Abstract
Vibratory signals of biotic and abiotic origin occur commonly in the environment of all living organisms. Many species deliberately produce such signals for communication purposes. Thus, it is not only useful but also advantageous and/or necessary to be able to detect and process vibratory signals with appropriate receptor organs. Mechanoreception is suggested to be evolutionarily ancient among animals (Kung 2005; Thurm 2001). Given the long evolutionary history, such receptors have very different anatomical structures and corresponding physiological properties. Responding to mechanical stress is a basic property of cells, even outside the nervous system. In the nervous system, specialized sensory cells and organs register mechanosensory signals and impart the information to higher centers. Structural and molecular adaptations in various mechanoreceptors can push these systems to a sensitivity at or near to the physical limits, e.g., with respect to the noise–stimuli relation. Here, we will deal with the vibratory receptor systems of insects, with a focus on the specialized scolopidial sensory organs from molecular mechanisms to systems analysis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Anatomical Diversity of Sensilla
In insects, some vibration receptor types are located at the external surface or embedded in the cuticle, like campaniform sensilla, hair sensilla, or hairplates (Fig. 14.1). Other receptors are internal sensilla, like scolopidial sensilla or multipolar/multidendritic sensilla. Among these types, the scolopidial sensilla are particularly sensitive to vibrational stimuli. However, a classification and discrimination between the different mechanical processes, like cell expansion, stretching of organs, touch, substrate vibration, and airborne sound, is sometimes difficult, and adequate stimuli may overlap in a given type of sensilla. In the following, different types of sensilla are briefly reviewed with respect to their properties for mechanoreception of vibration.
Different mechanoreceptive sense organs of insects. a, b Scanning electron microscopic photos of campaniform sensilla a and hair sensilla, b of the appendages of Decticus verrucivorus (Orthoptera). c, d Transmission electron microscopic photos of scolopidial sensilla. c Longitudinal section of a scolopidium in the prosternal organ of Homotrixa alleni (Diptera). Note the dendrite (d) running in the lumen of the scolopale (sc). ca cap, cd ciliary dilation, scn nucleus of the scolopale cell. d Cross section of scolopidia in Phormia regina (Diptera). Scales a 10 µm, b 50 µm, c 2 µm, and d 1 µm (Unpublished data)
1.1 Campaniform Sensilla
Campaniform sensilla (CS) are a type of external sensilla that is embedded in the cuticle. From the outside, the sensilla are characterized by a shallow dome (Fig. 14.1). Often, the anatomical structure is also indicative of the direction of stimulus perception. The campaniform sensilla react to mechanical stress in the cuticle—the stimuli can originate either from the animal’s own movements (proprioception) or from external sources (substrate vibration). CS are located at different positions on the body segments and on appendages, such as the wings or halteres, where mechanical deformations can be detected (Keil 1997). CS can occur as single sensilla, or they can be arranged in groups. CS of the legs have been found to possess minimum acceleration thresholds at frequencies of 50 Hz and lower (Kühne 1982). Stimuli with such frequencies were responded to with a phase-lock of the action potentials. The upper cutoff frequency is some hundred hertz. The CS of legs typically have an axonal projection that is ramified in the lateral part of the corresponding ganglion (Schmitz et al. 1991; Merritt and Murphey 1992; Mücke and Lakes-Harlan 1995) (see also Fig. 14.6). CS occurring dorsally at the proximal tibia are involved in feedback loops of leg movement in many insects (Burrows and Pflüger 1988; Zill et al. 2011). The spatial location of the CS relative to vibration-receptive organs, like the subgenual organ, raises the question of whether CS responses might also be integrated in the neuronal vibratory network for filtering or modulation of information.
1.2 Hair Sensilla
Hair sensilla (HS) come in various anatomical shapes of the hair shaft (and were consequently differently named) and with different physiological functions (Fig. 14.1). Mechanosensory bristles can be found on all body parts. They are, just as CS, usually constructed by four cells during development: a sensory neuron, a sheath cell, a socket cell, and a hair shaft cell. The mechanosensory neuron has ultrastructural specializations in its dendrite, like the tubular body (Keil 1997). The tubular body is a massive complex of microtubules in the dendritic tip that is involved in mechanotransduction. Destruction of the tubular body leads to a decrease in the receptor potential (Erler 1983). The interaction between stimuli, structural elements, and ion channels is the subject of an ongoing analysis of transduction mechanisms (Liang et al. 2011) (see also Sect. 14.2.2). Hair sensilla typically react to touch stimuli. However, filiform hairs might also react to vibrational stimuli, either direct or indirect. An example of indirect measurements is a vibrating leaf that moves air particles above its surface, which in turn deflect the filiform hairs of the insect. Parasitoid wasps might locate their vibration-producing prey within the leaves by such a mechanism (Meyhöfer and Casas 1999). Hair sensilla (trichobotria) of water striders also serve as vibration receptors (Goodwyn et al. 2009). Neuronal networks involving HS have been studied with respect to touch perception (Burrows and Newland 1993). The central projections of hair sensilla are somatotopically ordered in the lateral neuropile of the respective ganglion (Mücke and Lakes-Harlan 1995) (see also Fig. 14.6) and correlate with the receptive field of interneurons (Burrows and Newland 1993).
1.3 Multipolar Sensilla
In comparison with other sensilla in insects, least is known about function and physiology of multipolar sensilla. In contrast to bipolar neurons, these sensilla have multiple processes, which can cover much of the body surface (Grueber et al. 2002); in Drosophila, they are known as multidendritic (md) neurons. Multidendritic neurons might react to a variety of stimuli: Subsets of md neurons might be involved in proprioception (Grueber et al. 2002), whereas other subsets might respond to temperature or nociception (Tracey et al. 2003). Multipolar sensilla of locusts occur on different body positions and as joint receptors. They can react to vibrational stimuli, but are relatively insensitive (Kühne 1982).
1.4 Scolopidial Sensilla
Scolopidial sensilla or chordotonal organs in general have already been subject of a detailed treatise (Field and Matheson 1998). Therefore, their basic features are briefly summarized in the present review, and the focus here is on recent findings of the transduction mechanisms and a discussion of the complexity of sensory organs, their position, and evolution. Scolopidial sensilla are internal sensilla homologous to external sensilla and are characterized by an electron-dense structure, the “scolopale.” The scolopale is formed by actin filaments inside the scolopale cell and surrounds an extracellular space into which the dendrite of the sensory cells extends (Fig. 14.1). The dendrite terminates at an electron-dense cap that is formed by an attachment cell (Fig. 14.1). Further characters of the dendrite are a ciliary dilation and rootlets at its base in the cell body. The sensory cell is surrounded by a glial cell. Such a unit with basically a sensory cell, a glial cell, a scolopale cell, and an attachment cell is termed a scolopidium. Based on ultrastructural data, two basic types are distinguished. Type 1 scolopidia have cilia with constant diameter and an extracellular cap. The proposed stimulus is an axial extension, and these sensilla are probably most important for vibration perception. Type 2 scolopidia have an enlarged distal segment in the cilium and an elongated tube. They occur often in crustaceans, but have also been shown in the Johnston’s organ of insects (for review see Moulins 1976). The cellular composition of scolopidia varies between organs, as some scolopidia possess two sensory cells or additional ligament cells. Within the insect body, the scolopidia are arranged as single units, in small groups or in large, complex sensory organs. In the latter cases, distinct groups of scolopidia, named scoloparia, can occur within the organ complex and might have distinct physiological properties.
2 Scolopidial Organs
Scolopidial organs are located at different positions in the insect body. They are part of the sensory complement in the basic bauplan of an insect’s segment (Fig. 14.2). For example, in Drosophila larvae, most segments contain three scolopidial organs, with a specific number of sensory units (Campos-Ortega and Hartenstein 1997; Hertweck 1931). Besides Drosophila, the scolopidial organs have been mapped in orthopteran body segments (Field and Matheson 1998; Meier et al. 1991), whereas in many other insects, only exemplary scolopidial organs have been described. The scolopidial organs are located within a specific segment or are connecting two segments. The position determines the physiological function and sensitivity, but the function may not be obvious on first sight. For example, it is possible that substrate vibrations are perceived with body scolopidial organs, although the body does not have direct contact with the substrate (see Sect. 14.2.3.4 on the prosternal organ). Among the head appendages, the Johnston’s organ is well known as a receiver of antennal motion in many insects. Within the insect legs, scolopidial organs have typical positions (Fig. 14.2): in the proximal femur (femoral chordotonal organ), in the proximal tibia (subgenual organ, complex tibial organ), and in the distal tibia and tarsae (chordotonal organs with only a few scolopidia). In the following sections, the development, mechanisms of transduction, as well as functional morphology and physiology of chordotonal organs are reviewed in more detail, with a focus on the scolopidial organs in the proximal tibia.
