Abstract
Infantile hemangioma (IH) is the most common tumor of infancy ranging from a tiny red papule to a giant mass. Its typical natural history is characterized by an early rapid growth following birth and a slow spontaneous involution which is complete before puberty but almost complete within the first 3–6 years of life. The cause underlying IH is still unknown, but the role of fetal hypoxic stress is strongly suggested as a triggering signal. A different hypothesis suggests that IH can be derived from embolized placental progenitor cells that lodge in privileged sites of the developing embryo. Alternatively, IH has been reported as an aberrant proliferation and differentiation of a primitive mesoderm-derived hemogenic endothelium regulated by the renin-angiotensin system (RAS) leading to propose angiotensin-converting enzyme (ACE) as a potential therapeutic target. While some angiogenic factors have been identified (e.g., mast cells, heparin), there are no data demonstrating a hereditary component. Immunohistochemical studies of IH confirm their vascular origin. During the proliferative phase, IH shows a high expression of cell proliferation nuclear antigen, vascular endothelial growth factor (VEGF), type IV collagen, urokinase, basic fibroblast growth factor (bFGF), and von Willebrand factor. These data demonstrate active angiogenesis and are not observed in vascular malformations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Infantile hemangiomas (IHs) are the most common tumors of infancy. Their typical natural history is characterized by an early rapid growth in the first months of life and by a slow spontaneous involution in the first years of life.
-
Even though spontaneous regression of IHs could suggest therapeutic abstention, systemic treatment is the therapy of choice in many patients in which these situations occur: (1) rapid growth of IHs; (2) location of IHs in aesthetically critical areas; (3) recurrent hemorrhages, ulcerations, or infections of IHs; (4) IHs interfering with important physiological functions (breathing, feeding, vision, hearing, etc.); and (5) large or multicentric IHs that can cause heart failure.
-
Since 2008, systemic administration of propranolol, an old nonselective beta-blocker, was found, serendipitously, to improve the treatment of IHs replacing older and more dangerous therapies like oral steroids, vincristine, interferon-alpha, or vascular lasers. At present, oral propranolol has dramatically changed the approach of IHs because its efficacy is almost 100 % and its action is rapid, without important side effects. The formal approval by the FDA and EMA has been declared in 2014.
General Principles
Infantile hemangioma (IH) is the most common tumor of infancy ranging from a tiny red papule to a giant mass. Its typical natural history is characterized by an early rapid growth following birth and a slow spontaneous involution which is complete before puberty but almost complete within the first 3–6 years of life. The cause underlying IH is still unknown, but the role of fetal hypoxic stress is strongly suggested as a triggering signal. A different hypothesis suggests that IH can be derived from embolized placental progenitor cells that lodge in privileged sites of the developing embryo. Alternatively, IH has been reported as an aberrant proliferation and differentiation of a primitive mesoderm-derived hemogenic endothelium regulated by the renin-angiotensin system (RAS) leading to propose angiotensin-converting enzyme (ACE) as a potential therapeutic target. While some angiogenic factors have been identified (e.g., mast cells, heparin), there are no data demonstrating a hereditary component. Immunohistochemical studies of IH confirm their vascular origin. During the proliferative phase, IH shows a high expression of cell proliferation nuclear antigen, vascular endothelial growth factor (VEGF), type IV collagen, urokinase, basic fibroblast growth factor (bFGF), and von Willebrand factor. These data demonstrate active angiogenesis and are not observed in vascular malformations.
IHs occur in up to 4–5 % of neonates, although up to 30 % in premature babies with low birth weight (<1 kg). They are significantly more frequent in females, with a 3:1 to 5:1 female/male ratio. The majority of IHs are noticed in the postnatal period more often during the first to second week, even though few of them may already be visible at birth, although usually with very different clinical features. IHs are distinguished from other less common vascular malformations of infancy by their characteristic clinical course. Although each lesion may have a distinct growth pattern, most follow a typical course divided into four stages: (1) nascent, (2) proliferative, (3) steady state, and (4) involution.
