Abstract
Magnetite nanoparticles are produced in a continuous synthesis that allows the potentially unlimited production for large-scale applications. With the better control over the reaction parameters, the reproducibility is significantly improved. The particle size is tunable in a range of about 10–100 nm, and the distribution is significantly smaller than for those produced batch wise. The subsequent continuous surface modification of the pristine magnetite particles is difficult to date, especially if more than one chemical reaction is necessary. A batch preparation of kilogram of surface-modified ion-exchange magnetic particles for laboratory scale and pilot line purification experiments were realized. These ion-exchange particles could be recycled 50 times on an analytical scale without loss of efficiency. Several hundred grams of beads could be recovered 10 times with a stepwise efficiency of 99.9 %. A number of one-step protocols could be developed for the enrichment and purification of target proteins from different raw materials up to pilot-scale.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Atom Transfer Radical Polymerization
- Atom Transfer Radical Polymerization
- Magnetic Particle
- Cell Culture Supernatant
- Green Rust
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Surface-modified magnetic particles have been used for many years in the Life Science area, especially in diagnostics for the isolation and purification of proteins and nucleic acids on an analytical scale. In manual or automated high-throughput applications, milligram or even sub-milligram quantities of beads are consumed in at most one cycle of binding, washing, and elution.
The idea of using magnetic particles in downstream processing of proteins or other target molecules has existed for many years. The needs to combine different technologies and the associated high costs for large-scale equipment, materials, and samples have so far prevented appropriate marketable technologies. In addition, surface-modified magnetic particles have to compete against established conventional chromatography resins with respect to performance, price, and established purification procedures.
The obvious advantages of magnetic bead technology at least on an analytical or semi-preparative scale are the almost immediate binding and elution kinetics of target molecules, the possible use in unclarified raw materials and the fast separation of the particles in a magnetic field. This suggests applications in an early phase of downstream processes to capture and purify target molecules from untreated cell lysates, cell culture, supernatants, etc.
Compared to analytical one-time applications, the requirements for magnetic beads and the associated separation equipment for large-scale processing are significantly more challenging. Ideally, the synthesis of surface-modified magnetic particles is a continuous process with the least possible production steps and potentially unlimited quantities.
To be competitive with conventional chromatography resins, the particles need to have a high binding capacity in combination with sufficient chemical and mechanical stability to withstand manifold recycling. The specific surface area of nonporous resins is limited by the particle size itself, however, restrictions concerning harmful effects of nanomaterials require a particle size above 100 nm. For general purpose capturing of target molecules from large volumes, strong anion and cation exchange functionalities like those of trimethyl-ammonium and sulfonate are preferred. In addition, high surface charge with associated increased binding capacity can be achieved by tentacle-like structures. However, the polymer length is limited by the cumulative repulsive forces, which prevent an effective magnetic separation. The ideal method for fast protein purification is certainly affinity capture with specific antibodies, peptides, or other alternative binders. However, compared to chemical surface modifications these ligands are still rather expensive. Therefore, significant savings in time and equipment have to be weighed up against the costs of the separation medium, and the potential market value of the purified target molecule must also be considered. Depending on the application field in animal feed, food or pharma, the requirements on the quality of materials, equipment, procedures, and documentation must also to be accounted for.
2 Batch Synthesis of Magnetite
The classical synthesis of magnetite is typically carried out in batches by mixing aqueous solutions of iron salts under alkaline conditions as described (Massart 1981; Sugimoto and Matijewic 1980). Depending on the reaction conditions, this leads to a broad particle size distribution in the range of 10–150 nm (Fig. 7.1).
To protect the beads against oxidation and prepare them for further subsequent chemical modifications, they are usually coated with silica, silanes, organic acids, etc. (Schüth at al. 2007). For many analytical applications like manual and automated isolation and purification of nucleic acids, these kinds of particles fulfill most or even all requirements. More sophisticated procedures (e.g., lateral flow devices, biochips, therapeutic, or security applications) require preferably monodisperse particles with homogeneous magnetic and surface characteristics to ensure identical behavior for binding and elution of target molecules and during magnetic separation (van Amerongen et.al. 2009).