Schematic overview of the location of scolopidial organs in the body segments and legs. a In a typical segment, two to three nerves innervate scolopidial organs at two positions (in Orthoptera, the pleural chordotonal organ (plCO) and the sternal chordotonal organ (sCO); in Drosophila, the lateral chordotonal organ (lCO) and the ventral chordotonal organ (vCO). b In a typical insect leg, the femoral chordotonal organ (FCO) might have different subparts; the subgenual organ (SGO) is often accompanied by other scolopidial organs (see text), and chordotonal organs are located in the distal tibia (tiCO) and tarsae (taCO). (Modified from Campos-Ortega and Hartenstein 1997; Meier et al. 1991; Mücke 1991)
2.1 Development
For different types of sensilla, an underlying developmental sequence has been identified for the cellular differentiation of chordotonal cells, CS, and HS (microchaetes) of Drosophila (Lai and Orgogozo 2004). During that sequence, a sensory mother cell divides asymmetrically, and after further cell divisions, four to five cells are generated. Thereby, two accessory cells (e.g., socket cell and shaft cell) differentiate and one sensory neuron with glial or sheath cells. By differential gene expression, the different types of receptor organs are generated. For example, mutations in the cut gene transform a hair sensillum into a scolopidial sensillum (Bodmer et al. 1987).
By contrast, the development of complex scolopidial organs is only partially understood. For formation of sensory units in the relatively large femoral chordotonal organ of Drosophila, the epidermal growth factor receptor promotes continuous generation of sensory mother cells (zur Lage and Jarman 1999). Complexity is not only a question of cell numbers, but also a question of distinct subparts of an organ, as these subparts might have different physiological properties. The complex tibial organ (CTO) of some Ensifera consists of three parts with different functions (see Sect. 14.2.3). The CTO develops during embryonic development (Klose 1996; Lakes-Harlan and Strauß 2006; Meier and Reichert 1990), but at least in some species of Tettigoniidae, the sensory cells of the parts of the CTO have different developmental origins (Fig. 14.3, unpublished results). The cells of the subgenual organ (SGO) and the crista acustica homolog (CAH) arise from sensory mother cells that proliferate at separate positions in the epidermal cell layer. Later, during embryogenesis, the cells form the CTO together and establish their specific central projections. Scolopidial sensory neurons grow their axons during embryogenesis or metamorphosis to the CNS (Lakes-Harlan and Pollack 1993; Lakes and Pollack 1990). These axons orientate along preformed pathways in the embryo; in cases where the pathway is missing, the axons arrest their growth (Klose and Bentley 1989). For axonal guidance, the cell surface molecule Fasciclin I, which is expressed by the sensory cells and the target neuropiles in the CNS, could be important (Schäffer and Lakes-Harlan 2001). In the holometabolic Diptera, complex scolopidial organs like the femoral chordotonal organ (FCO) develop during metamorphosis. Interestingly, the axons of the FCO extend to the CNS, but after mid-metamorphosis, the organ is retracted within the femur and the axons are apparently shortened during this process (Lakes and Pollack 1990). By contrast, in the moth Manduca sexta, sensory units of the FCO are already present during larval stages (Kent et al. 1996). Vibration receptors are fully functional after hatching, and the sensitivity to vibration stimuli does not change during postembryonic development in tettigoniids and locusts (Rössler et al. 2006).
Model of the developmental sequence of the complex tibial organ of M. elongata (Ensifera). The model is based on sequential preparations of embryonic stages (indicated by percentages of developmental time to larval hatching) with immunolabeling of the sensory cells. The cells of the subgenual organ (SGO) and the crista acustica homolog (CAH) have different developmental origins (sense organ precursor cells) and form together the complex sensory organ, including the intermediate organ (IO). (Based on unpublished data)
2.2 Mechanotransduction
Transduction of mechanical stimuli is a multistep process ranging from mechanical force acting on the receptors to neuronal activity of sensory neurons (French 1992). The first step is a mechanical coupling between the stimulus, e.g., a substrate vibration, and the receptor structure. Such a structure could be the cuticle transmitting the external force. Consequently, embedded sensilla, the campaniform sensilla, can measure compression or strain of the cuticle that leads to activation of the receptor. In the case of the internal scolopidial sensilla, a longitudinal stretch of the dendrite is likely to be the adequate stimulus. The micromechanics of vibration inside a leg or other body parts are rarely investigated (see Sect. 14.2.3 for some discussion), although they might be decisive for the physiological function of the scolopidial organs. Subcellular structures in the scolopidial units, like a ciliary dilation in the dendrite of the sensory neuron, may also be important as suggested by mutant studies (reviewed in Field and Matheson 1998). The next steps in mechanotransduction are the opening of ion channels and changes in the membrane potential. In recent years, the molecular physiology of sensory transduction was pushed forward especially in the model system of the antennal Johnston’s organ of Drosophila (Kernan 2007; Lu et al. 2009). The Johnston’s organ consists of several hundred scolopidial units and is mainly an auditory and gravitation receptor.
Behavioral screens for flies defective in mechanotransduction identified candidate molecules for transduction gating, including ion channels of the transient receptor potential (TRP) ion channel family. TRP channels are cation channels with six transmembrane domains and cytoplasmic N- and C-terminals found in different sense organs; TRP channels can be classified into seven distinct groups based upon sequence similarities and structural characteristics (Christensen and Corey 2007; Matsuura et al. 2009).
Three TRP channels have been identified as candidates for the gating channel in mechanosensory cells: “No mechanoreceptor potential C” (NompC), “Inactive” (Iav), and “Nanchung” (Nan). These channels are specifically located in subcellular structures important for mechanotransduction: Iav and Nan are localized in the proximal cilia of chordotonal neurons (Gong et al. 2004); NompC is localized in the distal cilia of scolopidial neurons and the tubular body of campaniform sensilla (Lee et al. 2010; Liang et al. 2011; Keil 2012). Nan and Iav are expressed in most sensory neurons in the Drosophila Johnston’s organ. These channels form heterodimers, and Nan is dependent on Iav for proper localization within the scolopidia (Gong et al. 2004). If Iav is deleted, the neuronal response to acoustic stimuli is abolished. It was discussed whether the Nan-Iav dimers may be mechanically activated by ciliary deflections or have a function in signal control downstream of the transducing channel, as disruption of Nan-Iav increases the active amplification in the hearing organ (Göpfert et al. 2006). While the majority of the Johnston’s organ sensory neurons express NompC (Lee et al. 2010), only the neurons mediating hearing require it functionally for generating a regular neural response (Effertz et al. 2011). NompC includes an ankyrin spring of 29 ankyrin repeats (Howard and Bechstein 2004) and has been shown to be directly opened by mechanical stimuli (Yan et al. 2013). It may form the gating spring in campaniform sensilla (Liang et al. 2013) though not the gating channel in auditory neurons (Lehnert et al. 2013). The definite contribution of the respective molecules to mechanotransduction in Drosophila hearing is so far unresolved (Lehnert et al. 2013; Gong et al. 2013; Matsuo and Kamikouchi 2013). Given this functional specialization between Johnston’s organ neurons, the functional conservation or similarity of TRP channels in sensory organs for substrate vibrations like the subgenual organ or femoral chordotonal organ will be relevant to explore.
Related to the identity of the gating, channel is the question for the mechanism of channel opening ultimately causing mechanotransduction. According to a membrane force model, direct opening could result from transfer of forces on the neuron’s cell membrane and a pull on the ion channel, opening the pore (Chalfie 2009; Christensen and Corey 2007). Indirect tether models in general assume that force is transferred to specific molecules/proteins which are linked to the ion channel and activate it by induction of a conformational change. Such molecules could be linked to the intracellular cytoskeleton or extracellular matrix molecules or a combination thereof (Chalfie 2009; Christensen and Corey 2007). In scolopidia of Johnston’s organ of Drosophila, opening of the gating channel is supposedly direct and via gating springs (Albert et al. 2007). The short latencies in chordotonal organs in the submillisecond range support the hypothesis of a direct activation process (Albert et al. 2007).
In mechanoreceptors, the sheath cells produce a high concentration of potassium in the surrounding of the dendritic cilium compared to hemolymph (French 1988; McIver 1985). In scolopidial units, the scolopale space is filled by an endolymph with high potassium concentration, but low calcium concentration (Todi et al. 2004). The receptor potential of mechanoreceptors is proportionally graded to the stimulus intensity (French 1988). The primary sensory neurons are supposed to generate action potentials at the beginning of the axon. In the scolopidia of an orthopteran hearing organ, small non-propagating spikes are passively conducted back into the soma and the dendrites (Hill 1983).
