Abstract
The maize primary root is a cylindrical structure formed by consecutive zones: (a) the root apex contains the apical meristem, where cell divisions occur; (b) the elongation zone in which cells stop dividing and start to elongate; and (c) the maturation zone, where cells reach their definitive lengths, cell differentiation begins, and lateral roots initiate. In the root, three main tissue systems can be distinguished: the epidermis, the cortex, and the vascular cylinder. The first layer of the vascular cylinder is the pericycle. Cell cycle activation in pericycle cells is clearly connected with lateral root initiation.
Root grows basically by the elongation of its cells and branches through proliferation of pericycle founder cells. Auxin is the main hormone in regulating these both processes. Exogenous auxin inhibits root growth, increases transversal expansion, and enhances lateral root formation. As auxin also enhances ethylene production, it is difficult to know whether certain auxin effects are mediated by ethylene or not. Based on own results and on the specialized literature, we discussed on regulation by auxin and ethylene of the development of the maize root system. The emerging model is that auxin and ethylene regulate root elongation depending on concentration and that both regulators interact to regulate root growth. The role of auxin in regulating lateral root formation is clearly established. However, ethylene does not seem to have such a direct role in this process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Maize (Zea mays) is an herbaceous monocotyledon with an annual cycle. When the seed germinates, a primary or seminal root arises from the embryonic radicle. This root elongates rapidly and, as it grows, forms very many new lateral roots (LRs). The primary root may persist throughout the plant’s life, although frequently it ceases growing and branching (Feldman 1994). Consequently, it has relatively little functional importance after the first stages of seedling development (Hochholdinger et al. 2004). The mature plant has an extensive fibrous root system consisting of several whorls of adventitious roots, some of which become widely spread, horizontally growing roots, while others become deeply penetrating vertical roots. Because they originate from shoot nodal regions, these adventitious roots are often categorized as nodal roots. Post-embryonic nodal maize roots have been subdivided into crown and brace roots according to whether they initiated from underground or aboveground nodes, respectively (Hochholdinger et al. 2004).
Despite its relatively poor functional importance, the primary root is a wonderful system in which to analyse basic processes of development such as (i) organ polarity establishment, (ii) cell proliferation regulation, (iii) cell fate decisions, (iv) cell signalling, (v) cell growth coordination, etc. Together, these processes occur in a beautiful and highly ordered histological context and are affected by multiple physicochemical factors, some of which have long been well known, while others are the object of current research.
In order to draw rigorous conclusions, the study of the physiology and molecular biology which regulates root development requires an adequate analysis of the temporal evolution of the histological structure of the root. In this sense, the present study is an attempt to contribute to understanding how the primary root system proceeds in maize and what is the presumptive role played in this process by two specific phytohormones: auxin and ethylene.
The development of the maize root system has two main aspects which occur simultaneously although they are conceptually different. Firstly, it is necessary to understand how each individual root develops by a continuous contribution of new cells at the root apex and the subsequent growth of these cells. Secondly, one must consider how new root meristems destined to originate lateral and adventitious roots are formed endogenously in mature root or shoot tissues.
The present work is mainly concerned with the description of the development and branching of the primary roots. Adventitious roots follow paths of development which have been appropriately described in maize and other related species elsewhere (Erdelska and Vidovencova 1993; Hetz et al. 1996; Mergemann and Sauter 2000).
2 Morphology and Structure of the Primary Maize Root
2.1 General Morphology and Growth Zones
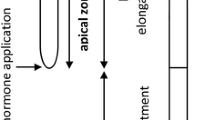
The maize primary root is a cylindrical structure, finished at the tip of its distal end by the presence of the root apex (Fig. 5.1a). It is generally considered to consist of a series of consecutive root zones. The root apex comprises approximately the distal-most 2 mm of the root extension (Fig. 5.1a). It contains the root apical meristem, consisting of undifferentiated meristematic cells that are in continuous cell division. Its function is to produce the cells destined to constitute the various tissues of the root.
Segments of the primary root of Zea mays showing the morphology of root apex and the development of lateral roots. (a) Root apex and elongation zone. (b) Maturation zone (distal). (c) Maturation zone (proximal). RC root cap, RM root meristem, DEZ distal elongation zone, EZ elongation zone, LRP lateral root primordia, ELR emerged lateral roots. Bar: 1 mm
Just above the root apex is located the elongation zone that extends approximately from 2 to 10 mm behind the tip (Fig. 5.1a). In this region, the cells stop dividing and begin to grow intensely and predominantly in a direction parallel to the longitudinal root axis in a process known as cell elongation. Initially, in the apical-most part of this zone, cell growth is both longitudinal and transverse, whereas subsequently, in the rapid elongation zone, it is only longitudinal (Ivanov 1997).
In the root zones located at more than 10 mm from the tip, one may consider the cells to have reached their definitive length, and cell differentiation begins. This region is defined as a maturation zone. In it, the vascular tissues progressively acquire maturity. The root hairs are formed and begin their development at this level of the root, and the LRs continue their growth (Fig. 5.1b, c).
2.2 Histology of the Primary Root
The arrangement of tissues that form the body of the primary root can be studied by means of transverse and longitudinal sections. In the root, as in the rest of the plant organs, three main systems of tissues can be distinguished: the epidermis, the cortex, and the vascular cylinder.
The outermost layer of cells of the root is the epidermis (Fig. 5.2a). The epidermis is uniseriate in maize, and the cell walls are asymmetrically thickened. Epidermal cells are elongated parallel to the long axis of the main root, and their internal content is very clear because almost all of the cell volume is occupied by a large vacuole. The cell nucleus is usually located away from the centre in a position adjacent to the cell wall (Fig. 5.2a).
Cross section of maize roots. (a) General view at the end of the elongation zone (toluidine blue staining, TBS). (b) Detail of the vascular cylinder and inner cortex at the end of the elongation zone (TBS). (c) Detail of the vascular cylinder and inner cortex at the proximal part of the maturation zone (safranin-fast green staining procedure). Note in (a) that the nucleus of the epidermal cells are displaced to the periphery of the cell (arrows) and in (c), the U-shaped wall of endodermal cells (arrowheads) and the early metaxylem elements (asterisk). ep epidermis, co cortex, en endodermis, pe pericycle, px protoxylem pole, lmx late metaxylem, ph phloem. Bars: 50 μm
The cortex consists of six to ten layers of parenchymatous tissue with small intercellular spaces at the angles of the cells (Fig. 5.2a). The outermost and innermost cortex layers have special characteristics and are called the exodermis and endodermis, respectively. The cells of these two layers are highly specialized, and in most higher plants, their cell wall development passes through three stages (Clarkson and Robards 1975). In Stage I, they deposit Casparian strips on their radial and transverse cell walls. Later in ontogeny (Stage II), a suberin lamella is deposited between the primary wall and the plasmalemma. Thanks to the Casparian strips and the suberin lamellae, the system of cortical extracellular spaces remains sealed, preventing the access of ions to the vascular cylinder via the apoplast (Enstone et al. 2002). In some species, these cells progress to a third stage of development (Stage III) with the deposition of a tertiary cellulose wall. This can be observed very clearly in the maize endodermis which, near the apex, presents a slightly thickened cell wall (Fig. 5.2b), while further away it presents the characteristic U-shaped tertiary cell wall with clearly discernible asymmetric thickening (Fig. 5.2c). The transition from Stage II to Stage III is not always sharply demarcated (Clarkson and Robards 1975).
The first layers of the vascular cylinder form the pericycle. The maize root pericycle is initially single layered, consisting of thin-walled parenchymatous cells (Fig. 5.2a–c) which may become thick-walled later in ontogeny (Sutherland and McCully 1976). Pericycle cells are capable of recovering their meristematic activity and of initiating the process of LR production.
The vascular cylinder has an alternating organization of xylem and phloem poles, which is typical of most roots. The number of protoxylem poles is variable in maize roots, ranging from 20 to 40. There are commonly two or three protoxylem strands to each large metaxylem vessel (Fig. 5.2c). The primary phloem poles alternate with the protoxylem poles and are in contact with pericycle cells with a particularly large cross-sectional area (Fig. 5.2c). Often, parenchymatous cells that are located between the xylem and phloem poles eventually present thickened and lignified cell walls. The centre of the root is occupied by a parenchymatous tissue called pith, which extends between the vascular tissues until it reaches the pericycle (Fig. 5.2a).
