Abstract
External beam radiotherapy is a commonly used treatment for the primary and post-operative treatment of head and neck cancer (HNC). The effects of ionising radiation on the parotid and submandibular glands have been investigated in preclinical studies and to a limited extent in human studies. These indicate acinar cells are more radiosensitive compared to ductal or adipose cells with post-radiotherapy recovery originating from the ductal cell region. The sequential development of radiotherapy (RT) delivery techniques, most recently with intensity-modulated radiotherapy (IMRT), has allowed increasing conformality of the dose delivered to the primary tumour in HNC patients. This has resulted in a significant reduction in the radiation dose to the parotid gland (PG) and thus better recovery of parotid and whole mouth saliva flow, with improved patient-reported xerostomia compared to conventional RT. The benefit of PG-sparing IMRT is now confirmed in four randomised controlled trials and a systematic review. Implementation of IMRT is a meticulous stepwise process for precise and safe treatment delivery. An associated quality assurance programme is also mandatory. A sizeable minority of HNC patients treated with IMRT still have persistent late xerostomia; therefore, further approaches to spare other salivary tissues may be of benefit. Further optimisation of IMRT delivery combined with complementary pharmacological strategies should continue to improve salivary gland function and reduce xerostomia rates following IMRT treatment for HNC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
External beam radiotherapy is a commonly used treatment for the primary and adjuvant (post-operative) treatment of head and neck cancer (HNC), where no distant metastases are present.
HNC including thyroid cancer comprises more than 15 primary tumour subsites above the clavicles, excluding brain tumours. It is the fifth commonest cancer diagnosis with approximately 10,000 new cases in England in 2009 [1].
Despite the benefit from ionising radiation of killing tumour cells, it can also have a deleterious effect on the normal tissues. These normal tissues such as salivary glands (SGs), spinal cord and optic nerves are frequently in close proximity to the site of primary tumour or regions of local lymph node metastases; therefore, the head and neck region is an ideal site to develop and apply improved radiotherapy (RT) delivery techniques.
The radiobiology of the SGs and historical development, current treatments and potential future approaches to using RT techniques to reduce SG toxicity will be discussed in this chapter.
Radiotherapy-Induced Pathology, Atrophy and Xerostomia
The SGs, particularly the acinar cells, are considered to be one of the most radiosensitive tissues in the body. This is in contrast to the logical assumption that a well-differentiated organ, with very low or no mitotic activity, should be relatively radioresistant, as is seen with central and peripheral nerves.
The understanding of how RT causes SG dysfunction is crucial to formulate a preventative or treatment strategy. Histopathological studies provide an insight into the structural changes caused by RT. Most studies have been in preclinical animal models; however, a few have been performed in humans. A summary of the data is presented below.
Preclinical – A recent comprehensive review provides a detailed summary of the preclinical data [2]. In most of the studies, a single large fraction of ionising radiation, 15–40 Gy, was delivered to the SGs of a variety of mammalian animal models. The most common acute changes were a significant reduction in saliva flow, decreased gland weight and reduced acinar cell volume. The association between acinar cell loss and a reduction in salivary flow would be expected as fluid and proteins are predominantly secreted from acinar cells, and these cells constitute ~80 % of the SG volume. Fractionated RT regimens have been less frequently investigated, but several studies, administering 2 Gy per fraction over 6 or 7 weeks (total dose 60–70 Gy), have reported the same acute changes associated with a single fraction.
Konings et al. [3] presented the hypothesis for four phases of radiation damage expression in the rat submandibular gland (SMG). Phase 1, the acute phase (0–10 days), is characterised by a rapid reduction in water excretion, but no cell loss and protein secretion are maintained. In phase 2 (10–60 days), there is a steady loss of damaged acinar cells with an associated reduction in the secretion of amylase. Phase 3 (60–120 days) is a plateau period with stable gland architecture and function, no change in cell number or fluid excretion. Finally phase 4 (120–240 days) is characterised by a late deterioration in function due to lack of stem cells and progenitor cells. Regenerated acinar cells exhibit poor function due to abnormal nerve, vascular and ductal structures.
Human – No prospective human studies have been performed to assess the histopathological changes in SGs induced by ionising radiation. Two retrospective reports of changes seen in the major SGs are published.
Sullivan et al. [4] identified ten patients who had received sequential induction chemotherapy then RT with concomitant chemotherapy followed by therapeutic neck dissection (ND). The ND was performed at a median of 10 weeks (range 42–123 days) after completion of RT. A control group of age- and sex-matched oral cancer patients (ND alone, no RT) was selected for comparison. The mean radiation dose to the irradiated SMG was between 50 and 72 Gy. Analysis of the SG morphology indicated pronounced acinar cell loss with ductal cell proliferation after treatment. This was confirmed on immunohistochemistry with an increase in ki-67 and p63 staining suggesting that ductal cells were attempting to proliferate and regenerate.
