Abstract
The genome of Xylella fastidiosa encodes the properties that enable it to alternately colonize its plant and insect hosts. In this chapter, we take a holistic approach and explore X. fastidiosa evolution, biology, and management based on information and insights that would not have been possible, or would have been technically challenging, during the pre-genomics period of plant pathology. Analysis of genome sequences illustrates the major physiological differences between X. fastidiosa and plant pathogens in the sibling genus Xanthomonas, which possess substantially larger genomes and a variety of genes that are essential for pathogenicity, yet absent from the X. fastidiosa genome. Genome sequence data have enabled reverse-genetic approaches to transfer knowledge from more genetically tractable organisms, along with examination of gene regulatory effects that are involved in colonization of the various hosts. The availability of reference genome sequences has also facilitated the examination of genetic diversity among X. fastidiosa found in different geographic regions and different host plants. Existing data demonstrates the importance of mobile genetic elements in producing genetic diversity among X. fastidiosa isolates. Genome-wide descriptions of diversity will be a powerful tool to identify the genetic changes that underlie the emergence of new agricultural diseases.
In late 2013 Xylella fastidiosa was reported in southern Italy (Saponari et al. 2013). This report highlights the importance of X. fastidiosa as a quarantine pathogen, and the need to better understand its ecology and evolution.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
The utilization of genomic data and widespread availability of genomics tools is still incipient in plant pathology. The first genome of a plant pathogen, that of the bacterium Xylella fastidiosa, was only completed in 2000 (Simpson et al. 2000). Since then, many bacterial plant pathogens have been sequenced, but much of the scientific knowledge extracted from these data is still limited, especially when compared to human pathogens. Nevertheless, significant advances have been made during the last decade, when genomics became widely available to the plant pathology community. Our understanding of the functional role of genes and pathogen taxonomy and evolution has improved significantly. The availability of genome sequences has served as the backbone for much of this work. In this chapter, we take a holistic approach and explore X. fastidiosa evolution, biology, and management based on information and insights that would not have been possible, or would have been technically challenging, during the pre-genomics period of plant pathology. Although X. fastidiosa is widely thought of as a plant pathogen, its biology is more complex and focus on its pathogenicity to crops clouds our broader understanding of its biology, ecology, and evolution. We predict that genomic data and further research on isolates that do not cause crop diseases will permit a more complete view of X. fastidiosa. This is especially important in the context of emerging diseases, as data suggest that novel X. fastidiosa isolates are particularly prone to emerge as crop pathogens of economic importance.
8.1.1 History
The first plant disease associated with the bacterium X. fastidiosa was described over one century ago (Pierce 1892). However, knowledge about this organism lags significantly behind other well-known plant pathogenic bacteria. Epidemics have been the major factor driving research on this pathogen. The first recorded epidemic caused by X. fastidiosa in grapevines in southern California in the late 1800s led to initial characterization of Pierce’s disease of grapevines (Pierce 1892). Later, parallel and inter-related epidemics of Pierce’s disease and alfalfa dwarf in the Central Valley of California resulted in the identification of insect vectors, but also in the conclusion that the etiological agent of these diseases was a virus (Hewitt et al. 1949). Only in the mid-1970s, after it was shown that ‘yellows’ diseases of plants were of bacterial origin (phytoplasmas and spiroplasmas; Doi et al. 1967), were diseases caused by X. fastidiosa determined to also have bacterial etiology (Hopkins and Mollenhauer 1973). That breakthrough led to its axenic culture in 1978 (Davis et al. 1978); and the bacterium was named X. fastidiosa one decade later (Wells et al. 1987). Despite its importance, research on this pathogen remained limited until a new disease in citrus emerged in Brazil in 1987 and another re-emerged in California in the late 1990s (Hopkins and Purcell 2002). These diseases, principally in Brazil, led to efforts aimed at sequencing the genome of these pathogens, with the expectation that this novel information would assist research aimed at the development of better and new disease management practices. That culminated in the sequencing of the first bacterial plant pathogen, X. fastidiosa, in the year 2000 (Simpson et al. 2000). It is fair to say that, other than data available for PCR-based detection of X. fastidiosa (Minsavage et al. 1994), very limited information derived from molecular tools was available for this bacterium prior to 2000. We direct readers interested in a more complete perspective of the history of X. fastidiosa research to a recently published review by Purcell (2013).
8.1.2 Geographical Distribution
Initially thought to be limited to North America (Hewitt 1958), X. fastidiosa is now known to be present throughout the Americas and Taiwan. There is one report of X. fastidiosa in Kosovo, Europe (Berisha et al. 1998); however, the bacterium is considered to be absent from the continent. Taiwan represents an interesting case, where two distinct phylotypes of X. fastidiosa occur, causing disease in pear trees and grapevines, respectively (Su et al. 2012, 2013). The grapevine disease is caused by isolates that are phylogenetically among those widely distributed in the United States, suggesting that it was recently introduced into the island, which is also evidenced by the lack of genetic diversity among Taiwanese isolates. The pear isolates, however, appear to have evolved in isolation, raising important questions regarding the worldwide distribution of X. fastidiosa and X. fastidiosa-like bacteria, which may have remained undetected due to significant biological, phenotypic, and genetic differences when compared to known taxa.
Within the Americas, X. fastidiosa occurs from the northeast region of the United States and Canada (Goodwin and Zhang 1997) to Argentina and southern Brazil (Hopkins and Purcell 2002). However, as discussed later, the distribution of individual subspecies is more limited, suggesting that geographical isolation has been important in the evolution of this bacterium. Furthermore, as with grapevine isolates in Taiwan, evidence indicates that at least some isolates causing disease in plum in Brazil originated from North America (Nunes et al. 2003) and that grapevine isolates in the United States originated from Central America (Nunney et al. 2010). Therefore, X. fastidiosa populations appeared to have remained largely isolated due to geographical barriers, but recent human activity has resulted in dispersal over continental distances.
8.1.3 Impact
X. fastidiosa causes disease in a wide range of host plants, from perennial fruit crops such as grapes and citrus to ornamental trees and shrubs. The economic impact of these diseases is poorly understood; to our knowledge, only Pierce’s disease of grapevines in Northern California has been carefully studied in this context (Fuller 2012). The production of sweet orange in Brazil is severely impacted by this pathogen, which now infects up to half of plants in some regions, over one-third of citrus plants in São Paulo State are estimated to be infected with the pathogen. In rare instances, such as oleander leaf scorch in southern California in the 1990s, most susceptible hosts were eliminated from the landscape due to X. fastidiosa infection. Control of X. fastidiosa diseases is dependent on host plant species, vector species, geographical location, and management practices, among other factors. We direct readers to other reviews that cover X. fastidiosa diseases, their impact, epidemiology, and management alternatives (Hopkins and Purcell 2002; Redak et al. 2004).
8.1.4 Quarantine Importance
This bacterium is of worldwide quarantine importance. Some countries where X. fastidiosa is established have enacted policies to avoid the introduction of new genotypes, while countries in which it is considered to be absent tend to prohibit the introduction of the species (i.e., Australia, New Zealand, and the European Union have strict regulations aimed at reducing the likelihood of X. fastidiosa introduction). It should be mentioned that these countries also list insect vectors of X. fastidiosa as quarantine organisms. The United States Department of Agriculture strictly regulates the introduction of foreign isolates and considered the genotype causing citrus variegated chlorosis (CVC), which is currently limited to South America, a bioterrorism agent until 2012 (Federal Register 2012).
The European Union considers X. fastidiosa an EPPO A1 pathogen, which are quarantine pests absent from the region. Strict guidelines exist for positive diagnostic and reporting of X. fastidiosa in Europe (European and Mediterranean Plant Protection Organization 2004). European governments routinely survey for this bacterium using ELISA and PCR. In addition to regulations on pathogen introduction and field surveys, some countries have been more proactive, assuming that an eventual introduction will occur. Australia and New Zealand scientists have performed research in California, USA, to determine the host range of X. fastidiosa and one of its vectors using native plant species from those countries (Rathé et al. 2012a; Sandanayaka and Backus 2008). Such efforts could have a significant impact on the eradication or management of X. fastidiosa introductions, as some biological knowledge would be already available to decision makers upon pathogen detection. Lastly, analysis of the potential threat of an introduction can provide important information to assess risk and develop science-based policies (e.g., Rathé et al. 2012b).
In addition to the above-mentioned risks, the introduction of X. fastidiosa into regions where the bacterium has already been detected increases chances of gene flow between endemic and invasive genotypes. The availability of novel loci and alleles can result in the emergence of epidemic isolates that have the potential to exploit host plants previously not susceptible to disease as a result of X. fastidiosa infection. Although not conclusively demonstrated, independent studies have suggested that X. fastidiosa diseases can emerge via gene exchange between isolates (Almeida et al. 2008; Nunes et al. 2003; Nunney et al. 2012). Therefore, increases in genetic diversity, driven by the introduction of foreign genotypes, carry risks that go beyond the potential host range of the newly arrived isolate.
8.1.5 Taxonomy
Xylella fastidiosa is a gamma-proteobacterium in the order Xanthomonadales, family Xanthomonadaceae (Wells et al. 1987). This is the single species in the genus Xylella, which is monophyletic, and has Xanthomonas ssp. as a sister clade (Fig. 8.1). X. fastidiosa is currently subdivided into four subspecies, largely based on DNA–DNA hybridization and multi-locus sequence typing data (Scally et al. 2005; Schaad et al. 2004). Although these groupings are phylogenetically robust, they can be further subdivided into groups with well-supported genetic and biological distinctions (i.e., different host ranges) (e.g., Almeida and Purcell 2003b; Almeida et al. 2008; Nunney et al. 2010, 2013). The taxonomy of X. fastidiosa is discussed in detail later within an evolutionary context.
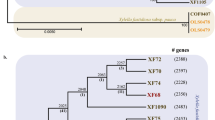
Evolutionary history and geographical distribution of X. fastidiosa subspecies in the Americas. a Relationship of X. fastidiosa to Xanthomonas species (after Rodriguez et al. 2012), rooted by Stenotrophomonas and Pseudoxanthomonas. The position of X. albilineans is ambiguous (star; see text). X. fastidiosa diversity (MLST) is shown in detail, with published genome sequences identified with squares; the Taiwanese pear isolate is not included due to limited sequence data. Gene flow has been observed between subsp. multiplex and others (arrows). b Approximate spatial distribution of subspecies; it is assumed that the species extends throughout Central and South America
8.2 General Biology
The biology of X. fastidiosa is complex due to its requirement to successfully colonize two very distinct hosts, plants and insects. This bacterium emerged from plant-colonizing Xanthomonas ssp., and its divergence is hypothesized to be driven by the novel utilization of insect vectors for dispersal (Killiny et al. 2010). Therefore, a discussion of the biology of X. fastidiosa must consider its plant and vector colonization, in addition to mechanisms necessary to switch from one host to another. This section provides an introduction to the general biology of plant and insect colonization by X. fastidiosa; genome-derived insights are addressed later in this chapter.
8.2.1 Colonization of Host Plants
Unlike many other bacterial plant pathogens, X. fastidiosa has a large host range, both as a species and for each individual subspecies. Hill and Purcell (1995) compiled published data and concluded that plants in twenty-nine families were hosts of this bacterium. This bacterium is capable of multiplying in almost all tested plant species under greenhouse conditions when mechanically inoculated (Purcell and Saunders 1999). It can also be recovered from a wide range of plants in the field (Lopes et al. 2003), although X. fastidiosa does not cause disease in most of these species (Purcell and Saunders 1999). Thus, X. fastidiosa colonization of plants does not necessarily equal disease development. The list of host plants known to be susceptible to this pathogen has grown during the last decade, as has the even longer list of non-symptomatic host plants.
