L, Boneschi V, De Blasio A, Chiappino G, et al. Mediterranean Kaposi’s sarcoma. Associated pathology in a case study of 100 patients. G Ital Dermatol Venereol. 1988;123:477–80.
Caccialanza M, Marca S, Piccinno R, Eulisse G. Radiotherapy of classic and human immunodeficiency virus-related Kaposi’s sarcoma: results in 1482 lesions. J Eur Acad Dermatol Venereol. 2008;22:297–302.
Chang Y, Cesarman E, Pessin MS, Lee F, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–9.
Chokunonga E, Levy LM, Bassett MT, Borok MZ, et al. Aids and cancer in Africa: the evolving epidemic in Zimbabwe. AIDS. 1999;13:2583–8.
Chor PJ, Santa Cruz DJ. Kaposi’s sarcoma. A clinicopathologic review and differential diagnosis. J Cutan Pathol. 1992;19:6–20.
Cooper JS, Sacco J, Newall J. The duration of local control of classic (non-AIDS associated) Kaposi’s sarcoma by radiotherapy. J Am Acad Dermatol. 1988;19:59–66.
Dourmishev LA, Dourmishev AL, Palmeri D, Schwartz RA, Lukac DM. Molecular genetics of Kaposi’s sarcoma-associated herpesvirus (human herpesvirus-8) epidemiology and pathogenesis. Microbiol Mol Biol Rev. 2003;67:175–212.
Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and do not know. AIDS. 2003;17:1717–30.
Dutz W, Stout AP. Kaposi’s sarcoma in infants and children. Cancer. 1960;13:684–94.
Gottlieb JJ, Washenik K, Chachoua A, Friedman-Kien A. Treatment of classic Kaposi’s sarcoma with liposomal encapsulated doxorubicin. Lancet. 1997;350:1363–4.
Hermans P, Lundgren J, Sommereijns B, Pedersen C, et al. Epidemiology of AIDS-related Kaposi’s sarcoma in Europe over 10 years. AIDS. 1996;10:911–7.
Ioachim HL, Adsay V, Giancotti FR, Dorsett B, Melamed J. Kaposi’s sarcoma of internal organs: a multiparameter study of 86 cases. Cancer. 1995;75:1376–85.
Iscovich J, Boffetta P, Franceschi S, Azizi E, Sarid R. Classic Kaposi’s sarcoma: epidemiology and risk factors. Cancer. 2000;88:500–17.
Kirova YM, Belembaogo E, Frikha H, Haddad E, et al. Radiotherapy in the management of epidemic Kaposi’s sarcoma: a retrospective study of 643 cases. Radiother Oncol. 1998;46:19–22.
Lim ST, Tupule A, Espina BM, Levine AM. Weekly docetaxel is safe and effective in the treatment of advanced-stage acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2005;103:417–21.
Martin-Carbonero L, Barrios A, Saballs P, Sirera G, et al. Pegylated liposomal doxorubicin plus highly active antiretroviral therapy versus highly active antiretroviral therapy alone in HIV patients with Kaposi’s sarcoma. AIDS. 2004;18:1737–40.
Mocroft A, Kirk O, Clumeck N, Gargalianos-Kakolyris P, et al. The changing pattern of Kaposi sarcoma in patients with HIV, 1994–2003: the EuroSIDA study. Cancer. 2004;100:2644–54.
Nobler MP, Leddy ME, Huh SH. The impact of palliative irradiation on the management of patients with acquired immune deficiency syndrome. J Clin Oncol. 1987;5(1):107–12.
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16:2445–51.
O’Mahony D, Gandjbakhche AH, Hassan M, Vogel A, Yarchoan R. Imaging techniques for Kaposi sarcoma. J HIV Ther. 2008;13(3):65–71.
Penn I. Kaposi’s sarcoma in organ transplant recipients. Transplantation. 1997;64:669–73.
Schwartz RA, Micali G, Nasca MR, Scuderi L. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008;59:179–206.
Sharpe M, Easthope SE, Keating GM, et al. Polyethylene glycol-liposomal doxorubicin: a review of its use in the management of solid and haematological malignancies and AIDS-related Kaposi’s sarcoma. Drugs. 2002;62(14):2089–126.
Shiels MS, Pfeiffer RM, Hall HI, Li J, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA. 2011;305:1450–9.
Stallone G, Schena A, Infante B, Di Paolo S, et al. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–23.
Tschachler E. Chapter 128: Kaposi’s sarcoma and angiosarcoma. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick’s dermatology in general medicine. 8th ed. USA: The McGraw-Hill Companies, Inc; 2012.
Walmsley S, Northfelt DW, Melosky B, Conant M, et al. Treatment of AIDS-related cutaneous Kaposi’s sarcoma with topical alitretinoin (9-cis-retinoic acid) gel. Panretin Gel North American Study Group. J Acquir Immune Defic Syndr. 1999;22:235–46.
Weninger W, Partanen TA, Breiteneder-Geleff S, Mayer C, et al. Expression of vascular endothelial growth factor receptor-3 and podoplanin suggests a lymphatic endothelial cell origin of Kaposi’s sarcoma tumor cells. Lab Invest. 1999;79:243–51.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tschachler, E., Tiplica, GS. (2015). Kaposi’s Sarcoma. In: Katsambas, A.D., Lotti, T.M., Dessinioti, C., D’Erme, A.M. (eds) European Handbook of Dermatological Treatments. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45139-7_46
Download citation
DOI: https://doi.org/10.1007/978-3-662-45139-7_46
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45138-0
Online ISBN: 978-3-662-45139-7
eBook Packages: MedicineMedicine (R0)