Abstract
Colloids and crystalloids are frequently use to restore intravascular volume. Both solutions interfere with the coagulation process in different ways. Increasing amounts of fluids result in dilutional coagulopathy. Artificial colloids exert additive effects on fibrin polymerisation and platelet function. In vitro and in vivo studies revealed that starches and dextrans show the greatest negative impact on hemostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In hemorrhagic shock, intravascular hypovolemia is one of the key factors contributing to insufficient oxygen delivery and subsequent tissue hypoxemia. Thus, in order to restore tissue perfusion, restoration of intravascular volume is one of the main aims of shock therapy. Both crystalloid and colloid solutions are frequently used. Compared to colloids, higher volumes of crystalloids are required to exert an equal intravascular volume effect (Jacob et al. 2012). It is noteworthy that large quantities of crystalloids have significant side effects, such as tissue edema, diminished blood viscosity, and hemostatic alterations. Colloids provide larger increases in intravascular volume, resulting in faster hemodynamic stabilization. However, artificial colloids can also cause adverse effects such as anaphylactic reactions, impairment of renal function, and alteration of hemostasis, which is potentially associated with an increased tendency to bleed (Choi et al. 1999).
Controversy regarding the optimal choice and composition of resuscitation fluids is ongoing. Randomized controlled trials (RCTs) have failed to prove that resuscitation with colloid solutions provides survival benefits compared to fluid therapy with crystalloids (Perel and Roberts 2012). The use of colloids is associated with an increased mortality compared to crystalloids in patients with severe sepsis and in trauma patients (Perner et al. 2012). This finding could be related to the negative effects of colloids on blood coagulation and platelet function (Schierhout and Roberts 1998). There is a paucity of evidence that one colloid is superior to another with regard to survival (Bunn and Trivedi 2012).
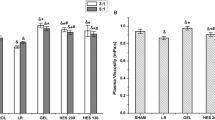
Infusion of large amounts of fluid results in nonspecific dilution of coagulation factors, coagulation inhibitors, and platelets (Table 9.1). In addition, the administration of artificial colloids leads to specific alterations of coagulation factors. Acquired von Willebrand syndrome and low FVIII activity can be observed, impairing both primary and secondary hemostasis (Kozek-Langenecker 2009). Viscoelastic tests such as thromboelastometry (ROTEM®) or thrombelastography (TEG®) have shown that clot strength (i.e., clot quality) is reduced following dilution with artificial colloids both in vitro and in vivo (Fig. 9.1) (Jones et al. 2003; Mittermayr et al. 2007; Fenger-Eriksen et al. 2009). This effect is due to the inhibition of fibrin polymerization (Fries et al. 2002; Fenger-Eriksen et al. 2009). Scanning electron microscopy revealed impairment of the reticular network of fibrin strands following dilution of blood samples with artificial colloids (Fig. 9.2) (Mardel et al. 1998; Fries et al. 2005; Sorensen and Fries 2012). Platelet dysfunction has also been reported after infusion of artificial colloids (Gamsjager et al. 2002; Deusch et al. 2004).
ROTEM® results from an in vitro model of 33 % hemodilution. Two ROTEM® tests were performed: an extrinsically activated test using tissue factor (EXTEM) and an extrinsically activated test without platelet component (FIBTEM). The most pronounced reduction in maximum clot firmness (MCF) was observed with 6 % HES 130/0.4. Dilution of whole blood with 5 % human albumin and 4 % gelatin produced similar reductions in MCF. Normal saline induced only moderate changes in MCF
In vitro effects on clotting of 50 % dilution of whole blood with different fluids: (a) no dilution (whole blood), (b) Ringer’s lactate, (c) 6 % HES 130/0.4, and (d) 4 % gelatin. 6 % HES 130/0.4 disturbed the fibrin meshwork more than Ringer’s lactate or gelatin (With permission from Sorensen and Fries (2012))
2 Crystalloid Solutions
Crystalloid fluids are widely used for volume resuscitation. These solutions are safe and well tolerated at low volumes, but their intravascular volume expansion effect is low. Within minutes, 80 % or more of the infused volume can cross the capillary membrane from the intravascular compartment into the interstitial space (McIlroy and Kharasch 2003). Therefore, large amounts of crystalloids are often necessary to provide a sufficient increase in intravascular volume, introducing a significant risk of soft tissue edema.
Normal saline (NS) is a solution with 0.9 % of sodium chloride, with an osmolality of 308 mOsm/l. It contains 154 mmol/l of sodium and 154 mmol/l of chloride. It can therefore be considered neither physiological nor balanced, yet NS is frequently used as a plasma substitute. In contrast, balanced electrolyte solutions are isotonic and have electrolyte compositions close to that of plasma (Guidet et al. 2010).
2.1 Effect on Coagulation Factors and Thrombin Generation
In vivo and in vitro studies have suggested a hypercoagulable state following moderate hemodilution with crystalloids (Ruttmann 2002; Ruttmann et al. 1996, 2001, 2006; Petroianu et al. 2000; Ng et al. 2002). Other investigators refute such a pro-hemostatic effect and suggest that these findings are an in vitro phenomenon only (Innerhofer et al. 2002).
High-volume crystalloid replacement therapy results in dilution not only of pro-hemostatic coagulation factors but also of coagulation system inhibitors, such as antithrombin III (AT III) and proteins S and C (Ruttmann et al. 1996). A variety of positive feedback loops in the clotting mechanism are regulated by AT III; therefore, a small change in its concentration may have a profound effect on the initiation and amplification of the clotting process – potentially much greater than that suggested by the change in absolute concentration (Jesty 1986). Indeed, Dunbar and Chandler measured thrombin generation in plasma diluted to 40 % of normal, which resulted in an increase in thrombin generation. Dilution in excess of 40 %, however, caused a decline in thrombin generation. Thus, when all plasma proteins decrease at the same time, including inhibitory factors such as AT III, thrombin generation initially increases (Dunbar and Chandler 2009).
2.2 Viscoelastic Findings
Ng et al. studied the effect of hemodilution with NS in patients undergoing major hepatobiliary surgery. Seven milliliter per kilogram of blood were withdrawn and simultaneously replaced with 14 ml/kg of NS. They observed decreases in r-time and in k-time indicating hypercoagulability (Ng et al. 2002). Ruttmann et al. reported comparable results in patients scheduled for vascular surgery. Randomly allocated patients received either 1,000 ml Ringer’s lactate (RL) or 500 ml 6 % hydroxyethyl starch (HES) 200/0.5. In the crystalloid group, TEG measurements revealed a faster onset of coagulation, an increased rate of clot formation, and an increase in clot strength. The observed shortening of r- and k-times suggests an imbalance between activated procoagulants and a reduction in anticoagulants, particularly AT III (Ruttmann et al. 2006).