The details of pathogenicity mechanisms are yet to be fully understood, but disease appears to be a consequence of bacterial multiplication and movement within the xylem vessel network, leading to clogging of water flow through the plant (Chatterjee et al. 2008a; Newman et al. 2003). Although the bacterium is xylem limited, populations are not homogenous in the entire plant (Hopkins 1985). Bacterial accumulation at specific tissues within the plant are also host dependent; as an example, in Pierce’s disease-infected grapevines, X. fastidiosa is found at higher populations in symptomatic leaf veins and petioles (Baccari and Lindow 2011; Krivanek and Walker 2005). A positive correlation between symptom severity and pathogen populations within the plant has also been shown (Krivanek and Walker 2005). X. fastidiosa genomic analyses revealed a number of genes that encode plant cell wall-degrading enzymes; X. fastidiosa is strictly xylem limited, and these enzymes are predicted to solely function in pit membrane degradation. Using a GFP-tagged X. fastidiosa strain, symptom development was shown to be highly correlated with the number of vessels clogged (Newman et al. 2003). While structural differences in xylem vessels of susceptible versus nonsusceptible grape varieties did not correlate with probability of disease development (Chatelet et al. 2011), pit membrane degradation was more successful in susceptible versus resistant grapevine varieties (Sun et al. 2011). In addition, defensive responses of susceptible grapevines to X. fastidiosa colonization may further exacerbate restrictions on xylem fluid flow and symptom development (Sun et al. 2013).
8.2.2 Colonization of Insect Vectors
Severin (1949) showed that X. fastidiosa is persistently transmitted by adult sharpshooter leafhoppers (Hemiptera, Cicadellidae), and Freitag (1951) demonstrated lack of transovarial transmission. Persistence of infection in adults has been confirmed in other studies (Almeida and Purcell 2003a; Hill and Purcell 1995b); although transovarial transmission was not tested further, it is unlikely that vertical transmission of X. fastidiosa occurs. The lack of transstadial transmission and absence of a detectable latent period for acquisition or inoculation (Almeida and Purcell 2003a; Purcell and Finlay 1979) are strong indicators that this bacterium does not circulate within vectors. In addition, microscopic studies on the colonization of the foregut of vectors (Almeida and Purcell 2006; Brlansky et al. 1983; Purcell et al. 1979) supported the results obtained from transmission experiments.
After acquisition from source plants, X. fastidiosa attaches to and multiplies in the foregut of vectors; multiplication was first shown by culturing (Hill and Purcell 1995b) and more recently by quantitative PCR (Killiny and Almeida 2009a), and inferred by microscopy at different time points after acquisition from plants (Almeida and Purcell 2006). The generation time of X. fastidiosa cells within vectors as estimated by quantitative PCR was 7–8 h and remained constant for up to 4 days (Killiny and Almeida 2009a). We estimate that the foregut of vectors may house ~50,000 cells. Two regions of the foregut have been implicated in X. fastidiosa transmission based on spatial colonization patterns. Purcell et al. (1979) observed cells in the cibarium, the distal region of the precibarium, and the anterior region of the esophagus; Brlansky et al. (1983) confirmed that the precibarium was colonized by X. fastidiosa. However, those studies did not correlate bacterial visualization with vector transmission to plants. A more recent microscopy-based study showed an association between bacterial colonization of the precibarium and transmission to plants (Almeida and Purcell 2006), suggesting that colonization of the esophagus and cibarium was not directly associated with inoculation events. However, the specific site(s) in the foregut as well as the vector probing behavior(s) associated with inoculation is yet to be determined.
The precibarium and cibarium of leafhoppers are highly turbulent environments, and cell attachment is not a trivial process. Although fluid flow dynamics in this system have not been experimentally determined, xylem sap has been estimated to flow through the precibarium at average speeds of 8 cm/s (Purcell et al. 1979). Turbulence is likely present as the muscle connected to the cibarium’s diaphragm creates enough tension to pump sap from plants into the midgut by contracting and relaxing approximately every second during ingestion events (Dugravot et al. 2008). Thus, it is possible that very few colonization events of the precibarium occur given the number of cells or cell aggregates that may be ingested by a vector through feeding on sap from an infected xylem vessel. While few colonization events have been suggested based on microscopy data (Almeida and Purcell 2006), quantitative PCR data indicate that approximately one to five thousand cells can be detected in sharpshooter heads after feeding on infected plant material (Rashed et al. 2011). Many of the ingested cells likely do not adhere to the insect cuticle and pass through the digestive tract, and therefore are considered to not be involved in vector colonization and X. fastidiosa transmission to plants. This discrepancy highlights the fact that successful attachment of X. fastidiosa to the foregut of vectors is probably a rare event requiring high-affinity ligand–receptor interactions.
8.2.3 Vector Transmission
Transmission experiments identified sharpshooter leafhoppers (Hemiptera, Cicadellidae, subfamily Cicadellinae) and spittlebugs (Hemiptera, Cercopoidea) as insect vectors of this pathogen (Severin 1949, 1950). A third group of xylem sap-sucking insects, cicadas (Hemiptera, Cicadidae), has been reported as a vector of X. fastidiosa (Paião et al. 2002; Krell et al. 2007), although more work is necessary to determine the contribution of this group to overall epidemiology and transmission dynamics. An important aspect associated with the transmission of X. fastidiosa is the lack of pathogen genotype and vector species specificity (Almeida et al. 2005). Frazier (1965) summarized the current state of knowledge at the time by concluding that all sharpshooter leafhoppers should be considered vectors of the Pierce’s disease etiological agent until proven otherwise. The addition of various pathogen genotypes to this statement occurred later, after the advent of molecular tools that eventually allowed the split of the species into subspecies, when various studies showed that all tested vector species were capable of transmitting various X. fastidiosa genotypes (Almeida et al. 2005). The best example was the demonstration that a North American vector species transmitted a South American isolate to plants (Damsteegt et al. 2006). Ultimately, more vector–pathogen combinations must be tested, but so far Frazier’s 50-year-old statement remains true.
Various factors affect X. fastidiosa vector transmission efficiency. The length of time insects are allowed to acquire or inoculate X. fastidiosa into plants is proportional to overall transmission efficiency, up to approximately four days (Almeida and Purcell 2003a; Daugherty and Almeida 2009; Purcell and Finlay 1979). The most parsimonious interpretation of these results is that the probability of insects probing into xylem vessels with X. fastidiosa increases over time (Almeida and Backus 2004; Backus et al. 2005). In fact, the overall population of X. fastidiosa within host plant tissue has been the only parameter consistently correlated with transmission efficiency. Hill and Purcell (1997) were the first to demonstrate such relationship, which has been used to explain why specific vector–pathogen–plant combinations result in higher or lower transmission efficiency [e.g., Lopes et al. (2009)]. Furthermore, within-plant differences in pathogen population also affect transmission efficiency (Daugherty et al. 2010). Thus, host plants harboring larger X. fastidiosa populations generally result in higher transmission efficiency, and vice versa. Vector age and sex do not affect overall transmission efficiency (Krugner et al. 2012).
8.3 Genome Structure
As of August 2013, five finished X. fastidiosa genome sequences have been published (PubMed Genomes Database; http://www.ncbi.nlm.nih.gov/genome/genomes/173), representing three of the four described subspecies (subspecies sandyi being the exception; Fig. 8.2) (Chen et al. 2010; Simpson et al. 2000; van Sluys et al. 2003). These genomes range from 2.5 to 2.7 megabase pairs (Mbp) and include a single chromosome along with zero to two plasmids (ranging from 1.3 to 51 kb). The nucleotide sequences are 51.8–52.6 % GC. Published annotations indicate that these genomes each contain from 2,066 to 2,294 protein-coding sequences, 2 ribosomal RNA operons, 49–50 tRNA genes (43 different anti-codons), and 1 tmRNA gene (van Sluys et al. 2003). Draft quality genomes are available for three other isolates, each appearing to be very similar to one of the finished genomes at the coarse scale (Bhattacharyya et al. 2002; Schreiber et al. 2010; Zhang et al. 2011), as are contigs from the shotgun sequencing of a mixed sample of subspecies multiplex and sandyi (Bhattacharyya et al. 2002; Nunney et al. 2012). The diversity of available sequences will increase rapidly in the near future, as several projects are underway that are sequencing tens of genomes from isolates collected around the world.
Venn diagram for the number of predicted coding sequences that are shared and unique from representative genomes of subspecies multiplex, fastidiosa, and pauca calculated with EDGAR (Blom et al. 2009), using a protein–protein BLAST e-value cutoff of 10−6 and 70 % amino acid identity
8.3.1 Core Genome
The location of the origin of replication can be confidently predicted from three different lines of genomic evidence: the location of the dnaA gene, a cluster of DnaA boxes, and inversion of the G/C and A/T skews (Mackiewicz et al. 2004; Simpson et al. 2000). The terminus can be identified by similar methods: Both the predicted dif sequence and oligonucleotide asymmetry analysis point to the same region of each sequenced chromosome (Yen et al. 2002; Kono et al. 2011). Based on the predicted origin and terminus of replication, we estimate that 59–60 % of genes are encoded on the leading replication strands (Rocha 2008). Notably, the chromosome arms of strain 9a5c are heavily imbalanced such that one replichore is approximately half the length of the other; in contrast, the origin and terminus of replication for the other complete X. fastidiosa chromosomes are approximately opposite from each other. Based on studies in Escherichia coli, this degree of replication imbalance is expected to be a substantial impediment to growth (Esnault et al. 2007).
We identified 1982 protein-encoding genes that are shared among subspecies fastidiosa, multiplex, and pauca (Fig. 8.2). This accounts for 71–86 % of the largest X. fastidiosa genome, 9a5c, depending on how conservative one is in predicting genes (e.g., Simpson et al. 2000; van Sluys et al. 2003). Only one study addressed this question at the subspecies level (subsp. pauca); it showed that the pan-genome of X. fastidiosa is potentially large and that differences among isolates were associated with genome island-like fragments (da Silva et al. 2007). However, representative genomes of different subspecies that have been fully sequenced are largely syntenic (Fig. 8.3). Several short sequence repeats (SSR) have been identified in the chromosome (Coletta-Filho et al. 2001). These loci tend to evolve quickly, and several have been confirmed as having variable number of tandem repeats (VNTR), making them useful for high-resolution studies of population structure (Coletta-Filho et al. 2011).
8.3.2 Mobile Elements
Several self-replicating genetic elements are present among X. fastidiosa genomes, including plasmids and prophages. These contribute to the gene diversity of X. fastidiosa and provide potential avenues for horizontal gene transfer (HGT) both within and between species. Due to the ability of HGT to confer new phenotypes on bacteria and modify their ecological niche, there is intense interest in exploring the contribution of these elements to the distinct virulence traits of different isolates, including the ability to colonize novel host plants (Moreira et al. 2005; Nunes et al. 2003).
Despite the abundance of putative prophages embedded within the chromosome, the isolation of active phage particles has been difficult, with the first lysogen confirmed only in 2010 (Summer et al. 2010). The prevalence of prophages and phage remnants within X. fastidiosa genomes contrasts with the absence of identifiable transposons, which are abundant in Xanthomonas genomes (Monteiro-Vitorello et al. 2005). Prophages are also candidates for inducing rearrangements within the X. fastidiosa genome, due to the activity of their integrase genes (Moreira et al. 2005; Nunes et al. 2003).
Among subspecies, it is evident that X. fastidiosa’s flexible genome is primarily composed of laterally transferred phage-like elements, including prophages (Nunes et al. 2003; van Sluys et al. 2003; Varani et al. 2008). Interestingly, transcription studies have suggested that this laterally transferred gene pool is differentially regulated in X. fastidiosa in comparison with genes in the core genome, suggesting that their regulation is yet to be tightly linked to the rest of the genome (Nunes et al. 2003). The finding that most obvious differences among X. fastidiosa genomes are connected to phage-like sequences has led several groups to propose that the divergence of lineages leading to host specificity is mediated by laterally transferred elements (e.g., van Sluys et al. 2003; Nunes et al. 2003; Varani et al. 2008). Thus, host adaptation in X. fastidiosa could be the result of one or few changes with large fitness benefits, despite the fact that overall pathogenicity mechanisms are similar regardless of host plant. This model is comparable to the effector–host resistance genes evolutionary model for most bacterial pathogens (Lindeberg et al. 2009; Ma et al. 2006). However, a competing hypothesis proposes that quantitative rather than qualitative differences are the major drivers of host specificity (Killiny and Almeida 2011).
