Abstract
Honeybee nests result from interactions among numerous bees performing different comb-building operations ranging from construction of new cells, shaving and thickening edges of cells, capping brood, and capping removals. The single major construction is comb-building. At the onset of comb-building, nascent cells are circular but soon after acquire a more crystalline structure; regular hexagons appear that are products of the physical properties of wax, equal pressure from adjacent cells, and the flow of the visco-elastic wax. The structure and formation of cells result from wax being a thermoplastic material while, the hexagonal structure is the result of the wax reaching a liquid equilibrium, changing from a crystalline state to an amorphous state at nest temperatures. The building ‘instincts’ of bees are labile and are supported by several possible subroutines in their total building programme; but the rather wide tolerances seen among cells show that bees cannot make precise measurements. In commercial beeswax foundation, both the cell base and the hexagonal rims of the cells have a pronounced taper to them. However, the natural outermost limits of cell patterns, and not the cell base, determine what pattern bees follow in cell construction. The antennae may play a role in maintaining tolerances on cell thickness because milling of the cell wall is controlled by individual workers at single sites, and antennectomy significantly increases wall thickness. The shape of the honeybee cell does not have its celebrated regularity; its economy is a teleological myth. The entire history of the honeybee cell in natural history, geometry and philosophy is the story of centuries-old misconceptions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
12.1 Introduction
Honeybee nests are the result of the interactions and interplay among numerous individuals performing various building and construction operations. The behavioural plasticity among individuals, paired with the various aspects of the construction of combs, makes for a fascinating and complex field of study. The end product, the comb, is used for storing resources, rearing the next generation of brood, and chemical and physical communication, which ensures the social cohesion of the honeybee colony.
12.2 Manipulation of Wax Scales
The first record on the handling of wax scales after they have been secreted is that of the famous 17th century work, The feminine monarchie, by Butler (1609) who wrote: “You may behold them (bees) working on the edges of their combs, and having blown their liquid and soft wax out of their mouths fasten and fashion it with their fanges and forefeet”. In the 19th century different findings and observations to Butler’s interpretations were proposed, particularly by Huber (1814), who noted that a worker removes a scale from the wax mirror with a hindleg and transfers it with a foreleg to its mouth. The scale is then thoroughly chewed and a frothy liquid added. He then mistakenly attributed the means for scale removal from the abdomen by the pollen press (the ‘wax-pliers’ of the old literature). Dönhoff (1854) disputed this, laid a counter claim for the basitarsal setae of the planta, but otherwise confirmed Huber’s basic observations.
It is extremely difficult to decide how to apportion credit when it concerns fine details, even when careful comparisons are made of the different authors’ works. While Huber (1814), admittedly, wrote the first extensive description of how bees handle wax scales, the most comprehensive account is by Casteel (1912), who meticulously followed the movements of bees in an observation hive with a binocular microscope. To briefly summarize, Casteel reported that wax scales are usually removed from the wax mirrors, passing them under the abdomen to the forelegs and finally to the mandibles, where they are chewed and then added to the comb. Lineburg (1924) extended Huber’s observations on the mandibulation of wax, and confirmed the detailed observations of Casteel (1912). Rösch (1927) and Gwin (1936) confirmed both the origin and the mandibulation of wax scales.
12.3 Comb Operations
Honeybee nests are the result of numerous kinds of building operations performed by many individual bees; they range from the construction of new comb (Fig. 12.1), to cell-shaving and cell edge-thickening, capping brood and the removal of cappings when adults eclose (Fig. 12.2).
Meyer (1952) and Meyer and Ulrich (1952) published concise accounts of comb-building for which all constructions in the nest were divided into either major or minor building operations. There is one major construction, the overall building of combs which is the foundation of a honeybee colony (Fig. 12.1), enabling them to communicate, store food and raise brood. There are several minor operations which tend to differ depending on whether they occur in areas of the brood comb or honeycomb (Figs. 12.2 and 12.3). Once the major task of constructing brood combs is completed, the cyclical rearing of multiple generations of brood occurs. Part of the cycle is when the larval cells are capped on the threshold of metamorphosis; the wax used for sealing these cells is generally recycled old wax, and not newly secreted wax. By using dyed waxes, Meyer and Ulrich (1952) reported that more than 60 % of the wax used to cap brood cells was salvaged from previously used brood cell cappings following the emergence of young adult bees. These minor constructions, based on recycling cappings wax (Lineburg 1923a, b), are generally performed by young bees, 3–9 days old, most of whom have not yet reached the peak of wax secretion (Rösch 1927). These nurse bees both feed the larvae and cap them in due course. Workers are able to identify the age of the larvae mainly based on pheromones emitted by the brood (cf. Chap. 5).
Capping behaviour of an individual A. mellifera worker over the course of 1 h (Smith 1959)
After the adult bees emerge, workers smooth and shave the walls of the empty cells and collect the remains of the cappings. By removing the wax cappings, they also removed part of the silken cocoons which are attached to the inside of the cappings. This material is either used as cappings for larvae in close proximity, which are ready to pupate, or gets attached to the rims of nearby cells; however, the addition of this material results in the thickening of the edges of cells, which then has to be thinned by workers (Fig. 12.2).