3 Continuous Production of Magnetite
The continuous precipitation method (Patent 2008) is based on the same principle as the above-mentioned batch production and has a number of significant advantages. It is a combination of the Messart precipitation and an oxidation method according to the ideas of Sugimoto and Matijewic. Two solutions are prepared in separate vessels and pumped through a mixing device. The precipitation takes place immediately in a volume of a few microliters at constant temperature, pH, and in absence of dissolved oxygen (Fig. 7.2).
Due to the controlled reaction conditions, the resulting crystals show a much narrower size distribution compared to the batch coprecipitation. The primary particles, an iron(oxo)hydroxide of mixed oxidation states also described as “green rust” (Bruun Hansen et al. 1994), is further dehydroxylated in a tempered loop to form pure magnetite. The mean particle size correlates with the composition of the educt solutions, especially the ratio of the oxidation states of iron. This is assumed to the speed of nucleation in dependency of the concentration of iron 2+ or 3+ species. As a result, the particle size can be fine-tuned between about 10 and 100 nm corresponding to a specific surface area of 200–20 m2/g (Fig. 7.3). Depending on the application needs, it must be taken into account that beads below about 20 nm are superparamagnetic and thus can no longer be efficiently handled in a magnetic field. They are usually embedded in larger polymer matrices to enable magnetic separation.
4 Surface Functionalization
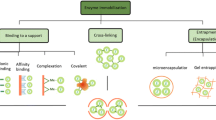
The basic particles need to be surface-modified with ion exchange and affinity functionalities to be able to capture the various target molecules. Anion and cation exchange resins usually only allow an unspecific adsorption of proteins from large volumes followed by high salt or stepwise elution. Such materials are normally used for capturing and concentrating target molecules in an early phase of downstream processing. However, as demonstrated in some applications within the project, the beads might even allow an almost affinity-like purification (e.g., monoclonal antibodies from cell culture supernatant, Fab fragments from bacterial cell lysate). The ultimate downstream processing is based on affinity particles using specific antibodies, peptides, or other ligands. These materials can be applied in a first step, e.g., for the capture of monoclonal antibodies from cell culture supernatant with protein A resins or as a final polishing procedure. Within MagPro2Life the use of antibody-based anti-BBI magnetic particles allowed a one-step purification of BBI directly from untreated soy whey with unsurpassed purity and high specific activity.
The surface modification with anion exchange functionalities is a multistep process based on the grafting from ATRP (Atom Transfer Radical Polymerization) technology. The continuously produced basic 100 nm magnetite crystals are first coated with TEOS (tetraethoxy-silane) to obtain a protective and reactive silica layer. Subsequently the ATRP initiator is covalently immobilized, followed by the polymerization with functionalized monomers. In addition to or instead of this, affinity ligands, especially antibodies, may be linked to the particle surface (Fig. 7.4).
4.1 Surface Modification by Atom Transfer Radical Polymerization
The strategy to produce nonporous, nano-sized anion and cation exchange magnetic particles is the generation of functional polymeric tentacles on the surface. This is possible in a “grafting from” or a “grafting to” approach (Rühe 2004). For the “grafting to” strategy, a ready-made functional polymer is linked with a reactive anchor group for immobilization on the particle surface. Due to the collapsed (mushroom-like) structure of most polymers, the grafting density is rather low and possible interactions of target molecules with the underlying accessible surface lead to unspecific binding. For the “grafting from” route, a high density of small molecules is anchored on the particle surface which acts as a polymerization initiator. Starting from those groups, the functional polymer chains are grown to a brush-like structure shielding the resins from unspecific binding (Fig. 7.5).
In a first step, the magnetite crystals are coated with silica (Brinker and Scherer 1990). The deposition of a thin layer of pure silica is achieved by hydrolysis of tetraethoxy-silane (TEOS) in a diluted, alkaline suspension of magnetite particles. The primary material formed by a sol-gel reaction deposits on the surface resulting in an amorphous silica coating (Fig. 7.6).
Further reactions of the nonbridged Si-OH groups with, e.g., (organo)-functionalized (mono- to tri)-alkoxysilanes are possible to create specific surface modifications. The silanization of silica surfaces with functional alkoxy-/chloro-silanes RnSiX4−n (X = Cl, OR’) offers an excellent method to covalently link new functionalities to the surface. The reaction proceeds along hydrolysis and condensation of these organosilanes, in the case of tri-reactive-silanes, creating a highly branched network.