The first stage is the newborn stage prior to emergence of lesions, which typically lasts a few days. In fact, when IH is present at birth as a precursor, it is very small and flat, usually represented by an erythematous dot or patch or, paradoxically, by an ischemic patch (“herald patch”) mimicking “nevus anemicus,” or by telangiectasias surrounded by an ischemic halo. The majority of IHs appear as a single lesion; although there is no limit to their numbers, it is very rare to see more than five or six lesions. Equally rare are visceral IHs, usually hepatic. Topographically, the head is the most affected region, accounting, alone, for about 50 % of total lesions. History and phenotype allow, in most cases, diagnosis of IHs without the need for additional intensive investigations.
During the following 3–6 months, proliferation occurs in an early proliferative stage which brings rapid growth in the first few months of life, followed by a late proliferative stage with less rapid growth. As compared to superficial or localized lesions (the so-called “strawberry angiomas”), deep or segmental lesions, as those affecting the parotid region, appear to have a mysterious and prolonged proliferative phase extending beyond 6 months and rarely beyond the first year of life; in addition, in these phenotypes, the precursors and the first steps of the proliferative phase cannot be clinically observed. However, most lesions still cease growth by 9 months of age. The steady state or stabilization phase is of variable duration, usually a few months. Involution is typically heralded by a change in color from bright red to gray or purple, as well as a softening texture and flattening or diminution in size. Thermographic studies show a higher temperature of IHs, rising in the proliferative phase and fading in the evolution stage. Approximately most of IHs involute by 5–7 years of age, but smaller lesions are not noted any more at the time of primary school.
It should be noted that the deep localization of IHs, in the absence of a superficial “sentinel” IH, delays greatly the observation of the lesion, which is not usually discovered until after its full proliferative stage. The spontaneous regression of IH leaves minimal sequelae in about half the cases; it can result in various degrees of lesions (scars, telangiectasias, yellowish hypoelastic patches, etc.), especially on the head. It is difficult to predict the quality of the final regression, which is not influenced by gender, race, site or size of the lesion, presence or absence at birth, and duration of the proliferating phase. Of course, usually, smaller lesions have a better outcome and deeper lesions more rarely produce sequelae that alter the skin surface.
In addition to the aesthetic changes, IHs may ulcer, producing hemorrhages that can obstruct the respiratory and digestive tract. Big IHs with rapid regression may form a scab with cicatricial sequelae. In periocular localizations, impaired vision may occur. Amblyopia and astigmatism are the most common ophthalmic complication of IHs. Orbital lesions are less common, but, secondary to their mass effect, they may result in strabismus, proptosis, exposure keratopathy, or compressive optic neuropathy.
Histologically, IHs are composed of a complex mixture of cells including multipotent stem cells, a majority of immature endothelial cells, pericytes, dendritic cells, and, in the late stage, adipocytes. In the proliferating phase, there is a new production of capillaries, sometimes mixed with larger vascular lacunae due to proliferation and swelling of endothelial cells, and mast cells are greatly increased in number. In the involuting phase, the cellular component becomes scarce, and vascular lakes regress and are replaced by fibroadipose structures. In the stable phase, under electron microscopy, the endothelial cell shows swollen mitochondria and activity of the rough endoplasmic reticulum; additionally, there is thickening of the basement membrane.
A critical consideration concerns the treatment of IH. Spontaneous regression of IHs could suggest therapeutic abstention but this attitude is not wise in the majority of cases. We agree with those authors who recommend therapeutic abstention, but only for very small IHs (<3 cm by the 3rd month of age, <6 cm by the 6th month of age) and for those whose locations do not create problems either objectively or subjectively. Otherwise, we recommend treating IHs, because, first, by limiting the expansion we can limit the symptoms, and, second, a small IH will have, in any case, a negligible fibroadipose residue. Finally, it should be considered that even a small IH located in an exposed site can cause variable psychological damage from the time of socialization onward. An early intervention is, therefore, recommended also in this case. In short, and especially after the rediscovery of propranolol, the sentence: “Leave it alone; it will go away” is no longer a universally acceptable advice for IH (Requena and Sangueza 1998). It is, however, better to continue to follow up the patients and their parents, reassuring them of the favorable outcome, with the aid of illustrations and photographs that show the evolution of these lesions. Local treatments (e.g., elastic compression, cryotherapy, laser therapy, ultra-potent topical corticosteroids, etc.) are often disappointing or impossible for various practical reasons, so that systemic treatment remains the therapy of choice in some situations: (1) rapid growth of the lesion; (2) location of the lesion in aesthetically critical areas; (3) recurrent hemorrhages, ulcerations, or infections; (4) in lesions interfering with important physiological functions (breathing, feeding, vision, hearing); and (5) large or multicentric IH that can cause heart failure.