The stored material on the rim can either be used to seal the cell if necessary, or may serve simply as a depot for the storage of capping material. Smith (1959) observed the capping activity of a single worker for an hour, and his results (Fig. 12.3) confirm that capping can be done either by a single or several bees (Meyer and Ulrich 1952).
The operation of capping is not restricted to brood, but is also performed on the honeycombs. It seems intuitively obvious that different stimuli trigger the bees to do these jobs in these two different areas. However, since the behavioural patterns involved in capping or uncapping cells are effectively the same as illustrated in Fig. 12.2, one might wonder if the stimuli are similar. An appreciation of the order of magnitude of the so-called minor operations can be gained from Lineburg’s (1923a) study on the turnover of cappings wax. Let’s assume that, in a colony in a standard Langstroth hive, half the cells are filled with brood at any given time. That would translate into workers having to cover an area with wax of about 800 cm2 (equal to the area of \( 1{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 3}}\right.\kern-0pt} \!\lower0.7ex\hbox{$3$}} \) of an an A4 sheet of paper) with wax on a 3-weekly basis, and they, or the newly emerged workers would have to remove the same amount at the end of a brood cycle. Eight-hundred cm2 converts to more than 10 g of wax, based on cappings of A. m. scutellata, or the production of nearly 1500 21-day old workers (Hepburn et al. 1984), which is built and removed every 3 weeks.
The huge turnover and shifting rate of wax is also evident given the fact that brood cell cappings in nests containing old and darkened combs are nearly as dark as the combs themselves. In the white combs of newly established swarms, the cappings are only slightly discoloured (Lineburg 1923a). The shifting of wax takes place within hours as was demonstrated by Darchen (1980) who showed that, by placing various radioactive labels within a colony and measuring their dispersal over 24 h the radioactivity spread to all the combs, although its intensity declined with increasing distance from the point of application of the tracer. Unfortunately, the passive transmission of label on the bodies of workers vis-à-vis real pieces of wax being moved could not be established.
12.4 Inception of the Nest
The processes that occur after a swarm settles at a new nest site are probably similar for cavity- and open-nesting Apis species when it comes to the inception of the comb or nest. One of the problems of studying comb-building has always been that of trying to see through a cluster of bees, often as much as 10 cm thick. Another problem with cavity-nesting species, like A. cerana or A. koschevnikovi, is that nests in cavities are extremely difficult to observe. One can address the problem in two ways; either let the bees construct a bit of comb and remove it for recording, which is easier in open-nesting bees, like Apis florea; or force the bees to construct in a way that exposes their progress. Huber (1814) constructed an experimental design that forced the bees to build from the bottom upwards on a lath, a rare but natural form of building behaviour (Bone 1952).
When following the first approach, similar building activities were observed in Asian honeybees. Both dwarf honeybees, A. florea and A. andreniformis, usually have a single, exposed comb, typically situated on a single branch of a bush or tree, in a shady location (cf. Chap. 2). The structure of the comb has been described in some considerable detail (Akratanakul 1977; Mossadegh 1990; Phiancharoen et al. 2011). However, all of the published reports on comb structure in the dwarf honeybees were made on mature nests collected in nature or purchased at markets. In all the interpretations of dwarf bee comb structure, it is implicit that the comb is built top-down, continuously, in the vertical plane, a point not established by observations. Moreover building, using hexagonal cells, poses serious geometrical problems because it is not possible to encircle a regular cylinder, like a twig, with hexagons. Close examination of such combs revealed a combination of various other polygons (Phiancharoen et al. 2011), so that real solutions to the problem of comb geometry are yet to be determined. The actual inception of comb construction from scratch, and its subsequent development in real time, have not previously been reported and are described here for the first time.
In recently settled A. florea colonies comb-building probably commences almost immediately after landing on the twig and settling because a small row of about seven nascent cells were subtending the twig at 2 h (Fig. 12.4a). After 6 h the addition of a second lower row of cells appeared (Fig. 12.4b). It is evident that these nascent cells are not polygonal but virtually circular. After 9 h (Fig. 12.4c) there are four rows of nascent cells. The row at the base of the twig consists of truncated hexagons and 2 rows of crude hexagons. The third row is exactly the same as in the first row, circular burrows in the wax. Twelve hours (Fig. 12.4d) after settling the comb acquires a more crystalline structure as regular hexagons begin to form, which is most likely as a result of the wax flowing into shape (Pirk et al. 2004; Karihaloo et al. 2013), with equal pressure from adjacent cells to shape the forming hexagon in the centre of the developing comb (Bergman and Ishay 2007). As a rule, the first row of cells appears anomalously different from the hexagons of comb cells with which we are familiar. It generally consists of regular pentagons and circles; the site of support forms one side from which two vertical walls are suspended and then two oblique ones. Ordinarily, the growth of the comb then progresses at a faster downward than lateral rate, so the combs tend to be ellipsoid in the early stages of construction (Figs. 12.4, 12.5 and 12.6).