The LRs originate as endogenous anlages named lateral root primordia (LRP). The LRP primarily consist of an arc of pericycle which more or less extends over three phloem and two intervening xylem poles (Fig. 5.3a). Although most of the cells that constitute the LRP derive from the pericycle, there is an endodermal cover which is clearly incorporated into the primordium (Fig. 5.3a). Longitudinal sections show that the early LRP consist of two short pericycle cells lying end to end in the same column, flanked above and below by two longer pericycle derivatives (Fig. 5.3b). More developed LRP gradually make their way through the cortex of the parent root (Fig. 5.3c, d) until they reach the level of the epidermis and break through it to emerge as young LRs (Fig. 5.1c).
Development of lateral root primordia (LRP) in Zea mays. (a) Transverse section of an arc of basophilic cells from the pericycle and the endodermis that are initiating an LRP (toluidine blue staining technique, TBS). (b) Longitudinal section of a column of pericycle where two founder cells have undergone the first transverse divisions during LRP initiation (Feulgen staining technique). Note that four cells, two short central and two long peripheral, can be distinguished. Arrows indicate the transverse walls of each cell. (c) Longitudinal section of a maize root segment along a xylem pole (TBS). Note that the LRP of the image has a perfectly organized apical meristem and has crossed more than half of its way towards the external surface of the parent root. (d) Transverse section of maize root showing an LRP in a more advanced stage of development (just emerging) which extends covering an arc of pericycle of approximately three phloem poles and two intervening xylem poles (TBS). co cortex, en endodermis, pe pericycle, xy xylem strand, lmx late metaxylem, ph phloem. Bars: 50 μm
3 Root Development
In general terms, biological development can be defined as a series of gradual changes in size, structure, and function affecting individuals, organs, or cells. The development of a root basically consists of the processes of growth and differentiation that represent, respectively, quantitative and qualitative changes (Segura 2000). In the study of the development of the root system of any plant, it is necessary to consider two specific aspects: the growth and differentiation of each individual root and how new LRs are formed.
The classical concept of the development of an individual root considers successive zones of division, elongation, and maturation. The source of cells for the body of the growing root is the root apex. Cells produced in the root apex are usually regarded as members of a larger unit, undergoing more or less abrupt transitions between different developmental stages at the same time as they transit specific root zones.
In most angiosperms, root branching occurs far from the apical meristem when specific founder cells initiate a sequence of events that leads them to create a new apical meristem inside the parent root. Because founder cells belong to the outer cell layer of the vascular cylinder, i.e., the pericycle, LRP are initially endogenous structures (Lloret and Casero 2002). Nevertheless, they quickly grow through the parent root cortex (Fig. 5.3c, d) and break the epidermis to emerge as young LRs (Fig. 5.1c).
In the following, we shall discuss some basic aspects of the development of the root system of plants, with special emphasis on the situation in maize.
3.1 The Root Apex
The structure and developmental dynamics of the apex of both the main root and the LRs are similar. There is always a terminal root cap and a sub-terminal root meristem (Figs. 5.4 and 5.5).
Schematic representation of the maize root apex including an explanation of the different orientation planes of cell divisions in meristematic cells. ca root cap, qc quiescent centre, ic initial cells, pw periclinal wall, aw anticlinal wall, tw transverse wall, pe pericycle, en endodermis, pc procambium, pr protodermis, gm ground meristem
(a) Longitudinal section of a maize root apex showing the overall organization (toluidine blue staining technique, TBS). (b) Detail of the promeristem and the quiescent centre as well as the base of the root cap. The cell columns of the ground meristem can be followed through the quiescent centre, leaving the initial cells of the root cap at a lower typical position of closed meristems (TBS). (c) The application of an immunocytochemical technique to detect proliferative cells (BrdU labelling technique) in the promeristem region of the corn root apex delimits clearly the location of the quiescent centre (arrows). (d–f) Transversal sections of maize root apex taken at the middle of the meristematic region (TBS). Note in (e) a mitotic figure in a cell of the ground meristem (arrow). qc quiescent centre, rc root cap, pe pericycle, en endodermis, lmx late metaxylem, ph phloem, pc procambium, pr protodermis, gm ground meristem, pxp protoxylem pole. Bars: 50 μm
The root cap forms a cover at the tip of the apex and consists of cells that differentiate quickly, secrete mucilaginous compounds, and slough off as they reach the organ’s surface. In addition to its protective function, the root cap also serves to sense the direction of gravity and other environmental signals (Sievers et al. 2002).
Interposed between the root cap and the meristem, there is a special region which is very important for both the organization and the physiology of roots: the quiescent centre (QC). The QC is discoid in shape (Fig. 5.4) and consists of cells which, on average, divide relatively infrequently (Fig. 5.5c). Clowes (1958) showed that, in the root apex, the cells undergoing DNA synthesis and mitoses are located around the QC. This means that the functional initials of root tissues are really two groups of cells located above and below the QC. The initial cells located below the QC will, by repeated cell divisions, form the root cap, whereas those located above the QC will form the rest of the root tissues. Often this latter cell population is considered to constitute the promeristem, from which originate the true primary meristematic tissues of the root apex: protodermis, ground meristem, and procambium (Fig. 5.4). To prevent depletion of the initials, after every division of each initial cell, the distal daughter retains its status of initial cell. Therefore, the initial cells are in theory capable of self-perpetuating indefinitely.
It has been found that there is a very high rate of auxin release in the QC by the mechanism of polar auxin transport (Kerk et al. 2000). Also, high auxin concentrations in the QC have been correlated with high ascorbic acid oxidase activity, which in turn results in low ascorbic acid concentrations and the establishment of the characteristic quiescence of this region (Kerk et al. 2000). Functionally, there is growing evidence that the QC is an organizing structure, and it has been shown to maintain the stem cell’s niche necessary for continuous root growth via the expression of the homeobox gene WOX5 (Sarkar et al. 2007).
Most cell divisions in the root meristem occur with the new cell wall in a plane perpendicular to the root’s long axis (transversal divisions), and the cells are arranged in long columns (Fig. 5.4). The outer columns of the meristem constitute the protodermis which, when it matures, forms the epidermal covering of the root. Further inwards is the ground meristem which gives rise to the cortex in the mature root, and in the centre is the procambium which gives rise to the vascular cylinder (Fig. 5.4).
The development of the primary root includes the processes of cell division, elongation, and differentiation. In principle, cell divisions are restricted to the promeristem and the meristem in the proximal part of the root apex and to the initial cells of the root cap and their immediate derivatives in the distal face of the QC. All the cells which will eventually form the root body are generated through the proliferation of the procambium, ground meristem, and protodermis. In maize roots, longitudinal growth occurs within the region extending 12 mm from the tip (Silk 2002). Although the maximum growth rate is attained at a distance of 4–6 mm from the apex (Silk 2002), cells in the meristematic region also elongate along the apical-basal axis at a slower growth rate. Consequently, one may assume that the processes of cell division and elongation overlap at the root apex.
Within the apical meristem, two types of cell division take place: formative and proliferative (Barlow 1984). The formative divisions follow an anticlinal (i.e., perpendicular to the root surface) or periclinal (parallel to the root surface) plane and have the function of producing new columns of cells (Fig. 5.4). New tissues can be established by means of this kind of cell division. For example, in most angiosperms the epidermis and cortex share a common ontogenetic origin in the root apex initial cell zone (Clowes 1994). The proliferative divisions have the equatorial plane oriented transversely with respect to the root’s long axis, thereby giving rise to daughter cells that belong to the same column. Consequently, it becomes possible for the number of cells constituting a given column to increase while the total number of columns remains unaltered (Fig. 5.4).
Anatomically, the apical meristem of the maize root is considered to be “closed” because it is possible to continue along all cell columns up to the groups of initial cells located around the QC (Fig. 5.5b). Closed root apices are also characterized by having a clear separation between the root cap and the rest of the root body. In other species, the meristem is considered as “open” because there is no such clear continuity of columns to the QC region, and the boundaries between cortex, epidermis, and cap are unstable (Clowes 1994).
The meristematic cells of the maize root apex are very small isodiametric cells, with there being scarcely any intercellular spaces observable between them (Fig. 5.5a). They present a very thin primary cell wall and middle lamella. Their cytoplasm is dense, with many small vacuoles, and the nucleus is located centrally (Fig. 5.5d–f).