To answer the question that parents have regarding the current stage of IH growth or involution, it is important to consider all available data: anamnestic, photographical, morphological (usually, IHs that are easily and frequently compressed against a bony surface, such as the back, stop earlier, while those that occur in very extensible areas, such as parotid, lateral-cervical, or mammary regions, expand much more), and especially the three parameters of color, temperature, and consistency. The color (not detectable only in subcutaneous IH) is connected with the temperature (that is related with metabolic activity) and with the consistency (in the proliferating phase, most of the mass is formed by endothelium that proliferates and forms solid cords with a small lumen and therefore difficult to compress). The increase of color, temperature, and consistency therefore indicates the proliferating phase of IH, while their reduction indicates the beginning of the involuting phase.
Infantile Hemangiomas and Beta-Blockers
Infantile hemangiomas (IHs) are vascular tumors expressing intrinsically benign proliferations with a spontaneous tendency to regression. So, when lesions are small and located in body regions that are aesthetically indifferent, a “wait-and-see” policy is the best management (Chandran et al. 2013). In many cases, the size and/or the localization of the lesion cause significant cosmetic deformity or functional compromise requiring a prompt treatment. Unfortunately IHs are mainly located in the head and neck area. In any case, besides aesthetic risks, the main indications for treatment of IHs are: life-threatening conditions (heart failure, respiratory distress), functional risks (amblyopia, swallowing disorders, etc.), and painful ulcerations.
For almost half a century, corticosteroids have been the first-line therapy for complicated IHs. Fortunately, this treatment was effective in the majority of patients affected by IHs and led to a progressive disappearance of older and more dangerous treatments like radiotherapy, cryotherapy, chemotherapy (like vincristine or cyclophosphamide), interferon-alpha 2a, therapeutic embolization, laser, and surgery.
Systemic corticosteroids, however, have a low safety profile, especially in infancy. Parents of children with IHs under treatment with systemic steroids are more worried during treatment and perceive a negative impact on normal life issues, including work and vaccination of their child. Not enough, the response to treatment is not homogenous, and rebound growth upon cessation of treatment is quite common.
At the end of 2007, by serendipity, the pediatric dermatologist Christine Léauté-Labrèze who works in the team of Professor Alain Taïeb in Bordeaux (France) realized that a common beta-blocker, the propranolol, could be used in the treatment of IHs. Indeed the propranolol was prescribed to treat hypertrophic obstructive cardiomyopathy, in a young infant with coexisting nasal IH, and the IH regressed rapidly. In the following year, she published the first two observations together with nine additional cases. Let’s quote the original text (Leauté-Labrèze et al 2008):
The first child had a nasal capillary hemangioma. Despite corticosteroid treatment, the lesion was stabilized but obstructive hypertrophic myocardiopathy developed, so the patient was treated with propranolol. The day after the initiation of treatment, the hemangioma changed from intense red to purple, and it softened. The corticosteroids were tapered, but the hemangioma continued to improve. When the corticosteroids were discontinued, no regrowth of the hemangioma was noted. When the child was 14 months of age, the hemangioma was completely flat. The second child had a plaque-like infantile capillary hemangioma involving the entire right upper limb and part of the face. At 1 month of age, a subcutaneous component developed, and despite corticosteroid treatment, the hemangioma continued to enlarge. Magnetic resonance imaging revealed intraconal and extraconal orbital involvement, as well as an intracervical mass causing compression and tracheal and esophageal deviation …. Ultrasonography showed increased cardiac output, and treatment with propranolol, at a dose of 2 mg per kilogram of body weight per day, was initiated. Seven days later, the child was able to open his eye spontaneously, and the mass near the parotid gland was considerably reduced in size. Prednisolone was discontinued …. without any regrowth of the hemangioma; at 9 months of age, the eye opening was satisfactory, and no major visual impairment was noted. After …, propranolol was given to nine additional children who had severe or disfiguring infantile capillary hemangiomas….. In all patients, 24 hours after the initiation of treatment, we observed a change in the hemangioma from intense red to purple; this change was associated with a palpable softening of the lesion. After these initial changes, the hemangiomas continued to improve until they were nearly flat, with residual skin telangiectasias. Ultrasound examinations in five patients showed an objective regression in thickness associated with an increase in the resistive index of vascularization of the hemangioma…
Mechanism of Action, Indications, and Other Uses
Propranolol is a member of a class of medications called beta-blockers. It works by relaxing blood vessels and slowing heart rate to improve blood flow and decrease blood pressure. Propranolol is an old drug classically used for decades to treat high blood pressure, abnormal heart rhythms, heart disease, pheochromocytoma, and certain types of tremor but also to prevent angina and migraine headaches and to improve survival after a heart attack. Several mechanisms may be involved in the control of IH growth by propranolol. Propranolol is responsible for a vasoconstriction of the microvessels of the IH resulting in a rapid change in color and softening. It lowers the rate of renin and thus has a modulating effect on angiotensin II. In addition, β-adrenergic receptors belong to the family of G-protein-coupled receptors, which, when activated by adrenergic catecholamines, can promote a series of intracellular signal transduction pathways including that of angiogenic factors such as VEGF or bFGF and some metalloproteinases such as MMP2 and MMP9. Finally, propranolol could lead to an early apoptosis of endothelial cells that make up the majority of IHs.