The existence of plasmids in X. fastidiosa cultures has been demonstrated in two manners: Some plasmids have been purified directly from X. fastidiosa cultures, while the existence of others have been inferred based on circular contigs generated during genome sequencing projects. These extra-chromosomal DNA molecules are commonly associated with bacteria, encoding accessory modules conferring selective advantage in specific environments (e.g., resistance to antibiotics or heavy metals, or an ability to degrade toxic organic compounds; Van der Auwera et al. 2009). Conjugative plasmids encode modules for DNA transfer, allowing for their own propagation independent of the host and potentially transferring chromosomal DNA. Complete nucleotide sequences are currently available for several plasmids of X. fastidiosa. Below, a brief summary of plasmids associated with X. fastidiosa is presented.
Known plasmids of X. fastidiosa represent a broad range of diversity. A 51 kilobase pair (kb) circular contig designated pXF51 was generated from genome sequencing of X. fastidiosa subspecies pauca strain 9a5c from citrus (Simpson et al. 2000; van Sluys et al. 2003). A smaller 6 kb plasmid (pXF5823) has been characterized from another citrus-infecting strain (Qin and Hartung 2001). Four X. fastidiosa strains isolated from mulberry in Southern California harbor closely related 25 kb plasmids (pXFRIV11, pXFRIV16, pXFRIV19, and pXFRIV25) that have been assigned to incompatibility group P1 (IncP1) (Stenger et al. 2010). A 38 kb plasmid (pXFRIV5) has been characterized from a subspecies multiplex strain (RIV5), isolated from ornamental plum in Southern California (Rogers and Stenger 2012; Chen et al. 2010). A nearly identical plasmid (pXFAS01), varying from pXFRIV5 at only six nucleotide positions, was discovered as a circular contig during genome sequencing of subspecies fastidiosa strain M23 (Chen et al. 2010) isolated from almond in California. Multiple strains of X. fastidiosa contain a small (1.3 kbp) plasmid that utilizes rolling circle replication (Guilhabert et al. 2006; Pooler et al. 1997). All of the plasmids mentioned above have been completely sequenced. Other, likely distinct, plasmids are present in X. fastidiosa strains isolated from a variety of hosts and locations but so far have been characterized only for restriction endonuclease patterns (Hendson et al. 2001).
Beyond inferences from homology, there is limited information regarding function(s) of genes encoded by X. fastidiosa plasmids. These plasmids appear to lack modules that would provide selective advantages to their hosts. Interestingly, the only type IV secretion systems in X. fastidiosa are found on conjugative plasmids, where they presumably facilitate DNA transfer. Among the sequenced plasmids of X. fastidiosa, only those of the 38 kb class (pXFAS01 and pXFRIV5) encode what appear to be a complete type IV secretion system: tra and trb modules necessary for conjugative transfer and mating pair formation, respectively. Indeed, the occurrence of nearly identical 38 kb plasmids in subspecies fastidiosa (pXFAS01) and multiplex (pXFRIV5) may be due to recent conjugative transfer among strains representing different subspecies (Rogers and Stenger 2012).
Several genes of the 25 kb (Inc-P1) class of plasmids from mulberry-infecting strains of X. fastidiosa (Stenger et al. 2010) have been characterized for function. Sequence comparisons indicate that extensive regions (~75 %) the 25 kb IncP-1 plasmids of X. fastidiosa are most closely related to an IncP-1 plasmid (pVEIS01) from the earthworm symbiont Verminephrobacter eiseniae (Pinel et al. 2008). IncP-1 plasmids initiate DNA replication through a plasmid-encoded protein (TrfA), which binds to iterative elements of the vegetative origin of replication (oriV) (Mei et al. 1995). Phylogenetic analysis revealed that the TrfA homologues of X. fastidiosa and V. eiseniae plasmids represent a newly discovered and divergent lineage of IncP-1 plasmids (Stenger and Lee 2011). Replication modules (trfA and oriV), derived from IncP-1 plasmids of X. fastidiosa or V. eiseniae, placed into a standard E. coli cloning vector allowed plasmid replication in X. fastidiosa (Lee et al. 2010). Furthermore, hybrid Inc-P1 replication modules (e.g., heterologous combinations of trfA and oriV from X. fastidiosa and V. eiseniae) conferred the ability to replicate in X. fastidiosa (Stenger and Lee 2011).
Plasmids bearing IncP-1 replication modules were stable in X. fastidiosa only when cultured under antibiotic selection. Growth of X. fastidiosa transformants in the absence of antibiotic selection resulted in extinction of plasmids bearing trfA and oriV. However, addition of the pemI/pemK toxin–antitoxin(TA) system homologue encoded by pXFRIV11 conferred stable inheritance of plasmids bearing the X. fastidiosa IncP-1 replication module in the absence of antibiotic selection (Lee et al. 2010). Functional analysis of the X. fastidiosa pemI/pemK TA system indicated that PemK toxin is an endoribonuclease and that PemI is the cognate antitoxin that blocks PemK ribonuclease activity via direct and reversible binding (Lee et al. 2012). These results, and the effects of unbound PemK toxin on cell growth, indicate that the X. fastidiosa pemI/pemK TA system is a classic plasmid addiction system (stable toxin, labile antitoxin) in which daughter cells not containing plasmid are killed by residual toxin. The construct pXF20-PEMIK (Fig. 8.4) bearing the X. fastidiosa Inc-P1 replication module and pemI/pemK TA system represents a stable shuttle vector able to replicate in both E. coli and X. fastidiosa.
Of what benefit are plasmids to X. fastidiosa fitness as a plant pathogen? At present, the answer to this question is not known. Currently, no pathogenicity or virulence factors of X. fastidiosa are known to be plasmid-encoded. In some cases (e.g., the 1.3 kb rolling circle replicon), plasmids simply may be acting as selfish DNA. In another case (e.g., the 38 kb plasmids), encoding a functional type IV secretion system may allow for (or has facilitated) acquisition of new traits, potentially including pathogenicity islands or other genetic modules of benefit to X. fastidiosa.
8.3.3 Comparative Sequence Analysis
Like other xanthomonads, X. fastidiosa has a circular chromosome and a variety of plasmids, none of which are present among all isolates. A notable difference between Xanthomonas and Xylella genomes is size; X. fastidiosa shares ~ 74 % of its genome with Xanthomonas strains (Moreira et al. 2004), but X. fastidiosa genomes range from 2.5 to 2.7 megabase pairs (Mb), the smallest Xanthomonas genome (X. albilineans) consists of 3.7 Mb and other genomes range from 4.5 to 5.5 Mb. Unlike X. fastidiosa, Xanthomonas ssp. genomes are highly plastic and there is very high diversity within the genus.
X. fastidiosa appears to be derived from a form of Xanthomonas (Fig. 8.1); the relationship between X. fastidiosa, Xanthomonas albilineans, and the remainder of Xanthomonas species is unclear, but the most comprehensive published analysis identifies X. albilineans as an outgroup with low confidence (Rodriguez et al. 2012). Our own analysis supports this relationship, but Xylella has also been inferred to be the sister clade to Xanthomonas albilineans (Pieretti et al. 2009). The family Xanthomonadaceae is very diverse, but there is no indication that plant association is common outside of Xanthomonas and Xylella. The family (and order) itself has a complicated evolutionary history; while it is nominally within the gamma-proteobacteria, its relationship to that group is not straightforward. Phylogenies generally depict the lineage as splitting from the rest of the gamma-proteobacteria, and a gene-by-gene analysis has revealed that many genes are more similar to genes of the alpha or beta proteobacteria, implying that much of the genome was acquired from these other lineages and the placement within gamma-proteobacteria may not be reliable (Comas et al. 2007).
Like X. fastidiosa, X. albilineans is considered to have a reduced form of the Xanthomonas genome. However, gene loss occurred subsequent to their divergence from each other, indicating that the lineage leading to X. fastidiosa likely underwent the loss of 2 Mb of genome content independent of any known lineage (Pieretti et al. 2009). This reduction in genome size is consistent with the stereotypical genome degradation process that has been described for assorted host-dependent bacteria (Moran and Plague 2004). The genome erosion syndrome is also revealed in the absence of detectable selection on codon usage and the slow growth rate of X. fastidiosa (Sharp et al. 2005; Rocha 2004), relative to Xanthomonas species. Two explanations are available for this evolutionary pattern. The ecological explanation posits that bacteria growing in a stable, nutrient-limited environment do not benefit from a diverse gene repertoire or from the ability to rapidly produce proteins (Vieira-Silva and Rocha 2010). Alternatively, the genetic explanation appeals to a reduction in selective efficacy due to reduction in effective population size that results from frequent population bottlenecks associated with transmission between hosts (Sharp et al. 2010). Combined with the deletion-biased mutation processes that are typical of bacterial genomes, this results in a reduction in genome size as genes that provide little fitness advantage are lost.
Only a small number of genes distinguish X. fastidiosa from Xanthomonas species. Due to evolutionary association of such genes with the defining ecological traits of X. fastidiosa (particularly insect transmission), they are candidates for participation in these functions. The pear leaf scorch pathogen from Taiwan appears to represent a lineage of X. fastidiosa that branched from the American lineages prior to their diversification. If it is confirmed to exhibit the same basic ecological and physiological traits of X. fastidiosa, then its genome sequence will help us to narrow in on the genomic changes responsible for the evolution of those traits.
Although four subspecies are commonly recognized within X. fastidiosa, there are reports of variants that may belong to two other subspecies, though these have yet to be well described (Su et al. 2012; Randall et al. 2009). Although various approaches have been used to study X. fastidiosa genetic diversity, multi-locus sequence typing (MLST) has provided the most valuable insights into the evolutionary history of this pathogen (Yuan et al. 2010). MLST is based on the sequencing of seven housekeeping genes showing neutral variation and are distributed throughout the chromosome of X. fastidiosa such that they are unlinked during recombination. This approach has been used to characterize the genetic diversity of X. fastidiosa subspecies, providing insights into the history of pathogen dispersal and genetic recombination between subspecies, as described below.
Subspecies fastidiosa and sandyi in the United States are composed of isolates with very little diversity when typed using MLST (Yuan et al. 2010), but subsp. fastidiosa isolates from Costa Rica colonizing various plant species have more allelic diversity than those causing disease in grapevines in the United States (Nunney et al. 2010). These results suggest that Pierce’s disease in the United States was introduced from Central America. The genetic structure of subsp. multiplex is somewhat distinct in that there are multiple clusters of isolates, with indications of host-range differences among clusters (Nunney et al. 2013; Scally et al. 2005; Almeida and Purcell 2003b). Lastly, subsp. pauca is limited to South America; while most studied isolates colonize either citrus or coffee sympatrically, there is no overlap in host range and the groups are phylogenetically distinct (Almeida et al. 2008). Interestingly, isolates recovered from plum in Brazil fall within subsp. multiplex, which is otherwise limited to North America, or fall between subsp. multiplex and pauca (Nunes et al. 2003), suggesting it was likely introduced from North America via contaminated plant material.
Horizontal gene transfer between subspecies is a recurring theme in X. fastidiosa evolution, as shown by both MLST and whole-genome data, along with distribution of plasmids as described above. Both the coffee and citrus isolates of subspecies pauca appear to have acquired alleles from subspecies multiplex (Nunney et al. 2012). Likewise, subspecies multiplex has participated in recombination with subspecies fastidiosa (Nunney et al. 2010; Kung et al. 2013). This process of allele conversion has even gone as far as to produce new lineages with roughly equal contributions from subsp. multiplex and fastidiosa, as found in the strains isolated from mulberry (Nunney et al. 2014). Overall MLST has proven to be a robust tool for reconstructing the relationship between X. fastidiosa isolates despite gene flow between subspecies and has therefore become the paradigm for X. fastidiosa taxonomy (Fig. 8.1).
In contrast to effector-mediated host/microbe interactions shown for Xanthomonas ssp. and most other bacterial plant pathogens, host specificity in X. fastidiosa violates the dominant paradigm of host–pathogen specificity among bacterial plant pathogens, as this bacterium does not have effector-encoding genes or a type III secretion system (Simpson et al. 2000). X. fastidiosa is further distinguished from Xanthomonas ssp. In that genome comparisons do not reveal gene content differences that could be responsible for the observed host specificity (van Sluys et al. 2003).