In a more recent study by Teshima and colleagues [5], parotid gland (PG) and SMG specimens were analysed from six patients, who had received low-dose, preoperative conventional 2D-RT with concomitant chemotherapy (30 Gy in 2 Gy per fraction over 3 weeks). The concomitant chemotherapy was S-1, a novel 5-fluorouracil analogue. All patients had a diagnosis of oral cavity squamous cell carcinoma, and histological specimens were compared to a control cohort (n = 10, ND alone, no RT). A homogenous radiation dose was delivered to both the PG and SMG (range 29.2–31.1 Gy). Functionally the median whole mouth saliva flow rate (ml/min) was reduced to ~60 % after RT (0.65 vs. 1.5). The ipsilateral whole SMG and part of the ipsilateral PG were resected at ND 3–4 weeks after RT for histology assessment.
The morphological appearance showed a significant decrease in the percentage of acinar cells between control and RT groups for the PG (31.5 % vs. 1.1 %, p = 0.001) and SMG (43.3 % vs. 19.0 %, p = 0.002). No significant difference in other SG cell types was noted, but interestingly a nonsignificant increase in the proportion of ductal cells was seen with no difference in the proportion of adipose cells. It was concluded that the loss of salivary flow was related to acinar cell loss.
A limitation of these studies is that they are retrospective and only assess SGs in the early period median of 3.5 and 10 weeks after RT; however, the lack of data in this field is testament to the ethical and logistical difficulties of collecting normal tissue samples in RT-treated HNC patients.
Dose-Response Relationship and PG Tolerance to Radiation
Several studies have investigated the dose-response relationship of the PG to radiation. Most have compared the PG mean dose to either WMS or individual ductal flow rates. The recent QUANTEC report presented SG dose constraint guidelines based on a detailed review of published literature [6]. The recommendations were that if one whole PG is spared with a mean dose of ~20 Gy or if both are spared with a mean dose of ~25 Gy, then severe PG hypofunction (PG flow rate <25 % of baseline) can be avoided in most cases. This is supported by estimates from combined multicentre databases [7] which indicate that a mean whole PG dose limit of 25–30 Gy is associated with 17–26 % normal tissue complication probability (PG flow rate <25 % of baseline) at 1 year (Fig. 10.1). In addition, there is a 50 % probability of PG flow reduction to <25 % of the pre-RT flow rate with a mean PG dose of 40 Gy. The QUANTEC guidelines have recently been validated in an independent patient cohort [8].
Normal tissue complication probability curves (NTCP) as a function of the mean PG dose for Michigan (dashed line) and Utrecht (solid line). Clinical NTCP values (using mean dose bins of 20 Gy) are shown for Michigan (open squares) and Utrecht (black squares), including 95 % confidence intervals (Reproduced with permission from Dijkema et al. [7])
Van Luijk et al. [9] have suggested, in a preclinical study, that there is a differential PG dose response dependent on the distribution of RT dose across the PG. Irradiation of the whole PG, with what is regarded as a sub-tolerance dose of 10 Gy, did not result in functional loss (recovery of salivary function). However, when 10 Gy is delivered to the entire PG (“bath dose”) and an additional 30 Gy is delivered to a smaller sub-volume (“shower dose”), specifically to the caudal half of the gland, then this resulted in greater functional loss. Conversely the administration of the shower dose (30 Gy) to the cranial half of the gland was shown to not cause additional functional impairment [10]. Buettner et al. [11] showed that a radiobiological model describing the distribution of dose across the PG was better than a mean whole gland dose parameter alone for predicting physician-graded late xerostomia (LENT-SOMA).
How IMRT Improves Radiation Dose Delivery
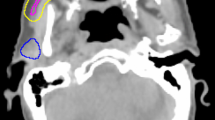
The delivery of therapeutic radiation for HNC has changed significantly over the last two decades. The original technique of conventional 2D radiotherapy (2D-RT) delivered a homogenous dose to both the malignant and normal tissues using paired, opposed radiation beams with limited ability to shape the dose distribution (Fig. 10.2). This led to a very high frequency of early and late normal tissue toxicity [12]. The first RT technique that was investigated to reduce SG toxicity, specifically PG dysfunction, was 3D conformal RT (3D-CRT). This technique uses a multi-leaf collimator (MLC) comprising many narrow, mobile lead leaves, to shape the radiation beam and produce convexities in the dose distribution; however, it is not possible to produce concavities.
Intensity-modulated radiotherapy (IMRT), first described in 1997 [13], is an advanced form of 3D-CRT with the MLC used to define the radiation dose intensity independently for different regions of the target volume. This is achieved by using multiple beam directions, commonly five or seven equi-spaced fields. The shape defined by the MLC is then varied over time. The most frequent methods used are step and shoot, with multiple static fields of different shapes or dynamic MLC with continuous, automated movement of MLCs without treatment interruption. IMRT may also be delivered using arc therapy delivering IMRT with one (360°) or two (720°) continuous rotations of the radiation source around the patient. Examples such as VMAT (Elekta), RapidArc or tomotherapy have the main benefit of a reduction in treatment time [14].
IMRT will therefore define concave and convex shapes (Fig. 10.3) thus allowing high-dose treatment of tumour sites but avoidance of adjacent nontarget normal tissues. The use of IMRT means that delineation of the target and nontarget tissues, patient immobilisation and verification of patient and tumour positions during a course of treatment become even more important. This is to avoid missing the edge of the tumour, which may lead to an increase in recurrence rates (see section “Local disease control with PG-or SMG-sparing IMRT”) with possible overdose of normal tissues.
