Abstract
It has been established in the last century that the skeletal muscle cells of vertebrates originate from the paraxial mesoderm. However, recently the lateral plate mesoderm has been identified as a novel source of the skeletal muscle. The branchiomeric muscles, such as masticatory and facial muscles, receive muscle progenitor cells from both the cranial paraxial mesoderm and lateral plate mesoderm. At the occipital level, the lateral plate mesoderm is the sole source of the muscle progenitors of the dorsolateral neck muscle, such as trapezius and sternocleidomastoideus in mammals and cucullaris in birds. The lateral plate mesoderm requires a longer time for generating skeletal muscle cells than the somites. The myogenesis of the lateral plate is determined early, but not cell autonomously and requires local signals. Lateral plate myogenesis is regulated by mechanisms controlling the cranial myogenesis. The connective tissue of the lateral plate-derived muscle is formed by the cranial neural crest. Although the cranial neural crest cells do not control the early myogenesis, they regulate the patterning of the branchiomeric muscles and the cucullaris muscle. Although satellite cells derived from the cranial lateral plate show distinct properties from those of the trunk, they can respond to local signals and generate myofibers for injured muscles in the limbs. In this review, we key feature in detail the muscle forming properties of the lateral plate mesoderm and propose models of how the myogenic fate may have arisen.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Vertebrates have three sets of skeletal muscles: (1) axial muscles, which facilitate movement of the vertebral column and the skull, (2) the limb and shoulder girdle muscles, which operate the movement of the extremity, and (3) the head muscles, which execute the movement complex for the eye, mouth, tongue, and larynx in the head. According to the classical view, the skeletal muscle cells have two sources: the paraxial and the pre-chordal mesodermal (Table 1). In the trunk and limb, muscle cells originate from the somites, the segmental units of the paraxial mesoderm. The head muscles are derived from the unsegmented cranial paraxial mesoderm (CPM) and the prechordal mesoderm. Recently, skeletal muscle cells have been shown to be derived from the lateral plate mesoderm (LPM). In this review, we concentrate on the myogenesis in the LPM in vertebrates. Recent findings resulting mainly from the chick and mouse models are discussed.
We will first briefly describe the morphogenesis of the somatopleura and the splanchnopleura of the LPM. Then we will discuss the specification and regionalisation of the LPM. In the third part, we will refer the recent studies about the origin of skeletal muscle cells from the LPM. This is followed by the cellular and molecular regulation of their myogenesis. Finally, we will discuss the satellite cells from the LPM.
2 Formation of the Lateral Plate Mesoderm
During gastrulation, cells delaminate from the node and primitive streak and migrate into the space between the epiblast and hypoblast to form the middle germ layer, the mesoderm. The mesoderm compartmentalizes into an axial part (the prechordal mesoderm and the notochord), a paraxial, an intermediate, and a LPM (Selleck and Stern 1991; Psychoyos and Stern 1996; reviewed in Schoenwolf and Alvarez 1992; Schoenwolf et al. 1992). The paraxial mesoderm flanks the axial structure, the notochord, and the neural tube (Christ and Ordahl 1995). It can be subdivided into a pre- and post-otic portion. The pre-otic portion of the paraxial mesoderm termed in many references as cranial paraxial mesoderm (CPM) never undergoes segmentation, while its post-otic part forms segmental units, the somites. Somites are formed by primary segmentation and epithelialisation. The LPM is made of one layer of mesenchymal cells at early stages and then subdivided into a dorsal somatic (SoM) and a ventral splanchnic mesoderm (SpM). The SoM is also called the somatopleure and SpM the splanchnopleure. The subdivision of the LPM starts in the anterior-most region and progresses along the head-to-tail axis towards the caudal end of the embryo (Funayama et al. 1999). In chick embryos, for instance, the LPM is clearly subdivided into two layers at the cephalic level at Hamburger-Hamilton-stage 8 (HH-8). However, the subdivision proceeds only partly at the prospective otic level at the same stage. It furthermore remains one layer at the first somite level. The lateral plate at the first somite level becomes two layers after HH-stage 10 (Fig. 1). The formation of the SoM and SpM is accompanied by the appearance of a coelomic cavity. It appears first in the lateral part of the lateral plate and extends from lateral to medial. The formation of the coelom is controlled by the ectoderm (Funayama et al. 1999).
Formation of the somatopleure and splanchnopleure from the lateral plate mesoderm proceeds gradually in cranial to caudal direction. (A) Dorsal view of a chick embryo at Hamilton and Hamburger-stage 8 (HH-8). Four somites are formed. (a1) Transverse section at the cranial cephalic level indicated by line a1 in Fig. A. The neural tube (nt) is still not yet closed. The cranial paraxial mesoderm (CPM) is composed of loosely arranged mesenchymal cells. The somatic mesoderm (SoM) and splanchnic mesoderm (SpM) are formed in the lateral plate mesoderm. A coelomic cavity (co) is surrounded by these two cell layers. The SpM is made of cylindrical epithelial cells, while the cells in the SoM layer are flat. (a2) Transverse section through the level indicated by line a2. The lateral plate mesoderm is presented as a layer of cells on the left side. In contrast, the right lateral plate contains a dorsal and ventral layer, the SoM and SpM, surrounding a coelom (co). (a3) Transverse section through the first somite (1.so) level indicated by line a3 in the Fig. A. The wide neural plate lies on the somite. While the section is located at the level of the middle part of the first somite on the left side, the section is cut through the cranial edge of the right first somite. Only a small coelom (co) is present on the right side. (B) Dorsal view of a HH-9 chick embryo. Seven somites are formed. (b1) Transverse section through the cephalic level indicated by line b1 in the Fig. B. The neural tube is starting to close. A coelom can be seen on both sides in the lateral part of the lateral plate mesoderm. (b2) Transverse section through the first somite (1.so). Only a very small coelom can be seen in the most lateral part of the lateral plate mesoderm. The main part of the lateral plate has not yet been subdivided into a somatic and a splanchnic mesoderm. (C) Dorsal view of a HH-10 embryo with 10 somites. (c) Transverse section through the first somite (1.so) on the right side and the unsegmented CPM level on the left side. At this level, the neural tube is closed and the lateral plate mesoderm is differentiated into a dorsal somatic (SoM) and a ventral splanchnic mesoderm (SpM). The coelom on the left side is located more cranially and has advanced in development compared to the right side. Bar: 100 μm for the sections
The intermediate mesoderm is not formed at the head level and lies posterior to the cervical somite level. Due to the lacking of the intermediate mesoderm, the CPM and the occipital somites are continuous with the LPM. The boundary between them is difficult to identify morphologically. It can be visualised only by genetic markers. For instance, Pax3 marks only the somites, but not the LPM (Theis et al. 2010). In chick embryos, Alx4, Cyp26c1, and Twist are expressed in CPM and occipital somites (Nathan et al. 2008; Bothe and Dietrich 2006; Tirosh-Finkel et al. 2006; Dastjerdi et al. 2007). The LPM is characterised by the expression of FoxF1, a forkhead box F1 transcription factor, and of Hand1 and Hand2 expression (Srivastava et al. 1995; Charite et al. 2000; Yelon et al. 2000; Deimling and Drysdale 2009). After the subdivision, the FoxF1 expression becomes restricted to the ventral splanchnic layer, while the Irx3 (Iroquois class homeodomain transcription factor) expression appears in the dorsal somatic layer (Funayama et al. 1999; Mahlapuu et al. 2001). Expression of a set of second heart field genes is found also in the SpM of chick embryos at HH-8 (Nathan et al. 2008).