2.3 Functional Morphology and Physiology
Scolopidial organs differ largely in their functional morphology. Here, we focus on those found within the legs, and especially in the proximal tibia, that have been investigated for several insect taxa, with another example of a vibration-sensitive organ in the thorax of an insect.
2.3.1 Femoral Chordotonal Organ
The femoral chordotonal organ (FCO) is probably present in all insects, and in many species, it is a large mechanosensory organ with up to hundreds of sensory units (Debaisieux 1938). It is located dorso-proximally in the femur and attaches via a cuticular apodeme at the femur–tibia joint (Fig. 14.2). While the FCO is in general a proprioceptive organ involved in sensory feedback loops required for limb coordination, it is also a vibration receptor in some insects. The functional specialization may be associated with structural subdivision. For example, in orthoptera and stick insects, two distinct scoloparia are found, whereby the locust FCO and Carausius FCO contain heterodynal type I scolopidia with two sensory neurons each (Füller and Ernst 1973; Matheson and Field 1990). In the locust, the FCO of the middle leg contains 42 sensory neurons in the distal scoloparium and several hundred relatively small sensory neurons in the proximal scoloparium (Field and Pflüger 1989). Physiological investigations in the locust showed a functional distinction between the two groups: The distal scoloparium mediates the postural resistance reflex, while the proximal group does not affect this reflex (Field and Pflüger 1989). The latter group was suggested to be a functional receiver of vibrational stimuli between 50 and 300 Hz, as it responds to vibrations with displacements of 4-µm amplitude (Field and Pflüger 1989). Similarly, in the stick insect Carausius morosus, a proprioceptive function could be ascribed to the ventral scoloparium in the resistance reflex, leaving open the role of the larger dorsal scoloparium (Kittmann and Schmitz 1992). In the stick insect Cuniculina impigra, a high number of sensory neurons in the FCO were proven to be vibrosensitive (Sauer and Stein 1999; Stein and Sauer 1999), but a localization of the structural correlate was not given. A distinction into two scoloparia is also clearly present in crickets (Nishino 2000) and bush crickets (Theophilidis 1986), although in some species, the separation into two scoloparia may not be complete (Matheson and Field 1990).
In the green lacewing Chrysoperla (Neuroptera), the femoral chordotonal organ contains up to 26 scolopidia and it is vibroceptive with a maximum sensitivity at about 1 kHz with a threshold between 0.1 and 1 ms−2 (Devetak and Amon 1997). In some insects, the number of scolopidia can be as low as 12, e.g., in the stink bug, Nezara viridula (Michel et al. 1982). The number of sensory cells, however, is not size related, as in the small Drosophila melanogaster, the FCO has three subunits with 14–32 scolopidia each (Shanbhag et al. 1992). The FCO of D. melanogaster also reacts to low-frequency vibrational stimuli (Lakes-Harlan and Lefevre 2012). Together with a rather insensitive tibial scolopidial organ (Schneider 1950), the FCO might be an important source for vibration perception, as Diptera do not possess a subgenual organ (see below). The vibratory function of the FCO is further indicated by the central projection because parts of the dipteran FCO axons project in the mVAC (Merritt and Murphey 1992; see Sect. 14.2.4).
2.3.2 Scolopidial Organs in the Proximal Tibia
In many insect taxa, scolopidial organs are found in the proximal tibia; known exceptions are the Diptera and Coleoptera. The most widely distributed organ is the so-called subgenual organ (SGO), by which scolopidial sensilla are named according to their location just distally of the femur–tibia joint (Fig. 14.4). However, in a number of species, additional scolopidial organs in the proximal tibia are documented: a distal organ (in Caelifera and Blattodea: Friedrich 1929), an intermediate organ and a crista acustica or its atympanate homolog (in Ensifera), a Nebenorgan (Blattodea and Mantophasmatodea), and an accessory organ (in Mantodea, Blattodea, and others: Strauß and Lakes-Harlan 2013). In contrast to the SGO, the specific functions and the evolutionary relations of the other organs are so far largely unknown.
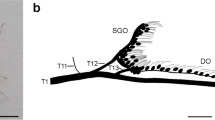
Photoplate of scolopidial sense organs of in the proximal tibia of different insects. a–c, Midleg of M. elongata (Ensifera; photos courtesy of Jan Häusler, unpublished). a View from proximal end into an opened tibia to visualize the localization of the subgenual organ (SGO). Anterior is to the left; staining with Janus Green. b Frontal view of a nerve backfill of the SGO with distinguishable cell bodies and dendrites. c Frontal view of the crista acustica homolog (CAH). d–f, Lateral view of the SGO and further scolopidial sense organs in different orthopteran species (unpublished). d Schistocerca gregaria (Caelifera), e Hierodula membranacea (Mantodea), and f Stenopelmatus spec. (Ensifera). at attachment of the SGO; cb cell bodies of sensory neurons; cu cuticle; de dendrites; DO distal organ; hc hemolymph channel; SGO subgenual organ. Scale 100 µm
The best-known vibration receiver is the subgenual organ, which has been studied in detail in a number of insect taxa and was documented in most Pterygota. It is not connected to leg joints and detects external stimuli transferred to the leg. The SGO is considered to be the most sensitive vibration receptor in insects (Cokl and Virant-Doberlet 2003). All subgenual organs contain type 1 scolopidia with a single sensory neuron per scolopidium (Field and Matheson 1998), but the organization of the subgenual organ is of notable diversity (Fig. 14.5): it is club shaped in some Hymenoptera species and in termites (Howse 1964), whereas in other Hymenoptera, the organ hangs in the hemolymph cavity (Vilhelmsen et al. 2001, 2008). In orthopteroid insects, the SGO is often sail shaped and suspended in the hemolymph (Lin et al. 1995; Schnorbus 1971). In this case, the cap and accessory cells span the hemolymph channel (Fig. 14.4); in other cases, the SGO is more like a “mass” of cells (Nishino and Field 2003). In Lepidoptera, it is diffusely organized and apparently the scolopidia are distally unattached (Howse 1968). In Hemiptera, like N. viridula or a species of Membracidae, Stictocephala bisonia, scolopidial cells are attached to a ligament stretching along the tibia from proximal to distal (Michel et al. 1982, Roye unpublished). This anatomy of scolopidial cells might even raise the question of whether those cells in Hemiptera and Neuroptera should actually be named subgenual cells. In other insects, scolopidial units adjacent to the SGO that extend into the longitudinal axis of the tibia are named intermediate organ or distal organ (see below). Further anatomical, physiological, and developmental studies are needed to clarify whether the differences are purely nomenclatorial or based on the possibly convergent evolution of tibial sense organs. The subgenual organ can be innervated by two different leg nerves. In orthopteroid insects, the sensory nerve 5B1 innervates one part of the subgenual organ and another part is innervated by a branch of the mixed leg nerve 5B2. In the tettigoniid Mecopoda elongata, the nerve 5B2 innervates the posterior portion of the subgenual organ and contains more axons of the subgenual scolopidia than the sensory nerve innervating the complex tibial organ.
Schematic overview of the sensory arrangement of scolopidial organs in the proximal tibia of different taxa. Drawn are sensory cell bodies with their dendrites and their attachment (stippled). Compare the overall similarities in Orthoptera (a–d), and in orthoptera-related groups (e–f), to more distantly related taxa (h–k). Note the large differences within the Hymenoptera (h: ant, i: parasitic wasp) that are probably caused by specialized functional adaptations. Drawings are based on the following: a Schistocerca gregaria (Lin et al. 1995), b Troglophilus neglectus (Jeram et al. 1995), c Comicus calcaris (Strauß and Lakes-Harlan 2010), d Hemideina femorata (Nishino and Field 2003), e Karoophasma biedouwense (Eberhard et al. 2010), f Sipyloidea sipylus (Strauß and Lakes-Harlan 2013), g Hierodula membranacea (unpublished), h Camponotus ligniperda (Menzel and Tautz 1994) and Apis mellifica (Schön 1911), i Orussus abietinus (Vilhelmsen et al. 2001), j C. carnea (Devetak and Pabst 1994), and k N. viridula (Michel et al. 1982) and S. bisonia (Roye, unpublished). AO accessory organ, CAH crista acustica homolog, DO distal organ, IO intermediate organ, NO Nebenorgan, and SGO subgenual organ. Anterior is to the left, proximal to the top. Drawn not to scale; cell numbers are represented, but not exactly depicted
The numbers of scolopidia in the SGO vary largely between species. In the stink bug N. viridula (Heteroptera), it consists of just two scolopidia (Michel et al. 1982), and in the neuropteran Chrysoperla carnea, it consists of three scolopidia (Devetak and Amon 1997), and many orthopteroid species have 20–80 scolopidia (Rössler 1992; Schnorbus 1971). For adult tobacco hornworm moths, M. sexta, about 30 neurons are found in the SGO (Kent and Griffin 1990). A large variance occurs within Hymenoptera: The SGO of ants contains 10–40 scolopidia (Howse 1964; Menzel and Tautz 1994), that of the honeybee Apis mellifera around 40 scolopidia (Kilpinen and Storm 1997), and in parasitoid wasps, females of certain species can have 300–400 SGO scolopidia (Vilhelmsen et al. 2008). The functional relevance of the SGO structure and cell numbers has only been discussed for the parasitoid wasps (see Sect. 14.2.4).