The maize root apex reaches a distance of about 2,000 μm from the distal end of the QC (root cap junction, RCJ). Most of the root apex is occupied by the meristematic region where cell divisions occur (Barlow 1987). The behaviour of cells in this region is affected by environmental changes monitored in the aerial part of the plant (Muller et al. 1998). Cell division by itself does not increase the length of the root. However, the cell division rate, the fraction of proliferating cells, and how long these cells remain mitotically active determine the rate at which cells are incorporated from the apex into the elongation zone, and therefore the root elongation rate (Beemster and Baskin 1998, 2000; Baskin 2000).
Beemster et al. (2003) divide the root apical meristem into two halves—the apical and the basal. In the apical half, cell divisions and root growth are correlated in such a way that the average cell length remains constant. Along the basal meristem, cells divide roughly at the same rate as in the apical meristem, but the elongation rate increases progressively. As a consequence, cell size also increases. The basal meristem cells share some common features with cells that are located at the beginning of the elongation zone (De Smet and Jürgens 2007). For example, it is assumed that these two areas are particularly important for integrating environmental and/or hormonal stimuli and that they modulate root growth (Ishikawa and Evans 1995; Beemster et al. 2003).
3.2 The Elongation Zone
In maize roots, the elongation zone (EZ) reaches to a distance of about 10–12 mm from the RCJ. It is characterized basically by the cessation of cell division, with the cells continuing their elongation at a variable rate that depends on their position. Ishikawa and Evans (1993) subdivide the EZ into five segments depending on the growth rate of the cells at each point. In the distal-most segment, the elongation growth rate is fairly slow (less than 0.3 times the maximum rate), and the cells seem to be particularly sensitive to auxin and to environmental changes. This root segment has been termed the transition zone (Baluška et al. 1996) or the distal elongation zone (Ishikawa and Evans 1993) and is located between the apical meristem and the region where rapid elongation takes place (Ivanov 1997). Some authors argue that the distal EZ, the transition zone, and the basal meristem are terms referring to the same region of the root (Beemster et al. 2003). The key to choosing which term to use would be whether or not cell division has ceased at this level of the root.
In the segment termed the rapid elongation zone, which lies at between 3,000 and 6,000 μm from the RCJ, major growth takes place. The beginning of this segment coincides with the cessation of transverse growth (García-Sánchez et al. 1991). Further away from the RCJ, the elongation gradually diminishes, until the expansion of the wall completely ceases at about 10 mm from the RCJ (Ishikawa and Evans 1993).
3.3 The Maturation Zone
In their transition through the three zones, as a result of polarized growth, the morphology of the root cells changes, and they gradually acquire traits of maturity through cell differentiation. From 10 mm from the RCJ onwards, the cell length remains constant, and those cells that acquire specific features of cellular differentiation gradually mature. This is particularly noticeable in cells such as those that constitute the hypodermis and endodermis that acquire Casparian strips and suberin lamellae (Zeier et al. 1999). In this zone, the conductive elements of the xylem, and even pericycle and stellar parenchyma cells, increase the thickness of their walls and deposit lignin (Sutherland and McCully 1976). The root hairs are also formed and eventually wither (Wen and Schnable 1994). Another remarkable phenomenon that occurs in this region is the beginning of the formation of the LRP. This is initiated by a series of cell divisions in some cells of the parent root pericycle. The first divisions are transversal and significantly reduce the length of the LRP’s founder cells (Fig. 5.3b) (Dubrovsky et al. 2000; Casimiro et al. 2003).
3.4 Lateral Root Ontogeny
The initiation of LRs is a fascinating developmental process because it involves the production of an entire organ from a small number of differentiated cells in response to intrinsic and environmental cues (Malamy 2005). In Zea mays, LR initiation occurs endogenously and far from the root apex, in particular, at a distance of approximately 12–15 mm behind the tip (Casero et al. 1995) when a few pericycle cells are stimulated to dedifferentiate and proliferate to form an LR primordium. The first sign of this process that is visible under light microscopy is that, by means of coordinated asymmetrical transverse divisions, one column of pericycle cells produces two very short cells (about 25 μm long, Fig. 5.3b) lying end to end and flanked above and below by two longer cells (Fig. 5.3b). The initiation of LRs by this procedure has been described in many species, and it can be assumed that this type of LR initiation is the commonest case among angiosperms (Lloret and Casero 2002).
In the circumferential plane, the columns which start the development of LR primordia are always located just in front of the protophloem poles of the parent root (Fig. 5.3a). Later, successive rounds of transversal root divisions extend longitudinally along the same column and transversally to adjacent columns, and a small plate of short cells is formed. This plate has been termed the rhizogenous plate (Van Tieghem and Douliot 1888) and extends transversally to an arc of approximately 12 columns of pericycle cells which cover three phloem poles and the two intervening xylem poles (Fig. 5.3a). In the longitudinal plane, the long founder cells of the LR primordium undergo a series of transverse divisions which give rise to many new short cells [see Fig. 5.6 in Lloret and Casero (2002)].
The pairs of cells of adjacent columns that give rise to the primordial rhizogenous plate are known as founder cells (Laskowski et al. 1995). Once these founder cells have undergone the first asymmetric transverse divisions, the process of LR development continues quickly. The following events are a radial enlargement of cells located at the centre of the rhizogenous plate, a reduction in the size of vacuoles, and, finally, periclinal divisions. In maize LR primordia, these cell divisions are asymmetrical, giving rise to an inner cell larger than the outer cell (Casero et al. 1995). The ulterior development of LRs in Raphanus sativus and Arabidopsis thaliana has been subdivided into seven and six developmental stages, respectively (Blakely et al. 1982; Malamy and Benfey 1997).
As the LRP pass through successive stages of development, they become ever more complex. During this process, the number of cells and the size of the LRP increase (Malamy and Benfey 1997), and the LRP acquire the capacity for autonomous growth, i.e., their growth becomes self-sufficient, no longer requiring growth regulators from the parent root. This phenomenon apparently occurs when the primordium organizes its own root apex (Laskowski et al. 1995).
The LRP must break through the parent root cortex and epidermis to emerge as young LRs. As the primordia cross these parental tissues, the primordial cells of pericyclic origin are covered by a cap of cells deriving from the endodermis termed the endodermal covering (Seago 1973). From the beginning of LR development, the endodermal covering cells present morphological features like those of pericyclic-derived cells, including increased protoplast stainability and transverse divisions which produce very short cells, etc. (Bell and McCully 1970; Seago 1973). Periclinal divisions are scarce, however, so that this layer does not constitute a permanent covering of the primordia, but must instead be regarded as a temporary structure to be replaced by a true root cap formed from cells of pericyclic origin (Seago 1973; Clowes 1978).
The outgrowth of the LRP through parent root tissues requires the “dissolution” of cells located in the way of the young developing primordium. In the case of the parent root cortex, it has been proposed that cells are pushed out by a combination of pressure (Pond 1908) and the secretion of hydrolases by the young primordium (Bonnett and Torrey 1966; Sutcliffe and Sexton 1968; Keller and Lamb 1989; Peretto et al. 1992). Also, it has been demonstrated recently that auxin promotes LR emergence through the regulation of the spatial and temporal distribution of aquaporin channels (Péret et al. 2012).
The final stage of LR development is emergence (Fig. 5.1c). In order to be fully functional, the vascular systems of the young LR and the parent root must be connected. This vascular connection matures either at about the time of LR emergence or later in freshly emerged LRs. Phloem and xylem connector elements are formed from the pericycle derivatives and vascular parenchyma cells of the parent root vascular cylinder (Peterson and Peterson 1986). It is generally accepted that maturation of the vascular connectors proceeds acropetally into the LRs (Peterson and Peterson 1986). These maturing vascular elements connect with newly formed vascular elements that have originated from the root apex of the young LR which is already active at this time.
4 Role of Auxin, Ethylene, and Other Phytohormones in the Development of Root Systems
An important challenge for plant developmental biologists is to understand the mechanisms that control the patterned development of more or less complex systems. The focus of the present section will be on the hormonal mechanisms that shape the growth, differentiation, and architecture of the maize root system. In particular, we shall analyse the effects on maize’s primary root development of treatments with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) and with the synthetic auxin 1-naphthaleneacetic acid (NAA), alone or in combination. Experiments have also been carried out using an ethylene antagonist and inhibitors.