3 Balanced Fluids
When using large quantities of crystalloid solutions, such as NS, development of dilutional hyperchloremic acidosis has been described (Rehm and Finsterer 2003). It is well known that acidosis impairs coagulation, particularly platelet function and thrombin generation (Kermode et al. 1999; Meng et al. 2003). Therefore, it has been speculated that balanced solutions may potentially avoid or minimize these effects. However, there is poor evidence that high volumes of NS have a clinically relevant, negative impact on coagulation, bleeding tendency, or the need for allogeneic blood transfusion (Gamsjager et al. 2002).
3.1 Effects on Coagulation Factors and Thrombin Generation
Brummel-Ziedins et al. measured thrombin production in a dilution model using different fluids. Fifty percent dilution with NS rapidly decreased thrombin generation, whereas RL dilution was associated with a constant thrombin generation level. The authors suggested that this might be explained by RL’s higher Ca++ content (Brummel-Ziedins et al. 2006).
3.2 Clinical Studies
In vivo studies comparing both fluids reported only minor differences between balanced and non-balanced solutions. Waters et al. compared RL with NS in major aortic surgery. Only minor, nonsignificant differences in blood loss, in favor of RL, were observed. There were no differences in transfusion of red blood cells (RBCs) or fresh frozen plasma (FFP) (Waters et al. 2001).
Studies comparing colloids in NS with balanced electrolyte solutions have not reported relevant differences in blood loss. For example, Casutt et al. revealed that HES 130/0.4 in a balanced solvent had similar effects on hemostasis (Casutt et al. 2010). Kulla et al. investigated patients undergoing abdominal surgery. No significant differences in coagulation parameters and blood loss were observed between balanced and unbalanced HES solutions (Kulla et al. 2008). Schaden et al. recently compared two different HES preparations containing tetrastarch in balanced solution, or NS, in healthy volunteers. Blood was subjected to a blood gas analysis, an assessment of platelet function by multiple electrode aggregometry, and thromboelastometry. After infusion of either 20 ml/kg HES in balanced solution or in NS, there was no significant change in calcium concentration, and only a minor impact on platelet aggregation and clot formation as assessed by ROTEM® (Schaden et al. 2012).
4 Colloid Solutions
4.1 Albumin
Albumin is the major serum protein synthesized in the liver. It contributes to 80 % of plasma colloid oncotic pressure. The molecular weight (MW) is approximately 66–69 kDa. Intravenous human albumin (HA) is derived from pooled human plasma, which introduces a theoretical risk of pathogen transmission.
HA is available for intravenous infusion either as an iso-oncotic 4–5 % solution or as a hyperoncotic 20–25 % solution. It is dissolved in isotonic saline. The volume efficacy of 4–5 % HA is low. In contrast, the high oncotic load of 25 % HA increases intravascular volume by approximately five times the volume administered (Hauser et al. 1980). The serum half-life is about 16–18 h (Scatchard et al. 1944).
The cost of HA is higher than that of colloids or crystalloids, which may limit its use for routine volume replacement. For patients with hypovolemic shock, there is no evidence that HA reduces mortality when compared with less expensive alternatives such as crystalloids (Roberts et al. 2011). A large double-blind RCT failed to demonstrate that 4 % HA confers a clear benefit over NS in critically ill patients (Finfer et al. 2004). In a subgroup of patients with severe brain trauma, higher mortality was observed among patients receiving 4 % HA, compared with those receiving NS (Myburgh et al. 2007).
4.1.1 Effects on Coagulation Factors and Thrombin Generation
Compared to artificial colloids, the effect of HA on coagulation factor and fibrinogen concentration is minor (Lucas et al. 1982; Denis et al. 1987; Myburgh et al. 2007). However, similarly to synthetic colloids, the oncotic pressure of HA can cause an efflux of plasma proteins to the interstitial fluid space which might affect coagulation (Lucas et al. 1982; Denis et al. 1987). An animal study revealed that, compared with crystalloid resuscitation, HA-supplemented fluid therapy significantly reduced fibrinogen and factor VIII concentration and increased prothrombin time (PT) (Johnson et al. 1979).
Kheirabadi et al. compared 5 % HA with 6 % HES 670/0.7 (Hextend™) and 6 % dextran 70 in a 40 % hemodilution model in rabbits. Thrombin generation assays revealed a shortened lag time in the HA group. Total thrombin generation and maximum thrombin concentration were unchanged in HA-diluted samples but decreased significantly following dilution with synthetic colloids. Total blood loss was lowest in the HA group (Kheirabadi et al. 2008).
4.1.2 Viscoelastic Findings
TEG analyses from the Kheirabadi study revealed a shortened r-time in the albumin group. Maximum clot strength was significantly reduced in all three groups but again decreased less in the HA group (Kheirabadi et al. 2008). Coats et al. studied in vitro dilution of whole blood to 40 % using different resuscitation fluids. A Sonoclot™ device was used to measure the clots’ viscoelastic properties. Of all the investigated colloids, 3.5 % urea cross-linked gelatin (Haemaccel®) had the fewest effects on dynamic clot formation, followed by HA. The latter reduced activated clotting time by 2 % and exhibited a 47 % reduction in clot rate (rate of fibrin formation) (Coats et al. 2006).
Our own group recently compared NS with 5 % HA, 4 % gelatin, and 6 % HES 130/0.4 in an in vitro dilution model investigating ROTEM® variables. Compared to undiluted whole blood, 33 % in vitro hemodilution with 5 % HA reduced maximum clot firmness in the extrinsically activated EXTEM test, and a more pronounced reduction was observed in the fibrin polymerization test (FIBTEM). Our in vitro study confirmed the observation that 5 % HA has less effect on ROTEM® test results than other colloids (Schlimp et al. 2013).
4.1.3 Effects on Platelets
Evans et al. investigated the effects of different fluids on platelet aggregation and agglutination in orthopedic patients. HA significantly reduced aggregation in response to collagen. In contrast, a small but significant increase in agglutination was also observed in the albumin group (Evans et al. 2003).
4.1.4 Clinical Studies
Niemi et al. studied the effect of 4 % HA and 6 % HES 120/0.7 in patients undergoing total hip arthroplasty. Postoperatively, Von Willebrand factor (vWF) was higher in the HA group, but no differences were observed in blood loss (Niemi et al. 2005). The same group investigated the effect of postoperative administration of different colloids on hemostasis in 45 patients following cardiac surgery. The most pronounced impairment of coagulation was found after administration of 6 % HES 200/0.5. HA appeared to have the least effect on hemostatic variables, including ROTEM® parameters (Niemi et al. 2006) (Table 9.2).
