Abstract
Foragers of several species of stingless bees (Apidae; Meliponini), a group of eusocial bees comprising more than 400 mainly tropical species, produce pulsed thoracic vibrations inside the nest when returning from a successful foraging trip. These vibrations do not provide navigational information on the direction and distance of a food source. Instead, both their occurrence and their temporal pattern correlate with the net gain during a foraging trip. The vibrations are therefore considered important information for potential foragers about the profitability of a food patch. Their repeated presentation lowers the foraging threshold of potential food collectors. The vibrations are considered as an alerting signal, which increases the colony’s foraging activity. So far, nothing is known about how foragers of stingless bees perceive the pulsed thoracic vibrations of the recruiters. Yet, consideration of the corresponding receptors and their thresholds in honeybees suggests three possible pathways for their transmission to the nestmates: (1) the substrate (vibrations), (2) the air (air particle movements), and (3) direct physical contact (tactile stimuli). The corresponding differ significantly. Whereas substrate vibrations will reach receivers up to ten bee lengths away (medium-range transmission), air particle oscillations and direct vibrations can be detected only by bees very close to, or in contact with, the forager (short-range transmission). Thus, depending on the transmission pathway and the recipient’s sensory capacity, the signal generated by thoracic vibrations will have different meanings. Indeed, substrate vibrations attract both food processors and potential foragers to the vibrating bee, whereas air particle oscillations and direct contact vibrations, in addition to important olfactory and gustatory information, may well be used by prospective recruits to evaluate the profitability of the advertised food source. In contrast to the honeybee waggle dance vibrations, there is no indication in stingless bees of an air jet potentially providing directional information.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
There are more than 18,000 described species of bees worldwide, and an estimate of the total number of species is near or even above 20,000 (Michener 2000). Thoracic vibrations not related to flight and generated by contractions of the indirect flight muscles (Simpson 1964; Esch and Wilson 1967) are widespread among bees (Michener 2000). They have been reported in a variety of behavioral contexts, such as nest construction (Michener 1974, 2000), nest defense (Vicidomini 1998; Hrncir et al. 2006a), and the detection of females by males (Larsen et al. 1986). There are male “sounds” during mating (Eickwort and Ginsberg 1980; Larsen et al. 1986; Roubik 1989; Conrad et al. 2010), vibrations used for pollen collection (Michener 1962; Wille 1963; Buchmann 1983; Harter et al. 2002; Nunes-Silva et al. 2010), and, in social bees, vibrations for the communication among nestmates (Hrncir et al. 2006a).
The term “bee communication” is most frequently associated with the honey bee’s famous waggle dance, the stereotyped figure-eight movements performed by successful food collectors on their return to the nest. Ever since the pioneering discovery by Karl von Frisch (1946) that these dances convey information about both the distance and the direction of the visited food source, scientists have been searching for similar forms of symbolic communication, that is, an abstract code providing information about an object without causal relation or similarity between signal and object (Menzel 2012), in closely related bee groups. Outstanding among these are the stingless bees (Apidae; Meliponini), which represent a group of highly eusocial bees with more than 400 species mainly found in the tropics (Michener 2000; Camargo and Pedro 2007). The degree of social organization of stingless bees is similar to that of the honeybees (Michener 1974), and above all, their impressive capacity to recruit nestmates to food sources (Lindauer 1956; Lindauer and Kerr 1958, 1960; Nieh and Roubik 1995; Jarau et al. 2000) furthered speculations about intranidal signals providing prospective recruits with navigational information about the position of a food patch (Esch et al. 1965; Esch 1967; Nieh and Roubik 1998).
Lindauer and Kerr (1958, 1960) were the first to investigate in detail the behavior of stingless bees within their nest during food exploitation processes. These authors observed three conspicuous behaviors shown by the foragers upon their return from a profitable food source: zigzag runs, jostling of nestmates, and buzzing sounds (Fig. 18.1). Of these, the best-studied displays related to recruitment communication are the buzzing sounds, which originate from thoracic vibrations generated by foragers collecting at a highly profitable food source (Hrncir 2009) (Fig. 18.1). The pulsed structure of these vibrations, reminiscent of a Morse code, promoted the idea that information about the food source may be encoded within the temporal pattern of the sounds. The first attempts to decode the message and meaning of the thoracic vibrations suggested that the duration of the pulses provides a measure of the distance to a food source (Melipona quadrifasciata, Melipona seminigra: Esch et al. 1965; Esch 1967; Melipona panamica: Nieh and Roubik 1998) or even its height (M. panamica: Nieh and Roubik 1998). Below, we reinterpret these results, taking into account additional factors that had not been considered in these early studies.
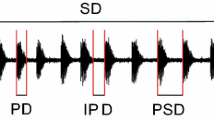
Intranidal behaviors of stingless bee foragers. When foragers (see empty/white bees and symbols) return from a profitable food source, they excitedly run through the colony (zigzag run), thereby jostling their nestmates (gray symbols and bees in inset). While running, but predominantly during trophallaxis (see filled/black bee and symbols), the foragers generate pulsed thoracic vibrations. F forager; D food receiver. Inset shows parameters of the temporal pattern of the vibratory signals recorded with a laser vibrometer: pulse duration, interval duration, pulse sequence, velocity magnitude. Symbols (circle head; line long axis of body) indicate change of position of the bees, video-taped at 25 frames per second [Adapted from Hrncir (2009)]
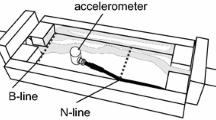
Unlike honeybees, which produce their communication signals exclusively during locomotion through the nest, the foragers of stingless bees generate thoracic vibrations predominantly when unloading their food to nestmates (trophallaxis) (Hrncir et al. 2006a, b; Barth et al. 2008; Hrncir 2009). Since the individuals move only slightly during the trophallactic food transfers, the recording of the thoracic vibrations is possible with high accuracy. Likewise, methodological innovations over the past decade, like using laser vibrometry instead of sound pressure microphones (Hrncir et al. 2004a, b, 2006b; Schmidt et al. 2006, 2008; Morawetz 2007) and the investigation of the vibrations generated by sling-tethered bees (Hrncir et al. 2008a, b) (Fig. 18.2), provided new insights about stingless bee vibratory signals. The present chapter outlines our current knowledge of the message and meaning of forager-produced thoracic vibrations, the mechanisms of their generation, and the possible pathways of transmission during recruitment communication.