Propranolol seems to act with a dose-dependent cytotoxic effect in IH endothelial cells with decreased cell viability, migration, and tubulogenesis. This cytotoxic effect has shown to be VEGF dependent. Decreased VEGF activity is mediated through the hypoxia-inducible factor (HIF)-1α pathway but not through NF-κβ signaling. Alternatively, propranolol may suppress IH growth by inhibiting expression of eNOS (endothelial nitric oxide synthase) protein and subsequent production of nitric oxide.
More recent investigations (Tu et al. 2013) demonstrated that propranolol induces apoptosis of IH endothelial cells through typical apoptotic changes, including shrinkage, formation of apoptotic bodies, and retention of plasma membrane integrity. The molecular and genetic mechanisms underlying the therapeutic effects of propranolol revealed that this beta-blocker drug led to a marked increase in the expression of caspase-8, cytochrome c, apoptosis-inducing factor, caspase-3, and poly(ADP-ribose) polymerase 1, as well as a concomitant reduction in lamin B1 expression. In short, these data demonstrate that propranolol induces apoptosis of IH endothelial cells through activation of the intrinsic and extrinsic apoptotic pathways, explaining its therapeutic effects against IHs. Nevertheless, some molecular mechanisms that could induce resistance to apoptotic stimuli and, therefore, justify the possible insensitivity of some IHs to propranolol have already been described (Caussé et al. 2013).
Treatment Regimen and Dosage
Most of the patients with IHs respond to propranolol more satisfactory and more quickly than systemic corticosteroids at 2 mg/kg per day in two divided doses. The average treatment duration with beta-blockers is 6 months continuously. However, the treatment can be more prolonged when the situation of the IH is problematic (Parikh et al. 2013). The latest available meta-analysis (Lou et al. 2014) concerning the efficacy of propranolol for IHs reviewed 35 studies involving 324 patients and 248 controls, comparing propranolol with steroids, vincristine, and laser in treating cutaneous IHs, periocular IHs, infantile airway hemangiomas, and infantile hepatic hemangiomas. The conclusions of these studies provide a strong evidence for propranolol as a first-line therapy for IHs.
Rebound growth, although mild, can occur in some patients. Late rebound can also be observed indicating a prolonged proliferation IHs even after the first year of age. Late proliferation can occur also after several months of a positive response to propranolol. However, a second course of propranolol is readily able to control the recurrence (Shehata et al. 2013).
In addition to propranolol, which is a lipophilic nonselective beta-blocker, other beta-blockers have been used in the treatment of IHs. Oral atenolol, a hydrophilic selective beta-1 blocker, compared with a control group treated with propranolol, seems to be equally effective and safe (de Graaf et al. 2013).
Topical propranolol in ointment or in gel and topical timolol have also been used with satisfactory results on superficial IHs (Mouhari-Toure et al. 2013).
In the last 5 years, some authors are evaluating whether IH treated with pulsed dye laser (PDL) and propranolol displayed more rapid and complete clearance than IH treated with propranolol alone. In a retrospective and blind review of facial-segmental IHs, those cases treated with a combination of propranolol and PDL displayed more rapid and complete clearance and required a lower cumulative propranolol dose to achieve near-complete clearance (Vigone et al. 2012).