One study has reported hemostatic alterations following HA administration. Johnson et al. found impaired coagulation in trauma patients after administering HA as a supplement to massive transfusion. During the first 5 postoperative days, patients receiving HA required more RBC units (7.1 vs. 3.8) and more FFP (455 ml vs. 317 ml). The authors suggested that HA may exert antihemostatic and platelet-lowering effects, with a risk of increased blood loss after surgery or trauma (Johnson and Criddle 2004).
4.2 Dextran
Dextrans are mixtures of polydisperse polysaccharides of various sizes and MWs. They are produced by the dextransucrase enzyme during growth of Leuconostoc bacteria in media containing sucrose. Between 50 and 70 % of dextran molecules are excreted unchanged via urine. Dextranase completely metabolizes the remaining dextran molecules to CO2 and H2O. Dextrans have MWs ranging from 40 to 70 kDa and are dissolved in NS. Dextran 70 is usually available as a 6 % solution, while dextran 40 is usually a 10 % solution. Due to its higher concentration, dextran 40 is a more potent volume expander than dextran 70, exerting higher colloid oncotic pressure. However, the volume expansion effect of dextran 40 is short lived due to the fact that it is eliminated by the kidneys more quickly than other colloids (de Jonge and Levi 2001).
In most European countries, dextrans have disappeared from the market due to potentially life-threatening side effects such as severe anaphylactic reactions, acute renal failure, and bleeding diatheses.
4.2.1 Effects on Coagulation Factors and Thrombin Generation
Both dextran 40 and dextran 70 produce dose-related hemostatic defects additional to the effects of hemodilution. Dextran 70 appears to cause a significantly greater impairment of coagulation than dextran 40 (Batlle et al. 1985; de Jonge and Levi 2001).
Dextran’s inhibitory effects on coagulation have been attributed to reductions in the activity or plasma levels of factors V, VII and VIII, and vWF (Batlle et al. 1985). Furthermore, increased fibrinolysis has been reported following dextran infusion. It has been suggested that increased plasma concentrations of tissue plasminogen activator (t-PA) and decreased levels of its most important antagonist, plasminogen activator inhibitor 1 (PAI-1), are responsible for these findings (Eriksson and Saldeen 1995).
4.2.2 Viscoelastic Findings
TEG measurements indicate that dextrans bind to fibrinogen and interfere with fibrin cross-linking, reducing clot elasticity or clot strength. Fifty percent dilution of blood with 10 % dextran 40 completely inhibited clot formation in TEG analysis (Mortier et al. 1997).
4.2.3 Effects on Platelets
Dextran’s effect on platelet function is multifactorial; they relate to reductions in platelet adhesion and aggregation and vWF inhibition (Batlle et al. 1985). Dextran molecules are understood to exert a coating effect on cellular elements of the blood, including platelets. Prolonged bleeding times, observed after dextran infusion, are in part related to diminished platelet function (Alexander et al. 1975).
4.2.4 Clinical Studies
Dextrans have been used for the prevention of postoperative venous thrombosis and pulmonary embolism (Clagett et al. 1995; de Jonge and Levi 2001). In order to minimize the risk of bleeding, dextran infusion should be restricted to patients with normal hemostasis. An upper limit of 20 ml/kg/day should not be exceeded. Patients with renal insufficiency face an increased risk of bleeding because of dextran’s prolonged intravascular half-life and the likelihood of uremic platelet dysfunction (de Jonge and Levi 2001).
4.3 Gelatin
Gelatins are polydisperse polypeptides with an MW of approximately 35 kDa, manufactured by degradation of bovine collagen (de Jonge et al. 1998). Two different formulations of gelatin are available: 4 % succinylated gelatin (Gelofusine®) and 3.5 % polygeline (Haemaccel®; degraded gelatin polypeptides cross-linked by urea). Due to their small MW, up to 50 % of gelatins are cleared within 4–6 h after administration, and complete plasma clearance is achieved within 3 days. Gelatins have the advantage of an unlimited daily dose. However, of all the artificial colloids, gelatins carry the highest risk of anaphylactic reaction (Laxenaire and Mertes 2001), and like other artificial colloids, they affect the coagulation system and platelet function (Van der Linden and Schmartz 1992).
4.3.1 Effect on Coagulation Factors and Thrombin Generation
Infusion of 1 l of gelatin-based solution in healthy volunteers decreases thrombin generation, as assessed by the measurement of thrombin–antithrombin complexes and prothrombin fragment F1 + 2. Compared with NS, a 1.7-fold increase in bleeding time has been observed at 60 min and a 1.4-fold increase at 120 min (de Jonge et al. 1998).
4.3.2 Viscoelastic Studies
Several studies have shown a significant reduction in maximum clot firmness (clot strength) in response to gelatin hemodilution; this is attributed to impaired fibrin polymerization (Egli et al. 1997; Mardel et al. 1998; Fenger-Eriksen et al. 2009; Jin and Yu 2010). The effect is most pronounced when using a fibrin polymerization test (e.g., the FIBTEM test in ROTEM®) (Schlimp et al. 2013).
In vitro experiments have shown that hemodilution with gelatin reduces maximum clot firmness to a greater extent than NS (de Jonge et al. 1998; Mardel et al. 1998). Mardel et al. compared Haemaccel® and Gelofusine® with RL and found significantly lower clot elasticity with both gelatin preparations (26.2 and 22.2 % lower than RL, respectively) (Mardel et al. 1998). One suggested mechanism is that gelatin binds with fibronectin and decreases the plasma concentration of this protein (Engvall et al. 1978; Brodin et al. 1984). Fibronectin contributes to clot stability by forming covalent cross-links with fibrin. Furthermore, it is suggested that gelatin might be incorporated into the clot as it forms, interfering with fibrin architecture and impairing the clot’s strength and stability (Mardel et al. 1998; Kheirabadi et al. 2008).
4.3.3 Effects on Platelets
Following infusion, Gelofusine® has been shown to exert a small, transient inhibitory effect on platelet aggregation (Evans et al. 1998). In the same study, Haemaccel® showed more significant inhibition of platelet aggregation through the glycoprotein (GP) IIb/IIIa receptors. Furthermore, gelatin administration decreases plasma levels of vWF. Aggregation studies have also revealed significant impairment of ristocetin-induced platelet aggregation, suggesting additional interference via the GP Ib receptor (Tabuchi et al. 1995). Overall, the prolonged bleeding times observed with gelatin could be a consequence of impaired primary hemostasis (de Jonge et al. 1998).