Three samples of freshly constructed A. mellifera cells. The newest cell on the left (round) to the oldest one on the right (hexagonal) (Pirk et al. 2004)
Construction of an A. florea comb over 121 days. For details, see text. (Duangphakdee et al. 2013)
Huber’s observations have been confirmed many times (Darwin 1856; Hubbe 1957; Lau 1959; Ulrich 1964; Darchen 1991). Figure 12.6 shows the development of an A. florea vertical, single comb nest over seventeen weeks once the swarm settled. By day 4 (b) the nest had already been partitioned into an area for honey (crown or top of comb), an underlying pollen layer, below which both capped and uncapped larval cells occurred. This basic pattern remained until the mature colony swarms some 4 months later. The sequence of photographs show: (a) on day 2, the darker wax honey crown was being developed above the brood area which contained eggs and larvae in a concentric pattern; (b) by day 4, a few brood cells had been capped with more eggs and larvae below, maintaining the concentric pattern; (c) on day 6, cell capping continued as did the expansion of the uncapped brood area; (d) by day 8, the concentric rings of capped and uncapped brood increased, workers were storing nectar in the crown; (e) on day 16, the first patch of brood emerged as adults and there was further extensive capping of brood cells (note that brood does not extend to the periphery of the comb); (f) on day 23, the empty cells of (e) contained capped brood from which the second generation of adults emerged, the cells in the surrounding area contained newly laid eggs, while the outer ring contained capped brood; (g–k) occurred sequentially between days 30 to 93, and the staggered distribution of concentric brood of various ages and generations are visible in each photograph, while drone cells were finally constructed by day 93; i) appearance of drone cells; (l) by day 100, drones emerged from their cells at the bottom of the comb; (m) on day 107, drones left the nest; (n) by day 114, there were no new eggs, no uncapped brood and only very few capped cells; (o) on day 121, the colony absconded (Duangphakdee et al. 2013).
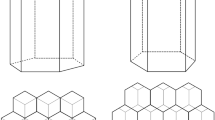
The method of attachment of cells and the subsequent extension of the comb have been analysed in some detail for around 200 naturally drawn A. mellifera combs (Hubbe 1957); nevertheless the problems also persist in open-nesting species. Hubbe (1957) found that the irregular nature of cells may well extend from the first row downwards, for another five or six or sometimes more rows. Similarly, the bees may begin their work on a horizontal plane where even greater irregularity will be encountered. Eventually some regularity, or at least patches thereof, can be found in feral nests. The irregularities not only result from attaching comb to an irregular substrate, but also by including drone and worker cells and different orientations (Fig. 12.7).
Natural patterns of cells constructed by A. mellifera. The vertical and horizontal patterns dominate combs built without foundation, and occur with similar frequency (Thompson 1930)
Thompson (1930) analysed the orientation of cells in 267 pieces of comb with the following results: 123 combs contained cells in the horizontal mode, the cells were vertical in 131 others, one comb contained both, and 13 contained only oblique or tilted combs (Fig. 12.7). The problem is exacerbated by the fact that combs also contain drone cells which are larger hexagons than worker cells and therefore the transition of workers cells to drone cells has to be architecturally solved. This, in fact, can only be achieved with the addition of various non-hexagonal polygons (usually pentagons and heptagons—Fig. 12.8).
Not only does the type of the cell play a role in pattern formation but also in the queen-status; cells constructed by queenless bees are less regular than those constructed by queenright bees (Taber and Owens 1970). The different types of cells and variations in comb-building behaviour are a precursor for the introduction of dislocations in the geometry of combs. Alternatively, instead of accepting the dislocations, the following was noted by Darwin (1859): “it was really curious to note in cases of difficulty, as when two pieces of comb met at an angle how often the bees would entirely pull down and rebuild in different ways the same cell, sometimes returning to a shape which they had at first rejected.” Therefore, when looking at the final comb many irregular cells (sometimes called ‘transition cells’—Dadant 1946) may still be found, others are retouched and hidden from view in the final product which we see (Darchen 1954; Hepburn and Whiffler 1991).
The interpretation of the geometry of combs has quite a respectable history that dates from the 4th century BP with the writings of the Alexandrian, Pappus. He held that bees had a certain geometrical forethought by which the most economical container to be made of wax was, in fact, the hexagonal configuration. Mathematical arguments about the comb cell were later advanced by that giant of 17th century science, Kepler, and were also debated in the 18th century at the Royal Society of London by such notables as Maclaurin and Lhuiller, and in Paris by Maraldi, Koenig, de Reaumur and Buffon. These mathematical discourses have been summarised by several authors over the years (Vogt 1911; Armbruster 1920; Thompson 1942; Meretz 1963).