The subgenual organ reacts to substrate vibration, but in cockroaches is so sensitive to mechanical stimuli that it might react to airborne stimuli despite lack of tympana (Shaw 1994). For physiological characterization of substrate vibration, an important parameter is the threshold: the lowest displacement or acceleration stimulus that elicits a neuronal response. For the cockroach Periplaneta americana, displacements of 0.22–5 nm at a frequency of 1.57 kHz can be detected by subgenual receptors (Shaw 1994). Similar values have been found in the green lacewing, which corresponds to an acceleration threshold of 0.02 m/s2 (Devetak and Amon 1997). The vibroceptors in N. viridula are tuned to different frequencies but share minimal thresholds around 0.01 m/s2 (Cokl 1983). The cricket Gryllus bimaculatus has been found to be highly sensitive: The threshold of midleg subgenual receptors was at only 0.0018 m/s2 at frequencies from 700 to 1,000 Hz (Dambach 1972). This sensitivity is not seen throughout the Ensifera, as the tree weta has a threshold of 0.015 m/s2 for vibrational signals at 1 kHz (McVean and Field 1996). Subgenual thresholds in both locusts and bush crickets range between 0.01 and 1 m/s2 with little species-specific variation (Kühne 1982).Whereas in crickets, the midleg reacts most sensitively to vibrational stimuli (Dambach 1972), in C. carnea, the metathoracic hindlegs were most sensitive (Devetak and Amon 1997). In Mantodea, the vibration sensitivity has been tested with respect to functional adaptations of the leg pairs. The foreleg is adapted for prey capture and typically held in the position known for mantis without contact to the substrate; the other legs are used for standing and foraging and are in contact with the substrate. However, no major functional adaptation could be found: The sensory organs in the three legs are highly similar, and furthermore, the legs do not have different physiological sensitivities (unpublished results). The characteristic frequency (CF), defined as the frequency with the lowest threshold, is in crickets around 700–1,000 Hz in the midlegs and 400–500 Hz in the hindlegs (Dambach 1972). In the midlegs of various species of bush crickets, the CFs range from 500 to 1,500 Hz (Kühne 1982). Tuning curves as well as intensity response curves vary between recorded neurons, suggesting that vibratory stimuli can be precisely fractioned and coded in the SGO. Even the few SGO neurons in Nezara may discriminate different frequencies (Cokl 1983). A major difficulty with the interpretation of some physiological data is that they cannot be unequivocally ascribed to the subgenual organ. Extracellular recordings could contain responses from unidentified elements; in intracellular recordings, SGO neurons were identified via (an assumed) physiology and by their probable central projection. Only in a very few cases has the peripheral cell body been labeled and its position unequivocally identified (see Fig. 14.6, recording and labeling by A. Stumpner, Göttingen).
Schematic overview of typical central axonal projections of mechanoceptive sensory fibers. The projections are outlined in a generalized segmental hemiganglion. The median ventral association center (mVAC) is outlined, to indicate which fibers project into this internal neuropile. a, b The femoral chordotonal (FCO) can have different central projections: a Fibers of the dorsal scoloparium of crickets (Nishino 2000) or the proximal part in locusts (Field and Pflüger 1989) project into the mVAC; b Other fibers of the FCO have a more lateral projection. c Projection of the midleg CTO of Stenopelmatus (Strauß and Lakes-Harlan 2008b). d Projection of single fiber originating in the SGO. This neuron has been recorded from and it has been stained completely with neurobiotin, thereby confirming unequivocally its origin in the SGO of Ancistrura nigrovittata, Ensifera (courtesy of Dr. Stumpner, Göttingen, unpublished). e Central projection of campaniform sensilla from the leg of locusts. f Central projection of hair sensilla on leg parts, which are somatotopically ordered within the neuropile (two positions are indicated by the two arborization areas). Drawings generalized after (Pflüger et al. 1988 for the ganglion outline) [a, b after (Nishino 2000), c after (Strauß and Lakes-Harlan 2008b), e after (Hustert et al. 1981) and f after (Mücke and Lakes-Harlan 1995)]
An important, but rarely addressed question is what are the mechanical forces and parameters driving the physiological reaction of the vibration receptors. It has been suggested that the SGO acts like an accelerometer (Schnorbus 1971). In the honeybee, it has been possible to document the vibrations of the SGO itself. Substrate vibrations are transferred to the hemolymph, and the sensory organ is actually oscillating with the hemolymph rather than in the hemolymph (Kilpinen and Storm 1997). Thus, the subgenual organ’s oscillations are matched with the hemolymph oscillations and model calculations show that it behaves as an overdamped system (Storm and Kilpinen 1998).The model suggests that the sensory cells of the SGO are displacement sensitive. Velocity threshold curves of SGO neurons from Nezara run in parallel with equal acceleration values below best frequency and in parallel with equal displacement lines above the best frequency (Cokl 1983). In addition, the SGO of honeybees was shown to have stimulus-direction-specific responses (Rohrseitz and Kilpinen 1997). These results indicate that careful control of the stimulus application is important and that further research is needed to understand the micromechanics of vibration perception in the SGO and other scolopidial sense organs. Interestingly, the structure of the SGO of aqueous larvae and land-living adults of Plecoptera is rather similar (Wittig 1955), although different mechanical forces might act on the legs.
In many taxa, the subgenual organ is not the only scolopidial organ in the proximal tibia, although vibration perception can obviously be well achieved with the SGO alone (Fig. 14.5). Thus, questions arise about the function and the evolution of the other sensory organs. As an example, this can be studied in the Ensifera, which possess the so-called complex tibial organ (CTO). Within the foreleg of Tettigoniidae, this organ complex is associated with sound-propagating structures and can perceive vibratory stimuli as well as acoustic stimuli. The CTO comprises the subgenual organ, an intermediate organ, and a crista acustica (in tympanate legs). The crista acustica is a conspicuous feature of the CTO due to its more or less linear arrangement of sensory cells, which correlates with physiological response properties of the auditory receptors (review: Stumpner and von Helversen 2001). Recently, such a tripartite CTO has also been described in atympanate Ensifera (Strauß and Lakes-Harlan 2008a, b), including a structure homologous to the crista acustica. The distinct parts of the CTO have commonalities, but also differences: The parts have different attachments, partly different adequate stimuli and different developmental origin. The scolopidia of the SGO are orientated circularly within the leg and clearly separated from the other two parts. The scolopidia of the intermediate organ point toward an attachment fixed at the dorsal tibia. The scolopidia of the crista acustica homolog (CAH) point toward a supporting structure that extends in the longitudinal axis of the leg. The intermediate organ and the CAH are probably vibration receivers, but physiological details are so far unknown. The different dendritic attachment in comparison with that of the SGO might indicate perception of other physiological parameter. For example, the CAH might not vibrate with the hemolymph as the SGO (see above). Thereby, other parameters, like different waveforms or different oscillation planes, might be perceived by the CAH. Biophysical measurements of the oscillations of the CAH will hopefully resolve the physiological properties. Interestingly, the CAH is present not only in deaf ensiferans, but also in the atympanate legs of hearing Tettigoniidae. Thus, it is likely that the organ complex has an important function in the sensory world of Ensifera. Generally, it might be more than a coincidence that the CTO has been formed in the proximal tibia. The position just distally of the femur–tibial joint seems to be well suited for perception of vibratory signals, due to filter properties of the leg (Cokl personal communication). Consequently, also other taxa have distinct scolopidial organs in the proximal tibia besides the SGO (Fig. 14.5): In Caelifera and Mantophasmatodea, a distal organ has been described (Eberhard et al. 2010; Lin et al. 1995). The Mantophasmatodea have a pronounced vibrational communication with species-specific signals (Eberhard and Picker 2008). The vibrational sensitivity of the leg nerves reaches thresholds of 0.01–0.001 m/s2 within a frequency range from 600 to 1,200 Hz (Eberhard et al. 2010). The precise origin of this physiological response is not known, although the SGO is likely to be the most sensitive vibration receptor. Phasmatodea possess an elaborated distal organ besides the SGO (Strauß and Lakes-Harlan 2013).