Plant development sciences have evolved from being mostly descriptive and comparative to a field dominated by genetic and molecular approaches. This change has been favoured by the systematization of experimental procedures and the emergence of ever more sophisticated research tools. Nevertheless, classical studies of root development using such simple techniques as the exogenous application of growth regulators are still useful in this context, while recent physiological studies using new tools such as auxin transport inhibitors, ethylene biosynthesis inhibitors, or ethylene action inhibitors, for example, continue to improve our knowledge of the process (Casimiro et al. 2001; Alarcón et al. 2009; Strader et al. 2009; Steinitz et al. 2010).
Usually, the exogenous application of auxin promotes three main alterations in root system development: inhibition of elongation, increased transversal expansion, and enhanced lateral root formation. Physiologically, these changes occur in association with increased ethylene production due to increased transcription of ACC synthase genes (Abel et al. 1995). This leads to the accumulation of the ethylene precursor ACC and its product, ethylene, in tissues as a result of the auxin treatment.
The morphological changes observed in dark-grown seedlings treated with ethylene or its metabolic precursor ACC have been termed the triple response syndrome: exaggerated curvature of the apical hook, radial swelling of the hypocotyl, and shortening of the hypocotyl and root (Ecker 1995).
Since the effects of auxin and ethylene on root development share some common features, it is difficult to assign the causality of these effects to one or the other of these growth regulators or to the interaction between the two. As auxin stimulates ethylene production by increasing the biosynthesis rate of ACC synthase, it has been posited that ethylene is the mediator of, and directly responsible for, the effects of auxin on root growth (Eliasson and Bollmark 1988; Jackson 1991). Hansen and Grossmann (2000) report that auxin-induced ethylene triggers the production of abscisic acid (ABA) and inhibits root growth through this growth regulator. The fact that there is some crosstalk between auxin- and ethylene-resistant mutations suggests at least that these two phytohormones interact in the regulation of root development (Stepanova et al. 2007). Consequently, there is a need for a precise dissection of the particular effects of each growth regulator on different aspects of root development.
4.1 Phytohormones and Root Elongation
The most obvious generalization about the effect of exogenous auxin on root elongation is that its inhibition of growth is dose dependent (Blakely et al. 1982; Lloret and Pulgarín 1992). Indeed, when it is applied at very low doses, it can even stimulate root growth (Díez et al. 1971). Such stimulation by exogenous auxin at very low concentrations has also been observed in maize roots pretreated with ethylene biosynthesis inhibitor (Mulkey et al. 1982). Moreover a negative correlation has been found between root growth and endogenous indole-3-acetic acid (IAA) content (Pilet and Barlow 1987). These results support the idea that the endogenous IAA level should be optimal or supraoptimal for elongation, and consequently any increment in the auxin level would result in root growth inhibition (Evans et al. 1994).
Auxin is mostly synthesized in the shoot, whence it is transported to the root. Inhibition of this transport from shoot to root reduces root elongation (Rashotte et al. 2003). In the root, IAA moves acropetally towards the root apex through the central cylinder and basipetally from the root apex towards the base through the epidermal and cortical cells (Ruzicka et al. 2009), controlling root elongation (Rashotte et al. 2000). In Arabidopsis, basipetal auxin transport is sufficient to control root elongation, and inhibition of this transport by N-1-naphthylphthalamic acid (NPA) reduces root elongation (Casimiro et al. 2001).
Auxin can regulate the elongation of root cells by modifying different aspects of their physiology. Elongation requires both a driving force and a modification of the cell wall elasticity by loosening cross-links between cell wall components. In coleoptiles, auxin has been demonstrated to promote a rapid increase in cell wall elasticity (Cleland 1992; Taiz 1994; Cosgrove 2000). The acid growth hypothesis (Rayle and Cleland 1970) is that hydrogen ions are the agents responsible for cell wall loosening during Avena coleoptile growth, and auxin-induced stimulation of growth in coleoptiles is driven by proton extrusion into the apoplastic space. The pH falls to below 5.5, and this acidification enhances cell wall extensibility and increases the activity of enzymes involved in cell growth. In roots, exogenous auxin usually inhibits proton extrusion and hence cell elongation (Evans et al. 1980).
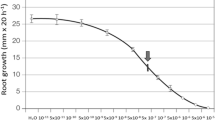
However, as was noted above, root elongation is stimulated by very low auxin concentrations if ethylene biosynthesis has been inhibited (Mulkey et al. 1981). In maize, exogenous NAA at concentrations greater than 0.001 μM inhibit root elongation, and complete inhibition is observed at 1 μM, with the inhibition correlating with the logarithm of the NAA concentration (Alarcón et al. 2012). This inhibitory effect is probably due to the greater sensitivity of roots than of stem tissues such as coleoptiles, in which these concentrations are stimulatory (Thimann 1936).
Similarly, ethylene has long been recognized as a growth inhibitor, but there has recently been reported evidence of its effects in promoting plant growth. In particular, the inhibitory effect of ethylene on root growth was described in 1901 by Neljubov (Dugardeyn and Van Der Straeten 2008), but today it is understood that while high concentrations inhibit root elongation and increase swelling, very low concentrations may promote root elongation (Chang et al. 2004; De Cnodder et al. 2005; Dugardeyn and Van Der Straeten 2008).
ACC reduces cell length in the rapid elongation zone, and ethylene also controls cell elongation (De Cnodder et al. 2005). However, a certain threshold of ethylene is also known to be necessary to maintain root growth in rice (Yin et al. 2011), and other evidence for ethylene’s stimulation of growth has been reported (Pierik et al. 2006). The stimulatory effects occur mostly at very low concentrations and are more pronounced in situations of stress. In P-sufficient white clover, low ACC concentrations stimulate root growth, while higher concentrations have no such effect (Dinh et al. 2012). In P-deprived seedlings, however, not only is root elongation enhanced, but ACC at higher concentrations super-stimulates growth, suggestive of an increased ethylene sensitivity in response to the low availability of P (Dinh et al. 2012). Arabidopsis root growth is promoted by ethylene under phosphorus stress (Ma et al. 2003). Also, ethylene is required for root development under non-stressful growth conditions. In particular, in Oryza sativa, the ethylene biosynthesis inhibitor aminoethoxyvinylglycine (AVG) reduces primary root elongation (Yin et al. 2011). In coherence with this result, AVG or cobaltous ions also cause a strong reduction in ethylene levels and inhibited root elongation in maize (Alarcón et al. 2009).
Pierik et al. (2006) propose a biphasic ethylene response model which integrates growth inhibition and stimulation, with low ethylene levels promoting, and high levels inhibiting, growth. The levels required to stimulate or inhibit growth depend on the species, plant organ, environmental conditions, and endogenous hormone levels. Four types of plant have been distinguished according to their ethylene dose–response relationship. In terrestrial plants, aerial organ growth can be stimulated by ethylene at low concentrations, but ethylene always has an inhibitory effect at high levels. In semi-aquatic plants, ethylene stimulates petiole elongation at high concentrations.
Ethylene is needed for the development of the root cap, the organ which facilitates the penetration of the roots into the growth medium (Zacarias and Reid 1992). In particular, high impedance triggers an increase in ethylene biosynthesis which then serves to facilitate the penetration of the root into the soil (Sarquis et al. 1992). Recently, it has been reported that a coaction between auxin and ethylene is required for root penetration during tomato seed germination (Santisree et al. 2011).
The inhibitory effect of aluminium on root elongation is well known, and it has recently been shown that ethylene mediates this effect in Arabidopsis (Sun et al. 2010). The inhibitory effects of aluminium are stronger in the wild type than in ethylene signalling-defective (etr1-3 and ein2-1) and auxin polar transport-defective (aux1-7 and pin2) mutants. Inhibitors of ethylene and auxin polar transport partially prevent the aluminium-induced inhibition. Aluminium and ACC increase the transcripts of aux-1 and pin-2, but this increase is not observed in the presence of ethylene inhibitors. It has therefore been suggested that aluminium-induced ethylene alters the auxin distribution by disrupting AUX1- and PIN2-mediated auxin polar transport, inhibiting root growth elongation (Sun et al. 2010).
Root development is not only regulated by auxin and ethylene. Gibberellins, cytokinins, and other phytohormones play a significant role (Hansen and Grossmann 2000). Also, ethylene biosynthesis is stimulated by other growth regulators: ACC synthase is up-regulated by cytokinin (Rodrigues-Pousada et al. 1999), abscisic acid (Wang et al. 2011), and brassinosteroids (Joo et al. 2006).