Annoyance buzzing in stingless bees. Stingless bees (shown: worker of Melipona rufiventris) generate pulsed thoracic vibrations when tethered by a sling around their neck. a Sling-tethering method: the sling (S) formed by a nylon thread (T) and guided through an injection needle (IN). Sy, syringe for fixing the thread. Using one or even two laser vibrometers, this method allows the detailed measurement of the vibrations at various body parts such as thorax (Tx) or distal mesothoracic femur (Fe) and the calculation of signal transmission. b The following parameters of the pulsed vibrations can be analyzed for a comparison with those of forager vibrations: velocity amplitude (VA), duration of single pulses (PD), pulse sequence (PS), and the main component (MF) of the frequency spectrum [Adapted from Hrncir et al. (2006b)]
2 Message of Thoracic Vibrations of Stingless Bee Foragers
The key to decoding the message of a putative signal is the unequivocal identification of all the factors that influence and shape the respective behavioral display (Seeley 1992). The first attempts to decode the message of meliponine vibratory signals (Esch et al. 1965; Esch 1967; Nieh and Roubik 1998) suffered from premature conclusions regarding the existence in stingless bees of a referential communication of a food source’s location. Probably biased by the expectation of finding a precursor of the honeybee’s “dance language,” these early studies searched for correlations between the temporal pattern of the vibratory pulses and the spatial parameters of a food patch. However, they did not consider numerous additional criteria potentially influencing the temporal pattern of the foragers’ sounds. In four Melipona species (M. quadrifasciata, M. seminigra: Esch et al. 1965; Esch 1967, Melipona bicolor, and Melipona mandacaia: Nieh et al. 2003), the duration of the vibratory pulses (pulse duration) was found to increase with increasing distance of the food source (Fig. 18.3). In M. panamica (Nieh and Roubik 1998), pulse duration during food unloading was longer when bees collected food at ground level than when collecting at the canopy top, whereas after unloading, pulse duration increased with increasing foraging distance (Fig. 18.3). In all these studies, pulse duration varied by up to 60 % (Esch 1967; Nieh et al. 2003) or even by more than 200 % (Nieh and Roubik 1998) at each investigated distance/height (Variation = Standard Deviation × 100/mean value; values obtained from the respective publication). This variability raises the question whether potential recruits could extract reliable information about food source position from the temporal pattern of the foragers’ vibrations. Furthermore, these results, which were interpreted to support the referential communication hypothesis, could never be replicated by other researchers studying the same (M. quadrifasciata: Hrncir et al. 2000, M. seminigra: Samwald 2000) or closely related bee species (M. costaricensis: Aguilar and Briceño 2002; just as M. panamica, M. costaricensis had formerly been classified as subspecies of M. fasciata: Camargo and Pedro 2007) (Fig. 18.3). The hypothesis that the thoracic vibrations of Melipona code the distance to a food source was also greatly weakened by the later finding that the visual flow (lateral image motion experienced by the bees during flight) used by foragers to estimate the distance to a food source (shown for M. seminigra: Hrncir et al. 2003, following the establishment of the “visual flow hypothesis” for the honeybee: Esch and Burns 1995; Srinivasan et al. 2000) does not affect the temporal pattern of the thoracic vibrations (Hrncir et al. 2004a).
Hypotheses concerning the message of vibratory signals. Shown is the phylogenetic relationship of stingless bee species [adapted from Ramírez et al. (2010) and Rasmussen and Cameron (2010)] studied in regard to recruitment activity and/or the message of their thoracic vibrations. Recruitment success (number of activated recruits) usually increased with increasing food profitability (red squares). Studies corroborating the profitability hypothesis found an increase in pulse duration with increasing sugar concentration (red squares). Studies corroborating the referential communication hypothesis found an increase in pulse duration with increasing foraging distance (black squares). If both hypotheses were true, pulse duration would not provide conclusive information for potential recruits. According to the profitability hypothesis, pulse duration should decrease with foraging distance (see text), whereas, according to the referential communication hypothesis, it increases with distance of the food source
According to recent studies, both the occurrence and the temporal pattern of the vibrations are related to the profitability of the food source experienced by the forager (Hrncir 2009) (Figs. 18.3, 18.4) rather than encoding spatial information about the food patch visited. The most obvious evidence supporting this conclusion is that foragers do not generate thoracic vibrations at all as long as the value of a food source is below a certain threshold (Esch 1967; Hrncir et al. 2000; Schmidt et al. 2006, 2008). Because in all experimental studies so far sugar solution had been offered ad libitum, this “excitement threshold” was determined by the sugar concentration of the collected food. However, the food profitability experienced by a forager and, consequently, her disposition to generate thoracic vibrations, may as well be determined by parameters different from sugar concentration, such as solution flow, handling time, and even the presence of competitors (Hrncir 2009; Hrncir et al. 2011) (Fig. 18.4).
Message of vibratory signals. a The thoracic vibrations generated by foragers of many stingless bee species correlate with the concentration of the collected sugar solution or nectar. To show this, an example is given of vibrations generated by a forager of Melipona seminigra collecting an aqueous solution containing 60, 40, or 20 % sugar weight on weight (w/w). b In addition to sugar concentration, other parameters determine the value of a food source for collecting bees and, consequently, influence the temporal pattern of the foragers’ thoracic vibrations: Increasing energetic gains at the food patch result in longer pulses, shorter intervals, and consequently, an increasing duty cycle (duty cycle = pulse duration/[pulse duration + interval duration]). Increasing energetic costs, by contrast, result in shorter pulses, longer intervals, and a decreasing duty cycle. F forager; D food receiver [Adapted from Hrncir (2009)]
Once the food profitability exceeds the “excitement threshold” of a forager, the temporal pattern of her vibrations is strongly influenced by the energy intake (sugar concentration). Pulse duration increases and the interval between pulses decreases with increasing profitability of the food source, which implies an increasing duty cycle as well (M. costaricensis: Aguilar and Briceño 2002; M. bicolor, M. mandacaia: Nieh et al. 2003; M. rufiventris: Hrncir et al. 2006a; M. seminigra: Hrncir et al. 2004a, b; N. testaceicornis: Allerstorfer 2004; Schmidt et al. 2008) (Figs. 18.3, 18.4). Along this line of thought, increased energetic expenses experienced during a collecting trip should reduce the “excitement” of a forager. And indeed, in M. seminigra, the effect of increased flight costs on the temporal pattern of the foragers’ thoracic vibrations was exactly the opposite of that of increased energetic gains (Hrncir et al. 2004a; Hrncir 2009).
In accordance with the profitability hypothesis, the temporal pattern of the thoracic vibrations should eventually be influenced by foraging distance because the energy expenditure increases linearly with flight distance (Hanauer-Thieser and Nachtigall 1995). Yet, in contrast to the increase in pulse duration postulated by the referential communication hypothesis (see above), the profitability hypothesis predicts a decrease in pulse duration (Hrncir et al. 2004a). In any case, the large differences in energy uptake at a food source among individual foragers (Hrncir et al. 2004b) would strongly disguise differences in energy consumption due to different food source distances.Footnote 1 It seems, therefore, unlikely that thoracic vibrations of Melipona bees contain reliable information about the distance of a food source.
3 Meaning of Thoracic Vibrations of Stingless Bee Foragers
For a comprehensive understanding of the vibratory signals produced by stingless bees, it is essential to decipher not only their message but also their potential meaning in recruitment communication (message: information provided by the sender, meaning: influence on the behavior of the receiver, Seeley 1992). Since the behavioral response to a signal depends both on the behavioral context and on the recipient’s motivation, revealing the signal’s meaning often is an even greater challenge than revealing its message.
Observations suggest that in meliponine bees, the foragers’ thoracic vibrations have a modulatory function, raising the activity level of nestmates and increasing their propensity to forage (Hrncir 2009). According to a detailed study of the intranidal case histories of individually marked recruits in M. seminigra (Kronberger 2000), the agitation of inactive foragers, measured as jostling contacts, abruptly increased after the first contact with an active collector (Hrncir 2009). The sudden increase of their locomotor activity is taken to indicate the increased motivation to forage in response to the interactions with the food collectors (Hrncir 2009).