3 Specification of the Lateral Plate Mesoderm
According to the developmental properties, the lateral plate can be subdivided into the anterior and the posterior LPM (Waxman et al. 2008; Zhao et al. 2009). The boundary of these two mesoderm regions lies at the level of somite 5–6 in mouse embryos (Waxman et al. 2008). While Hand1 and Hand2 genes are expressed throughout the entire LPM, NKx2.5 and Tbx20 expression is restricted to the anterior LPM (Buchberger et al. 1996; Kraus et al. 2001; Yamagishi et al. 2004; Deimling and Drysdale 2009). The pharyngeal LPM is characterised by the Tbx1 expression (Garg et al. 2001).
The anterior LPM contributes to the heart formation and is considered as cardiac mesoderm. Firstly, myocardial progenitor cells populate a region of the lateral plate on either side of the neural folds. This region is considered as the primary heart field. A primary heart tube forms from each side of the primary heart field. The bilateral symmetrical heart tubes fuse into one heart tube, consisting of a venous and an arterial pole. As development proceeds, further myocardial progenitor cells are recruited at the both poles of the heart tube. The progenitor cells for additional growth of the arterial pole, which gives rise to the outflow tract and right ventricle, was recently shown to arise from the SpM of the pharyngeal LPM known as the secondary or anterior heart field (SHF/AHF).
The entire posterior LPM at early stages of development has been reported to have limb-forming potential. Stephens et al. (1989) explanted lateral plate with overlying ectoderm and underlying endoderm from the neck (somites 10–14), wing (somites 15–20), flank somites 21–25), and leg (somite 26-end of the embryo) of stage 11–14 chick embryos. Each explant was allowed to grow in the coelom of 2.5–3 day-old-embryo for further 7–8 days. All explants formed limb structures. This means that the limb field is present throughout the posterior LPM.
Our previous studies show that the skeletal muscle forming potential is restricted to the anterior LPM. Only the lateral plate at the level of somites 1–3 (occipital region) contributes to cucullaris muscle in chick embryos (Theis et al. 2010). The lateral plate tissue which was grafted from the neck region into the occipital region could not form skeletal muscles. This indicates that the cervical lateral plate has no intrinsic myogenic potential and cannot be induced to form muscles through local cues in the occipital region. After the occipital LPM was grafted into the limb level, it could not form skeletal muscle cells. This indicates that the skeletal muscle forming potential of the cranial LPM is not cell-autonomous and its myogenesis requires local inductive signals.
4 Origin of Skeletal Muscle Cells from the Lateral Plate Mesoderm
The skeletomyogenic potential of the LPM was first observed by means of cell lineage tracing in mouse embryos. The murine myocyte enhancer factor-2C (MEF-2C) has been shown to be expressed in the secondary heart field and controls the heart looping and right ventricular chamber formation (Lin et al. 1997; Dodou et al. 2004). Verzi et al. (2005) used the mef2c, an anterior heart field promoter and enhancer, to direct the expression of cre recombinase exclusively in the anterior heart field. The Cre expression was reported by Cre-dependent lacZ activity (Soriano 1999). They showed that the mef2c–AHF–Cre transgene expression overlaps with markers of the secondary/anterior heart field. As development proceeds, not only the outflow tract and right ventricle but also the mesodermal component of the branchial arches are marked by the activity of the mef2c-AHF-Cre transgene (Verzi et al. 2005). It can be assumed that progenitor cells from the secondary heart field may migrate into the branchial arch to form skeletal head muscles.
This assumption was strengthened by the study using Isl1-Cre mice (Nathan et al. 2008). Isl1 is first expressed in the cranial splanchnic mesoderm, especially in the secondary heart field (Cai et al. 2003). During further development, Isl1+ cells are found in the mesenchymal core of both 1st and 2nd branchial arch. Finally, Isl1+ cells are identified in muscles derived from these both branchial arches, such as the mylohyoid, styloid, digastric, buccinator, and facial subcutaneous muscles. This observation predicts that the head muscle and cardiac muscle share a common cell lineage. This is confirmed by a retrospective clonal assay that cells derived from a single precursor are found in both branchiomeric head muscles and right ventricular and arterial pole myocardium (Lescroart et al. 2010).
The DiI labelling experiment in the chick confirmed the observation of the cre-lineage tracing experiment in the mouse (Nathan et al. 2008). After DiI was injected into the cranial SpM, cells of the outflow tract and the first branchial arch were labelled. Because cells from the CPM also migrate into the branchial arch to form head muscles (Noden et al. 1999; Tirosh-Finkel et al. 2006), the topographic relationship of the CPM and SpM descendants in the branchial arch were addressed. By means of injection of DiI and DiO into the CPM and SpM, respectively, of the same embryo, Nathan et al. (2008) showed that the CPM-derived precursor cells are located in the proximal part of the first branchial arch, while the SpM-derived cells reside in the distal part of the first branchial arch. Due to dilution of dye after several cell divisions, the cell fate of the precursor cells in the CPM and SpM cannot be followed directly. So Nathan et al. (2008) labelled cells in the proximal and distal mesenchymal core of the first branchial arch using DiI and DiO, respectively. Although neural crest cells in the mesenchymal core of the branchial arch were also labelled, these cells are known to never differentiate into myocytes. The dye labelled proximal myogenic population was found to contribute to the masseter muscle, while the distal myogenic population gives rise to the intermandibular muscles. The contribution of the CPM to the mastication muscle was confirmed by the retrospective clonal assay made by Lescroart et al. (2010), who observed that a cell lineage which gave temporalis and masseter muscle provided also muscle cells for the extraocular muscle.
A further LPM-derived muscle is a dorsolateral neck muscle, the cucullaris muscle in birds, corresponding to the trapezius and sternocleidomastoideus in mammals. The M. cucullaris in birds is a very broad and flat muscle, which can be subdivided into M. cucullaris capitis and M. cucullaris cervicis. The cucullaris capitis muscle has its origin in the lateral surface of the head, Os squamosum (a part of the temporal bone) and extends caudally to the neck. The muscle sheets of both sides touch each other in the dorsal region of the neck from the 2. to the 7. cervical segments, forming a hood. Then, the muscle is subdivided into three portions. The Pars interscapularis sends muscle fibres into the skin in front of the shoulder joint. The Pars propatagialis consists of a few muscle fibres, which reach the flight skin (propatagium). The Pars clavicularis draws ventrally over the crop and extends as a thin and triangular muscle sheet between the furcula, the forked clavicle bones. The terminal tendon of this muscle touches finally the rostrum sterni. The M. cucullaris capitis has its attachment in the shoulder girdle region. The M. cucullaris cervicis encompasses the caudal part of the neck and the shoulder region.