Contraindications and Side Effects
While cardiovascular diseases and asthma are absolute contraindications for propranolol therapy, relative contraindications include: hypersensitivity to propranolol in first-degree relatives, diabetes mellitus, chronic renal insufficiency, and cerebrovascular anomalies. Adverse events (hypotension, somnolence, wheezing, insomnia, agitation and/or nightmares, hypoglycemia) are rare and uneventful and do not require, usually, treatment suspension. As far it concerns the frequency of side effects, a systematic review of 1,264 patients treated with propranolol for IHs showed (besides a high rate of efficacy) a low rate of serious adverse events with reports of symptomatic hypotension in five patients (0.39 %), hypoglycemia in four (0.31 %), and symptomatic bradycardia in one (0.079 %). The milder and most common adverse events were changes in sleep (n = 136; 10.75 %) and acrocyanosis (n = 61; 4.8 %) (Marqueling et al. 2013).
Propranolol has also been proposed as a first-line therapy of thyroid dysfunction associated with IHs. Consumptive hypothyroidism is a rare condition related to massive IHs producing an excess of the thyroid-hormone-inactivating enzyme type 3 iodothyronine deiodinase. While corticosteroid treatment showed only partial benefit in a large parotid IH, introduction of propranolol instead led to normalization of thyroid hormones along with a dramatic involution of the hemangioma (Vigone et al. 2012). A similar positive result was observed in thyroid dysfunction associated with severe infantile hepatic hemangiomas (Vergine et al. 2012).
Discussion and Final Remarks
Even though some authors think that, despite the numerous papers already published, more reliable prospective studies are still needed in the field of IHs treated with beta-blockers, we think that the efficacy coupled with the safety of oral (and probably topical) beta-blockers in the treatment of IH is now a matter of fact: propranolol is the first-line treatment of most IHs.
We are aware that the vast majority of the published literatures concern case reports and that there is a lack of well-designed, high-quality randomized control trials (e.g., beta-blockers versus corticosteroids), and we consider also that the updated knowledge of the broad spectrum of vascular anomalies described by Mulliken and Glowacki (1982) and Enjolras et al. (2007) and better detailed by the International Society for the Study of Vascular Anomalies (ISSVA) in 2014 is not universal. In other words, it is likely that, in some cases, the selection of the patients has not been optimal or it will not be optimal in the next future. However, we agree that the present availability of the big international study on propranolol in IH (the HEMANGIOL® study; ClinicalTrials.gov Identifier: NCT01056341) will confirm the experience of hundreds of doctors that treated thousands of patients. The final approval of beta-blockers for the treatment of IH by the FDA and EMA in 2014 has been a major step for the medicine, allowing all the physicians and the pediatricians (not only the academic doctors!) to prescribe this treatment for the most common tumor of infancy: the IH. In addition, it is not surprising that beta-blockers have already been used successfully in other vascular proliferations such as pyogenic granulomas (Wine Lee et al. 2014).
Our protocol for the management of complicated IHs is summarized in Table 135.1a–c. Obviously, when a complex syndrome is suspected, e.g., PHACES and PELVIS or SACRAL or LUMBAR,Footnote 1 additional investigations should be considered such as eye examination, head ultrasound, MRI/CT scan of the brain, etc. Ultrasound examination of the abdomen for hepatic hemangiomas should be advised in case of neonatal diffuse hemangiomatosis or when cutaneous IHs are more than five.
In the meantime and in the next future, besides for a formal approval of the use of beta-blockers for the treatment of IHs by health authorities, we must always consider the potential side effects of this class of drugs. Thus, parents and caregivers should check the possible appearance of the following items:
-
Allergic reactions such as skin rashes (itching or hives), swelling in the face or hands, swelling or tingling in the mouth or throat, and chest tightness
-
Mood change, unusual tiredness or weakness, trouble sleeping (nightmares), trouble awakening, or losing consciousness
-
Cold sweats and/or bluish-colored skin
-
Slow, fast, or uneven heartbeat
In order to prevent hypoglycemic status (check: coldness, shakiness, and sweating), it is wise to give propranolol during mealtime or right after your child has eaten. In our experience (more than 200 children with IHs treated with oral propranolol from 2008 to 2013 – unpublished data), the side effects have always been mild, and in just two cases, the treatment has been stopped prudently and in no case severe side effects have been observed while the efficacy has always been good and, in some cases, spectacular (Fig. 135.1a–d). In addition, we were able to rule out hyperkalemia from the list of possible side effects. Indeed hyperkalemia and hyperphosphatemia observed in a 33-day-old female with an ulcerated IH undergoing oral therapy with propranolol were finally linked with tumor lysis syndrome that was diagnosed after excluding other causes of electrolyte imbalance in the diagnostic workup (Cavalli et al. 2012).