All Xanthomonas have six secretion systems (type I–VI) to export proteins. Within individual Xanthomonas ssp., pathovars show specific host associations, largely driven by secreted effectors (White et al., 2009), of which each strain secretes a unique blend of 20–40 directly into the host cytoplasm. While the exact function of many effectors in host cells is just beginning to be understood, many interfere or suppress induced plant defenses. X. albilineans is the only sequenced Xanthomonas ssp. without an Hrp-T3SS, but contains an SP1-T3SS. Mutations at the T3SS locus eliminate virulence among Xanthomonas isolates; therefore, the absence of a T3SS in X. fastidiosa indicates that its pathogenicity mechanisms are distinct and not yet fully described compared to most other bacterial pathogens (Simpson et al. 2000; Chatterjee et al. 2008a).
8.4 Applications from Genomic Data
8.4.1 Genetic Tractability
Determination of the complete genome sequence of X. fastidiosa (Simpson et al. 2000) raised questions about the putative function of several genes in plant pathogenicity and insect transmission, among other topics. Genetic analysis of these genes typically requires the ability to transform bacteria with appropriate plasmids carrying complementary wild-type genes. The first description of a system for genetic analysis was the transformation of a construct carrying ORF1 of X. fastidiosa along with its origin of replication, originally present in the sequence of one of its plasmids (Qin and Hartung 2001). Several studies described different systems used to transform X. fastidiosa (e.g., Silva Neto et al. 2002) or extracted from broad-host-range plasmids (Guilhabert and Kirkpatrick 2003). However, many of these early constructs were not stable once introduced in X. fastidiosa chromosome without antibiotic selection, and genetic analysis of knockouts within plants or insects was impossible. Development of tools allowing the stable transformation of X. fastidiosa by homologous recombination through the utilization of plasmids carrying its own chromosomal replication origin (oriC) circumvented this limitation (Monteiro et al. 2001; Newman et al. 2003, 2004). More recently, the pAX1 plasmid series carrying a colE1-like (pMB1) replicon associated with four different antibiotic selection markers allowed the recovery of double recombinants (Matsumoto et al. 2009). The pXF20-PEMIK plasmid with an addiction system, described above, may be successful in allowing for complementation of mutants, which has been a major limiting factor in X. fastidiosa research. To our knowledge, only Chatterjee et al. (2010) performed studies with complemented knockouts in both plants and insects using the broad-host-range plasmid pBRR-5 (Kovach et al. 1995), which was previously used by Reddy et al. (2007).
Xylella fastidiosa is naturally competent and able to transform and recombine linear and circular DNA during its growth phase (Kung and Almeida 2011). Transformation and recombination efficiencies increase with increments in the length of homologous flanking regions of inserts, up to 1 kb in size. In addition, efficiency reduces as the size of the non-homologous insert increases, with recombination not detected once the region was 6 kb in length (Kung et al. 2013). Competency, as in other bacteria, appears to be mediated by a type IV pilus-like apparatus, with associated com genes that transport DNA fragments through the cell membrane. The ease of transformation with this protocol, in addition to its high efficiency, should facilitate the genetic tractability of X. fastidiosa.
8.4.2 Genomics Opens New Research Venues
While there has been much less study of the virulence mechanisms utilized by compared to its closest relatives in the genus Xanthomonas, the availability of genome sequences has enabled several putative virulence factors to be investigated. Both the lack of an apparent type III secretion system, commonly used in other plant pathogens to suppress plant host defense responses, and the fact that this pathogen probably seldom encounters living plant cells while colonizing the xylem vessels suggests that X. fastidiosa differs in the factors that contribute to its virulence compared to other plant pathogens. Most attention has been directed toward the study of the role of other secretion systems in its virulence, and it appears that X. fastidiosa is capable of type I secretion. Analysis of the genome of X. fastidiosa reveals the presence of at least 23 systems comprising 46 proteins that belong to the ABC (ATP-binding cassette) superfamily of proteins (Meidanis et al. 2002). Type I secretion systems are often used in tolerance of toxic compounds and encode efflux pumps for small molecules as well as enabling the secretion of extracellular proteins such as hemolysins. A central component of many bacterial type I secretion systems is a protein similar to TolC that spans the inner and outer membranes of gram-negative species. Importantly, a tolC knockout mutant of X. fastidiosa exhibited nearly complete loss of virulence to grape (Reddy et al. 2007). Perhaps more importantly, no viable cells of the tolC mutant could be recovered after inoculation into grape. This latter observation suggests strongly that an efflux pump in which TolC participated was required for survival in plants after inoculation, perhaps by enabling export of toxic compounds found in the xylem of the plant. Support for this conjecture was provided by the observation that the tolC mutant was more sensitive to toxic phytochemicals such as berberine, certain detergents, as well as the variety of compounds found in crude plant homogenates than the wild-type strain (Reddy et al. 2007). While such efflux systems can also export toxins and proteins that might elicit a host plant response, no studies have appeared to suggest that X. fastidiosa uses such a process to interact with living plant cells. Clearly, more work is needed to understand to which extent X. fastidiosa interacts with living plant tissues. The fact that tyloses that invade the xylem vessel are induced in plants infected with this pathogen (Hopkins and Purcell 2002) suggests that some communication with living plant tissue does occur. Furthermore, others have posited that many of the water stress symptoms associated with X. fastidiosa infection are not due solely to plugging of xylem vessels by cells of the pathogen, but instead are a result of excessive self-induced blockage of vessels by tyloses that are induced by the presence of the pathogen (Fry and Milholland 1990; Hopkins 1989; Hopkins and Purcell 2002; Purcell and Hopkins 1996).
The type II secretion system appears to be particularly important for the virulence of X. fastidiosa in the absence of at type III secretion system. The type II secretory system is primarily involved in the export of extracellular enzymes, frequently for the hydrolysis of various plant structural features. The type II secretion system, encoded by a collection of genes commonly known as Xps (Xanthomonas protein secretion), has been widely studied in other Xanthomonas species. Not only does X. fastidiosa harbor a complete set of Xps homologues, but it also contains genes capable of encoding several different extracellular enzymes such as several β-1,4 endoglucanases, xylanases, xylosidases, and a polygalacturonase (Simpson et al. 2000; van Sluys et al. 2003). Only the role of the polygalacturonase, encoded by pglA on the virulence of X. fastidiosa, has been addressed experimentally. Importantly, a pglA mutant exhibited greatly reduced ability to colonize grape and therefore incited very little symptom development (Roper et al. 2007). While the mutant could be re-isolated from near the point of inoculation, it was severely restricted in its long-distance movement along the grape xylem vessels (Roper et al. 2007). It seems likely that its greatly reduced motility is attributable to its inability to degrade pit membranes that serve to restrict both lateral and longitudinal movement of the pathogen between xylem vessels. As both the growth and movement of the pglA mutant was suppressed compared to the parental strain, it seems possible that pectin may serve not only as a barrier to intercellular movement, but might also be a nutrient source for X. fastidiosa although pectin does not appear to affect bacterial growth in vitro (Killiny and Almeida 2009b). In fact, the differential polysaccharide composition of pit membranes has been suggested to account for the relative differences in susceptibility of different grape varieties to invasion by X. fastidiosa, with homogalacturans and xyloglucans playing an important role in the susceptibility of various varieties to infection (Sun et al. 2011). Further evidence for the role of pectin as a constituent of a physical barrier for intercellular movement of X. fastidiosa is provided by the observation that grape expressing a polygalacturonase-inhibiting protein from pear exhibited higher resistance to symptom development after inoculation with this pathogen than the parental line (Agüero et al. 2005). It is also important to note that rpfF mutants of X. fastidiosa that do not produce diffusible signaling factor (DSF) express pglA and genes encoding certain other extracellular enzymes at a higher level than that of the wild-type strain (Chatterjee et al. 2008b; Wang et al. 2012). Given that rpfF mutants are hyper-virulent to grape (Newman, et al. 2004), it is reasonable to assume that the elevated expression of these cell wall-degrading enzymes in this mutant background could have contributed to at least some of the enhanced virulence that they exhibit.
Active motility appears to be an important factor contributing to the virulence of X. fastidiosa. While X. fastidiosa is a non-flagellating bacterium, it harbors several genes that encode proteins involved in production and function of type IV pili (Simpson et al. 2000; van Sluys et al. 2003). These long, polar located pili have been shown to be involved in twitching motility in X. fastidiosa (Meng et al. 2005). Intriguingly, such twitching motility was demonstrated to enable the movement of X. fastidiosa not only along abiotic surfaces, but also along xylem vessels. Importantly, X. fastidiosa exhibits the ability to actively move against the flow of xylem fluids in vessels by twitching motility (Meng et al. 2005). The apparently high efficiency with which cells of X. fastidiosa move through the orifices of pits (Newman et al. 2004), which are apparently enlarged due to the action of extracellular enzymes secreted by this pathogen (Perez-Donoso et al. 2010), may be due at least in part to its ability to move along surfaces by retraction of the type IV pili. X. fastidiosa also produces relatively short type I pili, encoded by fimA, that apparently act to restrict motility (De La Fuente et al. 2007). fimA mutants exhibited a higher rate of twitching motility than that of the wild-type strain, suggesting that it may serve as an “anchor,” being involved in attachment but serving to repress motility (De La Fuente et al. 2007). Perhaps not surprisingly, the expression of the genes for type IV pili and that of fimA tend to be oppositely regulated by the accumulation of DSF signal molecule (Chatterjee et al. 2008b; Wang et al. 2012). It therefore would seem prudent for X. fastidiosa to balance the abundance of these two pilus types depending on those stages of its colonization to which they might primarily contribute. That is, the initial attachment of X. fastidiosa to surfaces such as insect vectors may be facilitated by fimA (Killiny and Almeida 2009b), but its presence would tend to suppress active movement by twitching within the plant after inoculation. Curiously, a tonB homologue was found to be required for twitching motility and appropriate biofilm formation and virulence of X. fastidiosa, phenotypes unexpected from its role in transport of non-permeable molecules across the outer membrane and other bacteria (Cursino et al. 2009). Further evidence of the need for complex regulation of type IV pili function in X. fastidiosa is the observation that a chemosensory system is operative. An operon named Pil-Chp containing genes homologous to those found in chemotaxis systems of other bacteria were found to be required for proper pilus function but not biogenesis in X. fastidiosa (Cursino et al. 2011). Such regulatory mutants were also deficient in colonization of plants, suggesting that complex regulation of pilus function may also occur in plants (Cursino et al. 2011). While such regulators are apparently required for motility, it remains unclear whether X. fastidiosa exhibits chemotactic movement toward or away from particular compounds. It is intriguing to consider that the host range or virulence of X. fastidiosa might be modulated by the differential presence of plant compounds that might anticipate in regulation of type IV pilus function.
The role of extracellular polysaccharide (EPS) production in the behaviors of X. fastidiosa is not completely understood. EPS production is an important virulence factor in many Xanthomonas species. X. fastidiosa contains homologues of many but not all of the so-called gum genes necessary for EPS production in Xanthomonas species (Simpson et al. 2000; van Sluys et al. 2003). It therefore has been suggested that X. fastidiosa makes an EPS molecule similar to that of xanthan gum made by Xanthomonas campestris, but one which is lacking terminal mannosyl residues (Silva et al. 2001). Antibodies that could recognize the EPS produced by X. fastidiosa were recently used to illustrate its production both in culture and in planta (Roper et al. 2007). Generation of gum mutants (Killiny et al. 2013) recently helped to better characterize the role of EPS in X. fastidiosa virulence. In addition to their reduced capacity to form biofilms in culture, gum mutants, once mechanically introduced in plants, were avirulent and did not result in the development of any symptoms in grapevines (Killiny et al. 2013). These traits were also associated with an apparent lack of plant colonization and an altered motility compared to that of the wild-type cells. In addition, EPS was also shown having an important role in vector transmission, probably due to its implication in biofilm formation, essential step for a successful insect colonization.