Another organ in the proximal tibia is the accessory organ, which has only a few sensory units attached to the dorsal cuticle. It is present in mantids as well as in some orthopterans in close association with the other parts of the sensory complex. The function of the organ is unknown; it might contribute to the information processing of relevant vibratory signals.
2.3.3 Tarsal Chordotonal Organs
Scolopidial organs can be found in different parts of tarsi of many insects (Mücke 1991; Goodwyn et al. 2009; Wiese 1972). The function of these scolopidial organs has been addressed in water striders, which detect water surface waves for prey location. The respective sensors of water vibrations are located in the tarsi, as cutting of the entire tarsi abolished the orientation of the animal (Murphey 1971). In Aquarius paludum, three scoloparia occur in the tarsi. Each scoloparium contains 2–3 scolopidia and is orientated in different directions, which might be seen as sensory adaptation to perception of complex water wave vibrations (Goodwyn et al. 2009). In the backswimmer Notonecta glauca, the tarsal chordotonal organ enables localization of the wave-producing prey (Wiese 1972). The tarsal chordotonal organ is located in the distal tarsomere and consists of two scoloparia (proximal and distal) with three and five sensory neurons, respectively. The sensory units respond in the behaviorally relevant frequency intensity range (Wiese 1972). However, not all tarsal chordotonal organs might serve as vibration receivers: In C. carnea, a sensitive vibration response was lacking (Devetak and Amon 1997), and in Mantophasmatodea, the destruction of the tarsal chordotonal organ did not change the vibration sensitivity (Eberhard et al. 2010).
2.3.4 Prosternal Chordotonal Organ
The prosternal chordotonal organ of Diptera is interesting for several aspects. This organ presents an example of a sense organ that is not immediately obvious for substrate vibration perception. The prosternal chordotonal organ is located in the prothorax, directly behind the head. It attaches to a neck membrane and has therefore been proposed to monitor head movements (Preuss and Hengstenberg 1992). However, it could be shown that the organ reacts sensitively to high-frequency vibrations not found in movements of the head or during flight (Lakes-Harlan et al. 1999; Stölting et al. 2007). The organ might therefore be able to pick up substrate vibrations. This finding is furthermore in accordance with the hypothesis that the prosternal organ was modified during evolution into a hearing organ (Lakes-Harlan and Heller 1992; Lakes-Harlan et al. 1999; Robert et al. 1992). The evolutionary scenario implicates that the animals first perceived vibratory signals and that modifications in the sound-propagating structures (enlargement of a tracheal chamber and thinning of tympanal membranes) resulted in the capacity to perceive airborne sound.
The physiology of the prosternal organ is also interesting with respect to unknown vibratory sense organs in other insects. For example, Membracidae have been shown to communicate with vibratory signals and to react very sensitively to vibratory stimuli (Cocroft 1996; Cocroft and McNett 2006). The vibratory stimuli might be sensed by the SGO, but perhaps also with scolopidial organs at other body locations. Many Membracidae have a spectacular morphology with protuberances and extensions of the body surface. These structures have been shown to vibrate in response to substrate vibration, whereby the maxima and minima of vibrations follow a complex system (Cocroft et al. 2000). If an internal scolopidial organ were located near the maximum of such vibrations, this organ could pick up the substrate vibrations, similar to the prosternal organ. Future experiments will certainly unravel the behaviorally relevant sensory organs.
2.4 Neural Networks and Neuroethology
The central projections of the sensory fibers are the first level in the neural networks processing vibrational information. Probably in order to facilitate efficient neuronal processing, these central projections are usually ordered within the central nervous system by type of the sensory neuron and/or by position of the sensory cell body (Fig. 14.6). Consequently, the central projection of the sensory neuron may be indicative of its function. In each segmental ganglion of insects, a neuropile area, the median ventral association center (mVAC), is known for processing of vibratory, auditory, and proprioceptive information (Pflüger et al. 1988). The projection into this neuropile may also be indicative of a vibration-receptive function of its sensory cells.
As described above, the FCO often contains two distinct parts (scoloparia) and these parts also have different projections in the corresponding ganglion. In locusts, the proximal part has a dense projection close to the midline within the mVAC and the distal part has a rather loose projection, not merging with that of the proximal scoloparium (Field and Pflüger 1989; Mücke and Lakes-Harlan 1995). In crickets and in wetas, neurons of dorsal parts of the FCO project into the mVAC (Fig. 14.6; Nishino 2000, 2003). In these species, even a more detailed order in the central projection of small groups of neurons could be shown. Furthermore, distinct central projections also correlate with different physiological properties (Matheson 1992). In the Diptera, the FCO projects into the mVAC as well (Merritt and Murphey 1992). Given that the mVAC is often the first-order neuropile involved in vibration processing, the projection in Diptera could indicate that at least parts of the FCO play a role in sensing vibrations. The sensory neurons of the prosternal chordotonal organ of Diptera project among other areas into the mVAC of all three thoracic neuromeres, in both atympanate and tympanate Diptera (Stölting et al. 2007; Stumpner et al. 2006).
On the other hand, the sensory cells of the vibrosensitive SGO do not project completely in the mVAC. The central projection of the SGO has unequivocally been documented in orthopterans either by labeling a complete single cell during recording (Stumpner 1996) or by careful anterograde backfills (Nishino and Field 2003). The fibers of the tettigoniid SGO might project into the mVAC, but not close to the midline (Fig. 14.6; Stumpner 1996). Axons with a bifurcating morphology project anteriorly in the mVAC, whereas subparts of the SGO also have posterior projecting fibers that do not reach the mVAC, but establish an ordered projection outside the mVAC (Nishino and Field 2003). The complex tibial organs of the atympanate Ensifera also have a projection within the mVAC, but so far it has not been unequivocally resolved whether the cells of the CAH project into the neuropile (Fig. 14.6; Strauß and Lakes-Harlan 2008a, b). Nevertheless, in the different atympanate Ensifera analyzed so far, the distinct overall projections in the taxon-specific anatomies of the mVAC are likely to originate at least partly in the CAH (Strauß and Lakes-Harlan 2008a, b, 2010). The accessory chordotonal organ in wetas also projects into the mVAC (Nishino and Field 2003). A CO that does not project in the mVAC is the proprioceptive tarsal CO in locusts (Mücke and Lakes-Harlan 1995). It will be interesting to study the central projection of the tarsal CO in the water strider, which is supposed to register water vibrations (Goodwyn et al. 2009).
In contrast to the scolopidial sensilla, mechanosensitive hair sensilla have somatotopically ordered, often tufted-like projections mainly in the lateral neuropiles of the respective ganglion (Fig. 14.6; Burrows and Newland 1993; Mücke and Lakes-Harlan 1995). Campaniform sensilla often have a widely arborized projection in lateral neuropiles, as shown in locusts and flies (Fig. 14.6; Hustert et al. 1981; Merritt and Murphey 1992). Some of the CS may reach the median ventral association center with single axonal branches. As mentioned above, external sense organs (es) like sensory hairs can be transformed into chordotonal sensilla in Drosophila embryos. Such transformed sensilla exhibit a variety of central projection anatomies ranging from those of es neurons to those of chordotonal neurons (Merritt et al. 1993). Thus, the formation of a central projection is probably controlled by a number of genes.
The mechanosensory neurons synapse onto vibratory interneurons that distribute and compute the information in the CNS (Rössler et al. 2006). One of the first features of such networks is probably the localization of the source of the stimuli. Such directional discrimination is possible by calculation of the input from the receptors of different legs (Virant-Doberlet et al. 2006). However, it has to be kept in mind that the sensitivity of the different legs can be different (see above) and that additional input from scolopidial organs in various parts of the body could contribute to the networks. Biologically relevant answers of discrimination are given by the behavior of the animal: ant lions that locate their prey in sandy pits (Devetak 1998); toktok beetles in the Namib Desert tap on the sand surface with their abdomen and attract each other (unpublished observations). In other species, vibrotaxis might supplement phonotaxis. In crickets or tettigoniid, species-specific vibratory signals can facilitate orientation in a complex 3D habitat, like bushes, toward conspecifics (Latimer and Schatral 1983; Stiedl and Kalmring 1989; Weidemann and Keuper 1987). Holometabolous caterpillars can detect and discriminate vibratory signals occurring on leaves with so far unidentified sense organs (Guedes et al. 2012).These exemplary observations show the ability of various species to locate vibrational stimuli.
2.5 Evolution
Vibration receptor organs have evolved in relation to selective pressures on vibration perception, and some organs have later been further modified for perception of airborne sound. The receptor organs have to match the distinct parameters of vibratory signals relevant for reproduction and offspring. But can receptor organ complexity be related to vibratory signaling or parameters of vibratory signals, like frequency, displacement, and others?