4.3.4 Clinical Studies
It is uncertain whether gelatin infusion increases bleeding tendency. To date, only one study in cardiac surgery patients has reported increased blood loss following gelatin infusion, in comparison with albumin (Tabuchi et al. 1995).
Karoutsos et al. studied 42 ASA I patients undergoing total hip or knee replacement. The effects of moderate intraoperative hemodilution with gelatin, HES, or 5 % HA were assessed using TEG. Findings differed from the abovementioned studies, as they found clear evidence of a state of hypercoagulability in the gelatin group, with significant decreases in r- and k-time and an increase in alpha angle. There were no significant between-group differences in intraoperative blood loss and RBC transfusion (Karoutsos et al. 1999).
4.4 Starches
HES are derived from amylopectin and consist of highly branched polysaccharides, similar to glycogen. HES solutions are available either as iso-oncotic 6 % or hyperoncotic 10 % fluids. The volume effect of a 10 % solution exceeds the infused volume, and it is therefore considered a plasma expander (Westphal et al. 2009). Compared to gelatin and crystalloids, the initial volume expansion of HES is greater and lasts longer (Van der Linden and Ickx 2006).
HES are polydisperse fluids with a wide range of MW. HES solutions can be categorized as high-MW (>400 kDa), medium-MW (200–400 kDa), and low-MW fluids (<200 kDa) (Jungheinrich and Neff 2005).
The most important physicochemical aspect of HES is the degree of substitution, defined as the proportion of hydroxyl groups per glucose molecule replaced by hydroxyethyl subunits. Increased substitution increases the solubility of starch in water and, more importantly, reduces the rate of destruction by serum amylase (Kozek-Langenecker 2005). Highly substituted (hetastarch 0.62–0.75), medium-substituted (pentastrach 0.5), and low-substituted (tetrastarch 0.4) HES preparations are available.
HES substitution is possible at different positions of the glucose molecule, with the C2 to C6 ratio describing the ratio of hydroxyethyl group substitutions at these positions. It has been shown that a high C2 to C6 ratio reduces degradation of the HES molecule by α-amylase and delays elimination from the bloodstream (Karoutsos et al. 1999). Newer HES preparations have low MW and low substitution of hydroxyethyl subunits, resulting in rapid metabolism and clearance.
The mechanisms of coagulopathy induced by HES infusion are multifactorial: slow degradation of HES enables interaction with platelets, the coagulation cascade, and the fibrin polymerization process (Kozek-Langenecker 2005). Thus, it has been suggested that HES solutions with a prolonged half-life produce more pronounced side effects than those with a short half-life. First- and second-generation HES solutions were associated with adverse effects on renal function, tissue storage, and coagulopathy. Modern, rapidly degradable HES formulations have less of an impact on the coagulation cascade, particularly the factor VIII–vWF complex (Jungheinrich et al. 2004).
4.4.1 Effect on Coagulation Factors and Thrombin Generation
Acquired von Willebrand syndrome, together with a decrease in circulating factor VIII of up to 80 %, has been reported in both healthy volunteers and patients receiving HES (Kapiotis et al. 1994; Conroy et al. 1996; Jamnicki et al. 2000; Jones et al. 2003; Jungheinrich et al. 2004; Jungheinrich and Neff 2005; Kozek-Langenecker 2005; Van der Linden and Ickx 2006). Kapiotis et al. compared the effect of 6 % HES 200/0.5 in isotonic saline with 5 % HA using healthy volunteers. The infusion of 500 ml significantly reduced levels of factor VIII:C in the HES group. However, there were no significant differences between the study groups regarding other coagulation and fibrinolytic parameters, such as activated partial thromboplastin time (aPTT), fibrinogen, thrombin–antithrombin complexes, d-dimers, t-PA, and PAI-1 (Kapiotis et al. 1994). Recent studies have suggested that rapidly degradable HES solutions have only minor effects on coagulation. Even high doses of tetrastarch (50–70 ml/kg) did not impair factor VIII activity (Kasper et al. 2003; Neff et al. 2003).
Fenger-Eriksen et al. studied cancer patients following 30 % hemodilution with HES 130/0.4. Fibrinogen, factor II, factor X, and factor XIII decreased significantly below levels typically expected from dilution. Endogenous thrombin potential, however, remained unchanged (Fenger-Eriksen et al. 2009).
4.4.2 Viscoelastic Studies
The study by Fenger-Eriksen et al. revealed that 30 % hemodilution with HES 130/0.4 had no significant effect on whole-blood clotting time or maximum velocity of clot formation, compared with baseline observations (Fenger-Eriksen et al. 2009).
However, both in vivo and in vitro studies have reported early, dose-dependent disturbances in fibrin polymerization following HES infusion (Treib et al. 1999; Kozek-Langenecker 2005). This results in a marked decrease in the velocity of clot formation, together with a decrease in the α-angle (Schramko et al. 2010; Schlimp et al. 2013).
More importantly, a pronounced and significant reduction in maximum clot firmness has been observed. In particular, fibrin-based assays, such as FIBTEM, reveal significantly diminished fibrin polymerization following dilution with HES products (Fries et al. 2002, 2005; Schramko et al. 2010; Schlimp et al. 2013). Only minor differences between HES 130/0.4 and HES 200/0.5 have been identified (Jamnicki et al. 1998; Entholzner et al. 2000; Sossdorf et al. 2009; Casutt et al. 2010).
Some studies suggest that HES solutions might also increase clot lysis (Mittermayr et al. 2008). In an in vivo study, Jamniki et al. investigated TEG findings following hemodilution with HES 200/0.5 and HES 130/0.4. Clot lysis after 60 min increased significantly after hemodilution with both fluids (Jamnicki et al. 1998).
4.4.3 Effects on Platelets
HES solutions have been shown to reduce the platelet contribution to hemostasis. Several mechanisms have been proposed, such as a decrease in expression and availability of the GP IIb/IIIa receptors (Stogermuller et al. 2000; Deusch et al. 2003; Thaler et al. 2005). Coating of platelets by HES macromolecules, nonspecific modification of cytoplasmic membrane structures, and consequent inhibition of conformational changes to GP IIb/IIIa receptors have all been discussed (Stogermuller et al. 2000). In vitro studies have identified nonspecific binding of HES molecules to platelet surfaces as a potential mechanism of HES-induced platelet inhibition (Deusch et al. 2003). In healthy volunteers, slowly degradable HES significantly prolongs PFA-100 closure times (Stogermuller et al. 2000). In addition to these direct effects, decreased vWF activity may further reduce platelet responsiveness after HES administration (Strauss et al. 2002). However, rapidly degradable HES has only minor effects on platelet function (Franz et al. 2001). Further, HES does not seem to affect intracellular signal transduction of platelets (Gamsjager et al. 2002).