4.2 Root Transversal Expansion
Generally, the inhibition of root elongation produced by auxin is associated with an increased rate of transverse expansion, altering the predominant direction of root growth from longitudinal to transversal (Burström and Svensson 1974).
This change in growth polarity may be related to changes in the orientation of the cortical microtubules, which pass from an essentially transversal orientation to one which is predominantly longitudinal. Such a relationship is proposed by the alignment hypothesis that there is a causal link between the orientation of cortical microtubules and the orientation of nascent cell wall microfibrils (Baskin 2001), which would in turn regulate cell expansion (Green 1984; Baskin and Williamson 1992). With respect to this hypothesis, it has been demonstrated that treatment with auxin affects the direction of cell expansion and the orientation of the cortical microtubules in the epidermal cells of maize coleoptiles (Bergfeld et al. 1988). It seems, however, that auxin treatment does not affect the orientation of microtubules in the cells of the root vascular cylinder in the same way (Blancaflor and Hasenstein 1997), indicating that the response to auxin treatment could be tissue dependent.
As with auxin, ethylene also increases the root’s radial expansion (Smalle and Van der Straeten 1997; Buer et al. 2003), which change in the growth pattern has also been related to a change in the disposition of the cortical microtubules (Baskin and Williamson 1992).
Treatments of maize roots with the synthetic auxin NAA or with the ethylene precursor ACC inhibit root elongation and increase radial growth, although the responses to the two treatments differ in degree (Alarcón et al. 2012). In particular, NAA enhances radial expansion by nearly 200 %, whereas ACC only increases it by about 40 %, so that NAA seems to be more effective than ACC as a root swelling promoter (Alarcón et al. 2012).
4.3 Auxin–Ethylene Interaction
Since auxin specifically stimulates ethylene biosynthesis, it is often unclear whether the effects observed are due to auxin alone, to ethylene alone, or to an interaction between the two. In maize roots, both hormones inhibit elongation and promote swelling in the root tips (Alarcón et al. 2012). Two hypotheses have been proposed to explain auxin’s regulation of root growth. One is that auxin’s action is mediated by ethylene, as indeed has been demonstrated in the case of light-promoted inhibition of root growth (Eliasson and Bollmark 1988; Jackson 1991). The other is that auxin directly affects root growth, as seems to be the case in Pisum sativum for which it has been demonstrated that auxin inhibits elongation without the mediation of ethylene (Eliasson et al. 1989). Recent studies in Z. mays and Oryza sativa are consistent with this concept of auxin exerting its effect directly (Yin et al. 2011; Alarcón et al. 2012). Nevertheless, the fact that auxin has a direct effect on root elongation and transversal growth does not imply that it cannot interact with other growth regulators (including ethylene). Indeed, our results show that auxin and ethylene can inhibit root elongation and increase radial growth cooperatively. The effect of combined treatments is synergistic when NAA or ACC are applied at very low concentrations and additive at slightly higher concentrations. Cooperation between the two hormones is absent, however, at high concentrations of either (Alarcón et al. unpublished results).
The synergy between auxin and ethylene has been described in processes affecting seedling development (Muday et al. 2012), root hair growth and differentiation (Pitts et al. 1998), root gravitropism (Buer et al. 2006), and root growth (Swarup et al. 2007). Particularly interesting for the present context is the work of Santisree et al. (2011) on the coactions between auxin and ethylene required for roots to penetrate into the soil during tomato seed germination.
Another possible mechanism of interaction could be related to the role of ethylene in the regulation of auxin transport and/or biosynthesis. It is known that ethylene stimulates the biosynthesis of auxin and its basipetal transport to the elongation zone (Ruzicka et al. 2007). This would increase the concentration of auxin in this zone and hence decrease the root elongation rate (Lee et al. 1990).
Molecular studies have demonstrated an interaction between auxin and ethylene at the genetic level. Auxin response factors ARF19 and ARF7 participate in auxin signalling and play a critical role in ethylene responses in the Arabidopsis root, indicating that ARFs serve as a point of crosstalk between the two hormones (Li et al. 2006a).
4.4 Lateral Roots
The generation of LRs has a decisive influence on the root system’s capacity to anchor the plant to the soil and to acquire water and nutrients. It is unsurprising therefore that the regulation of the formation of LRs is under redundant control mechanisms performed through both endogenous and exogenous agents (Lloret and Casero 2002).
It is generally accepted that, among the endogenous factors that regulate LR formation, the most important is the phytohormone auxin (Casimiro et al. 2003). Many lines of experimental evidence relate auxin to diverse aspects of LR development. In particular, it appears to be involved in LR initiation, in the organization of the apical meristem, and in the emergence and ulterior growth of LRs.
Increased production of LRs has been demonstrated in most species after treatment with exogenous auxins (Torrey 1962; Blakely et al. 1972, 1982; Webster and Radin 1972; Wightman et al. 1980; Zeadan and MacLeod 1984; Hinchee and Rost 1986; MacIsaac et al. 1989; Lloret and Pulgarín 1992; Baum et al. 1998; Vuylsteker et al. 1998; Zhang and Hasenstein 1999). Nevertheless, the experimental procedures involved in these exogenous auxin treatments have been subject to criticism (Lloret and Casero 2002). The results of more recent studies with mutants and transgenic plants strongly relate LR formation to the biosynthesis or metabolism of auxin (Casimiro et al. 2003). For example, the sur1 mutants of A. thaliana that overproduce auxins also show increased LR formation (Boerjan et al. 1995). Similarly, transgenic plants that overexpress bacterial iaa genes, and consequently have high auxin levels, also tend to form many LRs (Klee et al. 1987). On the contrary, the diageotropica (dgt) tomato mutant and the combination of two auxin resistance mutations (axr4 and axr1) both reduce the number of LRs (Hobbie and Estelle 1995; Muday et al. 1995). The common feature of these two kinds of mutation is their reduced sensitivity to auxins.
Auxin overproducers and auxin-resistant mutants show striking physiological alterations in addition to changes in LR production. For example, there are specific effects on LR formation in alf1-1, alf3-1, and alf4-1 mutants of A. thaliana (Celenza et al. 1995). The alf1-1 mutation promotes the formation of LRs, alf4-1 inhibits their initiation, and alf3-1 is defective in their maturation. The alf1-1 mutants are likely to have a defect in auxin catabolism that favours the accumulation of the hormone in the root, resulting in an increased number of LRs. The alf4-1 mutant seems to be affected in the perception or the response to auxin and does not produce LRs, and alf3-1 initiates the LRP but soon aborts its development. The treatment with auxin reverts alf-3 but not alf4-1 (Celenza et al. 1995). These findings suggest a model for LR formation in which IAA is required for at least two stages in LR development: (i) to initiate cell division in the pericycle and (ii) to promote cell division and maintain cell viability in the developing LR.
The regulation of the development of LRs is not just a matter of the concentration of auxin but is also related to the transport of this hormone. Auxin transport is a directional process. Applied to the stem, auxin is transported to the root (McDavid et al. 1972), where it moves acropetally through the vascular cylinder (Kerk and Feldman 1995) to accumulate in the LRP and in the apex of the main root (Rowntree and Morris 1979; Sabatini et al. 1999). When the hormone reaches the root apex, its transport is inverse, i.e., basipetally from the apex to the elongation zone (Muday and DeLong 2001). This transport seems to occur through the root epidermis or the external layers of the cortex (Tsurumi and Ohwaki 1978; Yang et al. 1990). The acropetal movement of auxin has been associated with the regulation of LRP growth (Reed et al. 1998), while the basipetal movement would be associated with the initiation of the LRs (Casimiro et al. 2001).
There are essentially three lines of experimental evidence concerning the transport of auxin in regulating the LR formation process. First, elimination of the plant’s aerial organs (the source of auxin) reduces the LR frequency, and then application of exogenous auxin to the aerial part of the mutilated plant reverses this effect (Hinchee and Rost 1986). Second, mutants with defects in the auxin transport system (tir3) present fewer LRs (Ruegger et al. 1997). And third, NPA, an inhibitor of auxin transport, suppresses the formation of LRs in tomato (Muday and Haworth 1994) and Arabidopsis (Reed et al. 1998; Casimiro et al. 1999, 2001). Together, these three lines of evidence clearly reflect a fundamental role for auxin transport in the formation of LRs.