In addition to the reduced normal tissue toxicity, IMRT has the potential to allow dose escalation to the tumour which will increase cell kill and may improve recurrence and cure rates. Dose escalation has been studied recently in a number of phase I/II studies [15–18], and a multicentre phase III RCT is currently recruiting in the UK [19].
Non-randomised Studies of PG-Sparing RT and IMRT for the Treatment of Head and Neck Cancer
In an early planning study where patients were treated with unilateral irradiation of the neck lymph nodes, 3D-CRT was shown to be superior to 2D-RT for both target volume coverage and contralateral PG sparing [20]. In addition, when treating the bilateral neck, a reduction in the mean radiation dose delivered to the contralateral PG (21 Gy vs. 58 Gy) was reported in a study by Eisbruch et al. [21] using 3D-CRT.
Eisbruch et al. subsequently pioneered the implementation of IMRT for the routine treatment in HNC. Their initial case series of 88 patients treated with IMRT reported that the PG mean dose should be limited to 26 Gy or 24 Gy, for stimulated or unstimulated flow, respectively, to maintain a substantial fraction of pre-IMRT PG saliva flow [22]. Furthermore they showed that patient-reported xerostomia significantly improved over time with the use of IMRT [23].
Further single-arm phase I and II prospective studies have shown that PG-sparing IMRT produces favourable xerostomia rates for several common subsites of HNC (Table 10.1).
RCTs of PG-Sparing IMRT
RCT of Conventional RT Versus IMRT
Four phase III randomised controlled trials (RCTs), one multicentre and three single institution, have compared conventional 2D-RT with IMRT.
The PARSPORT trial [32] is the largest study to investigate PG-sparing IMRT in non-nasopharyngeal carcinoma (NPC) patients; it is also the only multicentre IMRT RCT for squamous cell HNC. Ninety-four patients from six UK centres were randomised to IMRT vs. 2D-RT. The primary end point was patient-reported high-grade (≥G2) xerostomia by the LENT-SOMA scale at 12 months after RT. The secondary end points were global and xerostomia-specific quality of life scores, acute and other late radiation side effects, measurable PG and floor of mouth salivary flow and progression-free and overall survival. The salivary function outcomes at 12 months for contralateral measurable PG salivary flow were 47 % for IMRT compared to 0 % for conventional RT (p < 0.0001). The frequency of high-grade xerostomia was 38 % for IMRT and 74 % for conventional RT (p = 0.0027) (Fig. 10.4). In addition, the EORTC-HN35 subscale score for dry mouth showed deterioration from baseline (increased mean score) at all time points after RT. At 12 months the mean increases were 56.6 (2D-RT) and 48 (IMRT), and at 24 months the mean increases were 59.3 (2D-RT) and 34.8 (IMRT). Despite numerical improvement in the IMRT arm when compared with the 2D-RT arm, the result was not statistically significant (p > 0.01) (Fig. 10.5).
Proportion of patients reporting grade 2 or worse LENT-SOMA subjective xerostomia salivary gland side effects (primary trial end point ≥ grade 2 subjective xerostomia at 12 months) (Reproduced with permission from PARSPORT trial [32])
Mean EORTC QOL HN35 dry mouth subscale score changes from baseline (Reproduced with permission from PARSPORT trial [32])
Peng et al. [33] published the most recent and largest RCT investigating PG-sparing RT techniques. Patients with NPC and no distant metastases were randomised to IMRT (n = 315) or 2D-RT (n = 325). Administration of induction, concomitant and/or adjuvant CT was similar between treatment groups. The 6-month rate of xerostomia (any CTCAE grade) showed a significant benefit for IMRT vs. 2D-RT, 39.5 % vs. 99.4 % (p < 0.001).
Two smaller, single institution RCTs are reported for patients with early-stage NPC [34,35]. Pow et al. [34] (45 patients) reported stimulated whole mouth saliva (WMS) and unilateral stimulated parotid saliva (SPS) flow rates. At 12 months post-RT, the proportion of patients with stimulated WMS and SPS flow recovery to >25 % of the pre-RT level was significantly higher with IMRT vs. 2D-RT (stimulated WMS 50 % vs. 4.8 % and SPS 83.3 % vs. 9.5 %). Despite this finding, patient-reported oral quality of life (EORTC-HN35) scores for sticky saliva and dry mouth were not significantly different between the two techniques; this may be related to the small number of patients enrolled in the trial.
Kam et al. [35] (60 patients) reported a lower proportion of patients with ≥ G2 physician-reported xerostomia (RTOG) using IMRT compared to 2D-RT (39.3 % vs. 82.1 %, p = 0.001) at 1 year. This was comparable to the PARSPORT trial outcomes. As with Pow et al., the fractional recovery of flow from baseline for stimulated WMS, 0.41 vs. 0.2 (p = 0.01), and SPS, 0.9 vs. 0.05 (p < 0.001), was significantly better at 1 year posttreatment with the use of IMRT.
A systematic review has been published recently by O’Sullivan et al. [36] which assessed the benefit of IMRT over conventional 2D-RT for multiple adverse effects and disease outcomes and specific to this discussion, xerostomia.
They retrieved seven prospective, retrospective and case-controlled studies with xerostomia as an end point, which enrolled 567 patients between them. Five of the studies reported a statistically significant reduction in xerostomia at 6 months [37], 1 year [32,34,35] or 20 months [38] after RT. However, two other studies [39,40] showed no significant difference in xerostomia outcomes.