In our previous study, we demonstrated that this muscle originates from the LPM at the level of the occipital somites (Theis et al. 2010). To trace the LPM descendants, we replaced a piece of the LPM adjacent to the three first occipital somites of a host chick embryo with the same tissue part from either quail or transgenic chick embryos expressing cytoplasmic GFP under control of the beta-actin promoter (provided by Dr. H. Sang). While quail cells can be identified using a perinuclear antibody only on tissue sections, GFP cells can be seen in whole mounts of embryos. So we observed that GFP cells distributed in the neck region according to the same pattern as the cucullaris muscle. GFP cells extended from the head to the shoulder region. Furthermore, GFP cells populate also in the upper back region, corresponding to the trapezius muscle. In sections, GFP cells as well as quail cells were identified as muscle cells in the M. cucullaris.
In previous studies, it was reported that the cucullaris muscle is composed of myoblasts from somites in chicken (Noden 1983a, b, 1986a, b; Noden et al. 1999; Couly et al. 1993; Huang et al. 1997, 2000). The somitic origin of the cucullaris muscle was confirmed by a study in Ambystoma mexicanum embryos (Piekarski and Olsson 2007). They injected FITC-dextran into cranial somites and observed FITC-labelled cells in this muscle. In view of numerous studies evidencing the somitic contribution to the cucullaris, two scenarios were proposed. First, the cucullaris muscle could derive muscle progenitors from both somites and LPM, in a manner similar to the branchiomeric musculature (Harel et al. 2009). Second, the cucullaris muscle is derived only from one of these structures, as experiments leading to the aforementioned conclusion could have arisen due to tissue contamination during the transplantation. We improved the transplantation procedure by using dispase I to reduce the tissue contamination and quantified the cellular contribution of the somite and the lateral plate. We found that the somitic contribution was quite minor, whereas there was a very high density of tissue originating from the lateral plate in the cucullaris muscle. These results demonstrate that the cucullaris muscle is mainly derived from the LPM.
Our genetic cell lineage tracing study in mouse exclude the somitic contribution to the cucullaris muscle and substantiate the finding from the transplantation experiment in birds (Theis et al. 2010). In mammals, the trapezius and sternocleidomastoideus muscle are avian homologues of the cucullaris muscle. Both neural crest cells and somite cells express Pax3 (Goulding et al. 1991, 1993; Goulding and Paquette 1994). In Pax3 Cre :Rosa STOP/YFP embryos, these cells express Cre-recombinase under the endogenous Pax3 promoter, which ultimately initiates YFP expression from a floxed Rosa allele. YFP fluorescence marks all cells with a past or present history of Pax3 expression. We found YFP activity in most trunk muscles. However, we could not find YFP-positive cells in the muscle fibres of the trapezius and sternocleidomastoideus as well as other head muscles. YFP cells could be found only between the muscle fibres in the head. These were the neural crest-derived cells which form connective tissue of these muscles (Noden 1983a). These results confirm the sole contribution of the LPM to the cucullaris muscle in birds and its homologues in mammals.
Taken together, the LPM participates in the formation of branchiomeric muscles and dorsolateral neck muscles. While branchiomeric muscles are comprised of myoblasts from both CPM and LPM, the dorsolateral neck muscle is derived only from the lateral plate.
5 Lateral Plate-Derived Muscles Differentiate Later Than Other Muscles
After examining the formation of the skeletal musculature in head and trunk, we found that the cucullaris muscle developed in chick embryos very late compared to other skeletal muscles (Theis et al. 2010). First, the cucullaris myogenic cells require a long period to reach their destination. The cranial to caudal migration of the LPM cells from the level of somite 1-3 is detectable first by HH-14. By HH-20, the transplanted cells extended just to the sixth somite level. The caudal end of the grafted tissue reached the anterior limb base by HH-26. The morphological form resembling the adult muscle was achieved by HH-30.
The differentiation of the cucullaris muscle also occurs at a relatively late stage. MyoD expression was found to be initiated in somites, limb, and branchial arches at HH-24. The first faint expression of MyoD was detected in the cucullaris muscle at HH-26. Just after HH-30, MyoD had reached its entire extent of the muscle. Correspondingly, the terminal muscle markers could be detectable in somites, extremities, and heart at HH-24. Differentiated myoblasts could be seen in the second and third branchial arch at HH-26. However, the myoblasts were detected in the cucullaris muscle at HH-30.
The late differentiation of the cucullaris muscle predicts that the myogenic precursor required a longer period for proliferation than other muscles. The possible reason might be that the muscle is very long and large. So the progenitor cells require long time for generating a large pool of myogenic cells. It is still unknown how the cell proliferation is controlled in this process.
This feature of late development is conserved in vertebrates. For instance, in the turtle at stage 15, differentiated muscle was found in the head, trunk and limb with exception of the cucullaris muscle. The cucullaris muscle was clearly discernible at stage 17. It means that also in the turtle, the cucullaris develops later than the other muscles.
6 Molecular Regulation of the Myogenesis in the Lateral Plate Mesoderm
Although there are only few studies concerning the LPM myogenesis directly (Theis et al. 2010; Harel et al. 2009; Nathan et al. 2008; Dong et al. 2006; Verzi et al. 2005), numerous studies investigating head myogenesis provide some knowledge about myogenesis in the LPM (Harel et al. 2009; Sambasivan et al. 2009; Ericsson and Olsson 2004; Ericsson et al. 2004; Olsson et al. 2001; Bothe and Dietrich 2006; Rinon et al. 2007; Lescroart et al. 2010; Couly et al. 1992; Noden 1983a, b; Noden 1986a, b; Marcucio and Noden 1999; Noden et al. 1999; reviewed by Noden and Francis-West 2006; Tzahor 2009; Sambasivan et al. 2011; Buckingham and Vincent 2009; Buckingham et al. 2005; Kelly et al. 2004).
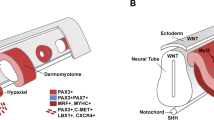
During the embryonic myogenesis in somites, Pax3 and Pax7 are expressed the dermomyotome and play pivotal roles for the cell proliferation and survival of the somitic myogenic progenitors (Relaix et al. 2005; Sambasivan and Tajbakhsh 2007). Pax3 and Pax7 act upstream of MyoD (Tajbakhsh et al. 1997). However, both these transcription factors are not involved in the myogenesis in the cranial mesoderm (Noden and Francis-West 2006). The cranial myogenesis is regulated by a series of different transcription factors, such as Pitx2, Tbx1, MyoR, Capsulin, and Isl1 (reviewed by Sambasivan et al. 2011; Tzahor 2009).