One study randomly assigned 30 patients with cerebrovascular disease to receive daily infusions of up to 1.5 l of 6 % HES 200/0.62, 10 % HES 200/0.5, or 6 % HES 40/0.5. Platelet count significantly decreased in all three groups, but the largest drop was observed in the HES 200/0.62 group. The authors speculate that HES macromolecules attach to platelets and are subsequently phagocytosed (Treib et al. 1996).
4.4.4 Clinical Studies
Despite their negative side effects on laboratory and viscoelastic parameters, modern, rapidly degradable HES 130/0.4 solutions have not been shown to increase blood loss. A double-blind RCT of 42 patients with severe blunt trauma compared resuscitation using 6 % HES 130/0.4 versus NS. The HES group required significantly more blood products than patients receiving NS; however, the mean injury severity score was significantly higher in the HES group. The effect of HES on blood loss thus remains unclear (James et al. 2011). Studies in orthopedic and cardiac surgery patients show that 6 % HES 130/0.4 is associated with lower blood loss and transfusion requirements than 6 % HES 200/0.5 (Langeron et al. 2001; Niemi et al. 2005).
Studies comparing 3.5 % urea-linked gelatin and 6 % HES 130/0.4 reported no significant differences in either the number of patients receiving allogeneic blood products or the volume of RBCs, FFP, or platelets administered (Van der Linden et al. 2005; Schramko et al. 2010).
Mittermayr and coworkers investigated 61 orthopedic patients who received 6 % HES 130/0.4, 4 % gelatin, or RL solution. The RL group had the lowest number of patients receiving allogeneic blood products. The highest number of RBCs was transfused to the gelatin group. However, significant differences in baseline hemoglobin levels between the study groups made interpretation of these results difficult (Mittermayr et al. 2007).
5 Hypertonic–Hyperoncotic Solutions
In experimental and preclinical studies, rapid infusion of small amounts (4 ml/kg) of hypertonic 7.2–7.5 % saline (HS) solutions have proved to be effective in hypotensive patients, with rapid restoration of cardiovascular function and tissue blood flow (Velasco et al. 1980; Tollofsrud et al. 1998). For the restoration of intravascular volume, the infusion of 4 ml/kg 7.5 % HS was reported to be as effective as an infusion of 2–3 l of crystalloids. Improvement in cardiovascular function has been attributed to the redistribution of body water from the extravascular to the intravascular compartment by the osmotic forces of HS (~2,400 mosm/l) (Smith et al. 1985). However, the vascular volume expansion is relatively transient unless an oncotically effective colloid (e.g., dextran 70 or a starch) is combined with HS to hold the fluid in circulation. These fluids are called hypertonic–hyperoncotic solutions (HHS) (Angle et al. 1998). Numerous animal studies have revealed attenuated inflammation, enhanced organ function, and improved survival rates following hemorrhagic shock and resuscitation with HS/HHS (Smith et al. 1985; Angle et al. 1998).
A recent RCT investigated the effect of out of hospital infusions of HS, HHS, and NS in hypotensive trauma patients. This study was unable to demonstrate a clinically significant survival benefit of HS when it was infused during the early phase of hemorrhagic shock treatment. It is noteworthy that, in the subgroup of patients who received no blood transfusions in the first 24 h, infusions of HS increased mortality (Bulger et al. 2011).
Similar results have been found in patients with severe isolated brain injury. Among patients with severe traumatic brain injury, but not in hypovolemic shock, initial resuscitation with either HS or HHS provided no benefit over NS, in terms of 6-month neurological outcome (Bulger et al. 2010).
5.1 Effects on Coagulation Factors and Thrombin Generation
Several different HS preparations, from 1.6 to 29.9 %, with and without the addition of artificial colloids, have been used in experimental and clinical studies (Johnson and Criddle 2004). However, sound conclusions regarding the influence of HS/HHS are impossible due to the heterogeneity of the fluids.
Coats et al. investigated the in vitro effect of HHS (including dextran) on coagulation. A mild pro-hemostatic effect was observed up to a dilution of 11 %, but anticoagulant properties were evident above this level. At 15 % dilution, coagulation was grossly deranged (Coats and Heron 2004).
Wilder et al. reported that HS has strong anticoagulant and antiplatelet effects. Dilution with HS to a level of only 5 % caused a significant prolongation of PT. In contrast, there was no significant change in PT following 5 % dilution with hypertonic sorbitol or mixtures based on glycine (Wilder et al. 2002).
A 20 % dilution with 3 % saline resulted in a 52 % reduction of thrombin generation and 11 % reduction of fibrin formation. Increasing the dilution to 30 % decreased fibrin formation by 89 % (Brummel-Ziedins et al. 2006).
5.2 Viscoelastic Tests
In vitro, 10 % dilution of blood with HHS has been shown to cause significant impairment of fibrin polymerization (Hanke et al. 2011). Haas et al. studied the effect of HHS in vivo in an uncontrolled bleeding model in pigs. Median fibrinogen polymerization was significantly higher among animals receiving HHS, compared with those receiving 4 % gelatin or 6 % HES 130/0.4. Furthermore, median blood loss after liver incision was significantly lower in the HHS group (Haas et al. 2008a).
5.3 Effects on Platelets
In one study, platelet activation, as determined by α-granule release, decreased by 40 % following 10 % dilution of whole blood with HHS. Complete cessation of platelet activation was observed after 20 % dilution (Brummel-Ziedins et al. 2006). The authors suggested that platelets shrink and become dysfunctional, diminishing the surface required for propagation of thrombin generation (Brummel-Ziedins et al. 2006).
Wilder et al. reported that platelet function was critically impaired by HS hemodilution at a level of just 5 % (Wilder et al. 2002). Hanke et al. used multiple electrode aggregometry to study platelet function in whole blood following HS and HHS dilution in vitro. With 5 % dilution, both dilutants impaired platelet aggregation (Hanke et al. 2011).
6 Restoring Coagulation After Dilution Coagulopathy
All artificial colloids interfere with the process of fibrinogen polymerization, reducing maximum clot firmness in viscoelastic coagulation tests. To overcome this problem, fibrinogen supplementation has proved effective both in vitro and in vivo (Fries et al. 2005; Schramko et al. 2009; Grottke et al. 2010; Schlimp et al. 2013). Using a 33 % in vitro dilution model, Schlimp et al. recently showed that fibrinogen concentrate was effective in increasing clot firmness in blood samples diluted with either albumin or gelatin. In samples diluted with HES, however, the effect was poor (Schlimp et al. 2013). Fries et al. reported improved clot firmness following fibrinogen administration in a porcine model of uncontrolled bleeding (Fig. 9.3) (Fries et al. 2005). Grottke et al. replaced 80 % of blood volume with HES 130/0.4, RL, and retransfused erythrocytes. The animals randomly received 70 mg/kg fibrinogen concentrate, 200 mg/kg fibrinogen concentrate, or a placebo before blunt liver injury was induced. Total blood loss was significantly lower, and survival was significantly higher, in both fibrinogen groups as compared to controls. Microscopy revealed no thrombosis in either group (Grottke et al. 2010).