The capacity of accessible functionalities on magnetic particles is broadly enlarged by the “grafting from” polymerization. The first step of this strategy is the immobilization of an ATRP initiator to the silica surface (Huang and Wirth 1997). This is generated directly with, e.g., ((chloromethyl)-phenylethyl)-trimethoxy-silane in THF at elevated temperature (Fig. 7.7). The grafting density is varied by mixing the initiator siloxane precursors with n-propyl-trimethoxy-silane.
The subsequent atom transfer radical polymerization (ATRP) is a “living” (controlled) radical polymerization technique (Matyjaszewski 1999; Matyjaszewski and Xia 2001). During the reaction, the molecular weight of the resulting polymer grows linearly with the conversion. The “living” character arises in part from the ability to reinitiate a second polymerisation reaction (also with another monomer) after the isolation of the first product, without additional initiator modification. Unlike conventional radical polymerisation, the concentration of free radicals within the reaction medium is so low that termination reactions (radical combination or disproportionation) rarely occur. Thereby, control over length is improved and the polydispersity of the polymer reduced.
For the generation of a strong cation exchange surface, the sodium salt of acrylamido-2-methylpropanesulfonic acid (AMPS) as a monomer can be used. The modification of magnetic particles with a strong anionic exchanger may, for example, be achieved with the monomer trimethylammoniumpropyl-acrylamide chloride (TMAPAA) (Fig. 7.8).
The resulting tentacle length has to be well controlled. Short tentacles have a low capacity and eventually allow an unspecific adsorption of proteins (or other target molecules) to the underlying, still accessible silica surface. Very long tentacles exhibit a high binding capacity, but the extreme negative surface charge strongly prevents an effective magnetic separation. An empirical length optimum for both polymers is about 30–50 monomer units (Fig. 7.9).
4.2 Immobilization of Antibodies
A monoclonal anti-BBI antibody provided by the project partner fzmb has been covalently immobilized on carboxylated magnetic particles via standard aqueous carbodiimide-chemistry. The beads enabled a highly specific adsorption of BBI without any detectable background (Fig. 7.10b).
a Lab-scale purification of BBI from untreated soy whey. 1 molecular weight marker; 2 untreated soy whey; 3 heat-treated soy whey; 4 heat + silica treated soy whey; 5 BBI purified with TMAP-modified magnetic particles. b Purification of BBI from untreated soy whey with anti-BBI immobilized on MagPrep beads. 1 molecular weight marker; 2 untreated soy whey; 3 eluted purified BBI
5 Applications
A number of applications have been developed with magnetic particles with TMAP, sulphonate, and anti-BBI modification for the capture and purification of proteins from different raw materials on a laboratory scale. The major target within MagPro2Life was BBI from untreated soy whey. The purification strategy involved an initial heating step at 70–80 °C (at which BBI is unaffected) to remove unwanted heat-sensitive components. Subsequently, a silica-treatment eliminates most remaining proteins, leaving BBI in the supernatant. Finally, BBI is captured with TMAP-modified anion exchange magnetic particles and eluted with 1 M sodium chloride (Fig. 7.10a). Alternatively, BBI can be purified in one step using anti-BBI affinity particles. Here, the target molecule is captured directly from untreated soy whey with an immobilized monoclonal antibody against BBI and eluted at pH 3 in a small volume (Fig. 7.10b). This results in a 20-fold purification with a specific activity of 1,500–2,000 CI units/g, which is well above the projected target level of 1,000. The maximum theoretical purity is 3,194 CI units/g.
TMAP-grafted magnetic particles have also been successfully used to purify monoclonal antibodies from cell culture supernatant (Fig. 7.11a) and IgG from human plasma (Fig. 7.11b) in one step by removing unwanted proteins. The remaining antibodies can subsequently be concentrated and desalted by cross-flow filtration or directly used for downstream applications.