Pituitary homeobox 2 (Pitx2), a paired-related homeobox gene, is expressed in the cranial mesoderm including the periocular mesenchyme and the core mesoderm of the first branchial arch (Gage et al. 1999; Kitamura et al. 1999). In Pitx2-null embryo, EOMs are missing (Gage et al. 1999; Kitamura et al. 1999). Pitx2 is required for MyoR expression in the first branchial arch muscle precursors. In the Pitx2 null mouse, cells derived from the SpM, which were marked with Mef2c-AHF-Cre LacZ expression (Verzi et al. 2005), could not migrate into the first branchial arch (Dong et al. 2006). Pitx2 regulates specifically the early muscle specification of the first branchial arch (Shih et al. 2007a, b).
The T-box containing transcription factor Tbx1 plays a critical role in the head muscle and cardiac out flow tract development (Kelly et al. 2004). Tbx1 is required for the activation of Myf5 and MyoD in all branchiomeric muscles including both CPM- and LPM-derived muscles, whereas Tbx1 is not involved in the regulation of extraocular and tongue muscles. In the Tbx1-mutant, the formation of branchiomeric muscles such as jaw, craniofacial and laryngeal muscles, as well as trapezius are affected.
MyoR (Msc, musculin) and Capsulin (Tcf21) are bHLH transcription factors expressed in the head and body muscles. Both are postulated to repress the myogenic differentiation. Mutations of these both genes lead to the absence of a subset first arch-derived jaw muscles (masseter, pterygoid, and temporalis muscles). However, distal muscles of the first branchial arch (anterior digastri and mylohyoid) were not affected (Lu et al. 2002). This suggests that MyoR and Capsulin specifically control the formation of the CPM-derived but not the SpM-derived muscles in the first branchial arch.
Isl1 (the LIM homeodomain protein Islet1) plays pivotal role for the proliferation, differentiation and lineage specification of distinct cardiovascular precursors (Cai et al. 2003; Laugwitz et al. 2005; Moretti et al. 2006). Isl1 is expressed also in the branchial muscle progenitors derived from the SpM (Nathan et al. 2008). After overexpression of Isl1 by means of RCAS-Isl1 in chick embryos, MyoD, Myogenin, and MyHC were blocked in CPM explants in vitro and in the first branchial arch in vivo (Harel et al. 2009). Since Isl1 expression could be induced by BMP4 which has been shown to inhibit myogenesis in both somites and head mesoderm (Tirosh-Finkel et al. 2006), one can assume that BMP4 may fulfil its inhibitory function on myogenesis via inducing Isl1 expression (Harel et al. 2009). Furthermore, Isl1 was inhibited by overexpression using electroporation of Wnt3-IRES-GFP into the surface ectoderm. In agreement, inhibition of Wnt pathway with sFrp2 and sFrp3 resulted in an expansion of Isl1 expression (Nathan et al. 2008).
As described above, the cucullaris muscle in birds represents a pure lateral plate-derived muscle (Theis et al. 2010). We showed that the trunk myogenic programme is not involved in the development of this muscle. Pax3 and Pax7 which drive somite myogenic progenitor cell proliferation while suppressing differentiation (Amthor et al. 1999; Amthor et al. 1998) were never expressed in this muscle in chick embryos. Instead of expressing Pax3 and Pax7, genes of the head myogenic programme, such as MyoR, Tbx1, and Capsulin were expressed in the anlagen of the cucullaris muscle during the early development in chick embryos. The role of the head myogenic programme in the development of the cucullaris muscle was confirmed by examining Pax3cre:Rosastop/YFP, Pax3sp/sp:Myf5nlacZ/nlacZ, and Tbx1−/− mouse mutants (Engleka et al. 2005; Kelly et al. 2004). We found that myoblasts of the trapezius and sternocleidomastoid, the cucullaris homologues in mammals, never expressed Pax3 . Furthermore, the trapezius and sternocleidomastoid muscles were present in the Pax3sp/sp:Myf5nlacZ/nlacZmutants, in which all somite-derived muscles were missing. This is concordant with the finding in the Tbx1−/− line, which failed to form both of these neck muscles. Additionally the trapezius has a molecular history for Isl1, which might repress the differentiation and promote the proliferation of the myogenic progenitors during the early development of head muscles (Harel et al. 2009). Our lineage tracing experiment suggests that these muscles require a very long period for generating enough number of myoblasts to form a large muscle.
The cucullaris muscle is a long muscle sheet which is located from the occipital to the thorax region. It is still unknown how myogenic cells originating from the occipital region reach the shoulder and thorax region. Recently, myotomal cells have been shown to extend from one segment to the next segment (Chankiewitz et al. 2014). This myotomal extension is controlled by a thymosin beta 15-like peptide. Myogenic cells of the cucullaris might extend from their origin to the thorax and shoulder region by means of the same mechanism.
7 Cranial Neural Crest Cells Form the Connective Tissue of the Lateral Plate-Derived Muscle
Cranial neural crest (CNC) provides a wider range of differentiation potential than trunk crest. Their derivatives include not only neurons, glia, and pigment cells, but also skeletal cells of the head. In addition, CNC has also been reported to form the connective tissue of branchiomeric muscles (Noden 1983a; Kontges and Lumsden 1996). Using the quail-chick cell lineage tracing technique, in which the CPM and cranial neural crest from quail to chick was transplanted, Noden (1983a) observed that the branchiomeric muscle has two components: myogenic cells are of CPM origin and the connective tissue is derived from the cranial neural crest. Using the same tracing technique, Kontges and Lumsden (1996) mapped the neural crest (NC) subpopulations of individual rhombomeres. They observed that each rhombomeric NC population forms both the connective tissues of specific branchiomeric and hypoglossal muscles and their attachment sites on the mandibular and lower jaw skeleton.
This relationship between neural crest and paraxial mesoderm was extended to the neck region (Matsuoka et al. 2005). The vertebrate neck is a mobile interconnection of the head and trunk (McGonnell 2001). The length of the neck varies from one segment to 76 segments. The primitive amphibians were in possession of the first cervical vertebra (Torrey 1978). The fossil diapsid Muraenosaurus had 76 cervical vertebrae (Young 1981). While mammals have 7 cervical vertebrae, the avian cervical spinal column contains 13 (pigeon) to 25 segments (swans) (Burke et al. 1995). In spite of the different length of the neck, the dorsal and ventral shoulder muscles can extend from head to trunk, operating the shoulder girdle. Using cre-recombinase-mediated Wnt1 and Sox10 transgenesis, Matsuoka et al. (2005) mapped the long-term cell fate of NC in mouse embryos. They revealed that the connective tissue of dorsal and ventral neck muscles at both head and shoulder attachment sites is of NC origin. This was confirmed by other research groups who used also the Wnt1 transgenic (Theis et al. 2010; Valasek et al. 2010). We further investigated the originating axial level of the NC in our previous study. Using quail–chick chimaeras, we found that the neural crest cells formed the cucullaris muscle connective tissue, the dorsal neck muscle in birds, are derived from the occipital level (Theis et al. 2010). This observation led us to predict that both NC-derived connective and LPM-derived myogenic precursor cells originate from the same axial level, the occipital level, and migrate from the head region caudally to the trunk during formation of the neck.