Xylella fastidiosa appears to have a surprisingly large number of adhesin and hemagglutinin-encoding genes compared to other bacteria (Simpson et al. 2000; van Sluys et al. 2003), and such adhesins appear to play a central role in its complex lifestyle. Initial results of studies of virulence factors in X. fastidiosa involving the screening of random insertional mutants revealed a surprising role for adhesins. Specifically, hxfA and hxfB mutants that no longer expressed these two related hemagglutinin-like proteins exhibited a hyper-virulent phenotype in grape (Guilhabert and Kirkpatrick 2005). Furthermore, these mutants exhibited reduced cell–cell aggregation and moved further in grape xylem vessels in the wild-type strain after inoculation (Guilhabert and Kirkpatrick 2005). Because such proteins would be expected to facilitate both cell–cell aggregation as well as cell surface attachment, it would be expected that their presence would tend to reduce the virulence of X. fastidiosa by impeding its movement along xylem vessels. Further evidence for the negative effects of a fimbrial adhesins on the virulence of X. fastidiosa has come from studies of various mutants altered in DSF-mediated cell–cell signaling. For example, rpfF mutants, which are blocked in the accumulation of DSF, express a variety of adhesins such as HxfA, HxfB, FimA, and XadA at a much lower level than that of the wild-type strain (Chatterjee et al. 2008b; Wang et al. 2012). The hyper-virulence of the rpfF mutant could be conferred by the lower level of these adhesins, accounting for the ability of such mutant cells to move more extensively within plants (Chatterjee et al. 2008c). Further support for the role of adhesins as anti-virulence factors comes from studies of rpfC mutants which accumulate excessive amounts of DSF and cgsA mutants of X. fastidiosa which are predicted to have relatively low concentrations of the intracellular signaling molecule cyclic di-GMP (Chatterjee et al. 2008b, 2010). In both mutant backgrounds, the expression of various fimbrial and afimbrial adhesins is higher than in a wild-type strain, accounting for their relatively low ability of these mutants to move throughout plants and therefore to cause disease symptoms (Chatterjee et al. 2010). In addition to the hemagglutinin-like proteins and other fimbrial and afimbrial adhesins that have been noted to affect the adhesiveness and cell–cell aggregation capabilities of X. fastidiosa, LPS also may play a similar role. Disruption of PD0914, which encodes a Wzy polymerase involved in biosynthesis of a high molecular weight O-antigen in the LPS of X. fastidiosa, led to measurable differences in the structure of biofilms formed by such a mutant as well as reducing its virulence (Clifford et al. 2013). It is also fascinating to find that several afimbrial adhesins produced by X. fastidiosa can be found in the extracellular milieu of this pathogen. For example, the adhesin XadA1 was found associated not only with the surface of X. fastidiosa cells, but was also often found in intercellular spaces of bacterial aggregates (Caserta et al. 2010). In contrast, the related molecule XadA2 was found associated only with intact cells (Caserta et al. 2010). Curiously, a portion of the population of the adhesins HxfA and HxfB produced by X. fastidiosa in culture were found in culture supernatants, presumably associated with membranous vesicles (Voegel et al. 2010). The role of such extracellular forms of these adhesins is unknown, as is the identity of any factors that may control the apparent release of these molecules from the producing cell.
It is probably quite significant that several different regulatory systems have been found to coordinate expression of various fimbrial and afimbrial adhesins in X. fastidiosa. Given that adhesins almost certainly play a strong but contextual role in the biology of X. fastidiosa, their proper temporal and spatial expression would be essential for this pathogen to maintain optimal fitness in its plant and insect niches. The response regulator XhpT, composed of a receiver domain and a histidine phosphotransferase output domain, was found to control surface attachment, cell–cell aggregation, and EPS production as well as virulence of X. fastidiosa (Voegel et al. 2013). While any signal that this response regulator might perceive remains unknown, it is intriguing to consider that it might be involved in habitat-specific expression of those genes such as hxfA, hxfB, and tonB which, as discussed above, play central roles in the behavior of X. fastidiosa in different settings. The global regulator GacA was also found to positively regulate several virulence factors in X. fastidiosa including the adhesins XadA and Hsf (Shi et al. 2009). GacA has been found to control a variety of physiological processes as well as pathogenicity factors in many other gram-negative bacteria (Heeb and Haas 2001). As such, it is thought to play an important coordinating role in context-dependent expression of virulence factors. The suppression of adhesins in X. fastidiosa provides further evidence that the proper temporal and spatial expression of these molecules is important in the context-dependent behavior of this pathogen.
8.4.2.1 Vector Colonization
The chemical composition of the external layer of an insect’s exoskeleton (the epicuticle) is not well understood for several insect groups; and to our knowledge, there is no information on its composition for X. fastidiosa insect vectors. The epicuticle of insects is composed of several layers: the inner and outer epicuticle covered by a wax layer; and in some insects an additional cement layer also exists above this wax layer. The thickest layer, the inner epicuticle, is 0.5–2 µm thick (Chapman 1998). The cement layer is a thin layer composed of mucopolysaccharides associated with lipids. The wax layer is largely composed of lipids with embedded proteins, and serves as a waterproofing element for the cuticle. In addition, proteinaceous molecules would also be present in the cuticle, along with other potential molecules. Although the chemical composition of the cuticular surface of arthropods is generally not well understood, interactions between bacteria and the exoskeletons have been successfully studied in other systems using chitin as a proxy, as this system mimics bacterial behavior on the surface of actual hosts (Tarsi and Pruzzo 1999).
The first step in determining the nature of X. fastidiosa-vector interactions was to learn whether X. fastidiosa surface proteins were involved in cell adhesion to vectors. Killiny and Almeida (2009a) demonstrated that X. fastidosa cells bind to carbohydrates, and that treating intact cells with proteases reduced adhesion to compounds such as chitin. Thus, surface proteins were involved in cell adhesion to carbohydrates; however, X. fastidiosa had variable affinity to different molecules. For example, competition assays showed that N-acetylglucosamine (GlcNAc, the monomer of chitin) acted as a strong competitor in binding assays where vector foregut extracts were used as a substrate, reducing cell adhesion. On the other hand, mannose and galactose did not affect binding. In addition, to look for specific X. fastidiosa proteins involved in adhesion, several mutants were tested in vitro for binding to foregut extracts (Killiny and Almeida 2009a). Only hemagglutinin and cell–cell signaling mutants were affected in adhesion. Altogether, these biochemical and other biological assays indicated that initial cell adhesion to vectors is mediated by carbohydrate–lectin interactions and that specific X. fastidiosa surface proteins can be identified in vitro as potential candidates for more comprehensive studies.
Since it was first cultured in the laboratory, attempts to deliver X. fastidiosa cells to vectors from growth media have been unsuccessful (Davis et al. 1978), until it was discovered that plant structural polysaccharides result in phenotypic changes in X. fastidiosa, inducing its transmissibility by leafhoppers (Killiny and Almeida 2009b). Plant polysaccharides induce phenotypic changes leading to higher degrees of adhesiveness and, consequently, attachment to vectors after acquisition from plants (Killiny and Almeida 2009b). The pattern of gene expression of X. fastidiosa exposed to pectin-supplemented media is also similar to that of X. fastidiosa cells occurring at high cell density (Newman et al. 2004; Chatterjee et al. 2008b).
The gene expression profile is also modified when media is supplemented with chitin, the main component of the insect foregut surface (Killiny et al. 2010). Adhesins involved in X. fastidiosa initial adhesion to insect cells are upregulated, and biofilm formation is also enhanced on chitinous surfaces. In addition, a functional chitinase (ChiA; Killiny et al. 2010) was recently discovered, as part of the machinery used by X. fastidiosa to degrade and assimilate chitin as its sole carbon source. Molecular mechanisms involved in chitin utilization are not completely understood. Until now, no known chitin-binding domains have been found in that enzyme, and the implication of other X. fastidiosa chitin-binding proteins are likely to be involved in the process (Labroussaa and Almeida, unpublished).
8.4.2.2 Host Switching and Cell–Cell Signaling
One intriguing aspect of X. fastidiosa’s biology unique among xanthomonads is that it is restricted to colonizing two very different, yet highly specialized environments—plants and insect vectors. Water conducting xylem vessels of plants and the foregut of vectors are extremely different environments. Cells of X. fastidiosa are attached to surfaces in the insect foregut and experience very rapid fluid flow (estimated > 5 cm/s, Purcell et al. 1979) caused by a powerful pumping system used by insects to suck sap under negative tension; turbulence is created in the mouthparts once every second when leafhoppers pull sap from plants and push it into the gut (Dugravot et al. 2008; Purcell and Finlay 1979). Sap flow conditions are not nearly as extreme in xylem vessels, where flow was calculated to achieve ca. 1 × 10−2 to 1 × 10−4 cm/s inside grapes growing in the field (Andersen and Brodbeck 1989; Greenspan et al. 1996). The dramatic differences in the flow speed of different environments (100 to 10,000 times faster inside insect foregut compared to xylem in plants) will have an impact on bacterial cell attachment to surfaces and to each other, since they need to overcome the external shear force stress. In plants, X. fastidiosa multiplies within individual vessels and moves actively to adjacent vessels in the xylem network by producing enzymes that degrade pit membranes separating individual xylem vessels (Perez-Donoso et al. 2010). In plant xylem vessels, X. fastidiosa also has a larger surface area to colonize compared to the foregut of leafhoppers (Newman et al. 2003).
In addition to responding to host or environment specific cues, many bacteria also utilize highly specific quorum-sensing signals that induce gene expression above a certain concentration threshold. By using density-dependent signaling, populations of bacteria can quickly coordinate the expression of metabolically costly traits in response to environmental cues that would be ineffective if expressed by low populations of cells. The role of cell–cell signaling toward host colonization is often not well documented (Bassler and Losick 2006) but is implicated in expression of colonization and virulence traits for an increasing number of plant and animal pathogenic bacteria (Ham 2013; Ryan and Dow 2008).
Like many other bacteria, X. fastidiosa has a regulatory system that responds to signal molecules produced by individual cells in a population; these diffusible molecules accumulate in the environment and trigger population-wide phenotypic changes, likely associated with global changes in gene expression when the signal threshold is reached (Wang et al. 2012). Many traits implicated in insect and host plant colonization are also under control of the regulation of pathogenicity factor (rpf) operon. In Xanthomonas ssp. and X. fastidiosa, rpf controls synthesis and detection of DSF, a medium-chain fatty acid that functions as a signaling molecule (Colnaghi Simionato et al. 2007; Dow et al. 2003). The rpf cluster in Xanthomonas ssp. contains 12 genes, 9 of which are conserved in X. fastidiosa. In both genera, rpfF produces DSF, which diffuses freely through cell membranes and is sensed by a two-component transmembrane receptor RpfC and RpfG (Barber et al. 1997; Newman et al. 2004), but many of the genes in this operon remain functionally uncharacterized.
The Xanthomonas campestris pv. campestris DSF molecule was described as cis-11-methyl-2-dodecanoic acid (Barber et al. 1997; Wang et al. 2004); while X. fastidiosa can respond to Xanthomonas ssp. DSF, the weaker response (Newman et al. 2004) suggested that X. fastidiosa synthesizes and responds to a distinct DSF molecule. Recently, the structure of an X. fastidiosa DSF was identified as 2(Z)-tetradecanoic acid and was shown to control DSF-dependent traits in X. fastidiosa, including biofilm formation and attachment to surfaces (Beaulieu et al. 2013).
Experiments with DSF deficient and blind mutants have shown that DSF signals are important in regulating X. fastidiosa phenotypes in plant and insect hosts. Disruption of DSF production (in an rpfF knockout mutant) results in hyper-virulence within plants, possibly due to up-regulation of plant colonization-related genes and down-regulation of adhesins (Newman et al. 2004; Wang et al. 2012) that decrease rates of both self-aggregation and attachment to xylem vessels. However, the rpfF mutant is not capable of colonizing the precibarium of vectors and is very poorly transmitted to plants. It appears that the induction of a vector-transmissible state occurs due to up-regulation of genes under control of the rpf system.