Further evidence for the effect of the forager’s agitation on the nestmates’ motivation to forage (both experienced, inactive foragers and novice foragers) comes from the observation that no newcomers arrive at the food source as long as the value of the food is below the foragers’ “excitement threshold” (Jarau et al. 2000). As soon as the profitability of the food source exceeds this threshold, however, the recruitment success increases with increasing sugar concentration of the collected food. This could be shown for several Melipona species already (M. bicolor, M. mandacaia: Nieh et al. 2003; M. panamica: Nieh and Sanchez 2005) and for Nannotrigona testaceicornis (Schmidt et al. 2008). Since in these species, the recruiters’ excitement correlates with their energetic gains at the food source (see above), it cannot be decided whether the recruitment success depends on either the sugar concentration of the collected and distributed food, or on the foragers’ “excitement,” or both. Scaptotrigona aff. depilis is the only meliponine species so far known where recruitment success does not directly depend on the concentration of the sugar water collected by the foragers (Schmidt et al. 2006). In this species, the recruiter’s thoracic vibrations depended on past foraging experiences rather than the current food profitability. A steadily increasing sugar concentration did not change the temporal pattern of the vibrations, nor the recruitment success (Schmidt et al. 2006). Hence, in this case, the quality of the received food samples did not influence the foraging motivation of the hive bees. Yet, when the profitability of the food source continuously decreased, both the recruiters’ agitation and their recruitment success decreased (Schmidt et al. 2006). From these findings it follows that (at least in S. aff. depilis) the foraging motivation of inexperienced bees does not depend on the quality of the food brought in by the foragers but, indeed, on the degree of “excitement” of the recruiters.
So far, no studies have been performed to specify whether the foragers’ vibrations cause a general increase in foraging activity, where individual recruits search for their own food source, or whether recruits use odor cues to find the same source as the one advertised by the vibrating bee. Recent studies indeed provide strong evidence that meliponine foragers use olfactory information received within the nest for their search for food (Jarau 2009; Roselino and Hrncir 2012). Therefore, as also proposed for honeybees (Grüter and Farina 2009), the combination of vibratory information about a profitable food source with olfactory/gustatory information appears to serve the coordination of foraging processes in two ways. First, it may alert experienced but inactive foragers and inform them that a known food source, identified through the scent, has become profitable, as indicated by the vibratory signals. Provided a sufficiently lowered foraging threshold, these experienced bees will resume their collecting activity at the known food patch (Biesmeijer et al. 1998; Biesmeijer and Slaa 2004). Second, the vibratory signals may lower the foraging threshold of new, inexperienced foragers. In this case, the olfactory information provided by the vibrating bee will bias the search of the naive foragers toward the advertised food source in the field (Jarau 2009; Roselino and Hrncir 2012).
4 The Generation of Thoracic Vibrations
Many groups of insects use airborne sounds and substrate vibrations to communicate by periodically oscillating specialized organs at their resonant frequency (Bennet-Clark 1999). Bees are not equipped with such structures (Snodgrass 1956; Schneider 1975), their thorax being the only body part capable of generating adequate rhythmic oscillations. As in many other insects, the most prominent purpose of rhythmic thoracic oscillations is to move the wings. The periodic up- and down-strokes of the wings are maintained through stretch activation of the antagonistic indirect flight muscles at the resonant frequency of the oscillating system (Snodgrass 1956; Nachtigall 2003).
Thoracic vibrations associated with nestmate communication or buzz pollination are characterized by fundamental frequencies significantly higher than that of flight vibrations (King 1993; King et al. 1996; Nachtigall 2003; Hrncir et al. 2008a; Burkart et al. 2011) (Table 18.1). According to a study on the thoracic flight and non-flight vibrations generated by M. seminigra, the average fundamental frequency of annoyance buzzing (produced by tethered individuals) was 305 Hz, whereas that of forager vibrations was 487 Hz, and 182 Hz was the value found during tethered flight (Hrncir et al. 2008a).Footnote 2 The cycle frequency of flight vibrations did not change significantly during the entire oscillation period. In both types of non-flight vibrations, by contrast, the cycle frequency dropped to 215 Hz (annoyance buzzing) and 225 Hz (forager vibrations), respectively, within the last four to six oscillation cycles (Fig. 18.5). This frequency change is explained by the fact that an oscillating system driven by a periodic force at a frequency higher than its natural frequency will vibrate at the excitation frequency as long as the force is applied. As soon as the force stops, however, the vibration magnitude will decay and the frequency drop to the system’s resonant frequency (Nocke 1971; Bennet-Clark 1999).
Comparison of flight and non-flight thoracic vibrations of stingless bees (Melipona seminigra). The first and the last 15–20 oscillation cycles of thoracic vibrations (measured with a laser vibrometer) during stationary flight (a, d filled squares, N = 15 individuals), annoyance buzzing (b, e filled circles, N = 15), and forager vibrations (c, f open circles, N = 15) were analyzed regarding velocity amplitude (a–c) and cycle frequency (d–f). Graphs show the means ± s.d. of relative values (percent of the maximum velocity or of the main frequency, MF). Shaded area indicates the buildup and decay of thoracic oscillations. Broken lines indicate 95 % of maximum. Medium values of velocity amplitude and main frequency are given in the respective plot [Adapted from Hrncir et al. (2008a)]
5 Transmission Pathways of Vibratory Signals
In order to justify the terms “signal” and “communication,” a crucial question has to be answered: Who understands these signals? The identification of potential recipients requires knowledge of the exact physical nature of the signal and of the mechanisms underlying both its transmission to and perception by the receiver. In stingless bee recruitment communication, three transmission pathways of the vibratory signals have been suggested and analyzed: (1) the substrate (substrate vibrations), (2) the air (air particle movements), and (3) direct physical contact (tactile stimuli) (Fig. 18.6). The degree of signal attenuation and, therefore, the range of signal transmission differ greatly between these pathways (Hrncir et al. 2006a, b, 2008b; Morawetz 2007). Whereas substrate-borne vibrations will reach receivers at a distance of up to ten bee lengths from the signaler (medium-range transmission), air particle oscillations and direct vibrations are only detected by bees very close to or in actual contact with the vibrating forager (short-range transmission). Thus, the meaning of the original signal may well differ depending on the type of transmission considered. Like in honeybees, Apis mellifera, substrate vibrations are believed to attract hive bees to the forager unloading the collected food (Tautz and Rohrseitz 1998). Air particle oscillations and direct vibrations, on the other hand, in combination with olfactory and gustatory information originating from the food collector, may serve the prospective recruits to evaluate the advertised food source (Michelsen 2003; Grüter and Farina 2009). In the following, arguments supporting these conjectures are given.
Possible pathways of vibratory signal transmission. A forager of Melipona scutellaris distributing food to nestmates. During trophallactic contacts, the vibratory signals generated by the forager (F) may be transmitted to nestmates as substrate vibrations (medium-range transmission pathway), air particle movements, or directly during trophallaxis as contacts between forager and receivers (short-range transmission pathways). Considering physiological thresholds of vibration receptors of honeybees (see text), S-bees should perceive only substrate vibrations, whereas A-bees perceive air particle oscillations and substrate vibrations, and D-bees direct vibrations, air particle oscillations and substrate vibrations
5.1 Substrate Vibrations: Medium-Range Transmission
When vibrating their thorax, meliponine foragers generate substrate vibrations that can be measured (Hrncir et al. 2000, 2006b), their legs representing the mechanical link between thorax and substrate (Rohrseitz 1998; Tautz et al. 2001; Hrncir et al. 2006a, b). The vibrations are transmitted from the forager’s thorax to her leg without loss in velocity amplitude, but are strongly attenuated when passing from the leg to the substrate (Fig. 18.7). In M. seminigra, an attenuation of about 50 dB was found between the signal amplitude on the forager’s femur and the substrate halfway between forager and food receiver, respectively (Hrncir et al. 2006b) (Fig. 18.7). However, albeit strongly reduced in amplitude, the signal’s temporal pattern and, thus, the information about the forager’s degree of “excitement” were well preserved in the substrate vibrations (Hrncir et al. 2006b).