The contribution of cranial neural crest cells to the connective tissue of cranial muscles was investigated also in amphibians (reviewed in Schmidt et al. 2013 and Ericsson et al. 2013). Using DiI labelling, green fluorescent protein (GFP) mRNA injection and transplantation of neural folds, Olsson’s group showed that cranial neural crest cells form the connective tissue but not the myofibers in the branchiomeric muscles in Bombina orientali (Olsson et al. 2001) and in Ambystoma mexicanum (Ericsson et al. 2004).
8 Cranial Neural Crest Cells Determine the Patterning of the Lateral Plate-Derived Muscle
The CNC origin of the connective tissue suggests an important function of the neural crest in the patterning of the LPM-derived muscle. Following heterotopic transplantation in which neural crest at the level of the presumptive first branchial arch was grafted to the level of the presumptive second and third branchial arch, grafted cells form a duplicated first branchial arch skeletal system in the ectopic location (Noden 1983b). Furthermore, the pattern of the branchiomeric muscle is dependent upon properties of the grafted neural crest. These results indicate that neural crest cells are prespecified prior to their migration into branchial arch regarding the patterning of the branchiomeric skeletal system and the form of the associated muscle. The patterning information of the neural crest is not only axial level specific but also species specific. Homotopic transplantation of neural crest from a duck into a quail embryo led to the formation of duck-specific beak in the quail host (Tucker and Lumsden 2004).
After the migration into the branchial arch, CNC cells and the muscle precursor cells are arranged in a highly organised fashion. The muscle progenitors are located in the core of the branchial arch, while CNC cells are located beneath the ectoderm. Hence, the CNC cells enclose the muscle precursor cells and separate them from the overlying surface ectoderm (Noden and Trainor 2005). The neural crest cells streaming into a given branchial arch form both attachment sites of the muscle which is derived from the same branchial arch. Thus, the connective tissue forming neural crest cells and the myogenic cells take the same migratory route. Thereby, the neural crest cells could provide guidance cues for the migratory myogenic progenitors (Kontges and Lumsden 1996; Olsson et al. 2001; Matsuoka et al. 2005).
Based on the observation that surgical removal of the neural crest did not interrupt the early myogenesis in the branchial arch, the early branchiomeric myogenesis is independent of the neural crest cells (Olsson et al. 2001; Tzahor et al. 2003; Ericsson et al. 2004; Ericsson and Olsson 2004; Rinon et al. 2007). In amphibian embryos, myogenesis is initiated in absence of neural crest cells. However, the myogenic progenitor cells cannot reach their destinations (Olsson et al. 2001; Ericsson et al. 2004; Ericsson and Olsson 2004). In chick embryos, Myf5, MyoD, Tbx1 and capsulin were expressed in the branchial arches after neural crest ablation. However, their expression pattern was interrupted (Tzahor et al. 2003; Rinon et al. 2007). These findings indicate that neural crest cells are essential for the pattern of myogenic gene expression. They are, however, dispensable for the initiation of the myogenesis.
The results from the surgical removal in avian and amphibian embryos might be influenced by the manipulation limitation and thus their interpretation could be very complex, since neural crest is known to regenerate following ablation (Saldivar et al. 1997; Scherson et al. 1993; Vaglia and Hall 1999). To avoid the problem with post-operative regeneration of neural crest in chick and amphibian embryos, Rinon et al. (2007) analysed mutant mice in which crest cells were genetically ablated. One of such mouse line was the Hoxa1/Hoxb1 double-mutant mouse, in which crest cells fail to migrate into the second branchial arch. Though early muscle markers, such as capsulin and Tbx1, were detected in the second branchial arch, their expression was broader in the mutant than those in the control mouse.
9 Lateral Plate Mesoderm and Muscle Stem Cells
The satellite cell located in the basal lamina of a muscle fibre is the resident stem cell of skeletal muscle, carrying out the routine maintenance, hypertrophy, and repair of damaged adult skeletal muscles (Mauro 1961; Buckingham 2007; Kuang et al. 2007; Zammit et al. 2006). Satellite cells maintain a stable stem cell pool by means of the primary self-renewal mechanism (Collins et al. 2005; Montarras et al. 2005; Sacco et al. 2008). Pax7 expressed by satellite cells controls the generation of embryonic myogenic precursor cells (Seale et al. 2000; Lepper et al. 2009). In double mutants of Pax3 and Pax7, muscle development was severely affected (Relaix et al. 2005). Once activated, satellite cells co-express Pax7 with MyoD. After the cell division, one of the daughter cell down-regulates Pax7 and maintains MyoD. As a result it induces myogenin and differentiates into myoblast. The other daughter cell down-regulates MyoD and maintains Pax7 and remains in a quiescent state (Halevy et al. 2004; Zammit et al. 2004).
In vertebrates, trunk and limb skeletal muscles originate from somites, segmented paraxial mesodermal structures (Christ and Ordahl 1995). Using the quail–chick cell lineage tracing system, Armand et al. (1983) reported for the first time the embryonic origin of the satellite cells of the trunk muscle from the somites. During the development, the somite undergoes a dorsoventral compartimentalisation, resulting in a ventral mesenchymal sclerotome and a dorsal epithelial dermomyotome. While the sclerotome is responsible for the formation of the axial skeleton, the dermomyotome gives rise to dermal and muscular tissues (Stockdale et al. 2000). Gros et al. (2005) and Relaix et al. (2005) demonstrated that the dermomyotome is also the source of satellite cells. In chick embryos, different somitic compartments were labelled by electroporation of GFP-vectors and by quail–chick chimaeras. Using these two complementary cell tracing techniques, Gros et al. (2005) concluded that the central part of the dermomyotome contributes to both embryonic myogenic precursors and adult satellite cells. In mice, cells of the central dermomyotome labelled by reporter genes targeted into Pax3 and Pax7 loci were found to delaminate and migrate into the early myotome. In the late development, these cells were found to integrate into the adult muscle as satellite cells (Relaix et al. 2005). The limb muscle satellite cells originate from the ventral dermomyotome which provides hypaxial muscle precursors for the limb and ventral body wall (Schienda et al. 2006).
In the head, branchiomeric muscle satellite cells were found to have two sources. Using quail–chick chimaeras, Harel et al. (2009) identified CPM-derived cells in the position of satellite cells in masticatory muscles. They found that 90 % of satellite cells of eye and masticatory muscles were of CPM origin. Islet1 is expressed in the splanchnic layer of the LPM. In the Islet1Cre mouse line, Islet1+/+ cells were found to contribute to 90 % satellite cells of masseter and anterior digastric muscles. It is hard to understand how 90 % of satellite cells of a muscle are of CPM origin in chick embryos and the same muscle receives 90 % satellite cells from the SpM in mouse. The reason might lie in the difference between species. The reason for this finding is possibly the continuum from the CPM to the LPM as described above. This could lead to the problem with contamination of CPM cells with LPM cells during the tissue transplantation in birds. Furthermore, the overlap expression of Isl1 in the CPM and SpM may be so strong that Isl1-positive cells not only represent cells derived from the SpM, but also the CPM.