The authors concluded that if a reduction of xerostomia and an improvement in quality of life are the main outcomes of interest, then IMRT is the recommended treatment for all nasopharyngeal, oropharyngeal, hypopharyngeal, laryngeal, oral cavity and unknown primary cancers when delivery of RT to lymph node regions, requiring inclusion in the treatment volume, would result in irreparable damage to SG function, if a less conformal RT technique is used due to their inability to maintain SG doses within their tolerance limits.
RCT of 3D-CRT Versus IMRT
Gupta and colleagues [41] report the only RCT of PG-sparing IMRT vs. 3D-CRT. Sixty patients were assessed for radiation dosimetry and physician-reported SG toxicity (RTOG, acute and late). The ≥ G2 acute SG toxicity using IMRT was lower, 59 % vs. 89 % (p = 0.03) and for late toxicity ~30 % vs. ~75 % (p = 0.001, data in histogram figure only). IMRT plans achieved higher dose conformality and PG sparing. The mean (95 % CI) contralateral PG dose for 3D-CRT vs. IMRT plans was 49.8 Gy (46.5–53.1 Gy) and 28.8 Gy (27–30.7 Gy), respectively.
Implementation of IMRT: National and International Experience
The implementation of IMRT into routine practice is a meticulous process requiring multidisciplinary teamwork to provide the safe and precise delivery of this highly conformal RT technique [42]. For implementation and ongoing routine treatment, the clinical/radiation oncologist, physicist, dosimetrist and therapy radiographer must work closely together. Once the primary tumour site of interest has been determined, a stepwise implementation plan should be developed [42].
Firstly, treatment planning requires reproducible immobilisation of the patient, commonly using a thermoplastic shell. IMRT no longer necessitates a straight cervical spine therefore allowing extension of the neck which can reduce dose to critical normal tissues. The departmental set-up errors and patient movements in the treatment position should be audited to determine margins that should be added to the outlined regions of tumour and normal tissue. Patients should be imaged in the treatment position with axial scans, currently CT+/−MRI or PET, and the tumour and normal tissues delineated using computerised planning software performed by an oncologist. Alongside this a quality assurance programme should be maintained to confirm the accuracy of IMRT treatment delivery, planning systems and software. Guidelines for the roles of each team member in the delivery of IMRT are outlined in a recent joint ACR and ASTRO publication [43].
UK – A survey of the use of IMRT in the UK was performed in 2008 [44]. Fifty of 58 UK centres responded (~89 % of all patients treated) with 46 of 50 centres having at least 2 IMRT-capable treatment machines but only 18 centres treating patients with it. Despite HNC and thyroid cancer being the fifth commonest cancer diagnosis, it was the third most common cancer to be treated radically with IMRT in the UK, behind breast and prostate cancer. This indicates the important role it has in HNC treatment.
In 2008, 1,237 of 7,219 patients (17.1 %) eligible for a radical course of RT received IMRT. The same study estimated that 57 % of these patients would benefit from IMRT. The relatively low utilisation of IMRT in 2008 has been addressed over the last 5 years. Improved funding and direct Department of Health guidelines that are in place to bridge the gap between the current and optimal use of IMRT have accelerated this process of IMRT implementation [45]. Such that recently updated data from 2012 show that 68 % of UK centres are now offering IMRT with 83.9 % of all UK HNC patients receiving their radical treatment using an IMRT technique [46].
Worldwide – IMRT was developed in the USA and due to the differences in health care funding has been implemented at a faster rate compared to the UK. Between 2002 and 2004, the proportion of radiation oncologists treating with IMRT was reported to increase from 40 % to 73 % [47]. As seen in the UK, the introduction of IMRT in Canada for HNC was slightly slower compared to the USA but has rapidly increased recently with 80 % of centres using IMRT for treatment of HNC in 2010 and 37 % of all centres using IMRT for “virtually all” HNC patients [48]. The reported barriers to IMRT use in Canada are now most frequently the lack of trained IMRT planners or oncologist and no longer the lack of technical capability or support staff to deliver IMRT. Other countries such as India are developing IMRT-capable facilities rapidly and recently reported that 60 of 280 centres in India are delivering IMRT treatment [49].
Potential Strategies to Improve IMRT-Induced Xerostomia
PG-sparing IMRT is now the standard treatment for HNC. However, residual xerostomia remains a clinical problem for a sizeable minority, and further improvements may be gained through avoidance of the SGs or by the further reduction in dose to the PG with the use of novel RT delivery techniques.
Submandibular Gland-Sparing IMRT
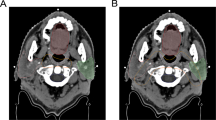
IMRT for PG sparing has now been extensively studied, but few trials have assessed the possibility of PG and SMG sparing. It is becoming apparent that the SMGs and the minor SGs may have an important role in the prevention of xerostomia as they are the sole source of salivary mucins for the oral cavity [50].
A prospective study [51] which investigated the dose response of the SMG also showed that for a subgroup, 8 of 148 recruited patients, using IMRT and applying a contralateral SMG (cSMG) dose constraint, the mean cSMG dose can be significantly reduced from 48 to 36 Gy (p = 0.001).