Vibration transmission to the substrate (Melipona seminigra). a Comparison of the velocity amplitudes (boxplot) of the forager’s thorax (TxF) and of the substrate (Su) close to the forager’s leg. Average signal attenuation on its way to the substrate was 43.7 dB in the given example. b–c Details of the vibration transmission from the forager’s thorax (TxF) to its femur (FeF) and from there to the substrate (Su). F forager; D food receiver. Data from simultaneous recordings with two laser vibrometers are presented as boxplots. Differences between vibration amplitudes picked up at the same body parts (compare TxF in a and b, and FeF in b and c) are due to differences between vibrating individuals [Adapted from Hrncir et al. (2006b)]
The propagation of substrate vibrations depends on the transmission properties of the respective substrate (Michelsen and Nocke 1974; Barth et al. 1988; Rohrseitz 1998; Sandeman et al. 1996; Barth 1998; Morawetz 2007). In stingless bees, trophallactic interactions and the generation of thoracic vibrations by foragers predominantly occur inside the nest’s entrance tunnel (Hrncir et al. 2006b; Morawetz 2007; Hrncir 2009). This is a narrow, tubular structure built from batumen, a mixture of mud, wax, and floral materials (Schwarz 1948; Wille and Michener 1973; Roubik 2006). Analysis of the transmission properties of diverse nest structures in M. scutellaris and M. bicolor showed that bee generated non-flight vibrations (tethered bees used as vibration generators) are propagated with an attenuation of between 1.5 and 2 dB/cm through the batumen of the entrance tube (Morawetz 2007). Given a velocity amplitude of bee-produced substrate vibrations of 0.37 mm/s right next to the vibrating individual (Fig. 18.7), the vibratory output at a distance of 1 cm from the forager would be at least 0.29 mm/s, at 4 cm 0.15 mm/s, and at 8 cm 0.06 mm/s (output calculated for an attenuation of 2 dB/cm).
To date, the reception of substrate vibrations has not been studied in stingless bees. The only way to get a preliminary idea about their detection by hive bees is through a comparison with the well-studied honey bee. In A. mellifera, the reception of substrate vibrations has been predominantly attributed to the subgenual organ, a chordotonal organ found in the proximal part of the tibia of each leg (Schön 1911; Autrum and Schneider 1948). This sensory organ responds to vibrations in the axial direction of the tibia. When the leg is accelerated by substrate vibrations, inertia causes the hemolymph and the subgenual organ suspended in it, to lag behind the movement of the leg, which mechanically stimulates the receptor cells (Autrum and Schneider 1948; Kilpinen and Storm 1997; Storm and Kilpinen 1998). When studied electrophysiologically, its sensory cells were most sensitive to vertical vibrations of the leg at frequencies between 150 and 900 Hz, with an average response threshold between 0.06 and 0.15 mm/s peak–peak (Kilpinen and Storm 1997; Rohrseitz and Kilpinen 1997). Assuming the threshold of the meliponine subgenual organ to be similar to that of the honeybee, the range of just noticeable vibrations would be between 4 and 8 cm from the forager generating them (Morawetz 2007). In case of Melipona bees with a body length of 0.8–1.4 cm (Schwarz 1948), this corresponds to between three and ten bee lengths.
5.2 Airborne Sound: Short-Range Transmission
Non-flight thoracic vibrations of stingless bees are transformed into airborne sound well audible for the human ear (Hrncir et al. 2004a, 2008b). Since, different from us, bees do not have sound pressure receivers (Snodgrass 1956; Hrncir et al. 2006a), the physical parameter most relevant for the perception of airborne sound is air particle movement. In dancing honeybees (A. mellifera), two different forms of air particle movement have been described. First, the oscillating wings create intense air particle oscillations close to their edges (Michelsen et al. 1987). Second, air that moves out from the space between the wings and the abdomen during wing vibrations creates an air jet moving away from the bee’s abdomen (Michelsen 2003). In the honeybee, both these forms of air particle movement depend on the wing oscillations that go along with the thoracic vibrations. In stingless bees, however, wings play a minor role for the transformation of thoracic vibrations into airborne sounds and medium flow, respectively. According to a detailed investigation in sling-tethered stingless bees (Melipona scutellaris), the sound field (particle movement) around a vibrating bee is predominantly generated by the oscillations of the thorax itself (Hrncir et al. 2008b). Although the wings vibrate with velocity amplitudes of close to 700 mm/s along with the thorax (measured in M. seminigra; Hrncir et al. 2008a), they significantly affect the vertically oriented particle velocity close to the abdomen only (Fig. 18.8). The different impact of the wings on the generation of air particle movement in A. mellifera and M. scutellaris, respectively, is believed to be due to a difference in their position when the bees are vibrating. Whereas stingless bees vibrate their thorax with their wings closely folded over the abdomen (Lindauer and Kerr 1958; Hrncir et al. 2006a, b, 2008a), honeybees do it with their wings splayed (wing tips 5–9 mm apart) when dancing (Michelsen 2003). This spreading of the wings increases the effective wing area (Schneider 1975). Consequently, the volume of air between the wings and the abdomen that is moved by every wing stroke is increased, as well, and most likely responsible for the air jet found in honeybees by Michelsen (2003).
Air particle oscillations generated by vibrating bees (Melipona scutellaris) and measured with airflow sensors. Ranges above and around (vertically or horizontally oriented) vibrating bees in which air particle velocities have the same mean amplitudes. Different colors indicate mean velocity amplitudes between 2 and 40 mm/s as explained by the logarithmic color scale. Left panels intact individuals; middle panels wingless individuals; and right panels fraction of particle velocity generated by wings only. Air particle oscillations cannot be accurately measured or estimated at distances below 1 mm from the vibrating bee (shaded area). For measurements of the air particle movement above bees, the airflow sensors were positioned at least 5 mm above the substrate. Therefore, no values are given for the region below 5 mm (shaded area) [Adapted from Hrncir et al. (2008b)]
In stingless bees, airborne sounds going along with the thoracic vibrations repeatedly have been assumed to transmit information (Esch 1967; Nieh et al. 2003). Whereas the temporal pattern of the thorax vibrations (pulse duration, pulse sequence, and main frequency component) is indeed well preserved in the air particle oscillations (Hrncir et al. 2004a, 2008b), the crucial question of whether the air particle velocity close to a vibrating bee is strong enough to be detected by the hive bees still awaits an answer.
The candidate mechanosensory organ detecting air particle velocity is Johnston’s organ in the antennal pedicel, which is stimulated when the flagellum is deflected by air movement (Snodgrass 1956; Heran 1959). Up to now, neither the physiological nor the mechanical properties of this mechanoreceptor are known in stingless bees. Again, a comparison with data available for A. mellifera may be helpful. Heran (1959) found that Johnston’s organ of the honeybee had physiological thresholds of 0.37 mm/s (oscillation velocity measured at the tip of the antenna) at a stimulation frequency of 200 Hz, 0.75 mm/s at 300 Hz, and 4.5 mm/s at 400 Hz. However, particle velocity around the antenna has to be about 100 times stronger (i.e., 37–75 mm/s) in order to generate such oscillation velocities of its tip (Kirchner 1994).
When adopting these physiological and mechanical properties for stingless bees, velocities of at least 37 mm/s are needed to effectively stimulate their Johnston’s organs. Vibrating stingless bees (M. scutellaris) indeed produce air particle velocities sufficiently strong close to their body surface (1 mm above the thorax; estimated particle velocity 43 mm/s) and to the wings (estimated particle velocity: 61 mm/s) (Hrncir et al. 2008b). Hive bees attending trophallactic events stay within less than 5 mm from the forager (distance between head of receiver and body of forager) with their splayed antennae close to or even touching the vibrating forager (Hrncir et al. 2008b). Similarly, in M. panamica, the antennal tips of hive bees were found to be only up to 2 mm away from the vibrating forager’s body during trophallaxis, and in about 30 % of the cases, the antennal tips were above the wings or the thorax of the forager (Nieh 1998). These behavioral observations taken together with the available measurements of air particle velocity and of the response thresholds of Johnston’s organ of the honeybee (Heran 1959) suggest that in stingless bees, hive bees can detect the air particle velocity induced by the forager’s thoracic vibrations within a range of 5 mm.