Electron microscopy scans of blood clots formed from (a) undiluted whole blood, (b) blood diluted 65 % with gelatin, and (c) blood diluted 65 % with gelatin and the addition of fibrinogen concentrate (With permission from Fries and Martini (2010))
Factor XIII supplementation, together with fibrinogen, partially restores clot formation in blood samples diluted in vitro with different colloids (Niemi et al. 2005). Again, the effect is significantly lower in the HES group compared to albumin and gelatin. The combination of factor XIII and fibrinogen effectively normalized maximum clot firmness in blood samples diluted with either albumin or gelatin. Haas and coworkers further investigated the effect of fibrinogen, factor XIII, and FFP in a 60 % hemodilution model. Fibrinogen together with factor XIII restored crystalloid-induced impairment of clot strength, while FFP only shortened coagulation times (Haas et al. 2008b).
As shown in elective surgery patients, desmopressin has the potential to reverse the colloid-induced decreases in levels of factor VIII and vWF (Conroy et al. 1996). Another potential option for such reversal is the administration of factor VIII/vWF concentrate, but there are no clinical data to support this strategy.
7 Conclusion
Crystalloids exert only minor effects on coagulation parameters, apart from the effects of hemodilution. Artificial colloids impair coagulation and platelet function to a greater extent than natural colloid albumin. Gelatin and rapidly degradable, short-acting HES seem to have similar effects on blood loss. There is no conclusive evidence that balanced solutions are superior to non-balanced fluids.
References
Alexander B, Odake K et al (1975) Coagulation, hemostasis, and plasma expanders:a quarter century enigma. Fed Proc 34(6):1429–1440
Angle N, Hoyt DB et al (1998) Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock 9(3):164–170
Batlle J, del Rio F et al (1985) Effect of dextran on factor VIII/von Willebrand factor structure and function. Thromb Haemost 54(3):697–699
Brodin B, Hesselvik F et al (1984) Decrease of plasma fibronectin concentration following infusion of a gelatin-based plasma substitute in man. Scand J Clin Lab Invest 44(6):529–533
Brummel-Ziedins K, Whelihan MF et al (2006) The resuscitative fluid you choose may potentiate bleeding. J Trauma 61(6):1350–1358
Bulger EM, May S et al (2010) Out-of-hospital hypertonic resuscitation following severe traumatic brain injury: a randomized controlled trial. JAMA 304(13):1455–1464
Bulger EM, May S et al (2011) Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg 253(3):431–441
Bunn F, Trivedi D (2012) Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev 7:CD001319
Casutt M, Kristoffy A et al (2010) Effects on coagulation of balanced (130/0.42) and non-balanced (130/0.4) hydroxyethyl starch or gelatin compared with balanced Ringer's solution: an in vitro study using two different viscoelastic coagulation tests ROTEMTM and SONOCLOTTM. Br J Anaesth 105(3):273–281
Choi PT, Yip G et al (1999) Crystalloids vs. colloids in fluid resuscitation: a systematic review. Crit Care Med 27(1):200–210
Clagett GP, Anderson FA Jr et al (1995) Prevention of venous thromboembolism. Chest 108(4 Suppl):312S–334S
Coats TJ, Heron M (2004) The effect of hypertonic saline dextran on whole blood coagulation. Resuscitation 60(1):101–104
Coats TJ, Brazil E et al (2006) The effects of commonly used resuscitation fluids on whole blood coagulation. Emerg Med J 23(7):546–549
Conroy JM, Fishman RL et al (1996) The effects of desmopressin and 6% hydroxyethyl starch on factor VIII:C. Anesth Analg 83(4):804–807
de Jonge E, Levi M (2001) Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med 29(6):1261–1267
de Jonge E, Levi M et al (1998) Impaired haemostasis by intravenous administration of a gelatin-based plasma expander in human subjects. Thromb Haemost 79(2):286–290
Denis R, Smith RW et al (1987) Relocation of nonalbumin proteins after albumin resuscitation. J Surg Res 43(5):413–419
Deusch E, Gamsjager T et al (2003) Binding of hydroxyethyl starch molecules to the platelet surface. Anesth Analg 97(3):680–683
Deusch E, Thaler U et al (2004) The effects of high molecular weight hydroxyethyl starch solutions on platelets. Anesth Analg 99(3):665–668
Dunbar NM, Chandler WL (2009) Thrombin generation in trauma patients. Transfusion 49(12):2652–2660
Egli GA, Zollinger A et al (1997) Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth 78(6):684–689
Engvall E, Ruoslahti E et al (1978) Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med 147(6):1584–1595
Entholzner EK, Mielke LL et al (2000) Coagulation effects of a recently developed hydroxyethyl starch (HES 130/0.4) compared to hydroxyethyl starches with higher molecular weight. Acta Anaesthesiol Scand 44(9):1116–1121
Eriksson M, Saldeen T (1995) Effect of dextran on plasma tissue plasminogen activator (t-PA) and plasminogen activator inhibitor-1 (PAI-1) during surgery. Acta Anaesthesiol Scand 39(2):163–166
Evans PA, Glenn JR et al (1998) Effects of gelatin-based resuscitation fluids on platelet aggregation. Br J Anaesth 81(2):198–202
Evans PA, Heptinstall S et al (2003) Prospective double-blind randomized study of the effects of four intravenous fluids on platelet function and hemostasis in elective hip surgery. J Thromb Haemost 1(10):2140–2148
Fenger-Eriksen C, Tonnesen E et al (2009) Mechanisms of hydroxyethyl starch-induced dilutional coagulopathy. J Thromb Haemost 7(7):1099–1105
Finfer S, Bellomo R et al (2004) A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med 350(22):2247–2256
Franz A, Braunlich P et al (2001) The effects of hydroxyethyl starches of varying molecular weights on platelet function. Anesth Analg 92(6):1402–1407
Fries D, Martini WZ (2010) Role of fibrinogen in trauma-induced coagulopathy. Br J Anaesth 105(2):116–121
Fries D, Innerhofer P et al (2002) The effect of the combined administration of colloids and lactated Ringer's solution on the coagulation system: an in vitro study using thrombelastograph coagulation analysis (ROTEG. Anesth Analg 94(5):1280–1287
Fries D, Krismer A et al (2005) Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. Br J Anaesth 95(2):172–177