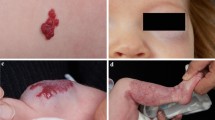
(a) This newborn, 17-day-old girl, presented with a large plaque-like IH showing a rapid enlargement with the involvement of the first trigeminal branch causing eye obstruction. (b) A cycle of oral corticosteroids for 1 month was almost ineffective and the left eye was still closed. (c) A cycle of oral propranolol was greatly effective within the first week of treatment. (d) At 6 months of life, the IH showed a complete regression with mild slack skin and fine telangiectasias
Conclusion
The serendipitous discovery of the efficacy of beta-blockers in the treatment of IHs has been a milestone in pediatric and in dermatologic practice and has revolutionized the approach to these neoplasms. Fortunately, this drug is now an approved therapy for IHs. There is great debate regarding appropriate monitoring protocols for initiating and maintaining propranolol therapy in infants with IHs as well as right parameters to follow, including the possible hospitalization of the patient. We are happy that precise treatment guidelines have recently come to help doctors and caregivers in this field. However, we strongly suggest, for the present time but also for the future, the constant need of a multidisciplinary approach for all vascular anomalies.
Notes
- 1.
PHACES is an acronym: P, posterior fossa malformation (brain); H, hemangiomas; A, arterial anomalies; C, coarctation of the aorta along with cardiac defects; E, eye abnormalities; and S, sternum and/or supraumbilical abdominal raphe clefts. PELVIS, SACRAL, and LUMBAR are acronyms that denote the association of local hemangioma and malformation in the pelvic region, e.g., PELVIS = perineal hemangioma, external genitalia malformations, lipomyelomeningocele, vesicorenal abnormalities, imperforate anus, and skin tag. SACRAL = spinal dysraphism, anogenital anomalies, cutaneous abnormalities, renal and urologic anomalies, and angioma of lumbosacral localization. LUMBAR = lower body hemangioma and other cutaneous defects, urogenital anomalies, ulceration, myelopathy, bony deformities, anorectal malformations, arterial anomalies, and renal anomalies.
References
Caussé S, Aubert H, Saint-Jean M, Puzenat E, Bursztejn AC, Eschard C, Mahé E, Maruani A, Mazereeuw-Hautier J, Dreyfus I, Miquel J, Chiaverini C, Boccara O, Hadj-Rabia S, Stalder JF, Barbarot S, Groupe de Recherche Clinique en Dermatologie Pédiatrique. Propranolol-resistant infantile haemangiomas. Br J Dermatol. 2013;169(1):125–9.
Cavalli R, Buffon RB, de Souza M, Colli AM, Gelmetti C. Tumor lysis syndrome after propranolol therapy in ulcerative infantile hemangioma: rare complication or incidental finding? Dermatology. 2012;224(2):106–9.
Chandran S, Ari D, Jose J. Use of propanolol for treatment of large infantile haemangiomas-a report of two cases and review of the literature. Ann Acad Med Singapore. 2013;42(5):253–6.
de Graaf M, Raphael MF, Breugem CC, Knol MJ, Bruijnzeel-Koomen CA, Kon M, Breur JM, Pasmans SG. Treatment of infantile haemangiomas with atenolol: comparison with a historical propranolol group. J Plast Reconstr Aesthet Surg. 2013;66(12):1732–40.
Enjolras O, Wassef M, Chapot R. Color atlas of vascular tumors and vascular malformations. Cambridge: Cambridge University Press; 2007.
Mouhari-Toure A, Azoumah KD, Tchamdja K, Saka B, Kombaté K, Tchangaï-Walla K, Pitche P. Rapid regression of infantile haemangioma with 2% propranolol ointment. Ann Dermatol Venereol. 2013;140(6–7):462–4.
Leauté-Labrèze C, DumasdelaRoque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–51.