5.3 Direct Transmission During Physical Contacts
Unlike honeybees, nectar-collecting foragers of stingless bees generate their vibratory signals predominantly during their trophallactic interactions with food receiving bees (Hrncir et al. 2006a, b; Hrncir 2009). By these mouth-to-mouth contacts, hive bees learn about the sugar concentration, the secretion rate, and the odor of a nectar source (Farina and Grüter 2009; Jarau 2009). In addition, the food receivers are vibrated by the foragers during trophallaxis (Fig. 18.9), thereby receiving information about the profitability of a food patch. The vibratory input received during direct contact with the forager by far exceeds the vibratory stimulation through the substrate (Fig. 18.9). Bees in the immediate vicinity of the vibrating bee but not touching it will detect these substrate vibrations despite their small amplitude (see above). However, it will be difficult for receiver bees to extract information from these substrate vibrations as soon as two or more foragers returning from different food sources are within their perceptive range. As soon as a hive bee has direct trophallactic contact with the forager, its vibratory input will drastically exceed stimulation by way of the substrate. Information about the profitability of a single food source will then be easy to recognize by the vibration’s magnitude.
Vibration transmission during trophallaxis (Melipona seminigra). a Comparison of velocity amplitudes (boxplot) of the vibrations recorded from both the forager’s thorax (TxF) and the receiver’s thorax (TxR) using laser vibrometers. b–e Transmission pathway in more detail: Boxplots of velocity amplitudes simultaneously measured on the forager’s thorax (TxF) and its head (HeF), on the forager’s head and the food receiver’s head (HeR), on the food receiver’s head and its thorax (TxR), and on the food receiver’s thorax and its femur (FeR). F forager; D food receiver. Differences between vibration amplitudes picked up at the same body parts (compare TxF in a and b, HeF in b and c, HeR in c and d, and TxR in d and e) are due to differences between vibrating individuals [Adapted from Hrncir et al. (2006b)]
Assuming similar properties for the subgenual organ of stingless bees and honeybees, the vibratory stimulation of the food receivers during trophallaxis (~10 mm/s) is well above the sensory threshold in stingless bees (average response threshold between 0.06 and 0.15 mm/s peak–peak at frequencies between 150 and 900 Hz; Kilpinen and Storm 1997; Rohrseitz and Kilpinen 1997). Yet, the subgenual organs are not the only vibration receptors in bees (Sandeman et al. 1996). An additional receptor had its highest sensitivity at low vibration frequencies between 20 and 100 Hz, with a displacement threshold of about 2 μm (corresponding to a velocity threshold between 0.5 and 1.5 mm/s at these frequencies; calculated from Sandeman et al. 1996). The unidentified receptor organ was suggested to be one of the other three chordotonal organs found in the femur, tibia, and tarsus of each leg (Snodgrass 1956). Additionally, a pair of small fusiform chordotonal organs in the head of honeybees and campaniform sensilla in the legs and the head potentially serve as vibration detectors (Snodgrass 1956).
A crucial question is whether potential recruits do actually have trophallactic contacts with the foragers or, alternatively, trophallaxis is restricted to hive bees unloading and storing the incoming food. According to studies of intranidal case histories of individually marked recruits of M. quadrifasciata and M. seminigra, prospective food collectors indeed do have trophallactic contacts with the foragers before they leave the nest to collect at an advertised food source (Hrncir et al. 2000; Kronberger 2000). The number of trophallactic food transfers and contacts even increases shortly before the prospective recruits leave the nest (M. quadrifasciata: Hrncir et al. 2000; M. seminigra: Kronberger 2000).
6 Conclusions and Outlook
Thoracic vibrations generated by foragers on their return from a profitable food source are a feature common among eusocial bees, that is the stingless bees (Meliponini), the honeybees (Apini), and the bumblebees (Bombini) (Hrncir et al. 2006a, 2011). To this day, few species have been studied in some detail. Yet, the available data all show that both the occurrence and the temporal pattern of the pulsed vibrations correlate with the profitability of the exploited food source (Meliponini: see above; Apini: A. mellifera; Esch 1962; Hrncir et al. 2011; Bombini: Bombus terrestris; Oeynhausen and Kirchner 2001). So far, it remains an open question whether these similarities in vibratory recruitment communication among eusocial bees derive from a common evolutionary origin or whether they have developed independently in the different bee groups. However, the dependence of the vibrational signals on the foragers’ motivation as well as their correlation with recruitment success (stingless bees: see above; honeybees: Esch 1962; Dyer 2002; Hrncir et al. 2011) suggests a similar function of the thoracic vibrations for the coordination of foraging processes in eusocial bees. At least in stingless bees, this function is not the transfer of navigational information but of information on the profitability of the food source. Similar interpretations exist for the honeybee (Tautz 1996; Hrncir et al. 2011). It may come as a surprise, however, that even in the well-studied honeybee the question of how exactly the recruits perceive the dance information is far from being fully answered (Esch 2012; Michelsen 2012).
Although foraging strategies differ significantly among social bees, a principal function of intranidal recruitment mechanisms like the generation of vibratory signals is the rapid mobilization of a colony’s foraging force. Among the Meliponini, a highly successful strategy is aggressive group foraging, described for many species of the genera Trigona and Oxytrigona (Hubbell and Johnson 1978; Johnson 1983; Biesmeijer and Slaa 2004). Here, large groups of aggressive foragers dislodge less aggressive species from a specific food patch and monopolize clumped and rich resources (Johnson 1983; Biesmeijer and Slaa 2004). The success of these aggressive species relies on the guidance of the entire group toward a specific goal. This is accomplished by the use of pheromone marks at and near the food patch (Lindauer and Kerr 1958; Schmidt et al. 2003; Jarau et al. 2004, 2006; Schorkopf et al. 2007, 2011; Barth et al. 2008; Jarau 2009). In addition, a quick activation of large numbers of individuals is fundamental to successfully chasing other species away from a food patch and to defending this patch against other aggressive colonies. The trade-off for this increased competitive ability is a reduced capacity to discover new food sources or even neighboring food patches independently (Hubbell and Johnson 1978; Biesmeijer and Slaa 2004). The foraging success of little or non-aggressive species, such as Melipona or Nannotrigona (Hubbell and Johnson 1978; Johnson 1983; Biesmeijer and Slaa 2004), relies on the quick detection of many food patches and a rapid activation of all available foragers. Thus, when dislodged from a food location by aggressive groups, these species are able to switch the colony’s foraging focus to another food patch. Hence, although aggressive and non-aggressive species employ fundamentally different foraging strategies, a quick mobilization of unemployed foragers is required in both cases.