Although it remains to be clarified whether the lateral plate at the occipital level gives rise to satellite cells of the dorsolateral neck muscles (cucullaris in birds and trapezius and sternocleidomastoideus in mammals), the above described observations predict that both myogenic and satellite cell progenitors arise from common embryonic origins. They travel along the same route to their destinations and maintain their spatial neighbourhood during development.
There are no studies investigating directly satellite cells being derived from the LPM. Based on the observation that Isl1-expressed SpM gave rise to a vast majority of satellite cells of the masseter muscle, results arising from the studies on masseter can be considered as representative for the lateral plate-derived satellite cells (Ono et al. 2010; Harel et al. 2009). After obtaining the percentage of satellite cell number on the total number of nuclei per myofiber, Ono et al. (2010) found that masseter muscle has fewer satellite cells than limb muscle. After counting the percentage of self-renewing satellite cells (Pax7+/MyoD-) and differentiating satellite cells (Pax7-/MyoD+, Pax7-/myogenin +) in relation to the total number of satellite cells per myofiber at different culture time points, they found that masseter-derived satellite cells differentiate later than those from a limb muscle. Furthermore, satellite cells of the masseter muscle have stronger proliferative ability than those from limb muscles. Gene expression profiles of satellite cell-derived myoblasts (after 4 h of isolated satellite cell culture) were compared between limb muscles and masseter muscle by measurements of gene expression intensity using quantitative RT-PCR. The results demonstrate that satellite cell-derived myoblasts maintain their molecular profile from their embryonic origin. Pax3 was robustly expressed in myoblasts from limb muscles, whereas it was not detectable in myoblasts from masseter. Pitx2b and Pitx2c were significantly higher in myoblasts from limb muscles than those from masseter. Pax7 and Mrf4 were more highly expressed in myoblasts from masseter muscle than in those from limb muscles. It is noteworthy that digastricus muscle-derived satellite cells (solely derived from the lateral plate) displayed a very high amplitude of gene expression of Nkx2.5 compared to those from limb muscle. Furthermore, masseter-derived cells expressed much stronger Tcf21 (Capsulin), which represents an important transcriptional factor for craniofacial muscle formation (Lu et al. 2002), than those from limb muscles (Ono et al. 2010; Harel et al. 2009).
During embryonic myogenesis, BMP4 induces cardiogenesis, while it blocks myogenesis in both head mesoderm and somites (Tirosh-Finkel et al. 2006; Reshef et al. 1998). BMP4 induces the proliferation of satellite cells derived from both trunk and head muscles. The inhibition of the myogenic differentiation through BMP4 was less potent in satellite cells derived from the masseter muscle than those from limb muscles (Harel et al. 2009). The proliferative state of satellite cells was revealed by Myf5 and Pax7 expression, while their differentiation activity was identified by MyoG and MyHC and also viewed by myofiber formation. In addition, BMP4 induces stronger expression of Isl1 and Tbx20 in satellite cells from masseter muscle than those from limb muscles. Isl1 and Tbx20 which are considered as cardiac markers were shown to repress myogenic differentiation in the head myogenesis, up-regulation of Isl1 by BMP4 predicts that BMP4 maintains the plasticity of satellite cells of head muscles (Harel et al. 2009).
In spite of their different embryonic origin and regulatory properties, lateral plate-derived satellite cells can regenerate somite-derived muscles (Harel et al. 2009; Ono et al. 2010). It has been shown that limb-derived satellite cells transplanted into an irradiated limb muscle can generate hundreds of muscle fibres (Collins et al. 2005). Ono et al. (2010) performed transplantation of satellite cells isolated from the masseter and the extensor digitorum longus (a limb muscle), respectively, into the tibialis anterior muscle (a limb muscle). In the fourth week of posttransplantation, they observed that the amount of donor-derived newly formed muscle fibres was not significantly different between muscles receiving either masseter- or extensor digitorum longus-derived satellite cells. By means of single myofiber transplantation, Harel et al. (2009) obtained similar findings. These findings indicate that independent upon their origin, satellite cells can respond to local signals in the limb to generation myofibers.
10 Perspectives
It is interesting to speculate on the mechanisms that imbued myogenic properties on the anterior lateral plate mesoderm. We discuss here two possibilities that may lead to this outcome. In the first scenario, we propose that the head mesoderm, that has myogenic properties, extends posteriorly adjacent to the first three somites. This is feasible since the head and lateral plate mesoderm are continuous. Alternatively, the occipital lateral plate mesoderm may have been patterned to gain characteristics of head mesoderm by the posterior extension of a molecular boundary that confers myogenic properties. There are numerous examples in the animal kingdom where molecular boundary shifts regulate the development of tissues, both in invertebrates and vertebrates. Further investigation using a combination of cell tracing and molecular analysis of key genes especially members of the Hox family of transcriptional factors will be needed to determine which of these possibilities is responsible for the lateral plate myogenicity.