In contrast, DSF-blind rpfC mutants overproduce hxfA and hxfB. This ‘stickier’ phenotype is less virulent than wild type in planta due to reduced movement through and colonization of xylem. While rpfC is not impaired in attachment to vector foreguts, transmission is reduced due to increased adhesin expression and lower rates of cell detachment (Chatterjee et al. 2008b). In X. campestris, DSF regulates the production of an enzyme that controls cell dispersal from a biofilm (Dow et al. 2003). The inability of rpfC to detach from the biofilm in the insect foregut implicates DSF signaling as an important but uncharacterized regulator of expression controlling cell detachment from biofilm bound cells in the insect foregut. Consequently, afimbrial adhesins are over-expressed, while genes associated with plant host colonization are down-regulated (see Chatterjee et al. 2008a for discussion). A complex picture of X. fastidiosa gene regulation in relation to vector transmission is emerging based on this research.
Although adhesion is essential for retention in insects, it limits colonization of plants compared to the rpfF mutant, highlighting the distinct requirements for life in such different hosts (Newman et al. 2004; Guilhabert and Kirkpatrick 2005; Chatterjee et al. 2008c). Thus, X. fastidiosa’s conflicting life history is framed by the contrasting requirement to move within plants to increase its population size and thus its chances of being acquired by insects, but at the same time, it must increase its adhesiveness so it can attach to insects, which consequently reduces within-plant movement.
Recent characterization of rpfB in both the rice blight pathogen Xanthomonas oryzae pv. oryzae (Xoo) (He et al. 2010) and X. fastidiosa (Almeida et al. 2012) have demonstrated that both species produce at least three distinct DSF signals, that they accumulate at different cell densities, and that rpfB has a role in DSF processing (Almeida et al. 2012). In X. fastidiosa and Xoo, an rpfB mutant only produced one of these signals, indicating a regulatory role in DSF processing for RpfB (Almeida et al. 2012). rpfB is spatially separated from the rpf operon in the X. fastidiosa genome compared to Xanthomonas ssp., suggesting a more complex role for rpfB in X. fastidiosa, which is vector transmitted compared to Xanthomonas ssp., which are not. The production of multiple DSF signals in wild-type cells and the ability of rpfB mutants, which produce only one of these signals, to adhere to but not colonize insect suggests the intriguing but unexplored possibility that host cues influence the production of different ratios of DSF to modulate complex host colonization behaviors in alternating plant and insect hosts (Almeida et al. 2012).
8.5 Disease Management Strategies
The availability of a collection of approaches to control X. fastidiosa diseases is highly desirable, as integration of various strategies will likely be necessary to sustainably control this pathogen. Unfortunately, disease spread in these systems is controlled primarily through the extensive use of pesticides, which often have negative short- and long-term consequences to integrated disease and pest management. In addition, this approach has obvious negative impacts on the environment and communities that rely on agricultural activities. Alternative, efficient, pathogen-specific, environmentally friendly, and safe approaches to control these diseases would lead to long-term sustainability of crop systems. Strategies to control X. fastidiosa currently in development, based on genomics-derived knowledge and the production of transgenic plants, focus on either the pathogen (e.g., Chatterjee et al. 2008c; Dandekar et al. 2012) or the vector (such as RNAi to impact insect development, not discussed here; Rosa et al. 2012), or on both partners and their interactions during insect transmission (Killiny et al. 2012). We limit our discussion to these approaches, although it should be mentioned that genomic data has provided great insights into the biology, ecology, evolution, and taxonomy of X. fastidiosa, much of which has been useful to devise other disease management strategies and for detection and quarantine purposes.
First, the DSF signaling system of X. fastidiosa has been used as the basis to develop a confusion strategy, where presence of DSF in the environment (i.e., xylem stream) at all times should limit bacterial movement and plant colonization. Chatterjee et al. (2008c) showed that the rpfC mutant strain is indeed limited within plant movement and multiplication, suggesting that the presence of DSF functions as a suppressor of population growth. If constitutively expressed in transgenic plants, DSF molecules should lead to adherent X. fastidiosa populations with limited movement within plants and, as a result, the expression of disease symptoms should be reduced.
Another strategy concerns the utilization of cecropin A or B as antibacterial peptides for limiting the growth of X. fastidiosa (Ishida et al. 2004). Recently, the introduction of a construct carrying such a peptide into transgenic grapevines, allowing its specific expression into the xylem of plants, was efficient protecting grapes against the development of Pierce’s disease (Dandekar et al. 2012). Other concepts leading to the constitutive expression of exogenous proteins in transgenic plants include the expression of polygalacturonase-inhibiting proteins (PGIPs) that inhibit X. fastidiosa polygalacturonase (pglA) responsible for the systemic movement of X. fastidiosa in plants (Agüero et al. 2005). Strategies may also emerge from research on the identification of xylem compounds produced during plant exposure to low temperatures (Wilhelm et al. 2011; Meyer and Kirkpatrick 2011). Grapevines with X. fastidiosa and expressing symptoms of Pierce’s disease are cured of infections when subject to cold winters (Purcell 1977, 1980), via a yet to be determined mechanism.
Lastly, a strategy following the hypothesis that blocking interactions between both partners occurring during initial adhesion could lead to the impossibility for the bacteria to successfully colonize its vectors is also being pursued (Killiny et al. 2012). X. fastidiosa proteins identified as able to bind to insect receptors will be used as transmission-blocking molecules. Once expressed in grapevines, those molecules will compete for insect receptors with X. fastidiosa preventing the attachment of the bacteria on insect cells, essential step for its transmission and consequently, disrupting bacteria transmission from plant to plant.
8.6 Conclusion
The availability of X. fastidiosa genome sequences have allowed research on this fastidious organism to move significantly faster than in the past. In addition to improving diagnostic tools and promoting research on the functional role of genes, which has led to the incipient technologies briefly discussed here, it has provided insights into its evolution that would not have been possible otherwise. Because advances in technology will continue to make sequence data available at a larger scale with diminishing costs, we foresee that it will become an integral part of X. fastidiosa research. The complete integration of this tool with others now routinely used in plant pathology is yet to be realized, but efforts to use genome sequences have already generated exciting findings and will continue to do so.
References
Agüero CB, Uratsu SL, Greve C et al (2005) Evaluation of tolerance to Pierce’s disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol Plant Pathol 6:43–51
Almeida RPP, Backus EA (2004) Stylet penetration behaviors of Graphocephala atropunctata (Signoret) (Hemiptera, Cicadellidae): EPG waveform characterization and quantification. Ann Entomol Soc Am 97:838–851
Almeida RPP, Purcell AH (2003a) Transmission of Xylella fastidiosa to grapevines by Homalodisca coagulata (Hemiptera: Cicadellidae). J Econ Entomol 96:264–271
Almeida RPP, Purcell AH (2003b) Biological traits of Xylella fastidiosa strains from grapes and almonds. Appl Environ Microbiol 69:7447–7452
Almeida RPP, Purcell AH (2006) Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Ann Entomol Soc Am 99:884–890
Almeida RPP, Blua MJ, Lopes JRS et al (2005) Vector transmission of Xylella fastidiosa: applying fundamental knowledge to generate disease management strategies. Ann Entomol Soc Am 98:775–786
Almeida RPP, Nascimento FE, Chau J et al (2008) Genetic structure and biology of Xylella fastidiosa strains causing disease in citrus and coffee in Brazil. Appl Environ Microbiol 74:3690–3701
Almeida RPP, Killiny N, Newman KL et al (2012) Contribution of rpfB to cell-to-cell signal synthesis, virulence, and vector transmission of Xylella fastidiosa. Mol Plant Microbe Interact 25:453–462
Andersen P, Brodbeck B (1989) Temperature and temperature preconditioning on flux and chemical composition of xylem exudate from muscadine grapevines. J Am Soc Hort Sci 114:440–444
Baccari C, Lindow SE (2011) Assessment of the process of movement of Xylella fastidiosa within susceptible and resistant grape cultivars. Phytopathology 101:77–84
Backus EA, Habibi J, Yan FM et al (2005) Stylet penetration by adult Homalodisca coagulata on grape: Electrical penetration graph waveform characterization, tissue correlation, and possible implications for transmission of Xylella fastidiosa. Ann Entomol Soc Am 98:787–813
Barber CE, Tang JL, Feng JX et al (1997) A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol 24:555–566
Bassler BL, Losick R (2006) Bacterially speaking. Cell 125:237–246
Beaulieu ED, Ionescu M, Chatterjee S et al (2013) Characterization of a diffusible signaling factor from Xylella fastidiosa. mBio 4:e00539-12
Berisha B, Chen YD, Zhang GY et al (1998) Isolation of Peirce’s disease bacteria from grapevines in Europe. Eur J Plant Pathol 104:427–433
Bhattacharyya A, Stilwagen S, Ivanova N et al (2002) Whole-genome comparative analysis of three phytopathogenic Xylella fastidiosa strains. Proc Natl Acad Sci USA 99:12403–12408
Blom J, Albaum SP, Doppmeier D et al (2009) EDGAR: a software framework for the comparative analysis of prokaryotic genomes. BMC Bioinf 10:154
Brlansky RH, Timmer LW, French WJ et al (1983) Colonization of the sharpshooter vectors, Oncometopia nigricans and Homalodisca coagulata, by xylem-limited bacteria. Phytopathology 73:530–535
Caserta R, Takita MA, Targon ML et al (2010) Expression of Xylella fastidiosa fimbrial and afimbrial proteins during biofilm formation. Appl Environ Microbiol 76:4250–4259
Chapman RF (1998) The insects, structure and function, 4th edn. Cambridge University Press, Cambridge, p 770
Chatelet DS, Wistrom CM, Purcell AH et al (2011) Xylem structure of four grape varieties and 12 alternative hosts to the xylem-limited bacterium Xylella fastidious. Ann Bot 108:73–85
Chatterjee S, Almeida RPP, Lindow SE (2008a) Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Ann Rev Phytopathol 46:243–271
Chatterjee S, Wistrom C, Lindow SE (2008b) A cell–cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc Natl Acad Sci USA 105:2670–2675
Chatterjee S, Newman KL, Lindow SE (2008c) Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol Plant Microbe Interact 21:1309–1315
Chatterjee S, Killiny N, Almeida RPP et al (2010) Role of cyclic di-GMP in Xylella fastidiosa biofilm formation, plant virulence, and insect transmission. Mol Plant Microbe Interact 23:1356–1363
Chen J, Xie G, Han S et al (2010) Whole genome sequences of two Xylella fastidiosa strains (M12 and M23) causing almond leaf scorch disease in California. J Bacteriol 192:4534
Clifford JC, Rapicavoli JN, Roper MC (2013) A rhamnose-rich O-antigen mediates adhesion, virulence, and host colonization for the xylem-limited phytopathogen Xylella fastidiosa. Mol Plant Microbe Interact 26:676–685