Karl von Frisch (1974) emphasized the amazing level of precision in comb-building and that man could not undertake work of this nature without the use of specialised tools. Unveiling the underlying mechanism(s) of how bees are able to construct and measure with such apparent precision took place for centuries. However, Pirk et al. (2004) argued on theoretical grounds and provided hard experimental evidence that the structure of honeybee combs results from wax being a thermoplastic material, and the hexagonal structure is as a result of the wax reaching a liquid equilibrium. Tautz (2008) provided support for the liquid equilibrium hypothesis based on the physical properties of beeswax. From a physical viewpoint beeswax is not a solid, but a fluid that changes from a crystalline state to an amorphous state at temperatures of 25–40 °C. Pirk et al. (2004) hypothesised that the round wax cells might naturally form hexagonal shapes due to the mechanical tension between adjacent cylindrical cells in the amorphous state, as subsequently confirmed by Karihaloo et al. (2013).
Honeybees form cells with their mandibles while palpating the comb surface constantly with their antennae. The mandibles are used with a left/right movement of the head or in a repeated movement of the head upwards into the neck, thereby sliding across the cell walls. All these observations have been previously described (Lau 1959; Martin and Lindauer 1966). Besides these points, Karihaloo et al. (2013) developed two scenarios of how comb patterns emerge, both of which support the idea that “the regular pattern of rounded hexagons is a result of the progressive fusion of the circular walls induced by the flow of the visco-elastic molten wax…” (Fig. 12.9). Both models partially or fully support the idea of a liquid equilibrium (Pirk et al. 2004) process being involved in the production of hexagonal cells (Fig. 12.9). Furthermore, hexagonal cells can also form if equal pressure is applied to the sides of a group of cells, the central cell then becomes hexagonal (Bergman and Ishay 2007). A similar phenomenon can be observed in basalt rocks which form the Giant’s Causeway located in County Antrim on the northeast coast of Northern Ireland (Thompson 1942).
The proposed mechanism for the transformation of a round cell to a hexagon in A. m. ligustica combs (Karihaloo et al. 2013)
When all is said and done, we concur with Vogt (1911), who performed the most exhaustive analyses of comb cells; that the shapes of worker and drone cells are, more or less, regular, hexagonal prisms. Each is closed by three quadrangular rhombs, the obtuse angles of which form a truncated pyramid which is the usual floor of a naturally built cell. As Vogt put it: “When judgement had to be passed on the way bees build, the metaphysical idea of perfection confused the issue for the great man (Darwin), impartial observer as he was, just as it had done for the eighteenth century teleologists. The shape of the bee cell does not have its celebrated regularity; its economy is a teleological myth. The entire history of the bee cell in natural history in geometry and philosophy is the story of a 200-year old mistake!”
Put another way, Vogt’s pronouncement, coupled with the results of Darwin’s second comb experiment and the measurements of Hubbe (1957), all point to the concept of behavioural plasticity. Not only is the instinct of the bee labile, but it evokes the notion of several possible subroutines in the total building programme of bees, to use an apt analogy from Gould and Gould (1983). The experimentalist may wish to view things slightly differently. It could well be that the rather wide tolerances one observes in their constructions simply show that bees cannot make very precise measurements; they are victims of the limitations inherent in their own neurophysiology. The latter interpretation certainly sides with Vogt (1911), but rather than reach a conclusion, it is more interesting to consider what bees have done under various experimental circumstances.
12.5 Recognition of Cell Patterns
If one considers the cells of freely-built combs, it is apparent that they are not really as uniform as they tend to appear at first sight. Indeed, in one study of such combs, no two cells were found to be identical (Darchen 1956). Nonetheless, the size of worker cells fall within fairly narrow limits, with the average dimensions varying in different species and races (Vogt 1911; Alber 1953; Taber and Owens 1970; Hepburn 1983; Phiancharoen et al. 2011). Most of the cells that exceed two standard deviations of the mean actually occur in the basal few rows of cells from where the nest began.
Returning to Thompson’s (1930) studies of the orientation of cells in three modes, a number of interesting questions emerge. For example, is there a genetic component to these different patterns? Do worker bees learn a cell type from whence they have come? Do workers learn, in the absence of cues, to work a particular pattern from those bees that come to a site with past experience? Oelsen and Rademacher (1979) considered these questions in an experimental way. In addition to vertical, horizontal, tilted (or oblique) cells, they also reported a rare form of a rosette pattern. All four of these kinds of cell patterns are shown in Fig. 12.7. On the assumption that newly emerged bees, deprived of their combs as reference cues, will demonstrate innate as opposed to learned behaviour, Oelsen and Rademacher (1979) reared bees in combs having vertical, horizontal or rosette cell patterns.
As the bees emerged they were collected and placed in a modified hive with space for the construction of three combs, to form small colonies of about 1000 bees. Each colony was given unembossed, pattern-free sheets of wax as sites to stimulate comb-building. The authors found that those bees bred from vertical cells constructed combs containing a mixture of vertical and oblique cells; bees reared from horizontal cells constructed a mixture of regular, vertical, oblique and horizontal cells; bees from rosette cells built a mixture containing all four patterns (Fig. 12.7).