Unlike auxin, ethylene is a negative regulator of LR formation in both A. thaliana and Solanum lycopersicum (Ivanchenko et al. 2008; Negi et al. 2008, 2010), with enhanced ethylene synthesis resulting in reduced LR initiation independently of whether the enhancement is due to ACC treatment or to eto1 or ctr1 mutations. Similarly, Prasad et al. (2010) report that the protein XBAT32 negatively regulates ethylene biosynthesis by modulating the abundance of the enzyme 1-aminocyclopropane-1-carboxylate synthase (ACS), and, as expected, xbat32 mutants produce an excess of ethylene and have a limited number of LRs.
Since auxins promote ethylene biosynthesis, it is difficult to evaluate any possible direct influence of ethylene on LR formation. The enzyme ACS is involved in the ethylene biosynthesis pathway. It is well known that, during the initiation of LRs, ACS activity increases in response to auxin (Rodrigues-Pousada et al. 1993). This is a perfect example of how these two hormones work together in regulating a biological process.
Another form of interaction between auxin and ethylene could be by the effect of ethylene on the regulation of auxin transport. It has been suggested that the increase in the concentration of ethylene in the root affects the distribution of proteins involved in auxin transport, causing two effects: accumulation of auxin at the root apex and depletion in the zone where LRs are initiating (Muday et al. 2012).
In contrast to LR initiation, the development of LRs is stimulated by treatments that raise ethylene production in the root (Ivanchenko et al. 2008). In the maturation and abscission processes, ethylene increases the hydrolytic activity of the enzymes that degrade the cell wall (Roberts et al. 2000). It has therefore been proposed that ethylene also favours the degradation of the cortical cells located just in front of the LRP during its development. Obviously, this would facilitate the emergence of the LRP (Bonfante and Peretto 1993).
LR development is regulated antagonistically by the plant hormones auxin and cytokinin. Indeed, cytokinins are considered to be good inhibitors of the formation of LRs because their exogenous application frequently reduces this formation (MacIsaac et al. 1989). However, very low concentrations of these hormones can stimulate auxin-promoted LR formation (Torrey 1962). Furthermore, the application of cytokinins not only inhibits the initiation of LRs but also their emergence (Van Staden and Ntingane 1996). The root apex is the main site in the plant at which cytokinins are synthesized (Van Staden and Davey 1979). From there, they are transported by the xylem to the rest of the root, inhibiting elongation and LR formation (MacIsaac et al. 1989).
Cytokinins inhibit LR formation both in wild A. thaliana plants and in mutants defective in auxin transport or response mechanisms (Li et al. 2006b). The application of exogenous auxin to these mutants does not reverse this inhibition. It is therefore posited that auxin and cytokinin act in this process via different signalling pathways, with the cytokinins exerting their inhibitory effect by blocking pericycle founder cells from progress in the cell cycle from G2 to mitosis (Li et al. 2006b). This does not mean, however, that there is no crosstalk between auxin and cytokinin in regulating the formation of LRs. Xylem-pole pericycle cells have been described as being sensitive to cytokinins, whereas young LRP are not (Laplaze et al. 2007). It appears that cytokinins perturb the expression of PIN genes in LR founder cells and inhibit the accumulation of auxin in them, which in turn promotes LR initiation and regulates cell spacing (Laplaze et al. 2007).
ABA is also considered to be an LR inhibitor. Recent studies by Guo et al. (2009) in Arachis hypogaea report that exogenous ABA treatments decrease root branching in a dose-dependent manner. In Arabidopsis, mutation in the Abscisic Acid Insensitive 4 (ABI4) transcription factor promotes an increase in the number of LRs. It has been demonstrated that the expression of ABI4 is enhanced by ABA and cytokinin but repressed by auxin. The production of LRs in abi4 mutants is not affected by cytokinin or ABA (Shkolnik-Inbar and Bar-Zvi 2010). As the expression of the auxin efflux carrier protein PIN1 is reduced in ABI4 over expressors and enhanced in abi4 mutants, it has been suggested that ABA and cytokinin counteract the effect of auxin in the regulation of LR formation by reducing polar auxin transport (Shkolnik-Inbar and Bar-Zvi 2010).
Gibberellins are reported to interact with auxin in the regulation of LR development in Populus. GA-deficient and GA-insensitive transgenic plants show increased LR proliferation and elongation which is reversed by exogenous GA. Microarray analyses suggest a crosstalk of GA with auxin and that GA modulates LR development by modifying polar auxin transport (Gou et al. 2010).
In sunflowers, the lipid-derived hormone jasmonic acid and its derivates (collectively named jasmonates), applied exogenously, inhibit primary root elongation and reduce LR growth and number. Treatment with ibuprofen, an inhibitor of jasmonate synthesis, enhances primary root and LR lengths, but auxin elicits its typical response even in the presence of ibuprofen. Jasmonates, therefore, may induce primary root and LR growth inhibition via an auxin-independent pathway (Corti-Monzón et al. 2012).
In contrast, recent studies on A. thaliana indicate that jasmonates are promoters of both the initiation and emergence of LRs (Raya-González et al. 2012). In addition, regulation of the development of the root system seems to operate through mechanisms of two types, some dependent on auxin and others independent of this hormone. These results indicate that it is still unclear how jasmonates contribute to many aspects of root development and that there may be differences between species.
In sum, while auxins seem to constitute the main regulator of LR formation, other growth regulators appear to collaborate with it in fine-tuning the process and accommodating it to environmental influences.
4.5 Root Hairs
Auxin and ethylene are required for the normal development of root hairs (Pitts et al. 1998; Rahman et al. 2002). Ethylene increases their growth in various species including pea, fava bean, and lupin (Abeles et al. 1992). Analysis of Arabidopsis mutants has shown that root hair elongation in plants with enhanced ethylene production (eto1-1) was greater than in wild-type plants, and the elongation was negatively affected in the ethylene-insensitive mutant etr1 (Pitts et al. 1998).
The constitutive ethylene mutant of Arabidopsis ctr1 has more root hairs than the wild-type phenotype, and the application of ethylene to the wild type produces root hairs with a morphology similar to that of the mutant. In addition, application of an inhibitor of ethylene biosynthesis (AVG) or action (silver thiosulfate, STS) to the wild-type phenotype reduces the production of root hairs (Tanimoto et al. 1995).
The root hair in Arabidopsis mutants insensitive to ethylene (etr1) or auxin (aux1) exhibits normal morphology, whereas double mutants show reductions in root hair formation (Smalle and Van der Straeten 1997). These reductions can be reverted by the application of IAA but not ACC, suggesting that ethylene may act via more than one route (Masucci et al. 1996).
5 Conclusions
The organs of higher plants are complex multicellular organizations which grow by means of coordinated activity from their constituent cell types. It has long been recognized that hormones play a crucial role in this coordination. The root is no exception in this respect. It has only been recently that researchers have begun to understand how different growth regulators interact to properly regulate the development of the root system and adapt it to changing environmental conditions. It is now clear that the root cells exchange signals and that the signalling network they form is far more intricate than was previously assumed. Over the past few decades, particular attention has been paid to analysing how individual tissues may contribute to the regulation of overall root growth, and there is a need to continue on this line of inquiry in even greater depth.
In genetic studies of higher plants, the material of choice has been A. thaliana. While such a concentration of research effort on this plant model has yielded impressive results, it may now be advisable to expand our vision somewhat to look at other species. In this sense, it would be very interesting to compare the situation in monocots and dicots. Even if it were only for being one of humanity’s largest volume crops, the maize plant merits particular attention in the immediate future.
The maize primary root is a cylindrical structure formed by consecutive zones: (a) the root apex contains the apical meristem, where cell divisions occur; (b) the elongation zone in which cells stop dividing and start to elongate; and (c) the maturation zone, where cells reach their definitive lengths, cell differentiation begins, and lateral roots initiate. In the root, three main tissue systems can be distinguished: the epidermis, the cortex, and the vascular cylinder. The first layer of the vascular cylinder is the pericycle. Cell cycle activation in pericycle cells is clearly connected with lateral root initiation.
Root grows basically by the elongation of its cells and branches through proliferation of pericycle founder cells. Auxin is the main hormone in regulating these both processes. Exogenous auxin inhibits root growth, increases transversal expansion, and enhances lateral root formation. As auxin also enhances ethylene production, it is difficult to know whether certain auxin effects are mediated by ethylene or not. Based on own results and on the specialized literature, we discussed on regulation by auxin and ethylene of the development of the maize root system. The emerging model is that auxin and ethylene regulate root elongation depending on concentration and that both regulators interact to regulate root growth. The role of auxin in regulating lateral root formation is clearly established. However, ethylene does not seem to have such a direct role in this process.
