Abstract
Esophageal stenting with a self-expandable metal stent (SEMS) or self-expandable plastic stent (SEPS) has increasingly been used and is currently the most common treatment modality for palliation of malignant dysphagia worldwide.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Key Summary-
Esophageal stenting with a self-expandable metal stent (SEMS) or self-expandable plastic stent (SEPS) has increasingly been used and is currently the most common treatment modality for palliation of malignant dysphagia worldwide.
-
SEMSs improve dysphagia in more than 90 % of patients with mid- and distal esophageal cancer.
-
Dysphagia relief is comparable between SEPSs and SEMSs. However, compared to SEMSs, SEPSs are associated with a higher rate of complications, including migration.
-
Previous retrospective studies have demonstrated the feasibility of stent placement for cervical esophageal cancer with effective palliation of dysphagia. Rates of complications and recurrent dysphagia are comparable to those in patients who undergo stent placement for mid- and distal esophageal cancer.
-
Dysphagia caused by extraesophageal malignancies can be safely and effectively treated with partially covered or fully covered SEMSs.
-
The complication rate associated with stent placement for malignant esophageal obstruction ranges from 30 to 50 % in most series. Placement of a second stent can effectively relieve recurrent dysphagia caused by stent migration or occlusion.
14.1 General Information
Esophageal stricture is a problem frequently encountered by gastroenterologists and can be subdivided into strictures of malignant origin and those with a benign origin. Malignant esophageal strictures are mainly caused by primary esophageal cancers but can also be caused by extraesophageal malignancies that compress the esophagus. More than 50 % of patients with esophageal cancer have incurable disease at presentation because of metastases, locally advanced disease, or poor medical condition. One of the major goals of palliative therapy in patients with incurable cancer is to relieve dysphagia. Various therapies have been used to palliate dysphagia in patients with esophageal carcinoma, including esophageal stenting, esophageal dilation, radiation therapy, chemotherapy, laser ablation, and photodynamic therapy. Among these modalities, esophageal stenting with a self-expandable metal stent (SEMS) or self-expandable plastic stent (SEPS) has increasingly been used and is currently the most common treatment modality for palliation of malignant dysphagia worldwide.
14.2 Types of Stents
-
SEMSs consist of woven, knitted, or laser-cut metal mesh cylinders that exert self-expansive forces until they reach their maximum fixed diameter. SEMSs are made of stainless steel and alloys, such as nitinol (nickel and titanium) and elgiloy (cobalt, nickel, and chromium), which have a high degree of flexibility and are capable of generating high radial forces to maintain stent patency and position. Most SEMSs have a proximal and/or distal flare to prevent migration [1].
-
SEPSs have a woven polyester skeleton and are completely covered with a silicone membrane. The silicone prevents tissue ingrowth through the mesh, and the polyester braids on the external surface anchor the stent to the mucosa to limit migration.
-
To prevent tumor ingrowth, the interstices between the metal mesh of esophageal SEMSs may be fully or partially covered by a plastic membrane or silicone.
-
Covered SEMSs are superior to uncovered SEMSs for the palliation of malignant dysphagia because uncovered SEMSs are associated with a higher rate of tumor ingrowth and consequent recurrent dysphagia after stent insertion [2]. Table 14.1 summarizes the advantages and disadvantages of the different stent types [3, 4].
-
There are only minor differences in efficacy and adverse event rates between the various commercially available SEMSs [2]. Therefore, the choice of stent should be determined by the location and anatomy of the malignant stricture in addition to the specific characteristics of the stent. Table 14.2 summarizes the features of SEMSs and SEPSs that are currently commercially available.
14.3 Stent Insertion Technique
-
The stricture to be stented is first identified endoscopically. The length of the stricture and degree of obstruction can be assessed by endoscopy or, in the case of non-traversable strictures, with fluoroscopic guidance [1].
-
A guidewire is advanced through the stricture, and the stent is positioned across the stricture and then deployed under fluoroscopic and/or endoscopic guidance by release of the constraining mechanism. There are two methods of stent delivery: through the scope and over the wire. The majority of deployment systems release the stent initially at the distal end of the catheter (Fig. 14.1).
What You Should Know Here: Stent Insertion Technique
-
During the transition from the compressed to the fully expanded state, most SEMSs and SEPSs undergo varying degrees of foreshortening. The endoscopist must anticipate and allow for this foreshortening to ensure appropriate stent placement.
-
During stent selection, it is important to choose a stent length that is 4 cm longer than the stricture being stented. This allows for 2 cm of stent on either end of the stricture to decrease the risk of migration [3].