References
Amthor H, Christ B, Weil M, Patel K (1998) The importance of timing differentiation during limb muscle development. Curr Biol 8:642–652
Amthor H, Christ B, Patel K (1999) A molecular mechanism enabling continuous embryonic muscle growth—a balance between proliferation and differentiation. Development 126:1041–1053
Armand O, Boutineau AM, Mauger A, Pautou MP, Kieny M (1983) Origin of satellite cells in avian skeletal muscles. Arch Anat Microsc Morphol Exp 72:163–181
Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C (1995) Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376:768–771. doi:10.1038/376768a0
Bothe I, Dietrich S (2006) The molecular setup of the avian head mesoderm and its implication for craniofacial myogenesis. Dev Dyn 235:2845–2860. doi:10.1002/dvdy.20903
Buchberger A, Pabst O, Brand T, Seidl K, Arnold HH (1996) Chick NKx-2.3 represents a novel family member of vertebrate homologues to the Drosophila homeobox gene tinman: differential expression of cNKx-2.3 and cNKx-2.5 during heart and gut development. Mech Dev 56:151–163
Buckingham M (2007) Skeletal muscle progenitor cells and the role of Pax genes. C R Biol 330:530–533. doi:10.1016/j.crvi.2007.03.015
Buckingham M, Vincent SD (2009) Distinct and dynamic myogenic populations in the vertebrate embryo. Curr Opin Genet Dev 19:444–453. doi:10.1016/j.gde.2009.08.001
Buckingham M, Meilhac S, Zaffran S (2005) Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet 6:826–835. doi:10.1038/nrg1710
Burke AC, Nelson CE, Morgan BA, Tabin C (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121:333–346
Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S (2003) Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell 5:877–889
Chankiewitz V, Morosan-Puopolo G, Yusuf F, Rudloff S, Pröls F, Kleff V, Hofmann DK, Brand-Saberi B (2014) A thymosin beta15-like peptide promotes intersegmental myotome extension in the chicken embryo. Histochem Cell Biol 141:275–87. doi:10.1007/s00418-013-1156-z
Charite J, McFadden DG, Olson EN (2000) The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development 127:2461–2470
Chevallier A, Kieny M, Mauger A (1977) Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol 41:245–258
Christ B, Jacob HJ, Jacob M (1977) [Experimental findings on muscle development in the limbs of the chick embryo]. Verh Anat Ges 1231–1237
Christ B, Ordahl CP (1995) Early stages of chick somite development. Anat Embryol (Berl) 191:381–396
Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122:289–301. doi:10.1016/j.cell.2005.05.010
Couly GF, Coltey PM, Le Douarin NM (1992) The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114:1–15
Couly GF, Coltey PM, Le Douarin NM (1993) The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development 117:409–429
Dastjerdi A, Robson L, Walker R et al (2007) Tbx1 regulation of myogenic differentiation in the limb and cranial mesoderm. Dev Dyn 236:353–363. doi:10.1002/dvdy.21010
Deimling SJ, Drysdale TA (2009) Retinoic acid regulates anterior-posterior patterning within the lateral plate mesoderm of Xenopus. Mech Dev 126:913–923. doi:10.1016/j.mod.2009.07.001
Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL (2004) Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development 131:3931–3942. doi:10.1242/dev.01256
Dong F, Sun X, Liu W et al (2006) Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development 133:4891–4899. doi:10.1242/dev.02693
Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA (2005) Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280:396–406. doi:10.1016/j.ydbio.2005.02.002
Ericsson R, Olsson L (2004) Patterns of spatial and temporal visceral arch muscle development in the Mexican axolotl (Ambystoma mexicanum). J Morphol 261:131–140. doi:10.1002/jmor.10151
Ericsson R, Cerny R, Falck P, Olsson L (2004) Role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Dev Dyn 231:237–247. doi:10.1002/dvdy.20127
Ericsson R, Knight R, Johanson Z (2013) Evolution and development of the vertebrate neck. J Anat 222:67–78. doi:10.1111/j.1469-7580.2012.01530.x
Evans DJ, Noden DM (2006) Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dyn 235:1310–1325. doi:10.1002/dvdy.20663
Funayama N, Sato Y, Matsumoto K, Ogura T, Takahashi Y (1999) Coelom formation: binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development 126:4129–4138
Gage PJ, Suh H, Camper SA (1999) The bicoid-related Pitx gene family in development. Mamm Genome 10:197–200
Garg V, Yamagishi C, Hu T, Kathiriya IS, Yamagishi H, Srivastava D (2001) Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev Biol 235:62–73. doi:10.1006/dbio.2001.0283
Goulding M, Paquette A (1994) Pax genes and neural tube defects in the mouse. Ciba Found Symp 181:103–113, discussion 113-107
Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P (1991) Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J 10:1135–1147
Goulding M, Sterrer S, Fleming J et al (1993) Analysis of the Pax-3 gene in the mouse mutant splotch. Genomics 17:355–363. doi:10.1006/geno.1993.1332
Gros J, Manceau M, Thome V, Marcelle C (2005) A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 435:954–958. doi:10.1038/nature03572
Halevy O, Piestun Y, Allouh MZ et al (2004) Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn 231:489–502. doi:10.1002/dvdy.20151
Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E (2009) Distinct origins and genetic programs of head muscle satellite cells. Dev Cell 16:822–832. doi:10.1016/j.devcel.2009.05.007
Huang R, Zhi Q, Ordahl CP, Christ B (1997) The fate of the first avian somite. Anat Embryol (Berl) 195:435–449
Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K (1999) Origin and development of the avian tongue muscles. Anat Embryol (Berl) 200:137–152
Huang R, Zhi Q, Patel K, Wilting J, Christ B (2000) Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anat Embryol (Berl) 202:375–383
Kelly RG, Jerome-Majewska LA, Papaioannou VE (2004) The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet 13:2829–2840. doi:10.1093/hmg/ddh304
Kitamura K, Miura H, Miyagawa-Tomita S et al (1999) Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126:5749–5758
Kontges G, Lumsden A (1996) Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development 122:3229–3242
Kraus F, Haenig B, Kispert A (2001) Cloning and expression analysis of the mouse T-box gene tbx20. Mech Dev 100:87–91
Kuang S, Kuroda K, Le Grand F, Rudnicki MA (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129:999–1010. doi:10.1016/j.cell.2007.03.044
Laugwitz KL, Moretti A, Lam J et al (2005) Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 433:647–653. doi:10.1038/nature03215
Lepper C, Conway SJ, Fan CM (2009) Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460:627–631. doi:10.1038/nature08209
Lescroart F, Kelly RG, Le Garrec JF, Nicolas JF, Meilhac SM, Buckingham M (2010) Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 137:3269–3279. doi:10.1242/dev.050674
Lin Q, Schwarz J, Bucana C, Olson EN (1997) Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science 276:1404–1407
Lu JR, Bassel-Duby R, Hawkins A et al (2002) Control of facial muscle development by MyoR and capsulin. Science 298:2378–2381. doi:10.1126/science.1078273
Mahlapuu M, Ormestad M, Enerback S, Carlsson P (2001) The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128:155–166
Marcucio RS, Noden DM (1999) Myotube heterogeneity in developing chick craniofacial skeletal muscles. Dev Dyn 214:178–194. doi:10.1002/(SICI)1097-0177(199903)214:3<178::AID-AJA2>3.0.CO;2-4
Matsuoka T, Ahlberg PE, Kessaris N et al (2005) Neural crest origins of the neck and shoulder. Nature 436:347–355. doi:10.1038/nature03837
Mauro A (1961) Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol 9:493–495
McGonnell IM (2001) The evolution of the pectoral girdle. J Anat 199:189–194
Montarras D, Morgan J, Collins C et al (2005) Direct isolation of satellite cells for skeletal muscle regeneration. Science 309:2064–2067. doi:10.1126/science.1114758
Moretti A, Caron L, Nakano A et al (2006) Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165. doi:10.1016/j.cell.2006.10.029
Nathan E, Monovich A, Tirosh-Finkel L et al (2008) The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development 135:647–657. doi:10.1242/dev.007989