Lou Y, Peng WJ, Cao Y, Cao DS, Xie J, Li HH. The effectiveness of propranolol in treating infantile hemangiomas: a meta-analysis including 35 studies. Br J Clin Pharmacol. 2014;78(1):44–57.
Marqueling AL, Oza V, Frieden IJ, Puttgen KB. Propranolol and infantile hemangiomas four years later: a systematic review. Pediatr Dermatol. 2013;30(2):182–91.
Mulliken JB, Glowacki J. Classification of pediatric vascular lesions. Plast Reconstr Surg. 1982;70:120–1.
Parikh SR, Darrow DH, Grimmer JF, Manning SC, Richter GT, Perkins JA. Propranolol use for infantile hemangiomas: American Society of Pediatric Otolaryngology Vascular Anomalies Task Force practice patterns. JAMA Otolaryngol Head Neck Surg. 2013;139(2):153–6.
Requena L, Sangueza OM. Cutaneous vascular proliferations. Part III. Malignant neoplasms, other cutaneous neoplasms with significant vascular component, and disorders erroneously considered as vascular neoplasms. J Am Acad Dermatol. 1998;38:143–75.
Shehata N, Powell J, Dubois J, Hatami A, Rousseau E, Ondrejchak S, McCuaig C. Late rebound of infantile hemangioma after cessation of oral propranolol. Pediatr Dermatol. 2013;30(5):587–91.
Tu JB, Ma RZ, Dong Q, Jiang F, Hu XY, Li QY, Pattar P, Zhang H. Induction of apoptosis in infantile hemangioma endothelial cells by propranolol. Exp Ther Med. 2013;6(2):574–8.
Vergine G, Marsciani A, Pedini A, Brocchi S, Marsciani M, Desiderio E, Bertelli S, Vecchi V. Efficacy of propranolol treatment in thyroid dysfunction associated with severe infantile hepatic hemangioma. Horm Res Paediatr. 2012;78(4):256–60.
Vigone MC, Cortinovis F, Rabbiosi S, Di Frenna M, Passoni A, Persani L, Chiumello G, Gelmetti C, Weber G. Difficult treatment of consumptive hypothyroidism in a child with massive parotid hemangioma. J Pediatr Endocrinol Metab. 2012;25(1–2):153–5.
Wine Lee L, Goff KL, Lam JM, Low DW, Yan AC, Castelo-Soccio L. Treatment of pediatric pyogenic granulomas using β-adrenergic receptor antagonists. Pediatr Dermatol. 2014;31(2):203–7.
Further Reading
Callahan AB, Yoon MK. Infantile hemangiomas: a review. Saudi J Ophthalmol. 2012;26(3):283–91.
Cavalli R, Novotna V, Buffon RB, Gelmetti C. Multiple cutaneous and hepatic infantile hemangiomas having a successful response to propranolol as monotherapy at neonatal period. G Ital Dermatol Venereol. 2013;148(5):525–30.
Chang LC, Haggstrom AN, Drolet BA, Baselga E, Chamlin SL. Hemangioma investigator group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122(2):360–7.
Dyer JA. Propranolol to treat hemangiomas of infancy: safety and side effect recognition comment on “retrospective review of adverse effects from propranolol in infants. JAMA Dermatol. 2013;149(4):481–504.
Enjolras O, Riché MC, Merland JJ, Mulliken JB. Hemangiomes et malformations vasculaires superficielles. Paris: MEDSI/McGraw-Hill; 1990.
Fette A. Propranolol in use for treatment of complex infant hemangiomas: literature review regarding current guidelines for pre-assessment and standards of care before initiation of therapy. Sci World J. 2013;2013:850193.
Fost NC, Esterly NB. Successful treatment of juvenile hemangiomas with prednisone. J Pediatr. 1968;72:351–7.
Gomulka J, Siegel DH, Drolet BA. Dramatic shift in the infantile hemangioma treatment paradigm at a single institution. Pediatr Dermatol. 2013;30(6):751–2.
Hartzell LD, Buckmiller LM. Current management of infantile hemangiomas and their common associated conditions. Otolaryngol Clin North Am. 2012;45:545–56.
Hermans DJ, Zweegers J, Evers AW, Van Der Vleuten CJ. Parental experiences with propranolol versus oral corticosteroids for complicated infantile hemangioma, a retrospective questionnaire study. Eur J Dermatol. 2013;23(6):857–63.