Insects that possess a subgenual organ as a sensitive vibration receiver have between two and several hundred sensory units. Orthoptera with elaborated acoustic and vibratory communication signals have about 20–80 scolopidia in each of the subgenual organs of their legs. So far, no correlation between cell number and signal has been worked out for Orthoptera. However, it has been shown that subgenual organs with few neurons are apparently sufficient to serve vibration communication and may even discriminate different frequencies (Cokl 1983). The subgenual organs of the neuropteran Chrysoperla contain only three scolopidia (Devetak and Pabst 1994), and only two scolopidia are in the SGO of the bug Nezara (Michel et al. 1982). Thus, from a strictly numerical perspective, a low number of receptor units might be functionally sufficient. It can be asked what the receptors in insects with more sensory neurons are used for, or whether they are functionally redundant.
On the other hand, an example for sensory adaptation has been proposed in some groups of parasitoid wasps. These female wasps tap on the substrate with their antenna to evoke “echoes” of vibration by which they locate their hosts. This behavior is termed vibrational sounding. Apparently, the receptor organ for the echoes is the subgenual organ, which contains 300–400 scolopidia in 55 species of Orussidae (Vilhelmsen et al. 2001) and five of 39 subfamilies of Ichneumonidae (Broad and Quicke 2000). This enlargement of the subgenual organ correlates with the vibrational behavior and ecological factors, like host size and substrate (Broad and Quicke 2000). The co-organization of sounding and signal detection indicates a coevolution between signal evocation and signal detection. The increase in number of receptors may functionally improve the ability to detect the hidden hosts. For specific taxa, phylogeny is helpful to infer the ancestral situation of a sensory system. However, the Orussidae are a basal group (Vilhelmsen et al. 2001), and therefore, the enlarged subgenual organ may not be apomorphic in this lineage.
The evolution of the tibial scolopidial organ in relation to vibrational signals has also been discussed for Ensifera. While crickets and bush crickets mainly use acoustic signaling (Bailey 1990), several taxa lack hearing organs but instead use vibrational signals by substrate drumming with hindlegs or abdomen (Field and Bailey 1997; Gwynne 2004; Weissman 2001). In the ensiferan phylogeny, the plesiomorphic situation seems to be the possession of a subgenual organ together with an intermediate organ (which might be related to the distal organ or Nebenorgan in other taxa). Such neuroanatomical organization is present in a cave cricket (Raphidophoridae: Jeram et al. 1995). Other taxa of atympanate Ensifera (Stenopelmatidae, Schizodactylidae, and Gryllacrididae) possess a tripartite CTO (Strauß and Lakes-Harlan 2008a, b, 2009, 2010) with receptor structures homologous to that in tettigoniids, but without adaptations to sound reception. Since the serial organization in all three leg pairs is similar (e.g., the numbers of scolopidia), it was presumed that the sensory organ structure represents the ancestral organization of hearing organs and therefore might have a vibroceptive function (Strauß and Lakes-Harlan 2009). In this case, it can be argued that the detection of vibration was the ancestral function of the subgenual organ, to which further receptor structures were added with a presumptive function in analyzing intraspecific signaling by substrate vibration. The additional receptor structures might be necessary for detecting vibration parameters independent from hemolymph oscillations that can be perceived by the SGO (see above for details).
The question of evolution of vibration receptors has rarely been analyzed in detail. Hopefully, future studies will show which selective pressures acted on the formation of scolopidial organs and what constraints influence the evolution of these interesting sense organs.
References
Albert J, Nadrowski B, Göpfert M (2007) Mechanical signatures of transducer gating in the Drosophila ear. Curr Biol 17:1000–1006
Bailey WJ (1990) Acoustic behaviour of insects. An evolutionary perspective. Chapman and Hall, London, New York, Tokyo
Bodmer R, Barbel S, Sheperd S, Jack J, Jan L, Jan Y (1987) Transformation of sensory organs by mutations of the cut locus of D. melanogaster. Cell 51:293–307
Broad G, Quicke D (2000) The adaptive significance of host location by vibrational sounding in parasitoid wasps. Proc R Soc Lond B 267:2403–2409
Burrows M, Newland PI (1993) Correlation between the receptive fields of locust interneurons, their dendritic morphology, and the central projections of mechanosensory neurons. J Comp Neurol 329:412–426
Burrows M, Pflüger H-J (1988) Positive feedback loops from proprioceptors involved in leg movements of the locust. J Comp Physiol A 163:425–440
Campos-Ortega J, Hartenstein V (1997) The embryonic development of Drosophila melanogaster. Springer, Heidelberg
Chalfie M (2009) Neurosensory mechanotransduction. Nature Rev Mol Cell Biol 10:44–52
Christensen A, Corey D (2007) TRP channels in mechanosensation: direct or indirect activation? Nature Rev Neurosci 8:510–521
Cocroft RB (1996) Insect vibrational defence signals. Nature 382:679–680
Cocroft RB, McNett GD (2006) Vibratory communication in treehoppers (Hemiptera: Membracidae). In: Drosopoulos S, Claridge MF (eds) Insect sound and communication. CRC Press, Boca Raton, pp 305–317
Cocroft RB, Tieu TD, Hoy RR, Miles RN (2000) Directionality in the mechanical response to substrate vibration in a treehopper (Hemiptera: Membracidae: Umbonia crassicornis). J Comp Physiol A 186:695–705
Cokl A (1983) Functional properties of vibroreceptors in the legs of Nezara viridula (L.) (Heteroptera, Pentatomidae). J Comp Physiol A 150:261–269
Cokl A, Virant-Doberlet M (2003) Communication with substrate-borne signals in small plant-dwelling insects. Ann Rev Entomol 48:29–50
Dambach M (1972) Der Vibrationssinn der Grillen. I. Schwellenmessungen an Beinen frei beweglicher Tiere. J Comp Physiol 79:281–304
Debaisieux P (1938) Organes scolopidiaux des pattes d’ìnsectes. La Cellule 47:77–202
Devetak D (1998) Detection of substrate vibration in Neuropteroidea: a review. Acta Zool Fennica 209:87–94
Devetak D, Amon T (1997) Substrate vibration sensitivity of the leg scolopidial organs in the green lacewing Chrysoperla carnea. J Insect Physiol 43:433–437
Devetak D, Pabst M (1994) Structure of the subgenual organ in the green lacewing, Chrysoperna carnea. Tiss Cell 26:249–257
Eberhard M, Picker M (2008) Vibrational communication in two sympatric species of Mantophasmatodea (Heelwalkers). J Insect Behav 21:240–257
Eberhard M, Lang D, Metscher B, Pass G, Picker M, Wolf H (2010) Structure and sensory physiology of the leg scolopidial organs in Mantophasmatodea and their role in vibrational communication. Arthrop Struct Devel 39:230–241
Effertz T, Wiek R, Göpfert MC (2011) NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol 21:592–597
Erler G (1983) Reduction of mechanical sensitivity in an insect mechanoreceptor correlated with destruction of its tubular body. Cell Tiss Res 234:451–461
Field LH, Bailey WJ (1997) Sound production in primitive Orthoptera from Western Australia: sounds used in defence and social communication in Ametrus sp. and Hadrogryllacris sp. (Gryllacrididae: Orthoptera). J Nat Hist 31:1127–1141
Field LH, Matheson T (1998) Chordotonal organs of insects. Adv Ins Physiol 27:1–228
Field L, Pflüger H-J (1989) The femoral chordotonal organ: a bifunctional orthopteran (Locusta migratoria) sense organ? Comp Biochem Physiol A 93:729–743
French AS (1988) Transduction mechanism of mechanosensilla. Ann Rev Entomol 33:39–58
French AS (1992) Mechanotransduction. Ann Rev Physiol 54:135–152
Friedrich H (1929) Vergleichende Untersuchungen über die tibialen Scolopalorgane einiger Orthopteren. Z wiss Zool 134:84–148
Füller H, Ernst A (1973) Die Ultrastruktur der femoralen Chordotonalorgane von Carausius morosus Br. Zool Jb Anat 91:574–601