Noden DM (1983a) The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat 168:257–276. doi:10.1002/aja.1001680302
Noden DM (1983b) The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol 96:144–165
Noden DM (1986a) Origins and patterning of craniofacial mesenchymal tissues. J Craniofac Genet Dev Biol Suppl 2:15–31
Noden DM (1986b) Patterning of avian craniofacial muscles. Dev Biol 116:347–356
Noden DM, Francis-West P (2006) The differentiation and morphogenesis of craniofacial muscles. Dev Dyn 235:1194–1218. doi:10.1002/dvdy.20697
Noden DM, Trainor PA (2005) Relations and interactions between cranial mesoderm and neural crest populations. J Anat 207:575–601. doi:10.1111/j.1469-7580.2005.00473.x
Noden DM, Marcucio R, Borycki AG, Emerson CP Jr (1999) Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn 216:96–112. doi:10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6
Olsson L, Falck P, Lopez K, Cobb J, Hanken J (2001) Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol 237:354–367. doi:10.1006/dbio.2001.0377
Ono Y, Boldrin L, Knopp P, Morgan JE, Zammit PS (2010) Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev Biol 337:29–41. doi:10.1016/j.ydbio.2009.10.005
Piekarski N, Olsson L (2007) Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum). Evol Dev 9:566–578. doi:10.1111/j.1525-142X.2007.00197.x
Psychoyos D, Stern CD (1996) Fates and migratory routes of primitive streak cells in the chick embryo. Development 122:1523–1534
Relaix F, Rocancourt D, Mansouri A, Buckingham M (2005) A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435:948–953. doi:10.1038/nature03594
Reshef R, Maroto M, Lassar AB (1998) Regulation of dorsal somitic cell fates: BMPs and Noggin control the timing and pattern of myogenic regulator expression. Genes Dev 12:290–303
Rinon A, Lazar S, Marshall H et al (2007) Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development 134:3065–3075. doi:10.1242/dev.002501
Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456:502–506. doi:10.1038/nature07384
Saldivar JR, Sechrist JW, Krull CE, Ruffins S, Bronner-Fraser M (1997) Dorsal hindbrain ablation results in rerouting of neural crest migration and changes in gene expression, but normal hyoid development. Development 124:2729–2739
Sambasivan R, Tajbakhsh S (2007) Skeletal muscle stem cell birth and properties. Semin Cell Dev Biol 18:870–882. doi:10.1016/j.semcdb.2007.09.013
Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S (2009) Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell 16:810–821. doi:10.1016/j.devcel.2009.05.008
Sambasivan R, Kuratani S, Tajbakhsh S (2011) An eye on the head: the development and evolution of craniofacial muscles. Development 138:2401–2415. doi:10.1242/dev.040972
Scherson T, Serbedzija G, Fraser S, Bronner-Fraser M (1993) Regulative capacity of the cranial neural tube to form neural crest. Development 118:1049–1062
Schienda J, Engleka KA, Jun S et al (2006) Somitic origin of limb muscle satellite and side population cells. Proc Natl Acad Sci U S A 103:945–950. doi:10.1073/pnas.0510164103
Schmidt J, Piekarski N, Olsson L (2013) Cranial muscles in amphibians: development, novelties and the role of cranial neural crest cells. J Anat 222:134–146. doi:10.1111/j.1469-7580.2012.01541.x
Schoenwolf GC, Alvarez IS (1992) Role of cell rearrangement in axial morphogenesis. Curr Top Dev Biol 27:129–173
Schoenwolf GC, Garcia-Martinez V, Dias MS (1992) Mesoderm movement and fate during avian gastrulation and neurulation. Dev Dyn 193:235–248. doi:10.1002/aja.1001930304
Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102:777–786
Selleck MA, Stern CD (1991) Fate mapping and cell lineage analysis of Hensen’s node in the chick embryo. Development 112:615–626
Shih HP, Gross MK, Kioussi C (2007a) Cranial muscle defects of Pitx2 mutants result from specification defects in the first branchial arch. Proc Natl Acad Sci U S A 104:5907–5912. doi:10.1073/pnas.0701122104
Shih HP, Gross MK, Kioussi C (2007b) Expression pattern of the homeodomain transcription factor Pitx2 during muscle development. Gene Expr Patterns 7:441–451. doi:10.1016/j.modgep.2006.11.004
Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21:70–71. doi:10.1038/5007
Srivastava D, Cserjesi P, Olson EN (1995) A subclass of bHLH proteins required for cardiac morphogenesis. Science 270:1995–1999
Stephens TD, Beier RL, Bringhurst DC, Hiatt SR, Prestridge M, Pugmire DE, Willis HJ (1989) Limbness in the early chick embryo lateral plate. Dev Biol 133:1–7
Stockdale FE, Nikovits W Jr, Christ B (2000) Molecular and cellular biology of avian somite development. Dev Dyn 219:304–321. doi:10.1002/1097-0177(2000)9999:9999<::AID-DVDY1057>3.0.CO;2-5
Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89:127–138
Theis S, Patel K, Valasek P et al (2010) The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development 137:2961–2971. doi:10.1242/dev.049726
Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E (2006) Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 133:1943–1953. doi:10.1242/dev.02365
Torrey TW (1978) Morphogenesis of the Vertebrates. Wiley, New York
Tucker AS, Lumsden A (2004) Neural crest cells provide species-specific patterning information in the developing branchial skeleton. Evol Dev 6:32–40
Tzahor E (2009) Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Dev Biol 327:273–279. doi:10.1016/j.ydbio.2008.12.035
Tzahor E, Kempf H, Mootoosamy RC et al (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17:3087–3099. doi:10.1101/gad.1154103
Vaglia JL, Hall BK (1999) Regulation of neural crest cell populations: occurrence, distribution and underlying mechanisms. Int J Dev Biol 43:95–110
Valasek P, Evans DJ, Maina F, Grim M, Patel K (2005) A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development 132:447–458. doi:10.1242/dev.01545
Valasek P, Theis S, Krejci E et al (2010) Somitic origin of the medial border of the mammalian scapula and its homology to the avian scapula blade. J Anat 216:482–488. doi:10.1111/j.1469-7580.2009.01200.x
Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL (2005) The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol 287:134–145. doi:10.1016/j.ydbio.2005.08.041
Wachtler F, Jacob M (1986) Origin and development of the cranial skeletal muscles. Bibl Anat 24–46
Wachtler F, Jacob HJ, Jacob M, Christ B (1984) The extrinsic ocular muscles in birds are derived from the prechordal plate. Naturwissenschaften 71:379–380
Waxman JS, Keegan BR, Roberts RW, Poss KD, Yelon D (2008) Hoxb5b acts downstream of retinoic acid signaling in the forelimb field to restrict heart field potential in zebrafish. Dev Cell 15:923–934. doi:10.1016/j.devcel.2008.09.009
Yamagishi T, Nakajima Y, Nishimatsu S, Nohno T, Ando K, Nakamura H (2004) Expression of tbx20 RNA during chick heart development. Dev Dyn 230:576–580. doi:10.1002/dvdy.20076
Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, Stainier DY (2000) The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 127:2573–2582
Young JZ (1981) The life of vertebrates, 3rd edn. Clarendon, Oxford
Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR (2004) Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol 166:347–357. doi:10.1083/jcb.200312007
Zammit PS, Partridge TA, Yablonka-Reuveni Z (2006) The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem 54:1177–1191. doi:10.1369/jhc.6R6995.2006
Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G (2009) Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol 19:1050–1057. doi:10.1016/j.cub.2009.04.059
Acknowledgements
This study was supported by the German foundation grant (DFG, Hu729/10).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Pu, Q., Patel, K., Huang, R. (2015). The Lateral Plate Mesoderm: A Novel Source of Skeletal Muscle. In: Brand-Saberi, B. (eds) Vertebrate Myogenesis. Results and Problems in Cell Differentiation, vol 56. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-44608-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-44608-9_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-44607-2
Online ISBN: 978-3-662-44608-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)