Gamsjager T, Gustorff B et al (2002) The effects of hydroxyethyl starches on intracellular calcium in platelets. Anesth Analg 95(4):866–869
Grottke O, Braunschweig T et al (2010) Effects of different fibrinogen concentrations on blood loss and coagulation parameters in a pig model of coagulopathy with blunt liver injury. Crit Care 14(2):R62
Guidet B, Soni N et al (2010) A balanced view of balanced solutions. Crit Care 14(5):325
Haas T, Fries D et al (2008a) Less impairment of hemostasis and reduced blood loss in pigs after resuscitation from hemorrhagic shock using the small-volume concept with hypertonic saline/hydroxyethyl starch as compared to administration of 4% gelatin or 6% hydroxyethyl starch solution. Anesth Analg 106(4):1078–1086
Haas T, Fries D et al (2008b) The in vitro effects of fibrinogen concentrate, factor XIII and fresh frozen plasma on impaired clot formation after 60% dilution. Anesth Analg 106(5):1360–1365
Hanke AA, Maschler S et al (2011) In vitro impairment of whole blood coagulation and platelet function by hypertonic saline hydroxyethyl starch. Scand J Trauma Resusc Emerg Med 19:12
Hauser CJ, Shoemaker WC et al (1980) Oxygen transport responses to colloids and crystalloids in critically ill surgical patients. Surg Gynecol Obstet 150(6):811–816
Innerhofer P, Fries D et al (2002) In vivo effect of haemodilution with saline on coagulation. Br J Anaesth 89(6):934
Jacob M, Chappell D et al (2012) The intravascular volume effect of Ringer's lactate is below 20%: a prospective study in humans. Crit Care 16(3):R86
James MF, Michell WL et al (2011) Resuscitation with hydroxyethyl starch improves renal function and lactate clearance in penetrating trauma in a randomized controlled study: the FIRST trial (Fluids in Resuscitation of Severe Trauma). Br J Anaesth 107(5):693–702
Jamnicki M, Zollinger A et al (1998) Compromised blood coagulation: an in vitro comparison of hydroxyethyl starch 130/0.4 and hydroxyethyl starch 200/0.5 using thrombelastography. Anesth Analg 87(5):989–993
Jamnicki M, Bombeli T et al (2000) Low- and medium-molecular-weight hydroxyethyl starches: comparison of their effect on blood coagulation. Anesthesiology 93(5):1231–1237
Jesty J (1986) The kinetics of inhibition of alpha-thrombin in human plasma. J Biol Chem 261(22):10313–10318
Jin SL, Yu BW (2010) Effects of acute hypervolemic fluid infusion of hydroxyethyl starch and gelatin on hemostasis and possible mechanisms. Clin Appl Thromb Hemost 16(1):91–98
Johnson AL, Criddle LM (2004) Pass the salt: indications for and implications of using hypertonic saline. Crit Care Nurse 24(5):36–38
Johnson SD, Lucas CE et al (1979) Altered coagulation after albumin supplements for treatment of oligemic shock. Arch Surg 114(4):379–383
Jones SB, Whitten CW et al (2003) The influence of crystalloid and colloid replacement solutions in acute normovolemic hemodilution: a preliminary survey of hemostatic markers. Anesth Analg 96(2):363–368
Jungheinrich C, Neff TA (2005) Pharmacokinetics of hydroxyethyl starch. Clin Pharmacokinet 44(7):681–699
Jungheinrich C, Sauermann W et al (2004) Volume efficacy and reduced influence on measures of coagulation using hydroxyethyl starch 130/0.4 (6%) with an optimised in vivo molecular weight in orthopaedic surgery : a randomised, double-blind study. Drugs R D 5(1):1–9
Kapiotis S, Quehenberger P et al (1994) Effect of hydroxyethyl starch on the activity of blood coagulation and fibrinolysis in healthy volunteers: comparison with albumin. Crit Care Med 22(4):606–612
Karoutsos S, Nathan N et al (1999) Thrombelastogram reveals hypercoagulability after administration of gelatin solution. Br J Anaesth 82(2):175–177
Kasper SM, Meinert P et al (2003) Large-dose hydroxyethyl starch 130/0.4 does not increase blood loss and transfusion requirements in coronary artery bypass surgery compared with hydroxyethyl starch 200/0.5 at recommended doses. Anesthesiology 99(1):42–47
Kermode JC, Zheng Q et al (1999) Marked temperature dependence of the platelet calcium signal induced by human von Willebrand factor. Blood 94(1):199–207
Kheirabadi BS, Crissey JM et al (2008) Effects of synthetic versus natural colloid resuscitation on inducing dilutional coagulopathy and increasing hemorrhage in rabbits. J Trauma 64(5):1218–1228
Kozek-Langenecker SA (2005) Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology 103(3):654–660
Kozek-Langenecker SA (2009) Influence of fluid therapy on the haemostatic system of intensive care patients. Best Pract Res Clin Anaesthesiol 23(2):225–236
Kulla M, Lampl L et al (2008) Hydroxyethyl starch 6% 130/0.42 in acetate-buffered Ringer's solution as a part of a balanced-volume resuscitation in abdominal surgery. Anästhesiol Intensivmed 1:7–18
Langeron O, Doelberg M et al (2001) Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg 92(4):855–862
Laxenaire MC, Mertes PM (2001) Anaphylaxis during anaesthesia. Results of a two-year survey in France. Br J Anaesth 87(4):549–558
Lucas CE, Ledgerwood AM et al (1982) Altered coagulation protein content after albumin resuscitation. Ann Surg 196(2):198–202
Mardel SN, Saunders FM et al (1998) Reduced quality of clot formation with gelatin-based plasma substitutes. Br J Anaesth 80(2):204–207
McIlroy DR, Kharasch ED (2003) Acute intravascular volume expansion with rapidly administered crystalloid or colloid in the setting of moderate hypovolemia. Anesth Analg 96(6):1572–1577
Meng ZH, Wolberg AS et al (2003) The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J Trauma 55(5):886–891
Mittermayr M, Streif W et al (2007) Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg 105(4):905–917
Mittermayr M, Streif W et al (2008) Effects of colloid and crystalloid solutions on endogenous activation of fibrinolysis and resistance of polymerized fibrin to recombinant tissue plasminogen activator added ex vivo. Br J Anaesth 100(3):307–314
Mortier E, Ongenae M et al (1997) In vitro evaluation of the effect of profound haemodilution with hydroxyethyl starch 6%, modified fluid gelatin 4% and dextran 40 10% on coagulation profile measured by thromboelastography. Anaesthesia 52(11):1061–1064