Gong Z, Son W, Chung YD, Kim J, Shin DW, McClung CA, Lee Y, Lee HW, Chang DJ, Kaang BK, Cho H, Oh U, Hirsh J, Kernan MJ, Kim C (2004) Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci 24:9059–9066
Gong J, Wang Q, Wang Z (2013) NOMPC is likely a key component of Drosophila mechanotransduction channels. Eur J Neurosci 38:2057–2064
Göpfert M, Albert J, Nadrowski B, Kamikouchi A (2006) Specification of auditory sensitivity by Drosophila TRP channels. Nat Neurosci 9:999–1000
Grueber WB, Jan LY, Jan YN (2002) Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129:2867–2878
Guedes R, Matheson S, Frei B, Smith M, Yack J (2012) Vibration detection and discrimination in the marked birch caterpillar (Drepana arcuata). J Comp Physiol A 198:325–335
Gwynne D (2004) Reproductive behavior of ground weta (Orthoptera: Anostostomatidae): drumming behavior, nuptial feeding, post-copulatory guarding and maternal care. J Kansas Entomol Soc 77:414–428
Hertweck H (1931) Anatomie und Variabilität des Nervensystems und der Sinnesorgane von Drosophila melanogaster (Meigen). Z Wiss Zool 139:560–664
Hill KG (1983) The physiology of locust auditory receptors. II. Membrane potentials associated with the response of the receptor cell. J Comp Physiol 152:483–493
Howard J, Bechstein S (2004) Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol 14:R224–226
Howse P (1964) An investigation into the mode of action of the subgenual organ in the termite, Zootermopsis angusticollis Emerson, and the cockroach, Periplaneta americana L. J Insect Physiol 10:409–424
Howse P (1968) The fine structure and functional organization of chordotonal organs. Symp Zool Soc Lond 23:167–198
Hustert R, Pflüger H-J, Bräunig P (1981) Distribution and specific central projections of mechanoreceptors in the thorax and proximal leg joints of locusts. III. The external mechanoreceptors: the campaniform sensilla. Cell Tiss Res 216:97–111
Jeram S, Rössler W, Cokl A, Kalmring K (1995) Structure of atympanate tibial organs in legs of the cave-living Ensifera, Troglophilus neglectus (Gryllacrididae, Raphidophoridae). J Morphol 223:109–118
Keil TA (1997) Functional morphology of insect mechanoreceptors. Microsc Res Tech 39:506–531
Keil TA (2012) Sensory cilia in arthropods. Arthrop Struct Dev 41:515–534
Kent KS, Griffin LM (1990) Sensory organs of the thoracic legs of the moth Manduca sexta. Cell tiss Res 259:209–223
Kent KS, Fjeld CC, Anderson R (1996) Leg proprioceptors of the tobacco hornworm, Manduca sexta: organisation of central projections at larval and adult stages. Micr Res Tech 35:265–284
Kernan M (2007) Mechanotransduction and auditory transduction in Drosophila. Pflügers Arch 454:703–720
Kilpinen O, Storm J (1997) Biophysics of the subgenual organ of the honeybee, Apis mellifera. J Comp Physiol A 181:309–318
Kittmann R, Schmitz J (1992) Functional specialisation of the scoloparia of the femoral chordotonal organ in stick insects. J Exp Biol 173:91–108
Klose M (1996) Development of leg chordotonal sensory organs in normal and heat shocked embryos of the cricket Teleogryllus commodus (Walker). Roux’s Arch Dev Biol 205:344–355
Klose M, Bentley D (1989) Transient pioneer neurons are essential for formation of an embryonic peripheral nerve. Science 245:982–984
Kühne R (1982) Neurophysiology of the vibration sense in locusts and bushcrickets: Response characteristics of single receptor units. J Insect Physiol 28:155–163
Kung C (2005) A possible unifying principle for mechanosensation. Nature 436:647–654
Lai E, Orgogozo V (2004) A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol 269:1–17
Lakes R, Pollack GS (1990) The development of the sensory organs of the legs in the blowfly, Phormia regina. Cell Tiss Res 259:93–104
Lakes-Harlan R, Heller K-G (1992) Ultrasound-sensitive ears in a parasitoid fly. Naturwissenschaften 79:224–226
Lakes-Harlan R, Lefevre C (2012) The femoral chordotonal organ of adult Drosophila melanogaster Meigen 1830. Mitt Dtsch Ges Allg Angew Ent 18:71–74
Lakes-Harlan R, Pollack GS (1993) Pathfinding of peripheral neurons in the central nervous system of an embryonic grasshopper (Chorthippus biguttulus). Cell Tiss Res 273:97–106
Lakes-Harlan R, Strauß J (2006) Developmental constraint of insect audition. Front Zool 3:27
Lakes-Harlan R, Stölting H, Stumpner A (1999) Convergent evolution of insect hearing organs from a preadaptive structure. Proc R Soc B 266:1161–1167
Latimer W, Schatral A (1983) The acoustic behaviour of the bushcricket Tettigonia cantans. I. Behavioural responses to sound and vibration. Behav Proc 8:113–124
Lee J, Moon S, Cha Y, Chung YD (2010) Drosophila TRPN(= NOMPC) channel localizes to the distal end of mechanosensory cilia. PLoS ONE 5:e11012
Lehnert BP, Baker AE, Gaudry Q, Chiang A-S, Wilson RI (2013) Distinct roles of TRP channels in auditory transduction and amplification of Drosophila. Neuron 77:115–128
Liang X, Madrid J, Saleh H, Howard J (2011) NOMPC, a member of the TRP channel family, localizes to the tubular body and distal cilium of Drosophila campaniform and chordotonal receptor cells. Cytoskeleton 68:1–7
Liang X, Madrid J, Gärtner R, Verbavatz J-M, Schiklenk C, Wilsch-Bräuninger M, Bogdanova A, Stenger F, Voigt A, Howard J (2013) A NOMPC-dependent membrane-microtubule connector is a candidate for the gating spring in fly mechanoreceptors. Curr Biol 23:755–763
Lin Y, Rössler W, Kalmring K (1995) Morphology of the tibial organs of Acrididae: comparison of the subgenual organ and distal organs in fore-, mid-, and hindlegs of Schistocerca gregaria (Acrididae, Catantopidae) and Locusta migratoria (Acrididae, Oedipodinae). J Morphol 226:351–360
Lu Q, Senthilan P, Effertz T, Nadrowski B, Göpfert M (2009) Using Drosophila for studying fundamental processes in hearing. Integr Comp Biol 49:674–680
Matheson T (1992) Morphology of the central projections of physiologically characterised neurones from the locust methathoracic femoral chordotonal organ. J Comp Physiol A 170:101–120
Matheson T, Field LH (1990) Innervation of the metathoracic femoral chordotonal organ of Locusta migratoria. Cell Tissue Res 259:551–560
Matsuo E, Kamikouchi A (2013) Neuronal encoding of sound, gravity, and wind in the fruit fly. J Comp Physiol A 199:253–262
Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T (2009) Evolutionary conservation and changes in insect TRP channels. BMC Evol Biol 9:228
McIver S (1985) Mechanoreception. In: Kerkut G, Gilbert L (eds) Comprehensive insect physiology, biochemistry and pharmacology. Pergamon Press, Oxford, pp 71–132
McVean A, Field LH (1996) Communication by substratum vibration in the New Zealand tree weta, Hemideina femorata (Stenopelmatidae: Orthoptera). J Zool 239:101–122
Meier T, Reichert H (1990) Embryonic development and evolutionary origin of the orthopteran auditory organs. J Neurobiol 21:592–610
Meier T, Chabaud F, Reichert H (1991) Homologous patterns in the embryonic development of the peripheral nervous system in the grasshopper Schistocerca gregaria and the fly Drosophila melanogaster. Development 112:241–253
Menzel J, Tautz J (1994) Functional morphology of the subgenual organ of the carpenter ant. Tiss Cell 26:735–746
Merritt DJ, Murphey RK (1992) Projections of leg proprioceptors within the CNS of the fly Phormia regina in relation to the generalized insect ganglion. J Comp Neurol 322:16–34
Merritt DJ, Hawken A, Whitington PM (1993) The role of the cut gene in the specification of central projections by sensory axons in Drosophila. Neuron 10:741–752
Meyhöfer R, Casas J (1999) Vibratory stimuli in host location by parasitic wasps. J Insect Physiol 45:967–971
Michel K, Amon T, Cokl A (1982) The morphology of the leg scolopidial organs in Nezara viridula (L.)(Heteroptera, Pentatomidae). Rev Can Biol Exp 42:139–150
Moulins M (1976) Ultrastructure of chordotonal organs. In: Mill PJ (ed) Structure and function of proprioceptors in the invertebrates. Chapman and Hall, London, pp 387–426
Mücke A (1991) Innervation pattern and sensory supply of the midleg of Schistocerca gregaria. Zoomorphology 110:175–187
Mücke A, Lakes-Harlan R (1995) Central projections of sensory cells of the midleg of the locust, Schistocerca gregaria. Cell Tissue Res 280:391–400