To test whether bees learn to follow a particular pattern and thereby become behaviourally entrained to follow it, Oelsen and Rademacher (1979) caught newly emerged bees and again constituted them in small colonies, and allowed them to work in the absence of any bees that might have had prior experience in building a particular cell type. Each colony was provided with three forms of wax. In one case, a colony was given a frame with a full sheet of beeswax foundation embossed with the rosette pattern, one frame with a small strip of the same wax, and the other frame with a sheet of unembossed, pattern-free wax. Other colonies received the same permutations based on the vertical cell pattern. On recovery of the combs, the authors found that in each case the bees built true to the form of pattern with which they were supplied; however, when they worked the unembossed wax into combs, they constructed a mixture of vertical and horizontal cells.
Oelsen and Rademacher (1979) then asked whether the cell pattern in which a bee is reared affects her subsequent proclivities as to cell orientation in a comb-building situation. To test this, they formed four colonies of bees reared in the vertical mode, and four other colonies reared from the rosette pattern. The colonies were assigned wax sheets as follows: of the ‘vertical’ bees, one colony was given the vertical pattern, two were given the rosette, and one was given unembossed wax. The ‘rosette’ colonies of bees were allocated wax sheets in the same way. Each of the colonies that subsequently constructed combs did so according to the pattern given, regardless of the type from which they were reared. It so happened that none of the unembossed sheets were worked during the experiment. In an addendum to this experiment, some colonies of bees reared in vertical cells were given only small strips of the rosette pattern, and these bees built horizontal and oblique cells as well as a patch of the rosette type.
12.6 Assessment of Cell Size
Following Gontarski’s (1935) experiments on how bees reacted to artificially enlarged cells, Hepburn (1983) analysed the tolerances in cell construction and the thresholds of acceptability for different cell sizes. He supplied colonies of the African honeybee, A. m. scutellata, with sheets of beeswax foundation manufactured in the laboratory of 170, 220, 275, 336, 390, 441, 462 and 522 cells/dm2. In addition, commercially manufactured foundation of 476, 493 and 1022 cells/dm2 were used. The foundation fashioned in the laboratory consisted of perfect hexagons with equilinear line segments, but the bottom of the cells were flat. In commercial foundation, the hexagons are very seldom equilinear and the cell bases consist of three rhombs. Six of the queenright A. m. scutellata colonies were tested on each of the different foundations. Each cell-type was tested using full foundation sheets given to the colonies nine times on a random basis. The resulting constructions (Figs. 12.10, 12.11 and 12.12) were divided into five different groups of building solutions.
170 cells/dm2. The bees characteristically began building from the bottom of the frame upwards—the reverse of normal building. There was considerable dry-working of the foundation wax, but the bees did not destroy the hexagonal patterns as they so often do when reworking worker foundation into drone comb. The bees generally worked within the constraints of these large hexagons by filling them with rosettes of irregular 5- to 7-sided polygons (Fig. 12.10a). Building commenced on a cell base by drawing a line of wax from the centre point of one wall line segment to just short of the cell centre. The construction of this initial small cell determined the size of the adjacent cells within the large hexagon, and so on.
Construction of cells by A. m. scutellata (formerly A. m. adansonii) on different sizes of beeswax foundation. a Is the result of 170 cells/dm2; b 220 cells/dm2; c 275, 336, 390 cells/dm2; and d diagrammatic projection of a comb shows the origin of false cells (Hepburn 1983)
220 cells/dm2. Although the cell bases in this foundation were smaller than those of 170 cells/dm2 foundation, the bees were unable to provide a symmetrical solution either within, or above, the constraints of the given hexagon. Figure 12.10b shows that the original pattern had been almost totally destroyed, and the comb built on this now disrupted foundation consisted of only highly irregular cells.
275, 336 and 390 cells/dm2. Here the construction solutions were uniformly the same. Each of the six corners forming the angles of the hexagons was used as the starting point for the construction of a new cell. As building progressed, a new, regular hexagonal pattern formed that was superimposed on, hence elevated above, the base pattern (Fig. 12.10c). In the finished comb all cells appear regular; however, there was a void in the centre, effectively a ‘false’ cell. The development of this false cell is shown in Fig. 12.10d from which it becomes apparent why the false cell cannot be easily detected in the finished comb.
The measurements of these new cells superimposed on the foundation cells are of considerable interest. Considering mid-wall to mid-wall diameters, it was found that these cells decreased in size in fixed proportion to the rate at which the foundation cell size decreased (Fig. 12.10). A. m. scutellata naturally drawn worker comb cells range in size from 4.37 to 5.39 mm, while those drawn on 275, 336 and 390 cells/dm2 foundation were 5.15, 4.80 and 4.34 mm respectively. This last dimension, 4.34 mm, is within 0.5 % of that occurring in the normal range of worker cells. This result implies that recognition and acceptance of a grossly large hexagon depends on the ability of the bees to superimpose a pattern of the cells which is within their natural working tolerances (Figs. 12.11 and 12.12).