Process of esophageal stent insertion. (a) A guidewire is advanced through the stricture. The proximal end of the stricture is marked with metal clips placed on the patient’s skin. (b) The stent is advanced over the guidewire under endoscopic and fluoroscopic guidance and positioned across the stricture. (c) The stent is then deployed. (d) Fluoroscopic image showing a deployed stent
14.4 Stent Placement for Mid- and Distal Esophageal Cancer
-
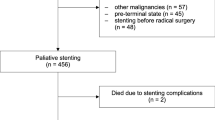
SEMSs improve dysphagia in more than 90 % of patients with mid- and distal esophageal cancer (Fig. 14.2).
-
Compared to brachytherapy, SEMSs more rapidly improve dysphagia, but brachytherapy yields better long-term control of dysphagia with fewer complications [5].
-
The dysphagia relief rate is comparable between SEPSs and SEMSs. However, compared to SEMSs, SEPSs are associated with a higher rate of complications, including migration.
Esophageal SEMS insertion for a patient with mid-esophageal cancer. (a) Endoscopic image showing luminal obstruction by a mid-esophageal cancer. (b) Fluoroscopic image showing narrowing of the esophageal lumen and a tracheoesophageal fistula. (c) Endoscopic image of a deployed esophageal SEMS covering both the stricture site and fistula opening. (d) Fluoroscopic image showing a deployed stent
14.5 Stent Placement Across the Gastroesophageal Junction
-
Stenting across the gastroesophageal junction is associated with a higher rate of migration compared to stenting for mid- and distal esophageal cancers. This is likely due to the distal stent end projecting freely in the gastric lumen and, therefore, not being fixed to the gastric wall [4].
-
Windsock anti-reflux stents use a polyurethane membrane that extends 8 cm beyond the metal portion of the stent to prevent gastroesophageal reflux (Fig. 14.3).
-
A specially designed stent with an anti-reflux valve yielded conflicting results in the prevention of esophageal acid reflux as determined by reflux-associated symptoms and 24-h pH monitoring.
Esophageal SEMS insertion for a patient with esophageal cancer involving the gastroesophageal junction. (a, b) Endoscopic image showing luminal narrowing of the distal esophagus and gastroesophageal junction by an esophageal cancer. (c) Endoscopic image of a deployed esophageal SEMS with an anti-reflux valve. (d) Fluoroscopic image showing a deployed stent
14.6 Stent Placement for Cervical Esophageal Cancer
-
Stent placement close to the upper esophageal sphincter in patients with cervical esophageal cancer (7–10 % of all esophageal cancer) may be limited by patient intolerance due to pain and globus sensation, as well as an increased risk of complications, such as perforation, aspiration pneumonia, and tracheoesophageal fistula [2].
-
Previous retrospective studies have demonstrated the feasibility of stent placement for cervical esophageal cancer with effective palliation of dysphagia. The occurrence of complications and recurrent dysphagia was comparable to that in patients who underwent stent placement for mid- and distal esophageal cancer (Fig. 14.4) [6].
-
To avoid adverse events, such as persistent globus sensation and proximal stent migration, there should be at least 2 cm distance between the proximal end of the stent and the upper esophageal sphincter during stent placement.
Esophageal SEMS insertion for a patient with cervical esophageal cancer. (a) Endoscopic image showing narrowing of the cervical esophageal lumen by an esophageal cancer. (b) Fluoroscopic image showing narrowing of the cervical esophageal lumen. (c) Endoscopic image of a deployed esophageal SEMS. (d) Fluoroscopic image showing a deployed stent
14.7 Stent Placement for Malignancies Compressing the Esophageal Lumen
-
Apart from being caused by primary esophageal cancers, dysphagia can also be caused by malignancies compressing the esophageal lumen, such as lung cancer or metastatic mediastinal lymph nodes.
-
Dysphagia caused by extraesophageal malignancies can be safely and effectively treated with partially covered or fully covered SEMSs (Fig. 14.5).
-
The occurrence of recurrent dysphagia and complications, including migration after stent insertion is comparable to that observed in patients with primary esophageal cancer.