Based on our current knowledge of both the message and the potentially relevant transmission pathways of the vibratory signals of stingless bee foragers, we attribute three behavioral functions to the thoracic vibrations in recruitment communication. (1) Medium-range transmission—attraction of hive bees to the forager. Nectar-uptaking bees and food processors wait close to the nest entrance (Sommeijer and De Bruijn 1994; Hart and Ratnieks 2002). An increased “excitement” of a forager returning from a high-profit food source, and the resulting increase in pulse duration and duty cycle of her vibratory signals (Fig. 18.4), increasingly attracts food receivers to the forager (honeybee: Tautz and Rohrseitz 1998; Hasegawa and Ikeno 2011; stingless bees: Hart and Ratnieks 2002). Thereby, the resulting nectar transfer will accelerate the colony’s food intake because foragers can resume their collecting activity faster. On the other hand, the gustatory and olfactory information about a profitable food source will spread more quickly through the colony, thereby arousing experienced but inactive foragers (Biesmeijer et al. 1998). (2) Short-range transmission—reactivation of temporarily inactive foragers. Just like the food receivers and nectar processors, unemployed experienced foragers stay close to the nest entrance (Nieh 1998; Hrncir 2009) and may be attracted toward the vibrating forager by the substrate vibrations received. Even without participating directly in the nectar transfer, these foragers receive confirming information about a known food source through the scents clinging to the forager’s body (honeybee: Grüter and Farina 2009). In addition, they will receive information about the current state of profitability of the resource through the temporal pattern of the vibratory signals transmitted through air particle movement close to the vibrator’s body. This latter information is thought important for the temporarily inactive individuals when deciding whether to resume their collecting activity or not (Biesmeijer et al. 1998; Biesmeijer and Slaa 2004). (3) Direct transmission during trophallaxis–activation. The quick activation of foragers to a particular food source helps to efficiently exploit ephemeral, high-profit food sources and necessitates the recruitment of collectors inexperienced regarding a particular food source. The novice foragers (Biesmeijer and de Vries 2001) receive multiple categories of information about a particular resource during trophallactic interactions: Once attracted to the forager, during the mouth-to-mouth food transfer, novices receive information about sugar concentration, nectar secretion rate, and the odor of a food source (Farina and Grüter 2009; Grüter and Farina 2009). In addition, they learn about the current profitability of the nectar source through the forager’s vibrations. The sum of the information received lowers the foraging threshold of the novice bees (Biesmeijer et al. 1998; Biesmeijer and Slaa 2004; Hrncir 2009), which then leave the nest and search for the advertised food source.
Although knowledge about the vibratory signals in stingless bees has advanced considerably during the past decade, we are still far from a complete understanding of this intriguing communication system, which so efficiently coordinates the foraging processes. So far, some of the conclusions drawn are based on knowledge derived from studies on honeybees (A. mellifera). Future research will have to investigate the sensory mechanisms underlying the perception and processing of vibratory signals in the Meliponini, themselves. Only after having determined the physiological thresholds of the sensory organs involved, we will be able to determine the actual range of signal transmission and, subsequently, focus behavioral observations on hive bees within this range. The fact that stingless bees generate thoracic vibrations when tethered (Hrncir et al. 2008a) (Fig. 18.2) will help considerably in designing key experiments. Using annoyance-buzzing bees, thoracic vibrations can be generated under controlled laboratory conditions. This in turn permits the detailed investigation of both the pathways and respective attenuation of the signals on their way to the receivers (Hrncir et al. 2008b) and of the mechanical and physiological responses of receptors to genuine bee-produced vibrations instead of synthetic airborne sounds or substrate vibrations.
Notes
- 1.
The individual variation in sugar intake of M. seminigra foragers collecting at an artificial food source was 3.32 mg (Hrncir et al. 2004b). Taking measurements in honeybees, which are of similar body size as M. seminigra, as reference, the bees spend 0.70 mg sugar for each 1,000 m of flight (Hanauer-Thieser and Nachtigall 1995). Nestmates receiving the thoracic vibrations of a forager would have to decide whether the forager loaded 3.32 mg less sugar at the food source (less energy intake) or spent more energy due to an additional 4,740 m of flight (consumption of additional 3.32 mg sugar). The energy budget, and thus thoracic vibrations reflecting it, would be the same under both conditions provided that thoracic vibrations are influenced to the same degree by energy intake and energy consumption.
- 2.
Wasps and bees produce thoracic vibrations when trying to escape from any form of confinement, such as when pushing through narrow nest entrances (Michener 2000), or when trying to escape from the grasp of predators or researchers (Esch and Wilson 1967; Schneider 1975; Larsen et al. 1986; Hrncir et al. 2008a). This form of thoracic vibrations (termed “disturbance buzzes”: Larsen et al. 1986; “annoyance buzzing”: Hrncir et al. 2008a) are known from both solitary bees (Colletes cunicularius: Larsen et al. 1986) and social bees (Bombini; Bombus terrestris: Schneider 1975; Meliponini; Melipona spp.: Esch and Wilson 1967; Hrncir et al. 2008a, b; Nunes-Silva 2011).
References
Aguilar I, Briceño D (2002) Sounds in Melipona costaricensis (Apidae: Meliponini): effect of sugar concentration and nectar source distance. Apidologie 33:375–388
Allerstorfer S (2004) Rekrutierung und Kommunikation bei Nannotrigona testaceicornis Lep. (1836) (Hymenoptera; Apidae; Meliponini). Diploma thesis, University of Vienna, Austria
Autrum H, Schneider W (1948) Vergleichende Untersuchungen über den Erschütterungssinn der Insekten. Z vergl Physiol 31:77–88
Barth FG (1998) The vibrational sense of spiders. In: Hoy RR, Popper AN, Fay RR (eds) Comparative hearing: insects. Springer, Berlin, pp 228–278
Barth FG, Bleckmann H, Bohnenberger J, Seyfarth EA (1988) Spiders of the genus Cupiennius Simon 1891 (Araneae, Ctenidae) II. On the vibratory environment of a wandering spider. Oecologia 77:194–201
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol A 194:313–327
Bennet-Clark HC (1999) Resonators in insect sound production: how insects produce loud pure-tone songs. J Exp Biol 202:3347–3357
Biesmeijer JC, de Vries H (2001) Exploration and exploitation of food sources by social insect colonies: a revision of the scout-recruit concept. Behav Ecol Sociobiol 49:89–99
Biesmeijer JC, Slaa EJ (2004) Information flow and organization of stingless bee foraging. Apidologie 35:143–157
Biesmeijer JC, van Nieuwstadt MGL, Lukács S, Sommeijer MJ (1998) The role of internal and external information in foraging decisions of Melipona workers (Hymenoptera: Meliponinae). Behav Ecol Sociobiol 42:107–116