Coletta-Filho HD, Takita MA, De Souza AA et al (2001) Differentiation of strains of Xylella fastidiosa by a variable number of tandem repeat analysis. Appl Environ Microbiol 67:4091–4095
Coletta-Filho HD, Bittleston LS, Almeida RPP (2011) Spatial genetic structure of a vector-borne generalist pathogen. Appl Environ Microbiol 77:2596–2601
Colnaghi Simionato AV, da Silva DS, Lambais M et al (2007) Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J Mass Spect 42:1375–1381
Comas I, Moya A, Gonzales-Candelas F (2007) From phylogenetics to phylogenomics: the evolutionary relationships of insect endosymbiotic & #x03B3;-proteobacteria as a test case. Syst Biol 56:1–16
Cursino L, Li Y, Zaini PA et al (2009) Twitching motility and biofilm formation are associated with tonB1 in Xylella fastidiosa. FEMS Microbiol Lett 299:193–199
Cursino L, Galvani CD, Athinuwat D et al (2011) Identification of an operon, Pil-Chp, that controls twitching motility and virulence in Xylella fastidiosa. Mol Plant Microbe Interact 24:1198–1206
Damsteegt VD, Brlansky RH, Phillips PA et al (2006) Transmission of Xylella fastidiosa, causal agent of citrus variegated chlorosis, by the glassy-winged sharpshooter, Homalodisca coagulata. Plant Dis 90:567–570
Dandekar AM, Gouran H, Ibáñez AM et al (2012) An engineered innate immune defense protects grapevines from Pierce disease. Proc Natl Acad Sci USA 109:3721–3725
Daugherty MP, Almeida RPP (2009) Estimating Xylella fastidiosa transmission parameters: decoupling sharpshooter number and feeding period. Entomol Exp Appl 132:84–92
Daugherty MP, Lopes JRS, Almeida RPP (2010) Vector within-host feeding preference mediates transmission of a heterogeneously distributed pathogen. Ecol Entomol 35:360–366
Davis MJ, Purcell AH, Thomson SV (1978) Pierce’s disease of grapevines: isolation of the causal bacterium. Science 199:75–77
De La Fuente L, Burr TJ, Hoch HC (2007) Mutations in Type I and Type IV pilus biosynthetic genes affect twitching motility rates in Xylella fastidiosa. J Bacteriol 189:7507–7510
Doi Y, Teranaka M, Yora K et al (1967) Mycoplasma or PLT group-like microorganisms found in the phloem elements of plants infected with mulberry dwarf, potato witches’ broom, aster yellows, or Paulownia witches’ broom. Ann Phytopath Soc Jpn 33:259–266
Dow JM, Crossman L, Findlay K et al (2003) Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc Natl Acad Sci USA 100:10995–11000
Dugravot S, Backus EA, Reardon BJ et al (2008) Correlations of cibarial muscle activities of Homalodisca ssp. sharpshooters (Hemiptera: Cicadellidae) with EPG ingestion waveform and excretion. J Insect Physiol 54:1467–1478
Esnault E, Valens M, Espéli O, Boccard F (2007) Chromosome structuring limits genome plasticity in Escherichia coli. PLoS Genet 3:e226
European and Mediterranean Plant Protection Organization (2004) Diagnostic protocols for regulated pests: Xylella fastidiosa. OEPP/EPPO Bulletin, pp 155–157
Federal Register (2012) Agricultural bioterrorism protection act of 2002; biennial review and republication of the select agent and toxin list; amendments to the select agent and toxin regulations
Frazier NW (1965) Xylem viruses and their insect vectors. Proceedings international conference on virus and vector on perennial hosts, with special reference to Vitis. University of California, Division of Agricultural Sciences, Davis, California, pp 91–99
Freitag JH (1951) Host range of Pierce’s disease virus of grapes as determined by insect transmission. Phytopathology 41:920–934
Fry SM, Milholland RD (1990) Response of resistant, tolerant, and susceptible grapevine tissues to invasion by the Pierce’s disease bacterium, Xylella fastidiosa. Phytopathology 80:66–69
Fuller KB (2012) The economics of Pierce’s Disease in the California winegrape industry. University of California, Davis, PhD, Dissertation
Goodwin PH, Zhang S (1997) Distribution of Xylella fastidiosa in southern Ontario as determined by the polymerase chain reaction. Can. J. Plant Pathol. 19:13–18
Greenspan MD, Schultz HR, Matthews MA (1996) Field evaluation of water transport in grape berries during water deficits. Physiol Plant 97:55–62
Guilhabert MR, Kirkpatrick BC (2003) Transformation of Xylella fastidiosa with broad host range RSF1010 derivative plasmids. Mol Plant Pathol 4:279–285
Guilhabert MR, Kirkpatrick BC (2005) Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol Plant Microbe Interact 18:856–868
Guilhabert MR, Stewart VJ, Kirkpatrick BC (2006) Characterization of putative rolling-circle plasmids from the Gram-negative bacterium Xylella fastidiosa and their use as shuttle vectors. Plasmid 55:70–80
Ham JJ (2013) Intercellular and intracellular signalling systems that globally control the expression of virulence genes in plant pathogenic bacteria. Mol Plant Pathol 14:308–322
He YW, Wu J, Cha JS, Zhang LH (2010) Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol 10:187
Heeb S, Haas D (2001) Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol Plant Microbe Interact 14:1351–1363
Hendson M, Purcell AH, Chen D et al (2001) Genetic diversity of Pierce’s disease strains and other pathotypes of Xylella fastidiosa. Appl Environ Microbiol 67:895–903
Hewitt WB (1958) The probable home of Pierce’s disease virus. Am J Enol Vitic 9:94–98
Hewitt WB, Frazier NW, Freitag JH (1949) Pierce’s disease investigations. Hilgardia 19:207–264
Hill BL, Purcell AH (1995a) Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology 85:1368–1372
Hill BL, Purcell AH (1995b) Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 85:209–212
Hill BL, Purcell AH (1997) Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 87:1197–1201
Hopkins DL (1985) Physiological and pathological characteristics of virulent and avirulent strains of the bacterium that causes Pierce’s disease of grapevine. Phytopathology 75:713–717
Hopkins DL (1989) Xylella fastidiosa: Xylem-limited bacterial pathogen of plants. Annu Rev Phytopathol 27:271–290
Hopkins DL, Mollenhauer HH (1973) Rickettsia-like bacterium associated with Pierce’s disease of grapes. Science 179:298–300
Hopkins DL, Purcell AH (2002) Xylella fastidiosa: cause of Pierce’s disease of grapevine and other emergent diseases. Plant Dis 86:1056–1066
Ishida ML, Andersen PC, Leite B (2004) Effect of Vitis vinifera L. cv. Chardonnay xylem fluid on cecropin B activity against Xylella fastidiosa. Mol Plant Pathol 64:73–81
Killiny N, Almeida RPP (2009a) Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl Environ Microbiol 75:521–528
Killiny N, Almeida RPP (2009b) Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc Natl Acad Sci USA 106:22416–22420
Killiny N, Prado SS, Almeida RPP (2010) Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl Environ Microbiol 76:6134–6140
Killiny N, Almeida RPP (2011) Gene regulation mediates host specificity of a bacterial pathogen. Environ Microbiol Rep 3:791–797
Killiny N, Rashed A, Almeida RPP (2012) Disrupting the transmission of a vector-borne plant pathogen. Appl Environ Microbiol 78:638–643
Killiny N, Hernandez Martinez R, Dumenyo CK et al (2013) The exopolysaccharide of Xylella fastidiosa is essential for biofilm formation, plant virulence and vector transmission. Mol Plant Microbe Interact 26:1044–1053
Kono N, Arakawa K, Tomita M (2011) Comprehensive prediction of chromosome dimer resolution sites in bacterial genomes. BMC Genom 12:19
Kovach M, Elzer P, Hill D et al (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Krell RK, Boyd EA, Nay JE et al (2007) Mechanical and insect transmission of Xylella fastidiosa to Vitis vinifera. Am J Enol Vitic 58:211–216
Krivanek AF, Walker MA (2005) Vitis resistance to Pierce’s disease is characterized by differential Xylella fastidiosa populations in stems and leaves. Phytopathology 95:44–52
Krugner R, Sisterson MS, Lin H (2012) Effects of gender, origin, and age on transmission of Xylella fastidiosa to grapevines by Homalodisca vitripennis (Hemiptera: Cicadellidae). Ann Entomol Soc Am 105:280–286
Kung SH, Almeida RPP (2011) Natural competence and recombination in the plant pathogen Xylella fastidiosa. Appl Environ Microbiol 77:5278–5284
Kung SH, Retchless AC, Kwan JY et al (2013) Effects of DNA size on transformation and recombination efficiencies in Xylella fastidiosa. Appl Environ Microbiol 79:1712–1717
Lee MW, Rogers EE, Stenger DC (2010) Functional characterization of replication and stability factors of an incompatibility group P-1 plasmid from Xylella fastidiosa. Appl Environ Microbiol 76:7734–7740
Lee MW, Rogers EE, Stenger DC (2012) Xylella fastidiosa plasmid-encoded PemK toxin is an endoribonuclease. Phytopathology 102:32–40
Lindeberg M, Cunnac S, Collmer A (2009) The evolution of Pseudomonas syringae host specificity and type III effector repertoires. Mol Plant Pathol 10:767–775
Lopes JRS, Daugherty MP, Almeida RPP (2009) Context-dependent transmission of a generalist plant pathogen: host species and pathogen strain mediate insect vector competence. Entomol Exp Appl 131:216–224
Lopes SA, Marcussi S, Torres SCZ et al (2003) Weeds as alternative hosts of the citrus, coffee, and plum strains of Xylella fastidiosa in Brazil. Plant Dis 87:544–549
Ma W, Dong FFT, Stavrinides J et al (2006) Type III effector diversification via both pathoadaptation and horizontal transfer in response to a coevolutionary arms race. PLoS Genet 2:e209
Mackiewicz P, Zakrzewska-Czerwinska J, Zawilak A et al (2004) Where does bacterial replication start? Rules for predicting the oriC region. Nucleic Acids Res 32:3781–3791
Matsumoto A, Young GM, Igo MM (2009) Chromosome-based genetic complementation system for Xylella fastidiosa. Appl Environ Microbiol 75:1679–1687
Mei J, Benashski S, Firshein W (1995) Interactions of the origin of replication (oriV) and initiation proteins (TrfA) of plasmid RK2 with submembrane domains of Escherichia coli. J Bacteriol 177:6766–6772
Meidanis J, Braga MDV, Verjovski-Almeida S (2002) Whole-genome analysis of transporters in the plant pathogen Xylella fastidiosa. Microbiol Mol Biol Rev 66:272–299
Meng Y, Li Y, Galvani CD et al (2005) Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J Bacteriol 187:5560–5567
Meyer MM, Kirkpatrick BC (2011) Exogenous applications of abscisic acid increase curing of Pierce’s disease-affected grapevines growing in pots. Plant Dis 95:173–177
Minsavage GV, Thompson CM, Hopkins DL et al (1994) Development of a polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology 84:456–461
Monteiro PB, Teixeira DC, Palma RR et al (2001) Stable transformation of the Xylella fastidiosa citrus variegated chlorosis strain with oriC plasmids. Appl Environ Microbiol 67:2263–2269
Monteiro-Vitorello CB, De Oliveira MC, Zerillo MM et al (2005) Xylella and Xanthomonas mobil’omics. OMICS 9:146–159
Moran NA, Plague GR (2004) Genomic changes following host restriction in bacteria. Curr Opin Genet Dev 14:627–633
Moreira LM, De Souza RF, Almeida NF Jr, Setubal JC, Oliveira JC, Furlan LR, Ferro JA, da Silva AC (2004) Comparative genomics analyses of citrus-associated bacteria. Annu Rev Phytopathol 42:163–184
Moreira LM, De Souza RF, Digiampietri LA, Da Silva AC, Setubal JC (2005) Comparative analyses of Xanthomonas and Xylella complete genomes. OMICS 9:43–76
Newman KL, Almeida RPP, Purcell AH et al (2003) Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl Environ Microbiol 69:7319–7327
Newman KL, Almeida RPP, Purcell AH et al (2004) Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc Natl Acad Sci USA 101:1737–1742
Nunes LR, Rosato YB, Muto NH et al (2003) Microarray analyses of Xylella fastidiosa provide evidence of coordinated transcription control of laterally transferred elements. Genome Res 13:570–578
Nunney L, Yuan X, Bromley R et al (2010) Population genomic analysis of a bacterial plant pathogen: novel insight into the origin of Pierce’s disease of grapevine in the US. PLos One 5:e15488