Saarilahti et al. [52] assessed the role of IMRT to spare the PG and the cSMG in 36 patients. Half the patients were treated with cSMG sparing, and a mean cSMG dose of 25.9 Gy (range 18–32 Gy) was achieved. At 12 months the ≥ grade 2 subjective xerostomia (LENT-SOMA) frequency was improved with cSMG sparing, 22 % vs. 61 % (p = 0.018). cSMG sparing significantly improved unstimulated but not stimulated relative-fractional WMS flow at 12 months, 0.6 vs. 0.25 (p = 0.006). Six-month flow was also significantly improved (p < 0.05).
Wang and colleagues [53] treated 52 patients with PG-sparing IMRT; 26 patients also had cSMG sparing. Mean unstimulated WMS flow was better with the addition of cSMG sparing compared to PG sparing alone, at all time points from 2 to 18 months (p < 0.0001 for all). However, any grade xerostomia (RTOG) was only significantly better in the cSMG-sparing group at 2 and 6 months (p = 0.036 and 0.046) but not at 12 and 18 months. No difference in late xerostomia score might be expected as the RTOG scale assesses xerostomia symptoms predominantly under stimulated conditions, whereas the most likely benefit of cSMG sparing would be found under unstimulated (resting) conditions.
More recently Little et al. [54] reported a study of PG-, cSMG- and oral mucosa-sparing IMRT in 78 patients with stage III or IV HNC. Significant sparing of the cSMG was not possible; mean cSMG dose was 62 Gy (range 29–75 Gy). The proportion of patients with >25 % of baseline flow at 12 months was 17 % (unstimulated) and 14 % (stimulated). When compared to the preceding two studies [52,53], patients in the study by Little et al. were not selected on their suitability for cSMG sparing, hence the high SMG dose of 62 Gy [54] vs. 25.9 Gy [52] or 20.4 Gy [53] and resultant poor, long-term SMG flow rates.
These reports make it apparent that careful selection of patients for cSMG sparing is needed, where the risk of contralateral level 1 lymph node disease is low. However, in a selected cohort good recovery of SG function can be achieved with this approach.
Oral Mucosa-Sparing IMRT
Within the oral mucosa (OM) are 600–1,000 minor SGs, and until recently there was no option to spare them; also the clinical relevance of these secretions was unclear. Despite contributing <10 % WMS they contribute the majority of mucins [50] which are associated with mucosal moisture retention and xerostomia at rest and at night.
The dosimetric and clinical benefits of IMRT for OM sparing, when compared to 3D-CRT, have been shown in a planning study of oropharyngeal carcinoma patients [55]. IMRT provides a reduction in the maximum OM dose, defined as the OM outside the target tumour volume, but significantly this is only possible when a dose constraint is applied to the OM. In this study a clinically relevant maximum OM dose was defined as 30 Gy (2 Gy per fraction, 6 weeks) to the spared region. When IMRT treatment was replanned for the same patient with no dose constraint applied, a significantly increased dose to the OM was seen, with significantly larger volume of OM receiving ≤39.3 Gy vs. 3D-CRT. This study also reported clinical correlation in 19 IMRT-treated patients by comparing RTOG acute mucositis score, in the spared region of the OM with the region within the PTV. This was significantly reduced from week 2 of treatment onwards (p < 0.01) compared to the unspared regions. Though not reporting on minor SG function or xerostomia, it is promising that OM may be spared with no significant impact on PG mean dose, target volume coverage or increase in other OAR doses.
A prospective RCT [56] (n = 48) of OM-sparing IMRT for post-operative treatment of oral tongue SCC showed a significant reduction in grade 2 and 3 acute oral mucositis (0 % and 25 % vs. 45.8 % and 54.2 %, respectively; p < 0.0001) and reduced mean dose to the OM (41.8 ± 7.4 Gy vs. 58.8 ± 2.2 Gy; p < 0.0001). Again no xerostomia end points were used in this study, but it shows that it is feasible to spare OM, and no increase in tumour recurrence rate was noted.
Two other prospective studies have examined the relationship of OM dose to patient-reported xerostomia. The first with no SG radiation dose constraints [57] and the second with pre-specified PG, SMG and OM radiation dose constraints [54].
The study by Jellema et al. [57], although treating with 2D-RT only, is the largest to date with 156 patients. It assessed for a correlation between the patient-reported symptoms of dry mouth (DM) and sticky saliva (SS) (EORTC HN35) in relation to mean RT dose to SMGs, PGs and OM. All tumours were squamous cell HNC, and the majority had a primary site in the hypopharynx or larynx (74 %). Seventy-six per cent received primary RT, and no chemotherapy was administered. The 6- and 12-month DM scores were associated with both SMG (OR 1.08, p = 0.02) and PG (OR 1.17, p = 0.002) mean doses. The rate of the 6-month moderate to severe DM was found to be reversible, dependant on SMG and PG mean dose such that if the mean SMG dose is 30 Gy or 40 Gy, the mean PG dose must be limited to 16 Gy or 10 Gy, respectively, for <20 % probability of moderate to severe DM. This was not seen at 12 months. The 6- and 12-month SS scores were only associated with SMG mean dose (OR 1.03, p < 0.001). The dose to the OM did not affect the probability of patient-reported xerostomia or sticky saliva at either time point in this patient cohort. Of note the majority of patients were on early-stage disease T1–2 ~ 85 % and N0 ~ 90 %, and the median radiation dose to bilateral SMG and the OM was low (46.7 and 9.1 Gy, respectively). This was despite no SMG or OM dose constraint. Much higher mean RT doses have been reported [55] when treating with IMRT and no SMG or OM constraint and also when treating more advanced disease stage.