Esophageal SEMS insertion for a patient with lung cancer compressing the esophageal lumen. (a) Endoscopic image showing narrowing of the esophageal lumen by extrinsic compression. (b) Fluoroscopic image showing narrowing of the esophageal lumen by extrinsic compression. (c, d) Fluoroscopic and computed tomography image showing a deployed stent
14.8 Bridge-to-Surgery Stenting
-
Some patients treated with neoadjuvant chemotherapy and radiotherapy experience severe dysphagia and weight loss while receiving treatment. Temporary stent placement during the neoadjuvant therapy period can be a reasonable option for relieving dysphagia and improving nutrition status before performing curative esophagectomy compared to placement of a nasogastric feeding tube or percutaneous endoscopic gastrostomy tube [4].
-
Stent migration occurs in 18–48 % of patients, especially when a fully covered SEMS or a SEPS is placed or there is tumor regression in response to neoadjuvant therapy.
14.9 Adverse Events Associated with Esophageal Stent Placement
14.9.1 Overview
-
The complication rate associated with stent placement for malignant esophageal obstruction ranges from 30 to 50 % in most series [2].
-
Stent placement for malignant esophageal obstruction is associated with severe life-threatening complications, including airway compression, perforation, and bleeding.
-
Other complications of esophageal stent placement include stent migration, stent occlusion caused by tissue hyperplasia or tumor ingrowth, chest pain, gastroesophageal reflux, aspiration pneumonia, and delayed tracheoesophageal fistula caused by pressure necrosis.
14.9.2 Airway Compression
-
Airway compression is an immediate life-threatening complication associated with esophageal stent insertion (Fig. 14.6).
-
Some have advocated bronchoscopy and possible tracheal stent placement simultaneously or before esophageal stent placement for bulky lesions in the upper esophagus that involve or compress the airways [1].
-
Smaller diameter stents for upper esophageal lesions might be helpful for avoiding excessive compressive forces, which can potentially cause airway compression or pressure necrosis with fistula formation.
Endoscopic removal of an esophageal SEMS due to airway compression in a patient with thymic cancer. (a) Computed tomography image showing luminal narrowing of the left main bronchus by an expanded esophageal stent. (b) Endoscopic image showing a blue-colored purse-string suture. (c) Endoscopic image showing the removal of an esophageal stent by pulling the purse-string suture with forceps. (d) Image of previously inserted esophageal stents successfully removed by endoscopy
14.9.3 Migration
-
Stent migration rates in partially covered SEMSs range from 4 to 23 % and those in fully covered SEMSs and SEPSs are higher. Embedding of the uncovered stent ends leads to better fixation of the stent to the esophageal wall.
-
Migrated stents can be easily removed by pulling the purse-string suture with forceps and collapsing the top of the stent.
-
The most frequently used method for reintervention after stent migration is placement of a second stent.
14.9.4 Tumor Ingrowth or Overgrowth
-
Stent occlusion occurs because of ingrowth of tissue through the uncovered mesh or overgrowth at the stent ends (Fig. 14.7).
-
The stent occlusion rate after insertion of partially covered SEMSs ranges from 10 to 14 %.
-
Secondary stent insertion through an occluded primary stent (stent-in-stent insertion) can effectively relieve recurrent dysphagia due to tumor ingrowth or overgrowth in most cases [6].
Occlusion of an esophageal SEMS due to tumor ingrowth and overgrowth in a patient with esophageal cancer. (a) Fluoroscopic image suggesting tumor ingrowth into a previously inserted esophageal stent. (b) Endoscopic image showing tumor overgrowth at the proximal end of the stent. (c) Endoscopic image showing tumor ingrowth into the stent. (d) Fluoroscopic image after placement of a second stent using the stent-in-stent insertion method
References
ASGE Technology Committee. Enteral stents. Gastrointest Endosc. 2011;74:455–64.
Practice Parameters Committee of the American College of Gastroenterology, Sharma P, Kozarek R. Role of esophageal stents in benign and malignant diseases. Am J Gastroenterol. 2010;105:258–73.
Kim S. Enteral stents: from esophagus to colon. Gastrointest Endosc. 2013;78:913–8.
Vleggaar FP, Siersema PD. Expandable stents for malignant esophageal disease. Gastrointest Endosc Clin N Am. 2011;21:377–88.
Homs MY, Steyerberg EW, Eijkenboom WM, et al. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet. 2004;364:1497–504.
Siersema PD. Treatment options for esophageal strictures. Nat Clin Pract Gastroenterol Hepatol. 2008;5:142–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Min, BH. (2015). Stent Placement for Malignant Esophageal Obstruction. In: Chun, H., Yang, SK., Choi, MG. (eds) Therapeutic Gastrointestinal Endoscopy. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55071-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-642-55071-3_14
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55070-6
Online ISBN: 978-3-642-55071-3
eBook Packages: MedicineMedicine (R0)