Nunney L, Yuan X, Bromley RE et al (2012) Detecting genetic introgression: high levels of intersubspecific recombination found in Xylella fastidiosa in Brazil. Appl Environ Microbiol 78:4702–4714
Nunney L, Vickerman DB, Bromley RE et al (2013) Recent evolutionary radiation and host plant specialization in the Xylella fastidiosa subspecies native to the United States. Appl Environ Microbiol 79:2189–2200
Nunney L, Schuenzel EL, Scally M, Bromley RE, Stouthamer R (2014) Large-scale intersubspecific recombination in the plant-pathogenic bacterium Xylella fastidiosa is associated with the host shift to mulberry. Appl Environ Microbiol 80:3025–3033
Paião FG, Meneguim AM, Casagrande EC et al (2002) Envolvimento de cigarras (Homoptera, Cicadidae) na transmissão de Xylella fastidiosa em cafeeiro. Fitopatol Brasil 27:S67
Perez-Donoso AG, Sun Q, Roper MC et al (2010) Cell wall-degrading enzymes enlarge the pore size of intervessel pit membranes in healthy and Xylella fastidiosa-infected grapevines. Plant Physiol 152:1748–1759
Pierce NB (1892) The California vine disease. US Dept Agric Div Veg Pathol Bull 2:22
Pieretti I, Royer M, Barbe V et al (2009) The complete genome sequence of Xanthomonas albilineans provides new insights into the reductive genome evolution of the xylem-limited Xanthomonadaceae. BMC Genom 10:616
Pinel N, Davidson SK, Stahl DA (2008) Verminephrobacter eiseniae gen. nov, sp nov, a nephridial symbiont of the earthworm Eisenia foetida (Savigny). Int J Syst Evol Microbiol 58:2147–2157
Pooler MR, Hartung JS, Fenton RG (1997) Sequence analysis of a 1296-nucleotide plasmid from Xylella fastidiosa. FEMS Microbiol Lett 155:217–222
Purcell AH (2013) Paradigms: examples from the bacterium Xylella fastidiosa. Annu Rev Phytopathol 51:339–356
Purcell AH (1977) Cold therapy of Pierce’s disease of grapevines. Plant Dis Rep 61:514–518
Purcell AH (1980) Environmental therapy for Pierce’s disease of grapevines. Plant Dis 64:388–390
Purcell AH, Finlay AH (1979) Evidence for noncirculative transmission of Pierce’s disease bacterium by sharpshooter leafhoppers. Phytopathology 69:393–395
Purcell AH, Hopkins DL (1996) Fastidious xylem-limited bacterial plant pathogens. Ann Rev Phytopathol 34:131–151
Purcell AH, Saunders SR (1999) Fate of Pierce’s disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis 83:825–830
Purcell AH, Finlay AH, McLean DL (1979) Pierce’s disease bacterium: mechanism of transmission by leafhopper vectors. Science 206:839–841
Qin X, Hartung JS (2001) Construction of a shuttle vector and transformation of Xylella fastidiosa with plasmid DNA. Curr Microbiol 43:158–162
Randall JJ, Goldberg NP, Kemp JD et al (2009) Genetic analysis of a novel Xylella fastidiosa subspecies found in the southwestern United States. Appl Environ Microbiol 75:5631–5638
Rashed A, Killiny N, Kwan J et al (2011) Background matching behaviour and pathogen acquisition: plant site preference does not predict the bacterial acquisition efficiency of vectors. Arthropod Plant Interact 5:97–106
Rathé AA, Pilkington LJ, Gurr GM et al (2012a) Potential for persistence and within-plant movement of Xylella fastidiosa in Australian native plants. Aus Plant Pathol 41:405–412
Rathé AA, Pilkington LJ, Gurr GM et al (2012b) Incursion preparedness: anticipating the arrival of an economically important plant pathogen Xylella fastidiosa Wells (Proteobacteria: Xanthomonadaceae) and the insect vector Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae) in Australia. Aus J Entomol 51:209–220
Redak RA, Purcell AH, Lopes JRS et al (2004) The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Ann Rev Entomol 49:243–270
Reddy JD, Reddy SL, Hopkins DL et al (2007) TolC is required for pathogenicity of Xylella fastidiosa in Vitis vinifera grapevines. Mol Plant Microbe Interact 20:403–410
Rocha EPC (2004) Codon usage bias from tRNA’s point of view: redundancy, specialization, and efficient decoding for translation optimization. Genome Res 14:2279–2286
Rocha EPC (2008) The organization of the bacterial genome. Ann Rev Genet 42:211–233
Rodriguez LM, Grajales A, Arrieta-Ortiz ML et al (2012) Genomes-based phylogeny of the genus Xanthomonas. BMC Microbiol 12:43
Rogers EE, Stenger DC (2012) A conjugative 38 kB plasmid is present in multiple subspecies of Xylella fastidiosa. PloS One 7:e52131
Roper MC, Greve LC, Warren JG et al (2007) Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol Plant Microbe Interact 20:411–419
Rosa C, Kamita SG, Falk BW (2012) RNA interference is induced in the glassy winged sharpshooter Homalodisca vitripennis by actin dsRNA. Pest Managem Sci 68:995–1002
Ryan RP, Dow JM (2008) Diffusible signals and interspecies communication in bacteria. Microbiology 154:1845–1858
Sandanayaka WRM, Backus EA (2008) Quantitative comparison of stylet penetration behaviors of glassy-winged sharpshooter on selected hosts. J Econ Entomol 101:1183–1197
Saponari M, Boscia D, Nigro F, Martelli GP (2013) Identification of DNA sequences related to Xylella fastidiosa in oleander, almond and olive trees exhibiting leaf scorch symptoms in Apulia (Southern Italy). J Plant Pathol 95:668
Scally M, Schuenzel EL, Stouthamer R et al (2005) Multilocus sequence type system for the plant pathogen Xylella fastidiosa and relative contributions of recombination and point mutation to clonal diversity. Appl Environ Microbiol 71:8491–8499
Schaad NW, Postnikova E, Lacy G et al (2004) Xylella fastidiosa subspecies: X. fastidiosa subsp piercei, subsp. nov, X. fastidiosa subsp. multiplex subsp. nov, and X. fastidiosa subsp. pauca subsp. nov. Syst Appl Microbiol 27:290–300
Schreiber HL, Koirala M, Lara A et al (2010) Unraveling the first Xylella fastidiosa subsp fastidiosa genome from Texas. Southwest Entomol 35:479–483
Severin HHP (1949) Transmission of the virus of Pierce’s diseasae of grapevines by leafhoppers. Hilgardia 19:190–206
Severin HHP (1950) Spittle-insect vectors of Pierce’s disease virus II. Life history and virus transmission. Hilgardia 19:357–382
Sharp PM, Bailes E, Grocock RJ et al (2005) Variation in the strength of selected codon usage bias among bacteria. Nucl Acids Res 33:1141–1153
Sharp PM, Emery LR, Zeng K (2010) Forces that influence the evolution of codon bias. Phil Trans Royal Soc London B 365:1203–1212
Shi XY, Dumenyo CK, Hernandez-Martinez R et al (2009) Characterization of regulatory pathways in Xylella fastidiosa: genes and phenotypes controlled by gacA. Appl Environ Microbiol 75:2275–2283
Silva FR, Vettore AL, Kemper EL et al (2001) Fastidian gum: the Xylella fastidiosa exopolysaccharide possibly involved in bacterial pathogenicity. FEMS Microbiol Lett 203:165–171
Silva VS, Shida CS, Rodrigues FB et al (2007) Comparative genomic characterization of citrus-associated Xylella fastidiosa strains. BMC Genom 8:474
Silva Neto JF, Koide T, Gomes SL et al (2002) Site-directed gene disruption in Xylella fastidiosa. FEMS Microbiol Lett 210:105–110
Simpson AJ, Reinach FC, Arruda P et al (2000) The genome sequence of the plant pathogen Xylella fastidiosa. Nature 406:151–159
Stenger DC, Lee MW (2011) Phylogeny of replication initiator protein TrfA reveals a highly divergent clade of incompatibility group P1 plasmids. Appl Environ Microbiol 77:2522–2526
Stenger DC, Lee MW, Rogers EE et al (2010) Plasmids of Xylella fastidiosa mulberry-infecting strains share extensive sequence identity and gene complement with pVEIS01 from the earthworm symbiont Verminephrobacter eiseniae. Physiol Mol Plant Pathol 74:238–245
Su CC, Chang CJ, Yang WJ et al (2012) Specific characters of 16 rRNA gene and 16S-23S rRNA internal transcribed spacer sequences of Xylella fastidiosa pear leaf scorch strains. Eur J Plant Pathol 132:203–216
Su CC, Chang CJ, Chang CM et al (2013) Pierce’s disease of grapevines in Taiwan: isolation, cultivation and pathogenicity of Xylella fastidiosa. J Phytopathol 161:389–396
Summer EJ, Enderle CJ, Ahern SJ et al (2010) Genomic and biological analysis of phage XFas53 and related prophages of Xylella fastidiosa. J Bacteriol 192:179–190
Sun Q, Greve LC, Labavitch JM (2011) Polysaccharide compositions of intervessel pit membranes contribute to Pierce’s disease resistance of grapevines. Plant Physiol 155:1976–1987
Sun Q, Sun Y, Walker MA et al (2013) Vascular occlusions in grapevines with pierce’s disease make disease symptom development worse. Plant Physiol 161:1529–1541
Tarsi R, Pruzzo C (1999) Role of surface proteins in Vibrio cholerae attachment to chitin. Appl Environ Microbiol 65:1348–1351
Van der Auwera GA, Król JE, Suzuki H et al (2009) Plasmids captured in C. metallidurans CH34: defining the PromA family of broad-host-range plasmids. Antonie Van Leeuw 96:193–204
van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB (2003) Comparative analyses of the complete genome sequences of Pierce’s disease and citrus variegated chlorosis strains of Xylella fastidiosa. J Bacteriol 185:1018–1026
Varani AM, Souza RC, Nakaya HI (2008) Origins of the Xylella fastidiosa prophage-like regions and their impact in genome differentiation. PloS One 3:e4059
Vieira-Silva S, Rocha EPC (2010) The systemic imprint of growth and its uses in ecological (meta)genomics. PloS Genet 6:e1000808
Voegel TM, Warren JG, Matsumoto A et al (2010) Localization and characterization of Xylella fastidiosa haemagglutinin adhesins. Microbiology 156:2172–2179
Voegel TM, Doddapaneni H, Cheng DW et al (2013) Identification of a response regulator involved in surface attachment, cell–cell aggregation, exopolysaccharide production and virulence in the plant pathogen Xylella fastidiosa. Mol Plant Pathol 14:256–264
Wang LH, He YW, Gao YF et al (2004) A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol 51:903–912
Wang N, Li JL, Lindow SE (2012) RpfF-dependent regulon of Xylella fastidiosa. Phytopathology 102:1045–1053
Wells JM, Raju BC, Hung HY et al (1987) Xylella fastidiosa gen. nov, sp. nov: Gram-negative, xylem-limited, fastidious plant bacteria related to Xanthomonas ssp. Int J Syst Bact 37:136–143
Wilhelm M, Brodbeck BV, Andersen PC et al (2011) Analysis of xylem fluid components in almond cultivars differing in resistance to almond leaf scorch disease. Plant Dis 95:166–172
White FF, Potnis N, Jones JB et al (2009) The type III effectors of Xanthomonas. Mol Plant Pathol 10:749–766
Yen MR, Lin NT, Hung CH et al (2002) oriC region and replication termination site, dif, of the Xanthomonas campestris pv. campestris 17 chromosome. Appl Environ Microbiol 68:2924–2933
Yuan X, Morano L, Bromley R et al (2010) Multilocus sequence typing of Xylella fastidiosa causing Pierce’s disease and oleander leaf scorch in the United States. Phytopathology 100:601–611
Zhang S, Flores-Cruz Z, Kumar D et al (2011) The Xylella fastidiosa biocontrol strain EB92-1 genome is very similar and syntenic to Pierce’s disease strains. J Bacteriol 193:5576–5577
Acknowledgments
We thank students, researchers, and colleagues that have contributed to the work discussed here. ACR is supported by a postdoctoral fellowship from the Miller Institute for Basic Research in Science. LRS is supported by a postdoctoral fellowship in biology from the NSF.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Retchless, A.C., Labroussaa, F., Shapiro, L., Stenger, D.C., Lindow, S.E., Almeida, R.P.P. (2014). Genomic Insights into Xylella fastidiosa Interactions with Plant and Insect Hosts. In: Gross, D., Lichens-Park, A., Kole, C. (eds) Genomics of Plant-Associated Bacteria. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55378-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-642-55378-3_8
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55377-6
Online ISBN: 978-3-642-55378-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)