The second prospective study by Little et al. [54], which addresses the limitations of the previous study specifically a cohort with more advanced disease stage and treated using IMRT, has been described earlier. In this study a multivariate analysis indicated that when bilateral PG and partial contralateral SMG-sparing IMRTs were used, then the OM dose was found to significantly correlate with late patient (XQ)-and physician-reported (CTCAE) xerostomia, such that if the mean OM dose <40 Gy, there were no cases of ≥ grade 2 xerostomia (CTCAE).
Local Disease Control with PG- or SMG-Sparing IMRT
With the increase in dose conformality, concerns have been raised that the risk of geographical miss of the tumour may increase local recurrence rates [58]. Treating the tumour must always remain the priority, and therefore this specific question has been assessed in a number of case series. The largest and most contemporary was Garden et al. [59] reporting the MD Anderson experience of PG-sparing IMRT for oropharyngeal carcinoma (n = 776). Promisingly only 12 patients (2 %) had a locoregional recurrence outside the high-radiation dose region, and no patients had a recurrence either within the PG or in the adjacent region of steep dose gradient between the spared PG and the treated nodal region. Smaller trials of SMG-sparing IMRT [52,53] have also shown no recurrences adjacent to the SMG, within the contralateral nodal level 1b region. However, this should be viewed with caution due to a limited number of patients treated with this technique, relatively short follow-up and the site of the SMG within the level 1b nodal region.
Novel Radiotherapy Techniques
Arc therapies (RapidArc, VMAT and helical tomotherapy) deliver IMRT with one (360°) or two (720°) continuous rotations of the RT source around the patient. Early data indicates they retain the good target tissue dose coverage with possibly better normal tissue sparing compared to static IMRT systems [14]. Arc therapy also benefits from significantly reduced treatment times which may improve patient QOL and increase patient throughput with the associated economic benefit.
Particle therapy with carbon ions or protons both have the benefit of minimal exit dose beyond the target tissue, unlike photons. A few small trials [60,61] in squamous cell HNC patients have been performed; however, the current role remains unclear, but it may have benefit as a focal boost dose when combined with IMRT. The routine use of carbon ions or protons is limited by very high set-up cost and a lack of facilities.
No data for particle therapies is available regarding their effect on SG dysfunction and oral symptoms, but with the extremely precise nature of the dose deposition, it would be hoped that no additional normal tissue toxicity would occur.
Conclusions
Major advances have been achieved over the last 15 years due to a better understanding of SG radiobiology, a clearer definition of PG tolerance to radiation and the development and implementation of IMRT for routine clinical use.
Despite the significant advances achieved with the reduction in PG radiation dose and hence long-term toxicity, with no reported detrimental effect on tumour control rates, a sizable minority of patients treated with IMRT for HNC continue to be affected by persistent, late xerostomia.
Future strategies for the use of IMRT to spare other SGs and the continued development of novel RT delivery techniques as outlined in this chapter show promise. Further optimisation for the delivery of RT and improving the therapeutic ratio are among several complementary approaches which when combined should continue to improve patient quality of life after RT for HNC over the coming years.
Abbreviations
- 2D-RT:
-
Two-dimensional radiotherapy
- 3D-CRT:
-
Three-dimensional conformal radiotherapy
- ACR:
-
American College of Radiology
- ASTRO:
-
American Society of Radiation Oncology
- CTCAE:
-
Common Terminology Criteria for Adverse Effects
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- IMRT:
-
Intensity-modulated radiotherapy
- LENT-SOMA:
-
Late effects on normal tissues, subjective, objective, management, analytical
- MLC:
-
Multi-leaf collimator
- NPC:
-
Nasopharyngeal carcinoma
- OM:
-
Oral mucosa
- PG:
-
Parotid gland
- RCT:
-
Randomised controlled trial
- RTOG:
-
Radiotherapy Oncology Group
- SG:
-
Salivary gland
- SMG:
-
Submandibular gland
- SS:
-
Sticky saliva
- WMS:
-
Whole mouth saliva
- XQ:
-
Xerostomia questionnaire
References
ONS. Cancer statistics – registrations, England, 2009. In: Office for National Statistics, Series MB1 no.40. London: Office for National Statistics; 2010.
Grundmann O, Mitchell GC, Limesand KH. Sensitivity of salivary glands to radiation: from animal models to therapies. J Dent Res. 2009;88(10):894–903.
Konings AWT, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62(4):1187–94.
Sullivan CA, Haddad RI, Tishler RB, Mahadevan A, Krane JF. Chemoradiation-induced cell loss in human submandibular glands. Laryngoscope. 2005;115(6):958–64.
Teshima K, Murakami R, Yoshida R, et al. Histopathological changes in parotid and submandibular glands of patients treated with preoperative chemoradiation therapy for oral cancer. J Radiat Res. 2012;53(3):492–6.
Deasy JO, Moiseenko V, Marks L, Chao KSC, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3):S58–63.