Murphey R (1971) Motor control of orientation to prey by the waterstrider Gerris remigis. Z vergl Physiol 72:150–167
Nishino H (2000) Topographic mapping of the axons of the femoral chordotonal organ neurons in the cricket Gryllus bimaculatus. Cell Tiss Res 299:145–157
Nishino H (2003) Somatotopic mapping of chordotonal organ neurons in a primitive ensiferan, the New Zealand tree weta Hemideina femorata: I. Femoral chordotonal organ. J Comp Neurol 464:312–326
Nishino H, Field LH (2003) Somatotopic mapping of chordotonal organ neurons in a primitive ensiferan, the New Zealand tree weta Hemideina femorata: II. Complex tibial organ. J Comp Neurol 464:327–342
Goodwyn PP, Katsumata-Wada A, Okada K (2009) Morphology and neurophysiology of tarsal vibration receptors in the water strider Aquarius paludum (Heteroptera: Gerridae). J Insect Physiol 55:855–861
Pflüger H-J, Bräunig P, Hustert R (1988) The organization of mechanosensory neuropils in locust thoracic ganglia. Phil Trans R Soc Lond B 321:1–26
Preuss T, Hengstenberg R (1992) Structure and kinematics of the prosternal organs and their influence on head position in the blowfly Calliphora erythrocephala Meig. J Comp Physiol A 171:483–493
Robert D, Amoroso J, Hoy RR (1992) The evolutionary convergence of hearing in a parasitoid fly and its cricket host. Science 258:1135–1137
Rohrseitz K, Kilpinen O (1997) Vibration transmission characteristics of the legs of freely standing honeybees. Zoology 100:80–84
Rössler W (1992) Funcional morphology and development of tibial organs in the legs I, II and III of the bushcricket Ephippiger ephippiger (Insecta, Ensifera). Zoomorphology 112:181–188
Rössler W, Jatho M, Kalmring K (2006) The auditory-vibratory sensory system in bushcrickets. In: Drosopoulos S, Claridge MF (eds) Insect sound and communication. CRC Press, Boca Raton, pp 35–69
Sauer AE, Stein W (1999) Sensorimotor pathways processing vibratory signals from the femoral chordotonal organ of the stick insect. J Comp Physiol A 185:21–31
Schäffer S, Lakes-Harlan R (2001) Embryonic development of the central projection of auditory afferents (Schistocerca gregaria, Orthoptera, Insecta). J Neurobiol 46:97–112
Schmitz J, Dean J, Kittmann R (1991) Central projections of leg sense organs in Carausius morosus (Insecta, Phasmida). Zoomorphology 111:19–33
Schneider W (1950) Über den Erschütterungssinn von Käfern und Fliegen. Z vergl Physiol 32:287–302
Schnorbus H (1971) Die subgenualen Sinnesorgane von Periplaneta americana: Histologie und Vibrationsschwellen. Z vergl Physiol 71:14–48
Schön A (1911) Bau und Entwicklung des tibialen Chordotonalorgans bei der Honigbiene und bei Ameisen. Zool Jb Anat 31:439–472
Shanbhag SR, Singh K, Singh RN (1992) Ultrastructure of the femoral chordotonal organs and their novel synaptic organization in the legs of Drosophila melanogaster Melgen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 21:311–322
Shaw SR (1994) Re-evaluation of the absolute threshold and response mode of the most sensitive known “vibration” detector, the cockroach’s subgenual organ: a cochlea-like displacement threshold and a direct response to sound. J Neurobiol 25:1167–1185
Stein W, Sauer A (1999) Physiology of vibration-sensitive afferents in the femoral chordotonal organ of the stick insect. J Comp Physiol A 184:253–263
Stiedl O, Kalmring K (1989) The importance of song and vibratory signals in the behaviour of the bushcricket Ephippiger ephippiger Fiebig (Orthoptera, Tettigoniidae): taxis by females. Oecologica 80:142–144
Stölting H, Stumpner A, Lakes-Harlan R (2007) Morphology and physiology of the prosternal chordotonal organ of the sarcophagid fly Sarcophaga bullata (Parker). J Insect Physiol 53:444–454
Storm J, Kilpinen O (1998) Modelling the subgenual organ of the honeybee, Apis mellifera. Biol Cybern 78:175–182
Strauß J, Lakes-Harlan R (2008a) Neuroanatomy and physiology of the complex tibial organ of an atympanate Ensiferan, Ametrus tibialis (Brunner von Wattenwyl, 1888) (Gryllacrididae, Orthoptera) and evolutionary implications. Brain Behav Evol 71:167–180
Strauß J, Lakes-Harlan R (2008b) Neuroanatomy of the complex tibial organ of Stenopelmatus (Orthoptera: Ensifera: Stenopelmatidae). J Comp Neurol 511:81–91
Strauß J, Lakes-Harlan R (2009) The evolutionary origin of auditory receptors in Tettigonioidea: the complex tibial organ of Schizodactylidae. Naturwissenschaften 96:143–146
Strauß J, Lakes-Harlan R (2010) Neuroanatomy of the complex tibial organ in the splay-footed cricket Comicus calcaris IRISH 1986 (Orthoptera: Ensifera: Schizodactylidae). J Comp Neurol 518:4567–4580
Strauß J, Lakes-Harlan R (2013) Sensory neuroanatomy of stick insects highlights the evolutionary diversity of the orthopteroid subgenual organ complex. J Comp Neurol (in press). doi:10.1002/cne.23378
Stumpner A (1996) Tonotopic organization of the hearing organ in an bushcricket. Naturwissenschaften 83:81–84
Stumpner A, von Helversen D (2001) Evolution and function of auditory system in insects. Naturwissenschaften 88:159–170
Stumpner A, Allen G, Lakes-Harlan R (2006) Hearing and frequency dependence of auditory interneurons in the parasitoid fly Homotrixa alleni (Tachinidae: Ormiini). J Comp Physiol A 193:113–125
Theophilidis G (1986) The femoral chordotonal organ of Decticus albifrons (Orthoptera: Tettigoniidae)—I. Structure. Comp Biochem Physiol A 84:529–536
Thurm U (2001) Evolutionary aspects of mechanoreception: from ciliates to man. In: Backhaus W (ed) Neuronal coding of perceptual systems. World Scientific Publishing, Singapore, pp 237–248
Todi S, Sharma Y, Eberl DF (2004) Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech 63:388–399
Tracey WJ, Wilson R, Laurent G, Benzer S (2003) Painless, a Drosophila gene essential for nociception. Cell 113:261–273
Vilhelmsen L, Nunzio I, Romani R, Basibuyuk H, Quicke D (2001) Host location and oviposition in a basal group of parasitic wasps: the subgenual organ, ovipositor apparatus and associated structures in the Orussidae (Hymenoptera, Insecta). Zoomorphology 121:63–84
Vilhelmsen L, Turrisi GF, Beutel RG (2008) Distal leg morphology, subgenual organs and host detection in Stephanidae (Insecta, Hymenoptera). J Nat Hist 42:1649–1663
Virant-Doberlet M, Cokl A, Zorovic M (2006) Use of substrate vibrations for orientation: from behaviour to physiology. In: Drosopoulos S, Claridge MF (eds) Insect sound and communication. CRC Press, Boca Raton, pp 81–97
Weidemann S, Keuper A (1987) Influence of vibratory signals on the phonotaxis of the gryllid Gryllus bimaculatus DeGeer (Ensifera: Gryllidae). Oecologia 74:316–318
Weissman D (2001) Communication and reproductive behaviour in North American Jerusalem crickets (Stenopelmatus) (Orthoptera: Stenopelmatidae). In: Field LH (ed) The biology of wetas, king crickets and their allies. CABI Publishing, Wallingford, pp 351–373
Wiese K (1972) Das mechanorezeptive Beuteortungssystem von Notonecta. I. Die Funktion des tarsalen Scolopidialorgans. J Comp Physiol 78:83–102
Wittig G (1955) Untersuchungen am Thorax von Perla abdominalis Burm. (Larve und Imago). Zool Jhrb Anat Ontog 74:491–570
Yan Z, Zhang W, He Y, Gorcuyca D, Xiang Y, Cheng LE, Meltzer S, Jan LY, Jan YN (2013) Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature 493:221–225
Zill S, Büschges A, Schmitz J (2011) Encoding of force increases and decreases by tibial campaniform sensilla in the stick insect, Carausius morosus. J Comp Physiol A 197:851–867
zur Lage P, Jarman AP (1999) Antagonism of EGFR and Notch signalling in the reiterative recruitment of Drosophila adult chordotonal sense organ precursors. Development 126:3149–3157
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lakes-Harlan, R., Strauß, J. (2014). Functional Morphology and Evolutionary Diversity of Vibration Receptors in Insects. In: Cocroft, R., Gogala, M., Hill, P., Wessel, A. (eds) Studying Vibrational Communication. Animal Signals and Communication, vol 3. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43607-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-662-43607-3_14
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-662-43606-6
Online ISBN: 978-3-662-43607-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)