a Lab-scale purification of monoclonal antibodies from cell culture supernatant. 1 molecular weight marker; 2 cell culture supernatant 1:3 diluted with water; 3 remaining antibodies in the supernatant; 4 proteins adsorbed to TMAP-modified magnetic beads; b Purification of IgG from human plasma. 1 molecular weight marker; 2 human plasma proteins; 3 remaining IgG in the supernatant
The main application of sulphonated magnetic particles within MagPro2Life is the purification of Fab fragments from Escherichia. coli cell lysate (Fig. 7.12a). Fab is enriched in the periplasma during fermentation and released by destruction of the outer cell membrane. Sulphonated magnetic beads allow the selective capture and release of the target protein with high purity on a laboratory scale. Similar to TMAP-modified magnetic particles, the cation exchange beads can be used for the enrichment and purification of monoclonal antibodies from cell culture supernatant by adsorption at pH 5.8, leaving unwanted protein components in the supernatant and release of the target IgG at 0.5 M sodium chloride (Fig. 7.12b).
a Lab-scale purification of Fab fragments from E. coli cell lysate. 1 molecular weight marker; 2 cell lysate; 3 remaining proteins in supernatant; 4 Fab eluate. b Purification of monoclonal antibodies from cell culture supernatant. 1 molecular weight marker; 2 + 4 different cell culture supernatants; 3 + 5 monoclonal antibody eluates
6 Summary
Magnetic particles could be an established alternative to conventional chromatography resins in automated, high-throughput protocols replacing centrifugation and filtration by simple magnetic separation steps. The basic magnetite crystals are typically synthesized in batches in limited amounts by aqueous coprecipitation. In contrast, continuous synthesis allows the potentially unlimited production of particles for large-scale applications. Moreover, the reliability and reproducibility is significantly improved as the reaction parameters can be controlled much more. In addition, the particle size and distribution is tunable in a range of about 10–100 nm. The subsequent desirable continuous surface modification of the basic magnetite crystals is difficult to date, especially if more than one chemical reaction is necessary. We succeeded in a batch preparation of kilogram of surface-modified ion-exchange magnetic particles for laboratory scale and pilot line purification experiments. The resins could be recycled 50 times on an analytical scale without loss of efficiency. Several hundred grams of beads could be recovered 10 times with a stepwise efficiency of 99.9 %.
A number of one-step protocols could be developed for the enrichment and purification of target proteins from different raw materials up to pilot-scale, the most effective being the capture and elution of BBI with anti-BBI coated MagPrep beads directly from untreated soy whey. Overall, the project has demonstrated the feasibility of pilot-scale downstream processing with magnetic particles as a potential alternative or complement to conventional chromatography.
References
Brinker CJ, Scherer GW (1990) Sol-gel science: the physics and chemistry of sol-gel processing. Academic Press, Chapter 3
Bruun Hansen H Ch et al (1994) Evaluation of the free energy of formation of Fe(II)–Fe(III) hydroxide-sulphate (green rust) and its reduction of nitrite. Geochimica et cosmochimica Acta 58:2599
Huang XY, Wirth MJ (1997) Surface-initiated radical polymerization on porous silica. Anal Chem 69:4577
Massart R (1981) Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans Magn 17:1247
Matyjaszewski K (1999) Polymers at interfaces: using atom transfer radical polymerization in the controlled growth of homopolymers and block copolymers from silicon surfaces in the absence of untethered sacrificial initiator. Macromolecules 32:8716
Matyjaszewski K, Xia J (2001) Atom transfer radical polymerization. Chem Rev 101:2921
Patent DE (2008) 102008015365
Rühe J (2004) Polymer brushes: on the way to tailor-made surfaces. In: Advincula RC (ed) Polymer brushes, Wiley-VCH, Weinheim, pp 1. doi:10.1002/3527603824.ch0
Schüth F et al (2007) Angew Chem 119:1242. doi:10.1002/ange.200602866
Sugimoto T, Matijewic K (1980) Formation of uniform spherical magnetite particles by crystallization from ferrous hydroxide gels. J Colloid Interf Sci 74:227
van Amerongen A et al (2009) Anal Bioanal Chem 393:569. doi:10.1007/s00216-008-2287-2
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Holschuh, K., Bauer, J. (2014). Industrial Production, Surface Modification, and Application of Magnetic Particles. In: Nirschl, H., Keller, K. (eds) Upscaling of Bio-Nano-Processes. Lecture Notes in Bioengineering. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43899-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-662-43899-2_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43898-5
Online ISBN: 978-3-662-43899-2
eBook Packages: EngineeringEngineering (R0)