Huoh KC, Rosbe KW. Infantile hemangiomas of the head and neck. Pediatr Clin North Am. 2013;60(4):937–49.
Itinteang T, Withers AH, Leadbitter P, Day DJ, Tan ST. Pharmacologic therapies for infantile hemangioma: is there a rational basis? Plast Reconstr Surg. 2011;128(2):499–507.
Ji Y, Chen S, Li K, Xiao X, Xu T, Zheng S. Up-regulated autocrine VEGF/VEGFR-2 loop prevents apoptosis in hemangioma-derived endothelial cells. Br J Dermatol. 2014;170(1):78–86.
Léauté-Labrèze C. Infantile hemangioma: update and treatment. Arch Pediatr. 2013;20(5):517–22.
Leonardi-Bee J, Batta K, O’Brien C, Bath-Hextall FJ. Interventions for infantile haemangiomas (strawberry birthmarks) of the skin. Cochrane Database Syst Rev. 2011;(5):CD006545.
Maguiness SM, Frieden IJ. Management of difficult infantile haemangiomas. Arch Dis Child. 2012;97(3):266–71.
Martin K, Blei F, Chamlin SL, Chiu YE, Frieden IJ, Frommelt PC, Garzon MC, Kwon EK, MacLellan-Tobert S, Mancini AJ, Seefeldt M, Sidbury R, Siegel DH, Drolet BA, Boucek RJ. Propranolol treatment of infantile hemangiomas: anticipatory guidance for parents and caretakers. Pediatr Dermatol. 2013;30(1):155–9.
Menezes MD, McCarter R, Greene EA, Bauman NM. Status of propranolol for treatment of infantile hemangioma and description of a randomized clinical trial. Ann Otol Rhinol Laryngol. 2011;120(10):686–95.
Mulliken JB, Young AE. Vascular birthmarks; hemangiomas and malformations. Philadelphia: WB Saunders Company; 1988.
Neri I, Balestri R, Patrizi A. Hemangiomas: new insight and medical treatment. Dermatol Ther. 2012;25(4):322–34.
Nozaki T, Matsusako M, Mimura H, Osuga K, Matsui M, Eto H, Ohtake N, Manabe A, Kusakawa I, Tsutsumi Y, Nosaka S, Kamo M, Saida Y. Imaging of vascular tumors with an emphasis on ISSVA classification. Jpn J Radiol. 2013;31(12):775–85.
Reddy KK, Blei F, Brauer JA, Waner M, Anolik R, Bernstein L, Brightman L, Hale E, Karen J, Weiss E, Geronemus RG. Retrospective study of the treatment of infantile hemangiomas using a combination of propranolol and pulsed dye laser. Dermatol Surg. 2013;39(6):923–33.
Ryan TJ, Cherry GW. Vascular birthmarks. Pathogenesis and management. Oxford: Oxford University Press; 1987. p. 203.
Saxena AK, Willital GH. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr. 2008;167(7):757–64.
Starkey E, Shahidullah H. Propranolol for infantile haemangiomas: a review. Arch Dis Child. 2011;96(9):890–3.
Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents’ photographs tell us. Pediatrics. 2012;130(2):e314–20.
Xu SQ, Jia RB, Zhang W, Zhu H, Ge SF, Fan XQ. Beta-blockers versus corticosteroids in the treatment of infantile hemangioma: an evidence-based systematic review. World J Pediatr. 2013;9(3):221–9.
Xue K, Hildebrand GD. Topical timolol maleate 0.5% for infantile capillary haemangioma of the eyelid. Br J Ophthalmol. 2012;96(12):1536–7.
Zhai YN, Song HT, Chen SQ, Zhang MX, Li CJ, Xia Y, Wang L. Effect of propranolol gel on infantile hemangiomas. Zhonghua Zheng Xing Wai Ke Za Zhi. 2013;29(1):25–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Gelmetti, C., Cavalli, R. (2015). Beta-Blockers for Hemangiomas. In: Katsambas, A.D., Lotti, T.M., Dessinioti, C., D’Erme, A.M. (eds) European Handbook of Dermatological Treatments. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-45139-7_135
Download citation
DOI: https://doi.org/10.1007/978-3-662-45139-7_135
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-45138-0
Online ISBN: 978-3-662-45139-7
eBook Packages: MedicineMedicine (R0)