The cell wall thickness of A. m. scutellata cells built naturally or on foundation. 461a and 461b differ in their basal diameters, but the diameter was kept constant (cf. Sect. 12.7, Cell Base)
441, 461 and 476 cells/dm2. This series included cells which constituted the upper limits on cell size construction, on which one particular colony would work, but the other colonies would not. Only a single colony drew comb on foundation with 441 cells/dm2; the other test colonies invariably began tapering the forming cells, and would then gap-fill the resulting voids. The 461 and 476 cells/dm2 were treated in the same way as the 441 cells/dm2 foundation; one colony would draw on them, the others would not. Just as the cells constructed on the 390 cells/dm2 foundation resulted in a 0.5 % extension of the range of worker cell sizes, this foundation size defined the upper limits of drone cells in naturally-built combs, which vary in size from 6.18 to 7.24 mm, with a mean of 6.66 mm. Experimental foundation of 441 cells/dm2 had a diameter of 7.20 mm, which was just within the limits of drone cell tolerances.
493 and 522 cells/dm2. Foundation with 493 cells/dm2 was the largest size which all six colonies would consistently draw into finished, regular combs. Workers easily filled these cells with honey and capped them, queens readily laid in them, and drones were reared from them. The appearances of such combs were the same as that of any comb drawn on commercially available foundation sheets, except that the cells were uniformly large. The cell walls were virtually identical to those of drone comb (Fig. 12.12).
Foundation of 522 cells/dm2 was almost identical to that of combs in wild colonies and was treated accordingly by the bees. In the absence of building foundation, the cell sizes of European races of A. mellifera bees are notoriously variable (Vogt 1911; Darchen 1956), while African A. mellifera construct cells that range from about 4.8 mm to 6.7 mm in diameter or 500–1100 cells/dm2. The reported lower limit on the cell size of African A. m. litorea from Tanganyika (presently, Tanzania) is 1243 cells/dm2 (Smith 1960) and the upper limit close to 441 cells/dm2 (Hepburn 1983). Considering drone and worker cells as separate entities that are recognised as such by bees, the variations in the tolerances of cell size approach some 40 %. Moreover, so-called intermediate or transitional cells are far from rare, as is shown when only a starting strip of embossed wax is given to honeybee colonies. The bees follow the pattern to the end of the strip, below which the cells become progressively larger. Over a distance of only 65 mm, the cells at the bottom of the comb were 20 % larger than those at the top, the rate of increase in cell size being about 3 % per mm (Hepburn 1983).
Against this background of seemingly immense variation there was, nonetheless, some control in comb-building. There were no strong correlation between cell diameter and cell wall thickness. Two groups appear with respect to these two variables (Hepburn 1986). One was a group of smallish cells centering around 5 mm in diameter with a mean wall thickness of about 0.25 mm, which were worker cells. In the other group, drone cells were around 6.8 mm, with an average wall thickness of about 0.43 mm. These two groups were discrete and there were no intermediate or bridging values between them (Figs. 12.11 and 12.12). The overall mean thickness of cell walls in naturally drawn combs is 0.36 ± 0.11 mm, whereas those drawn on foundation were marginally thicker, 0.41 ± 0.08 mm. However, the difference was not statistically significant. In view of the relatively large cell wall size from embossed foundation of about 0.60 mm (Figs. 12.11 and 12.12), it was significant that such large walls were planed by the bees and the thinning factor was about 30 % on average.
The limits on the acceptability of large cells by A. m. scutellata lie in the range of 441–493 cells/dm2 and provide a basis for assessing whether bees consistently apply a set of working limits. Tests were conducted using the same six colonies of bees, and different types of foundation were placed in the same frame. Thus, 1 dm2 sheets of foundation of one size were alternated with 1 dm2 sheets of another size, in the following combinations: 441 + 390, 336 + 522, 493 + 441 and 493 + 390 cells/dm2. The results of construction were astonishingly consistent, whatever the permutations of cell sizes, the bees consistently recognised 336 and 390 cells/dm2 sizes and built their combs accordingly. Similarly, when they encountered 493 and 522 cells/dm2 sizes, they built accordingly. The 441 cells/dm2 size presented the same problem in mixed-size foundation frames as in the whole frame experiment—one colony would work it, and the other colonies resorted to tapering and gap-filling. There was no distortion of the foundation and the metrological abilities of the bees remained constant.
12.7 The Cell Base: Changing from Rhombus to Hemisphere
It is very difficult to assess the importance of the cell base in relation to the construction of the rest of the cell. Huber (1814) assigned great importance to it, but only a few studies address the issue (Hepburn 1983; Pirk et al. 2004; Hepburn et al. 2007). In the earliest study, Hepburn moulded the wax so that the foundation consisted of perfectly equilinear hexagons and the bases of the cells were flat and of the different sizes mentioned above. In professionally made commercial beeswax foundation, both the cell base and the hexagonal rims of the cells have a pronounced taper to them. To assess what the bees might have measured, base width or wall taper, foundation of 461 cells/dm2 were manufactured in two different ways; cells were made in which the diameter was held constant at about 6.95 mm, but the bases varied. Type A cells had a base of 5.05 mm and type B cells with a basal diameter of 6.70 mm (Hepburn 1983). The results are shown in Figs. 12.11 and 12.12, from which it is evident that the finished cells differed, although not significantly, by about 0.1 %. One can conclude that the outermost limits of the pattern supplied to them, and not the nature of the cell base, determines what pattern the bees follow.