Buchmann SL (1983) Buzz pollination in angiosperms. In: Jones CE, Little RJ (eds) Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, pp 73–113
Burkart A, Lunau K, Schlindwein C (2011) Comparative bioacoustical studies on flight and buzzing of neotropical bees. J Pollination Ecol 6:118–124
Camargo JMF, Pedro SRM (2007) Meliponini Lepeletier, 1836. In: Moure JS, Urban D, Melo GAR (eds) Catalogue of bees (Hymenoptera, Apoidea) in the neotropical region. Sociedade Brasileira de Entomologia, Curitiba, pp 272–578
Conrad T, Paxton RJ, Barth FG, Francke W, Ayasse M (2010) Female choice in the red mason bee, Osmia rufa (L.) (Megachilidae). J Exp Biol 213:4065–4073
Dyer FC (2002) The biology of the dance language. Annu Rev Entomol 47:917–949
Eickwort GC, Ginsberg HS (1980) Foraging and mating behavior in Apoidea. Annu Rev Entomol 25:421–446
Esch H (1962) Über die Auswirkung der Futterplatzqualität auf die Schallerzeugung im Werbetanz der Honigbiene. Verh Deut Z 26:302–309
Esch H (1967) Die Bedeutung der Lauterzeugung für die Verständigung der stachellosen Bienen. Z vergl Physiol 56:199–220
Esch H (2012) Foraging honey bees: how foragers determine and transmit information about feeding site locations. In: Galizia CG, Eisenhardt D, Giurfa M (eds) Honeybee neurobiology and behavior. Springer, Dordrecht, pp 53–64
Esch HE, Burns JE (1995) Honeybees use optic flow to measure the distance of a food source. Naturwissenschaften 82:38–40
Esch H, Wilson D (1967) The sounds produced by flies and bees. Z vergl Physiol 54: 256–267
Esch H, Esch I, Kerr WE (1965) Sound: An element common to communication of stingless bees and to dances of the honey bee. Science 149:320–321
Farina WM, Grüter C (2009) Trophallaxis—a mechanism of information transfer. In: Jarau S, Hrncir M (eds) Food exploitation by social insects—ecological, behavioral, and theoretical approaches. CRC Press, Taylor & Francis Group, Boca Raton, pp 183–197
Frisch K von (1946) Die Tänze der Bienen. Österr Zool Z 1:1–48
Grüter C, Farina W (2009) The honeybee waggle dance: can we follow the steps? TREE 24:242–247
Hanauer-Thieser U, Nachtigall W (1995) Flight of the honey bee. VI. energetics of wind tunnel exhaustion flights at defined fuel content, speed adaptation and aerodynamics. J Comp Physiol B 165:471–483
Hart AG, Ratnieks FLW (2002) Task-partitioned nectar transfer in stingless bees: work organisation in a phylogenetic context. Ecol Entomol 27:163–168
Harter B, Leistikow C, Wilms W, Truylio B, Engels W (2002) Bees collecting pollen from flowers with poricidal anthers in a south Brazilian Araucaria forest: a community study. J Apicult Res 40:9–16
Hasegawa Y, Ikeno H (2011) How do honeybees attract nestmates using waggle dances in dark and noisy hives? PLoS ONE 6:e19619
Heran H (1959) Wahrnehmung und Regelung der Flugeigengeschwindigkeit bei Apis mellifica L. Z vergl Physiol 42:103–163
Hrncir M (2009) Mobilizing the foraging force—mechanical signals in stingless bee recruitment. In: Jarau S, Hrncir M (eds) Food exploitation by social insects—ecological, behavioral, and theoretical approaches. CRC Press, Taylor & Francis Group, Boca Raton, pp 199–221
Hrncir M, Jarau S, Zucchi R, Barth FG (2000) Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata II. Possible mechanisms of communication. Apidologie 31:93–113
Hrncir M, Jarau S, Zucchi R, Barth FG (2003) A stingless bee (Melipona seminigra) uses optic flow to estimate flight distances. J Comp Physiol A 189:761–768
Hrncir M, Jarau S, Zucchi R, Barth FG (2004a) Thorax vibrations of a stingless bee (Melipona seminigra). I. No influence of visual flow. J Comp Physiol A 190:539–548
Hrncir M, Jarau S, Zucchi R, Barth FG (2004b) Thorax vibrations of a stingless bee (Melipona seminigra). II. Dependence on sugar concentration. J Comp Physiol A 190:549–560
Hrncir M, Barth FG, Tautz J (2006a) Vibratory and airborne-sound signals in bee communication (Hymenoptera). In: Drosopoulos S, Claridge MF (eds) Insect sound and communication – physiology, behaviour, ecology and evolution. CRC Press, Taylor & Francis Group, Boca Raton, pp 421–436
Hrncir M, Schmidt VM, Schorkopf DLP, Jarau S, Zucchi R, Barth FG (2006b) Vibrating the food receivers: a direct way of signal transmission in stingless bees (Melipona seminigra). J Comp Physiol A 192:879–887
Hrncir M, Gravel AI, Schorkopf DLP, Schmidt VM, Zucchi R, Barth FG (2008a) Thoracic vibrations in stingless bees (Melipona seminigra): Resonances of the thorax influence vibrations associated with flight but not those associated with sound production. J Exp Biol 211:678–685
Hrncir M, Schorkopf DLP, Schmidt VM, Zucchi R, Barth FG (2008b) The sound field generated by tethered stingless bees (Melipona scutellaris): inferences on its potential as a recruitment mechanism inside the hive. J Exp Biol 211:686–698
Hrncir M, Maia-Silva C, McCabe SI, Farina WM (2011) The recruiter’s excitement—features of thoracic vibrations during the honey bee’s waggle dance related to food source profitability. J Exp Biol 214:4055–4064
Hubbel SP, Johnson LK (1978) Comparative foraging behavior of six stingless bee species exploiting a standardized resource. Ecology 59:1123–1136
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects—ecological, behavioral, and theoretical approaches. CRC Press, Taylor & Francis Group, Boca Raton, pp 223–249
Jarau S, Hrncir M, Zucchi R, Barth FG (2000) Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31:81–91
Jarau S, Hrncir M, Zucchi R, Barth FG (2004) A stingless bee uses labial gland secretions for scent trail communication (Trigona recursa SMITH 1863). J Comp Physiol A 190:233–239
Jarau S, Schulz CM, Hrncir M, Francke W, Zucchi R, Barth FG, Ayasse M (2006) Hexyl decanoate, the first trail pheromone compound identified in a stingless bee (Trigona recursa). J Chem Ecol 32:1555–1564
Johnson LK (1983) Foraging strategies and the structure of stingless bee communities in Costa Rica. In: Jaisson P (ed) Social insects in the tropics 2. Université Paris-Nord, Paris, pp 31–58
Kilpinen O, Storm J (1997) Biophysics of the subgenual organ of the honeybee, Apis mellifera. J Comp Physiol A 181:309–318
King MJ (1993) Buzz foraging mechanism of bumble bees. J Apicult Res 32:41–49
King MJ, Buchmann SL, Spangler H (1996) Activity of asynchronous flight muscle from two bee families during sonication (buzzing). J Exp Biol 199:2317–2321
Kirchner W (1994) Hearing in honeybees: the mechanical response of the bee’s antenna to near field sound. J Comp Physiol A 175:261–265
Kronberger E (2000) Futterplatzrekrutierung bei Melipona seminigra merillae. Diploma thesis, University of Vienna, Austria
Larsen O, Gleffe G, Tengö J (1986) Vibration and sound communication in solitary bees and wasps. Physiol Entomol 11:287–296
Lindauer M (1956) Über die Verständigung bei indischen Bienen. Z vergl Physiol 38:521–557
Lindauer M, Kerr WE (1958) Die gegenseitige Verständigung bei den stachellosen Bienen. Z vergl Physiol 41:405–434
Lindauer M, Kerr WE (1960) Communication between workers of stingless bees. Bee World 41(29–41):65–71
Menzel R (2012) Navigation and communication: commentary. In: Galizia CG, Eisenhardt D, Giurfa M (eds) Honeybee neurobiology and behavior. Springer, Dordrecht, pp 117–122
Michelsen A (2003) Signals and flexibility in the dance communication of honeybees. J Comp Physiol A 189:165–174
Michelsen A (2012) How do honey bees obtain information about direction by following dances? In: Galizia CG, Eisenhardt D, Giurfa M (eds) Honeybee neurobiology and behavior. Springer, Dordrecht, pp 65–76