Dijkema T, Raaijmakers CPJ, Ten Haken RK, et al. Parotid gland function after radiotherapy: the combined Michigan and Utrecht experience. Int J Radiat Oncol Biol Phys. 2010;78(2):449–53.
Moiseenko V, Wu J, Hovan A, et al. Treatment planning constraints to avoid xerostomia in head and neck cancer radiotherapy: an independent test of the QUANTEC criteria using a prospectively collected dataset. Int J Radiat Oncol Biol Phys. 2012;82(3):1108–14.
van Luijk P, Faber H, Schippers JM, et al. Bath and shower effects in rat parotid gland explain increased relative risk of parotid gland dysfunction after IMRT. Int J Radiat Oncol Biol Phys. 2009;74(4):1002–5.
Konings AWT, Faber H, Cotteleer F, Vissink A, Coppes RP. Secondary radiation damage as the main cause for unexpected volume effects: a histopathologic study of the parotid gland. Int J Radiat Oncol Biol Phys. 2006;64(1):98–105.
Buettner F, Miah AB, Gulliford SL, et al. Novel approaches to improve the therapeutic index of head and neck radiotherapy: an analysis of data from the PARSPORT randomised phase III trial. Radiother Oncol. 2012;103(1):82–7.
Vissink A, Jansma J, Spijkervet FKL, Burlage FR, Coppes RP. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14(3):199–212.
Boyer AL, Geis P, Grant W, Carol M. Modulated beam conformal therapy for head and neck tumors. Int J Radiat Oncol Biol Phys. 1997;39(1):227–36.
Van Gestel D, van Vliet-Vroegindeweij C, Van den Heuvel F, et al. RapidArc, SmartArc and TomoHD compared with classical step and shoot and sliding window intensity modulated radiotherapy in an oropharyngeal cancer treatment plan comparison. Radiat Oncol. 2013;8:37.
Miah AB, Bhide SA, Guerrero-Urbano MT, et al. Dose-escalated intensity-modulated radiotherapy is feasible and may improve loco-regional control and laryngeal preservation in laryngo-hypopharyngeal cancers. Int J Radiat Oncol Biol Phys. 2012;82(2):539–47.
Leclerc M, Maingon P, Hamoir M, et al. A dose escalation study with intensity modulated radiation therapy (IMRT) in T2N0, T2N1, T3N0 squamous cell carcinomas (SCC) of the oropharynx, larynx and hypopharynx using a simultaneous integrated boost (SIB) approach. Radiother Oncol. 2013;106(3):333–40.
Zaidi SH, Miah AB, Bhide SA, et al. Radiotherapy for high-risk thyroid malignancies – report of acute toxicities of a phase I sequential cohort dose-escalation IMRT study. Eur J Cancer. 2011;47:S560–S.
Cvek J, Kubes J, Skacelikova E, et al. Hyperfractionated accelerated radiotherapy with concomitant integrated boost of 70–75 Gy in 5 weeks for advanced head and neck cancer a phase I dose escalation study. Strahlentherapie Und Onkologie. 2012;188(8):666–70.
ARTDECO trial (ISRCTN 01483375). Accessed 20 May 2013, at http://www.controlled-trials.com/ISRCTN01483375/artdeco.
Hazuka MB, Martel MK, Marsh L, Lichter AS, Wolf GT. Preservation of parotid function after external-beam irradiation in head and neck cancer patients – a feasibility study using 3-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1993;27(3):731–7.
Eisbruch A, Ship JA, Martel MK, et al. Parotid gland sparing in patients undergoing bilateral head and neck irradiation: techniques and early results. Int J Radiat Oncol Biol Phys. 1996;36(2):469–80.
Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45(3):577–87.
Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50(3):695–704.
Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684–90.
Marucci L, Marzi S, Sperduti I, et al. Influence of intensity-modulated radiation therapy technique on xerostomia and related quality of life in patients treated with intensity-modulated radiation therapy for nasopharyngeal cancer. Head Neck. 2012;34(3):328–35.
Hunter KU, Schipper M, Feng FY, et al. Toxicities affecting quality of life after chemo-IMRT of oropharyngeal cancer: prospective study of patient-reported, observer-rated, and objective outcomes. Int J Radiat Oncol Biol Phys. 2012;85(4):935–40.
Eisbruch A, Harris J, Garden AS, et al. Multi-institution trial of accelerated hypofractionated intensity-modulated radiation therapy for early stage oropharyngeal cancer (RTOG 00-22). Int J Radiat Oncol Biol Phys. 2010;76(5):1333–8.
Richards TM, Bhide SA, Miah AB, et al. Phase 2 trial of total mucosal and bilateral neck intensity modulated radiotherapy in squamous cell cancer of unknown primary. Eur J Cancer. 2011;47:S559.
Toledano I, Graff P, Serre A, et al. Intensity-modulated radiotherapy in head and neck cancer: results of the prospective study GORTEC 2004-03. Radiother Oncol. 2012;103(1):57–62.
Scrimger R, Kanji A, Parliament M, et al. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. Am J Clin Oncol Cancer Clin Trials. 2007;30(3):271–7.
Munter MW, Karger CP, Hoffner SG, et al. Evaluation of salivary gland function after treatment of head-and-neck tumors with intensity-modulated radiotherapy by quantitative pertechnetate scintigraphy. Int J Radiat Oncol Biol Phys. 2004;58(1):175–84.
Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12(2):127–36.
Peng G, Wang T, Yang K-Y, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol. 2012;104(3):286–93.
Pow EHN, Kwong DLW, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66(4):981–91.
Kam MKM, Leung S-F, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25(31):4873–9.
O’Sullivan B, Rumble RB, Warde P. Intensity-modulated radiotherapy in the treatment of head and neck cancer. Clin Oncol. 2012;24(7):474–87.
Braam PM, Terhaard CHJ, Roesink JM, Raaijmakers CPJ. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66(4):975–80.
Lee NY, de Arruda FF, Puri DR, et al. A comparison of intensity-modulated radiation therapy and concomitant boost radiotherapy in the setting of concurrent chemotherapy for locally advanced oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2006;66(4):966–74.
Jabbari S, Kim HM, Feng M, et al. Matched case-control study of quality of life and xerostomia after intensity-modulated radiotherapy or standard radiotherapy for head-and-neck cancer: initial report. Int J Radiat Oncol Biol Phys. 2005;63(3):725–31.
Chen AM, Daly ME, Bucci MK, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: are we making improvement? Int J Radiat Oncol Biol Phys. 2007;69(1):141–7.
Gupta T, Agarwal J, Jain S, et al. Three-dimensional conformal radiotherapy (3D-CRT) versus intensity modulated radiation therapy (IMRT) in squamous cell carcinoma of the head and neck: a randomized controlled trial. Radiother Oncol. 2012;104(3):343–8.
McNair HA, Adams EJ, Clark CH, Miles EA, Nutting CM. Implementation of IMRT in the radiotherapy department. Br J Radiol. 2003;76(912):850–6.
Hartford AC, Galvin JM, Beyer DC, et al. American College of Radiology (ACR) and American Society for Radiation Oncology (ASTRO) Practice Guideline for Intensity-Modulated Radiation Therapy (IMRT). Am J Clin Oncol Cancer Clin Trials. 2012;35(6):612–7.
Mayles WPM. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol. 2010;22(8):636–42.
Radiotherapy Services in England 2012. 2012. Accessed 17 Aug 2013, at https://www.gov.uk/government/publications/radiotherapy-services-in-england-2012.
Mayles WPM, Cooper T, Mackay R, Staffurth J, Williams M. Progress with intensity-modulated radiotherapy implementation in the UK. Clin Oncol. 2012;24(8):543–4.
Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S, 2004. Cancer. 2005;104(6):1296–303.
AlDuhaiby EZ, Breen S, Bissonnette J-P, et al. A national survey of the availability of intensity-modulated radiation therapy and stereotactic radiosurgery in Canada. Radiat Oncol. 2012;7. Article 18.
Kumar R, Sharma SD, Amols HI, Mayya YS, Kushwaha HS. A survey on the quality assurance procedures used in Intensity Modulated Radiation Therapy (IMRT) at Indian hospitals. J Cancer Sci Ther. 2010;2(6):166–70.
Tabak LA. In defense of the oral cavity – structure, biosynthesis and function of salivary mucins. Annu Rev Physiol. 1995;57:547–64.
Murdoch-Kinch C-A, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72(2):373–82.
Saarilahti K, Kouri M, Collan J, et al. Sparing of the submandibular glands by intensity modulated radiotherapy in the treatment of head and neck cancer. Radiother Oncol. 2006;78(3):270–5.
Wang Z-H, Yan C, Zhang Z-Y, et al. Impact of salivary gland dosimetry on post-IMRT recovery of saliva output and xerostomia grade for head and neck cancer patients treated with or without contralateral submandibular sparing: a longitudinal study. Int J Radiat Oncol Biol Phys. 2011;81(5):1479–87.
Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-IMRT for head-and-neck cancer: beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83(3):1007–14.
Sanguineti G, Endres EJ, Gunn BG, Parker B. Is there a “mucosa-sparing” benefit of IMRT for head-and-neck cancer? Int J Radiat Oncol Biol Phys. 2006;66(3):931–8.
Wang Z-H, Zhang S-Z, Zhang Z-Y, et al. Protecting the oral mucosa in patients with oral tongue squamous cell carcinoma treated postoperatively with intensity-modulated radiotherapy: a randomized study. Laryngoscope. 2012;122(2):291–8.
Jellema AP, Doornaert P, Slotman BJ, Leemans CR, Langendijk JA. Does radiation dose to the salivary glands and oral cavity predict patient-rated xerostomia and sticky saliva in head and neck cancer patients treated with curative radiotherapy? Radiother Oncol. 2005;77(2):164–71.
Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):660–5.
Garden AS, Dong L, Morrison WH, et al. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(4):941–7.
Mizoe J-E, Tsujii H, Kamada T, et al. Dose escalation study of carbon ion radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;60(2):358–64.
Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62(2):494–500.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Richards, T.M., Nutting, C.M. (2015). New Radiotherapy Techniques for the Prevention of Radiotherapy-Induced Xerostomia. In: Carpenter, G. (eds) Dry Mouth. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55154-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-642-55154-3_10
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55153-6
Online ISBN: 978-3-642-55154-3
eBook Packages: MedicineMedicine (R0)