Therefore, the apparent regularity of comb cells derives from two sources: the abstractions of idealists (with the laudable exceptions of Vogt, Hubbe and Darchen), and the centuries-old use of beeswax foundation on which exact regularity has been embossed. The latter gives regularity to cells which masks the variability which one normally finds in feral honeybee nests. Based on that level of order, Martin and Lindauer (1966) thought that the diameter of cells must be measured by the bees in some way. The average measurements of the cells they studied (similar to others obtained by Taber and Owens 1970) are shown in Table 12.1.
Among the possible organs of measurement that came to mind were the inter-ommatidial setae, whose function is unknown. These were excluded because Neese (1965) had shown that their removal had no measurable effect on comb construction. These setae, while not essential for comb-building, are not precluded from a role in comb-building, as Martin and Lindauer (1966) rightly pointed out. Similarly, Lau (1959) had shown that bees could build extensive and normal combs after amputation of the tarsi of the forelegs. But to consider the more obvious things, Huber (1814) was emphatic that not a parcel of wax is removed in cell thinning before the antennae have palpated the surface to be planed. Likewise, Lau (1959) had shown that combs built by antennectomised bees contained several structural aberrations. On these notes, Martin and Lindauer (1966) went on to evaluate the antennae in determining cell size and wall thickness.
Martin and Lindauer (1966) performed an incredible series of important experiments in which they removed either one or both antennae, as well as different numbers of antennal segments from hundreds of bees (Table 12.2). The bees, despite the mutilations, constructed combs similar to the controls, providing negative results. However, returning to the palpations of the antennae, and because amputation did not prevent building (Lau 1959; Martin and Lindauer 1966), in future one just might have to re-define precisely the role of the antennae in comb-building. In both studies (Lau 1959; Martin and Lindauer 1966) it was noticed that the coping of the cell wall should show a deviation from the controls. The coping of cells built by antennectomised bees were wider and higher than those of the control groups (Martin and Lindauer 1966).
This suggests that the antennae play a role in maintaining tolerances on cell thickness. In building, the milling of the cell wall is controlled by a single worker working on a single side of the wall at a time. That the apical segment of the antenna is of great importance is shown by the effect of partially removing it, which increases the cell wall thickness significantly (Fig. 12.13). Martin and Lindauer (1966) observed that a building bee continuously executes planing movements with the curved edges of the mandibles during construction, while simultaneously ‘monitoring’ the forming or thinning wall with the antennae. As the mandibles are dragged over the wax, it is deformed. Presumably the controlled pressure on the mandibles could, in theory, be transmitted through the head capsule and onto the neck organ, whose response may inform the bee’s brain of quantitative changes in the wax. Part of the problem with the results reported above is that these questions have to be answered experimentally. Otherwise, it is only conjecture as to what is measured and how, if indeed, anything is measured at all.
References
Akratanakul P (1977) The natural history of the dwarf honey bee, Apis florea F in Thailand. Thesis, Cornell University
Alber M (1953) Favi naturali. Apic Ital 20:55–58
Armbruster L (1920) Zum Problem der Bienenzelle: eine vergleichende Instinkt-Biologie des Nestbaues bei Bienen und Wespen. Theodor Fisher, Leipzig
Bergman DJ, Ishay JS (2007) Do bees and hornets use acoustic resonance in order to monitor and coordinate comb construction? Bull Math Biol 69:1777–1790. doi:10.1007/s11538-006-9190-9
Bone D (1952) A rare architectural work of bees: comb built upwards. GleanBee Cult 80:216–217
Butler C (1609) The feminine monarchie, shewing their admirable nature, and properties, their generation, and colonies, their Government, loyaltie, art, industrie, enemies, warres, magnamimitie, &c. together with the right ordering of them from time to time. Joseph Barnes, Oxford
Casteel DB (1912) The manipulation of the wax scales of the honey bee. US Department of Agriculture, Bureau of Entomology, Washington
Dadant HC (1946) The honeycomb. In: Grout RA (ed) The hive and the honeybee. Dadant, Hamilton
Darchen R (1954) Quclquer regulations sociales dans la construction chez les abcille. lnsectes Soc 1:219–228
Darchen R (1956) La construction sociale chez Apis mellifica. Insectes Soc 3:293–301. doi:10.1007/bf02224312
Darchen R (1980) La cire, son recyclage et son rôle probable à l’interieur d’une colonie d’Apis mellifica. Apidologie 11:193–202
Darchen R (1991) The construction strategy of bees. In: Proceedings of 3rd conference on Italian section of the IUSSI Ethol Ecol Evol 3:107–110
Darwin C (1856) The origin of species by natural selection. Murray, London
Darwin C (1859) The origin of species by natural election. Murray, London
Dönhoff E (1854) Über den Übergang der Wachsblättchen in Wachslümpchen. Schweizerische Bienenzeitung 15:279
Duangphakdee O, Hepburn HR, Rodim P (2013) Seasonal movements of the red dwarf honeybee, Apis florea. (In ms.)