Michelsen A, Nocke H (1974) Biophysical aspects of sound communication in insects. Adv Insect Physiol 10:247–296
Michelsen A, Towne WF, Kirchner WH, Kryger P (1987) The acoustic near field of a dancing honeybee. J Comp Physiol A 161:633–643
Michener CD (1962) An interesting method of pollen collecting by bees from flowers with tubular anthers. Rev Biol Trop 10:167–175
Michener CD (1974) The social behavior of the bees: a comparative study. Harvard University Press, Cambridge
Michener CD (2000) The bees of the world. Johns Hopkins University Press, Baltimore
Morawetz L (2007) Reichweite und Übertragung vibratorischer Signale bei der Kommunikation stachelloser Bienen. Diploma thesis, University of Vienna, Austria
Nachtigall W (2003) Insektenflug. Springer, Berlin
Nieh JC (1998) The food recruitment dance of the stingless bee, Melipona panamica. Behav Ecol Sociobiol 43:133–145
Nieh JC, Roubik DW (1995) A stingless bee (Melipona panamica) indicates food location without using a scent trail. Behav Ecol Sociobiol 37:63–70
Nieh JC, Roubik DW (1998) Potential mechanisms for the communication of height and distance by a stingless bee, Melipona panamica. Behav Ecol Sociobiol 43:387–399
Nieh JC, Sanchez D (2005) Effect of food quality, distance and height on thoracic temperature in the stingless bee Melipona panamica. J Exp Biol 208:3933–3943
Nieh JC, Contrera FAL, Rangel J, Imperatriz-Fonseca VL (2003) Effect of food location and quality on recruitment sounds and success in two stingless bees, Melipona mandacaia and Melipona bicolor. Behav Ecol Sociobiol 55:87–94
Nocke H (1971) Biophysik der Schallerzeugung durch die Vorderflügel der Grillen. Z vergl Physiol 74:272–314
Nunes-Silva P (2011) Capacidade vibratória e polinização por vibração nas abelhas do gênero Melipona (Apidae, Meliponini) e Bombus (Apidae, Bombini). PhD thesis, University of São Paulo-Ribeirão Preto, Brazil
Nunes-Silva P, Hrncir M, Imperatriz-Fonseca VL (2010) A polinização por vibração. Oecolog Aust 14:140–151
Oeynhausen A, Kirchner WH (2001) Vibrational signals of foraging bumblebees (Bombus terrestris) in the nest. In: Proceedings of the meeting of the European sections of IUSSI, Berlin, Germany, p 31
Ramírez SR, Nieh JC, Quental TB, Roubik DW, Imperatriz-Fonseca VL, Pierce NE (2010) A molecular phylogeny of the stingless bee genus Melipona (Hymenoptera: Apidae). Mol Phylogenet Evol 56:519–525
Rasmussen C, Cameron SA (2010) Global stingless bee phylogeny supports ancient divergence, vicariance, and long distance dispersal. Biol J Linn Soc 99:206–232
Rohrseitz K (1998) Biophysikalische und ethologische Aspekte der Tanzkommunikation der Honigbienen (Apis mellifera carnica Pollm.). Doctoral thesis, Julius Maximilian University Würzburg, Germany
Rohrseitz K, Kilpinen O (1997) Vibration transmission characteristics of the legs of freely standing honeybees. Zoology 100:80–84
Roselino AC, Hrncir M (2012) Repeated unrewarded scent exposure influences the food choice of stingless bee foragers, Melipona scutellaris. Anim Behav 83:755–762
Roubik DW (1989) The ecology and natural history of tropical bees. Cambridge University Press, Cambridge
Roubik DW (2006) Stingless bee nesting biology. Apidologie 37:124–143
Samwald U (2000) Mechanismen der Futterplatzrekrutierung bei Melipona seminigra merillae CCKL (1919) (Hymenoptera; Apidae; Meliponinae). Diploma thesis, University of Vienna, Austria
Sandeman DC, Tautz J, Lindauer M (1996) Transmission of vibration across honeycombs and its detection by bee leg receptors. J Exp Biol 199:2585–2594
Schmidt VM, Zuchi R, Barth FG (2003) A stingless bee marks feeding site in addition to the scent path (Scaptotrigona aff. depilis). Apidologie 34:237–248
Schmidt VM, Zucchi R, Barth FG (2006) Recruitment in a scent trail laying stingless bee (Scaptotrigona aff. depilis): changes with reduction but not with increase of the energy gain. Apidologie 37:487–500
Schmidt VM, Hrncir M, Schorkopf DLP, Mateus S, Zucchi R, Barth FG (2008) Food profitability affects intranidal recruitment behaviour in the stingless bee Nannotrigona testaceicornis. Apidologie 39:260–272
Schneider P (1975) Versuche zur Erzeugung des Verteidigungstones bei Hummeln. Zool Jahrb allg Zool 79:111–127
Schön A (1911) Bau und Entwicklung des tibialen Chordotonalorgans bei der Honigbiene und bei Ameisen. Zool Jahr Anat 31:439–472
Schorkopf DLP, Jarau S, Francke W, Twele R, Zucchi R, Hrncir M, Schmidt VM, Ayasse M, Barth FG (2007) Spitting out information. Trigona bees deposit saliva to signal resource locations. P Roy Soc Lond B 274:895–898
Schorkopf DL, Morawetz L, Bento JM, Zucchi R, Barth FG (2011) Pheromone paths attached to the substrate in meliponine bees: helpful but not obligatory for recruitment success. J Comp Physiol 197:755–764
Schwarz HF (1948) Stingless bees (Meliponidae) of the western hemisphere. B Am Mus Nat Hist 90:1–546
Seeley TD (1992) The tremble dance of the honey bee: message and meanings. Behav Ecol Sociobiol 31:375–383
Simpson J (1964) The mechanism of honey-bee queen piping. Z vergl Physiol 48:277–282
Snodgrass RE (1956) Anatomy of the honey bee. Comstock Publishing Associates, Ithaca
Sommeijer MJ, De Bruijn LLM (1994) Intranidal feeding, trophallaxis and sociality in stingless bees. In: Hunt J, Nalepa C (eds) Nourishment and evolution in insect societies. Westview Press, Oxford, pp 391–418
Srinivasan MV, Zhang S, Altwein M, Tautz J (2000) Honeybee navigation: nature and calibration of the “odometer”. Science 287:851–853
Storm J, Kilpinen O (1998) Modelling the subgenual organ of the honeybee, Apis mellifera. Biol Cybern 78:175–182
Tautz J (1996) Honeybee waggle dance: recruitment success depends on the dance floor. J Exp Biol 199:1375–1381
Tautz J, Rohrseitz K (1998) What attracts honeybees to a waggle dancer? J Comp Physiol A 183:661–667
Tautz J, Casas J, Sandeman DC (2001) Phase reversal of vibratory signals in honeycomb may assist dancing honeybees to attract their audience. J Exp Biol 204:3737–3746
Vicidomini S (1998) Biology of Xylocopa (Xylocopa) violacea (L., 1758) (Hymenoptera: Apidae): female nest-defence. Annali del Museo Civico di Rovereto 12:85–100
Wille A (1963) Behavioral adaptations of bees for pollen collecting from Cassia flowers. Rev Biol Trop 11:205–210
Wille A, Michener CD (1973) The nest architecture of stingless bees with a special reference to those of Costa Rica (Hymenoptera, Apidae). Rev Biol Trop 21(Suppl 1):1–278
Acknowledgments
Our sincere thanks go to Ronaldo Zucchi, Stefan Jarau, Dirk-Louis P. Schorkopf, and Veronika Schmidt whose participation, help, and discussion were decisive for many of the studies described in this chapter. Financial support for the research came from grants FAPESP (2006/50809-7) and CNPq (304722/2010-3) to MH, and FWF (P 14328, P 17530) to FGB.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hrncir, M., Barth, F.G. (2014). Vibratory Communication in Stingless Bees (Meliponini): The Challenge of Interpreting the Signals. In: Cocroft, R., Gogala, M., Hill, P., Wessel, A. (eds) Studying Vibrational Communication. Animal Signals and Communication, vol 3. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43607-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-662-43607-3_18
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43606-6
Online ISBN: 978-3-662-43607-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)