Myburgh J, Cooper DJ et al (2007) Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med 357(9):874–884
Neff TA, Doelberg M et al (2003) Repetitive large-dose infusion of the novel hydroxyethyl starch 130/0.4 in patients with severe head injury. Anesth Analg 96(5):1453–1459
Ng KF, Lam CC et al (2002) In vivo effect of haemodilution with saline on coagulation: a randomized controlled trial. Br J Anaesth 88(4):475–480
Niemi TT, Silvanto M et al (2005) Albumin induced hypercoagulability does not reduce blood loss in patients undergoing total hip arthroplasty. Scand J Surg 94(3):227–232
Niemi TT, Suojaranta-Ylinen RT et al (2006) Gelatin and hydroxyethyl starch, but not albumin, impair hemostasis after cardiac surgery. Anesth Analg 102(4):998–1006
Perel P, Roberts I (2012) Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 6:CD000567
Perner A, Haase N et al (2012) Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 367(2):124–134
Petroianu GA, Liu J et al (2000) The effect of In vitro hemodilution with gelatin, dextran, hydroxyethyl starch, or Ringer's solution on Thrombelastograph. Anesth Analg 90(4):795–800
Rehm M, Finsterer U (2003) Treating intraoperative hyperchloremic acidosis with sodium bicarbonate or tris-hydroxymethyl aminomethane: a randomized prospective study. Anesth Analg 96(4):1201–1208, table of contents
Roberts I, Blackhall K et al (2011) Human albumin solution for resuscitation and volume expansion in critically ill patients. Cochrane Database Syst Rev (11):CD001208
Ruttmann TG (2002) Haemodilution enhances coagulation. Br J Anaesth 88(4):470–472
Ruttmann TG, James MF et al (1996) Haemodilution induces a hypercoagulable state. Br J Anaesth 76(3):412–414
Ruttmann TG, Jamest MF et al (2001) Haemodilution-induced enhancement of coagulation is attenuated in vitro by restoring antithrombin III to pre-dilution concentrations. Anaesth Intensive Care 29(5):489–493
Ruttmann TG, Lemmens HJ et al (2006) The haemodilution enhanced onset of coagulation as measured by the thrombelastogram is transient. Eur J Anaesthesiol 23(7):574–579
Scatchard G, Batchelder AC et al (1944) Chemical, Clinical, and Immunological Studies on the Products of Human Plasma Fractionation. Vi. The Osmotic Pressure of Plasma and of Serum Albumin. J Clin Invest 23(4):458–464
Schaden E, Wetzel L et al (2012) Effect of the carrier solution for hydroxyethyl starch on platelet aggregation and clot formation. Br J Anaesth 109(4):572–577
Schierhout G, Roberts I (1998) Fluid resuscitation with colloid or crystalloid solutions in critically ill patients: a systematic review of randomised trials. BMJ 316(7136):961–964
Schlimp CJ, Cadmoro J et al (2013) The effect of fibrinogen concentrate and factor XIII on thromboelastometry in 33 % diluted blood with albumin, gelatin, hydroxyethyl starch or saline in vitro. Blood Transfus 11(4):510–517
Schramko AA, Kuitunen AH et al (2009) Role of fibrinogen-, factor VIII- and XIII-mediated clot propagation in gelatin haemodilution. Acta Anaesthesiol Scand 53(6):731–735
Schramko A, Suojaranta-Ylinen R et al (2010) Hydroxyethylstarch and gelatin solutions impair blood coagulation after cardiac surgery: a prospective randomized trial. Br J Anaesth 104(6):691–697
Smith GJ, Kramer GC et al (1985) A comparison of several hypertonic solutions for resuscitation of bled sheep. J Surg Res 39(6):517–528
Sorensen B, Fries D (2012) Emerging treatment strategies for trauma-induced coagulopathy. Br J Surg 99(Suppl 1):40–50
Sossdorf M, Marx S et al (2009) HES 130/0.4 impairs haemostasis and stimulates pro-inflammatory blood platelet function. Crit Care 13(6):R208
Stogermuller B, Stark J et al (2000) The effect of hydroxyethyl starch 200 kD on platelet function. Anesth Analg 91(4):823–827
Strauss RG, Pennell BJ et al (2002) A randomized, blinded trial comparing the hemostatic effects of pentastarch versus hetastarch. Transfusion 42(1):27–36
Tabuchi N, de Haan J et al (1995) Gelatin use impairs platelet adhesion during cardiac surgery. Thromb Haemost 74(6):1447–1451
Thaler U, Deusch E et al (2005) In vitro effects of gelatin solutions on platelet function: a comparison with hydroxyethyl starch solutions. Anaesthesia 60(6):554–559
Tollofsrud S, Tonnessen T et al (1998) Hypertonic saline and dextran in normovolaemic and hypovolaemic healthy volunteers increases interstitial and intravascular fluid volumes. Acta Anaesthesiol Scand 42(2):145–153
Treib J, Haass A et al (1996) Influence of low and medium molecular weight hydroxyethyl starch on platelets during a long-term hemodilution in patients with cerebrovascular diseases. Arzneimittelforschung 46(11):1064–1066
Treib J, Baron JF et al (1999) An international view of hydroxyethyl starches. Intensive Care Med 25(3):258–268
Van der Linden P, Ickx BE (2006) The effects of colloid solutions on hemostasis. Can J Anaesth 53(6 Suppl):S30–S39
Van der Linden P, Schmartz D (1992) Pharmacology of gelatins. In: Plasma volume expansion. J. F. Baron/Arenette, Paris, pp 67–74
Van der Linden PJ, De Hert SG et al (2005) Hydroxyethyl starch 130/0.4 versus modified fluid gelatin for volume expansion in cardiac surgery patients: the effects on perioperative bleeding and transfusion needs. Anesth Analg 101(3):629–634
Velasco IT, Pontieri V et al (1980) Hyperosmotic NaCl and severe hemorrhagic shock. Am J Physiol 239(5):H664–H673
Waters JH, Gottlieb A et al (2001) Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg 93(4):817–822
Westphal M, James MF et al (2009) Hydroxyethyl starches: different products–different effects. Anesthesiology 111(1):187–202
Wilder DM, Reid TJ et al (2002) Hypertonic resuscitation and blood coagulation: in vitro comparison of several hypertonic solutions for their action on platelets and plasma coagulation. Thromb Res 107(5):255–261
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Schöchl, H., Schlimp, C., Voelckel, W. (2015). Intravenous Fluids and Coagulation. In: Marcucci, C., Schoettker, P. (eds) Perioperative Hemostasis. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-55004-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-55004-1_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-55003-4
Online ISBN: 978-3-642-55004-1
eBook Packages: MedicineMedicine (R0)