Gontarski H (1935) Wabenzellmasze bei Apis mellifica. Z Vergl Physiol 21:682–698
Gould JL, Gould CG (1983) Can a bee behave intelligently? New Sci 98:84–87
Gwin CM (1936) Further developments concerning wax production by the honeybee colony. 1. A study of the production of wax scales and comb-building. J Econ Entomol 29:318–321
Hepburn HR (1983) Comb construction by the African honeybee, Apis mellifera adansonii (= scutellata). J Ent Soc S Afr 46:87–101
Hepburn HR (1986) Honeybees and wax: an experimental natural history. Springer, Berlin
Hepburn HR, Whiffler LA (1991) Construction defects define pattern and method in comb-building by honeybees. Apidologie 22:381–388
Hepburn HR, Hugo JJ, Mitchell D, Nijland MJM, Scrimgeour AG (1984) On the energetic costs of wax production by the African honeybee, Apis mellifera adansonii (= scutellata). S Afr J Sci 80:363–369
Hepburn HR, Muerrle T, Radloff SE (2007) The cell bases of honeybee combs. Apidologie 38:268–271. doi:10.1051/Apido:2007005
Hubbe W (1957) Beobachtungen zum Wabenbau der Honigbiene Apis mellifica L. Arch Geflügel Kleintier Kd 6:343–358
Huber F (1814) Nouvelles observations sur les abeilles. [English translation, 1926] Dadant, Hamilton
Karihaloo BL, Zhang K, Wang J (2013) Honeybee combs: how the circular cells transform into rounded hexagons. J Roy Soc Interface 10(86). doi:10.1098/rsif.2013.0299
Lau D (1959) Beobachtungen und Experimente über die Entstehung der Bienenwabe (Apis mellifica L.). Zool Beitr 4:233–306
Lineburg B (1923a) Conservation of wax by the bees. Am Bee J 63:615–616
Lineburg B (1923b) What do bees do with brood cappings? Am Bee J 63:235–236
Lineburg B (1924) Comb-building. Am Bee J 64:271–272
Martin H, Lindauer M (1966) Sinnesphysiologische Leistungen beim Wabenbau der Honigbiene. Z Vergl Physiol 53:372–404
Meretz W (1963) Die Wabenzelle der Honigbiene. Bull Math Biophys 25:95–110. doi:10.1007/bf02477773
Meyer W (1952) Die Kleinbauarbeiten unserer Bienen. Deutsche Bienenwirts 3:237
Meyer W, Ulrich W (1952) Zur Analyse der Bauinstinkte unserer Honigbiene. Untersuchungen Åber die “Kleinbauarbeiten”. Naturwissenschaften 39:264
Mossadegh MS (1990) Nesting behaviour of Apis florea F (Hymenoptera: Apidae) in Khuzestan, Iran. Proc 11th Int Congr, IUSSI, New Delhi, pp 669–670
Neese V (1965) Zur Funktion der Augenborsten bei der Honigbiene. Z Vergl Physiol 49:543–585. doi:10.1007/bf00367159
Oelsen GV, Rademacher E (1979) Studies on the building behavior of the honeybee (Apis mellifica). Apidologie 10:175–209
Phiancharoen M, Duangphakdee O, Hepburn HR (2011) Biology of nesting. In: Hepburn HR, Radloff SE (eds) Honeybees of Asia. Springer, Berlin, pp 109–131
Pirk CWW, Hepburn HR, Radloff SE, Tautz J (2004) Honeybee combs: construction through a liquid equilibrium process? Naturwissenschaften 91:350–353
Rösch GA (1927) Über die Bautätigkeit im Bienenvolk und das Alter der Baubienen. Weiterer Beitrag zur Frage nach der Arbeitsteilung im Bienenstaat. Z Vergl Physiol 6:264–298
Smith FG (1960) Comb foundation: its use for African honeybees. Bee World 42:235–240
Smith MV (1959) A note on the capping activities of an individual honeybee. Bee World 40:153–154
Taber S, Owens CD (1970) Colony founding and initial nest design of honey bees, Apis mellifera L. Anim Behav 18:625–630. doi:10.1016/0003-3472(70)90005-9
Tautz J (2008) The buzz about bees, biology of a superorganism. Springer, Berlin
Thompson DAW (1942) On growth and form. Cambridge University Press, Cambridge
Thompson F (1930) Observations on the positions of hexagons in natural comb-building. Bee World 11:107
Ulrich W (1964) Geometrie und Entstehung der Bienenwabe. Zeit Bienenforsch 7:62–71
Vogt H (1911) Geometrie und Ökonomie der Bienenzelle. Trewendt and Granier, Breslau
von Frisch K (1974) Tiere als Baumeister. Frankfurt am Main, Ullstein
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hepburn, H., Pirk, C., Duangphakdee, O. (2014). Construction of Cells. In: Honeybee Nests. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54328-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-54328-9_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54327-2
Online ISBN: 978-3-642-54328-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)