References
Abel S, Nguyen MD, Chow W, Theologis A (1995) ACS4, a primary indole acetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin. J Biol Chem 270:19093–19099
Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in plant biology, 2nd edn. Academic, New York
Alarcón MV, Lloret-Salamanca A, Lloret PG, Iglesias DJ, Talón M, Salguero J (2009) Effects of antagonists and inhibitors of ethylene biosynthesis on maize root elongation. Plant Signal Behav 4:1154–1156
Alarcón MV, Lloret PG, Iglesias DJ, Talón M, Salguero J (2012) Comparison of growth responses to auxin 1-naphthaleneacetic acid and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid in maize seedling root. Acta Biol Crac 54:1–8
Baluška F, Volkmann D, Barlow PW (1996) Specialized zones of development in roots: View from the cellular level. Plant Physiol 112:3–4
Barlow PW (1984) Positional controls in root development. In: Barlow PW, Carr DJ (eds) Positional controls in plant development. Cambridge University Press, Cambridge, UK, pp 281–318
Barlow PW (1987) Cellular packets, cell division and morphogenesis in the primary root meristem of Zea mays L. New Phytol 105:27–56
Baskin TI (2000) On the constancy of cell division rate in the root meristem. Plant Mol Biol 43:545–554
Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215:150–171
Baskin TI, Williamson RE (1992) Ethylene, microtubules and root morphology in wild-type and mutant Arabidopsis seedlings. Curr Top Plant Biochem Physiol 11:118–130
Baum SF, Karanastasis L, Rost TL (1998) Morphogenetic effect of the herbicide cinch on Arabidopsis thaliana root development. J Plant Growth Regul 17:107–114
Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116:1515–1526
Beemster GTS, Baskin TI (2000) STUNTED PLANT 1 mediates effects of cytokinin, but not of auxin, on cell division and expansion in the root of arabidopsis. Plant Physiol 124:1718–1727
Beemster GTS, Fiorani F, Inzé D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8:154–158
Bell JK, McCully ME (1970) A histological study of lateral root initiation and development in Zea mays. Protoplasma 70:179–205
Bergfeld R, Speth V, Schopfer P (1988) Reorientation of microfibrils and microtubules at the outer epidermal wall of maize coleoptiles during auxin-mediated growth. Bot Acta 101:57–67
Blakely LM, Rodaway SJ, Hollen LB, Crocker SG (1972) Control and kinetics of branch root formation in cultured root segments of Haplopappus ravenii. Plant Physiol 50:35–42
Blakely LM, Durham M, Evans TA, Blakely RM (1982) Experimental studies on lateral root formation in radish seedling roots. I. General methods, developmental stages, and spontaneous formation of laterals. Bot Gaz 143:341–352
Blancaflor EB, Hasenstein KH (1997) The organization of the actin cytoskeleton in vertical and graviresponding primary roots of maize. Plant Physiol 113:1447–1455
Boerjan W, Cervera MT, Delarue M, Beeckman T, Desitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7:1405–1419
Bonfante P, Peretto R (1993) Cell-wall separation during the outgrowth of lateral roots in Allium porrum L. Acta Bot Neer 42:187–197
Bonnett HT Jr, Torrey JG (1966) Comparative anatomy of endogenous bud and lateral root formation in Convolvulus arvensis roots cultured in vitro. Am J Bot 53:496–507
Buer CS, Wasteneys GO, Masle J (2003) Ethylene modulates root-wave responses in Arabidopsis. Plant Physiol 132:1085–1096
Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic response in roots of Arabidopsis. Plant Physiol 140:1384–1396
Burström HG, Svensson SB (1974) Hormonal regulation of root development. In: Kolek J (ed) Structure and function of primary root tissues. Veda Publishing House of the Slovak Academy of Sciences, Bratislava, pp 121–135
Casero PJ, Casimiro I, Lloret PG (1995) Lateral root initiation by asymmetrical transverse divisions of pericycle cells in four plant species: Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma 188:49–58
Casimiro I, Calvo V, Marchant A, Bennett M, Casero PJ (1999) NPA reduces cell elongation and lateral root development in Arabidopsis thaliana. II Congress of the Spanish Society of Developmental Biology. Barcelona, Spain
Casimiro I, Marchant A, Bhalerao RP, Beeckmann T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M (2001) Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13:843–852
Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang HM, Casero P, Sandberg G, Bennett MJ (2003) Dissecting Arabidopsis lateral root development. Trends Plant Sci 8:165–171
Celenza JL, Grisafi PL, Fink GR (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9:2131–2142
Chang SC, Kim YS, Lee JY, Kaufman PB, Kirakosyan A, Yun HS, Kim TW, Kim SY, Cho MH, Lee JS, Kim SK (2004) Brassinolide interacts with auxin and ethylene in the root gravitropic response of maize (Zea mays). Physiol Plant 121:666–673
Clarkson DT, Robards AW (1975) The endodermis, its structural development and physiological role. In: Torrey JG, Clarkson DT (eds) The development and functions of roots. Academic, London, pp 415–437
Cleland RE (1992) Auxin-induced growth of Avena coleoptiles involves two mechanisms with different pH optima. Plant Physiol 99:1556–1561
Clowes FAL (1958) Development of quiescent centres in root meristems. New Phytol 57:85–88
Clowes FAL (1978) Chimeras and the origin of lateral root primordia in Zea mays. Ann Bot 42:801–807
Clowes FAL (1994) Origin of the epidermis in root meristems. New Phytol 127:335–347
Corti-Monzón G, Pinedo M, Lamattina L, de la Canal L (2012) Sunflower root growth regulation: the role of jasmonic acid and its relation with auxins. Plant Growth Regul 66:129–136
Cosgrove DJ (2000) Expansive growth of plant cell walls. Plant Physiol Biochem 38:109–124
De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reactions. New Phytol 168:541–550
De Smet I, Jürgens G (2007) Patterning the axis in plants-auxin in control. Curr Opin Genet Dev 17:337–343
Díez JL, de la Torre C, López-Sáez JF (1971) Auxin deficiency at the onset of root growth in Allium cepa. Planta 97:364–366
Dinh PTY, Roldan M, Leung S, McManus MT (2012) Regulation of root growth by auxin and ethylene is influenced by phosphate supply in white clover (Trifolium repens L.). Plant Growth Regul 66:179–190
Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL (2000) Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol 124:1648–1657
Dugardeyn J, Van Der Straeten D (2008) Ethylene: inhibitor and stimulator of plant growth. In: Bögre L, Beemster G (eds) Plant growth signaling (Plant Cell Monographs, 10). Springer, Heidelberg, pp 199–221
Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268:667–675
Eliasson L, Bollmark M (1988) Ethylene as a possible mediator of light-induced inhibition of root growth. Physiol Plant 72:605–609
Eliasson L, Bertell G, Bolander E (1989) Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol 91:310–314
Enstone DE, Peterson CA, Ma FS (2002) Root endodermis and exodermis: Structure, function, and responses to the environment. J Plant Growth Regul 21:335–351
Erdelska O, Vidovencova Z (1993) Development of adventitious seminal root primordia of maize during embryogenesis. Biologia 48:85–88
Evans ML, Mulkey TJ, Vesper MJ (1980) Auxin action on proton influx in corn roots and its correlation with growth. Planta 148:510–512
Evans ML, Ishikawa H, Estelle MA (1994) Responses of Arabidopsis roots to auxin studied with high temporal resolution: Comparison of wild type and auxin-response mutants. Planta 194:215–222
Feldman LJ (1994) The maize root. In: Freeling M, Walbot V (eds) The Maize Handbook. Springer, New York, pp 29–37
García-Sánchez C, Casero PJ, Lloret PG, Navascués J (1991) Morphological changes and transversal growth kinetics along the apical meristem in the pericycle cell types of the onion adventitious root. Protoplasma 160:108–114
Gou JQ, Strauss SH, Tsai CJ, Fang K, Chen YR, Jiang XN, Busov VB (2010) Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. Plant Cell 22:623–639
Green PB (1984) Analysis of axis extension. In: Barlow PW, Carr DJ (eds) Positional controls in plant development. Cambridge University Press, Cambridge, UK, pp 53–82
Guo DL, Liang JH, Li L (2009) Abscisic acid (ABA) inhibition of lateral root formation involves endogenous ABA biosynthesis in Arachis hypogaea L. Plant Growth Regul 58:173–179
Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124:1437–1448
Hetz W, Hochholdinger F, Schwall M, Feix G (1996) Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J 10:845–857
Hinchee MAW, Rost TL (1986) The control of lateral root development in cultured pea seedlings. I. The role of seedling organs and plant growth regulators. Bot Gaz 147:137–147
Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7:211–220
Hochholdinger F, Woll D, Sauer M, Debmbinsky D (2004) Genetic dissection of root formation in maize (Zea mays) reveals root-type specific development programmes. Ann Bot 93:359–368
Ishikawa H, Evans ML (1993) The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol 102:1203–1210
Ishikawa H, Evans MI (1995) Specialized zones of development in roots. Plant Physiol 109:725–727
Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55:335–347
Ivanov VB (1997) Relationship between cell proliferation and transition to elongation in plant roots. Int J Dev Biol 41:907–915
Jackson MB (1991) Ethylene in root growth and development. In: Matoo AK, Suttle JC (eds) The Plant Hormone Ethylene. CRC Press, Boca Raton, FL, pp 159–181
Joo S, Seo YS, Kim SM, Hong DK, Park KY, Kim WT (2006) Brassinosteroids induction of AtACS4 encoding auxin-responsive 1-aminocyclopropane-1-carboxylate synthase 4 in Arabidopsis seedlings. Physiol Plant 126:592–604
Keller B, Lamb CJ (1989) Specific expression of a novel cell wall hydroxyproline-rich glycoprotein gene in lateral root initiation. Genes Dev 3:1639–1646
Kerk NM, Feldman LJ (1995) A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development 121:2825–2833
Kerk NM, Jiang KN, Feldman LJ (2000) Auxin metabolism in the root apical meristem. Plant Physiol 122:925–932
Klee HJ, Horsch RB, Hinchee MA, Hein MB, Hoffmann NL (1987) The effects of overproduction of two Agrobacterium tumefaciens T-DNA auxin biosynthetic gene products in transgenic petunia plants. Genes Dev 1:86–96
Laplaze L, Benkova E, Casimiro I, Maes L, Vanneste S, Swarup R, Weijers D, Calvo V, Parizot B, Herrera-Rodriguez MB, Offringa R, Graham N, Doumas P, Friml J, Bogusz D, Beeckman T, Bennett M (2007) Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19:3889–3900
Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM (1995) Formation of lateral root meristems is a two-stage process. Development 121:3303–3310
Lee JS, Chang WK, Evans ML (1990) Effects of ethylene on the kinetics of curvature and auxin redistribution in gravistimulated roots of Zea mays. Plant Physiol 94:1770–1775
Li JS, Dai XH, Zhao YD (2006a) A role for auxin response factor 19 in auxin and ethylene signaling in Arabidopsis. Plant Physiol 140:899–908
Li X, Mo X, Shou H, Wu P (2006b) Cytokinin mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant Cell Physiol 47:1112–1123
Lloret PG, Casero PJ (2002) Lateral root initiation. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Dekker, New York, pp 127–155
Lloret PG, Pulgarín A (1992) Effect of naphthaleneacetic acid on the formation of lateral roots in the adventitious root of Allium cepa: number and arrangement of laterals along the parent root. Can J Bot 70:1891–1896
Ma Z, Baskin TI, Brown KM, Lynch JP (2003) Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiol 131:1381–1390
MacIsaac SA, Sawhney VK, Pohorecky Y (1989) Regulation of lateral root formation in lettuce (Lactuca sativa) seedling roots: Interacting effects of α-naphthaleneacetic acid and kinetin. Physiol Plant 77:287–293
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
Malamy JE, Benfey PN (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124:33–44
Masucci JD, Rerie WG, Foreman DR, Zhang M, Galway ME, Marks MD, Schiefelbein JW (1996) The homeobox gene GLABRA 2 is required for position-dependent cell differentiation in the root epidermis of Arabidopsis thaliana. Development 122:1253–1260
McDavid CR, Sagar GR, Marshall C (1972) The effect of auxin from the shoot on root development in Pisum sativum L. New Phytol 71:1027–1032
Mergemann H, Sauter M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 124:609–614
Muday GK, DeLong A (2001) Polar auxin transport: controlling where and how much. Trends Plant Sci 6:535–542
Muday GK, Haworth P (1994) Tomato root growth, gravitropism, and lateral development: Correlation with auxin transport. Plant Physiol Biochem 32:193–203
Muday GK, Lomax TL, Rayle DL (1995) Characterization of the growth and auxin physiology of roots of the tomato mutant, diageotropica. Planta 195:548–553
Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17:181–95
Mulkey TJ, Kuzmanoff KM, Evans ML (1981) Promotion of growth and shift in the auxin dose/response relationship in maize roots treated with the ethylene biosynthesis inhibitors aminoethoxyvinylglycine and cobalt. Plant Sci Lett 25:43–48
Mulkey TJ, Kuzmanoff KM, Evans ML (1982) Promotion of growth and hydrogen ion efflux by auxin in roots of maize pretreated with ethylene biosynthesis inhibitors. Plant Physiol 70:186–188
Muller B, Stosser M, Tardieu F (1998) Spatial distributions of tissue expansion and cell division rates are related to irradiance and to sugar content in the growing zone of maize roots. Plant Cell Environ 21:149–158
Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55:175–187
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Péret B, Li GW, Zhao J, Band LR, Voss U, Postaire O, Luu DT, Da Ines O, Casimiro I, Lucas M, Wells DM, Lazzerini L, Nacry P, King JR, Jensen OE, Schäffner AR, Maurel C, Bennett MJ (2012) Auxin regulates aquaporin function to facilitate lateral root emergence. Nat Cell Biol 14:991–998
Peretto R, Favaron F, Bettini V, De Lorenzo G, Marini S, Alghisi P, Cervone F, Bonfante P (1992) Expression and localization of polygalacturonase during the outgrowth of lateral roots in Allium porrum L. Planta 188:164–172
Peterson RL, Peterson CA (1986) Ontogeny and anatomy of lateral roots. In: Jackson MB (ed) New root formation in plants and cuttings. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 1–30
Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth inhibition and stimulation. Trends Plant Sci 11:176–183
Pilet PE, Barlow PW (1987) The role of abscisic acid in root growth and gravireaction: A critical review. Plant Growth Regul 6:217–265
Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16:553–560
Pond RH (1908) Emergence of lateral roots. Bot Gaz 46:410–421
Prasad ME, Schofield A, Lyzenga W, Liu HX, Stone SL (2010) Arabidopsis RING E3 ligase XBAT32 regulates lateral root production through its role in ethylene biosynthesis. Plant Physiol 153:1587–1596
Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S (2002) Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130:1908–1917
Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122:481–490
Rashotte AM, Poupart J, Waddell CS, Muday GK (2003) Transport of the two natural auxins, indole-3-butiric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133:761–772
Raya-González J, Pelagio-Flores R, López-Bucio J (2012) The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J Plant Physiol 169:1348–1358
Rayle DL, Cleland R (1970) Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 46:250–253
Reed RC, Brady SR, Muday GK (1998) Inhibition of auxin movement from the shoot into the root inhibits lateral root development in Arabidopsis. Plant Physiol 118:1369–1378
Roberts JA, Whitelaw CA, González-Carranza ZH, McManus MT (2000) Cell separation processes in plants. Models, mechanisms and manipulation. Ann Bot 86:223–235
Rodrigues-Pousada RA, De Rycke R, Dedonder A, Van Caeneghem W, Engler G, Van Montagu M, Van der Straeten D (1993) The Arabidopsis 1-aminocyclopropane-1-carboxylate synthase gene 1 is expressed during early development. Plant Cell 5:897–911
Rodrigues-Pousada R, Van Caeneghem W, Chauvaux N, Van Onekelen H, Van Montagu M, Van der Straeten D (1999) Hormonal cross-talk regulates the Arabidopsis thaliana 1-aminocyclopropane-1-carboxylate synthase gene 1 in a developmental and tissue-dependent manner. Physiol Plant 105:312–320
Rowntree RA, Morris DA (1979) Accumulation of 14C from exogenous labelled auxin in lateral root primordia of intact pea seedlings (Pisum sativum L.). Planta 144:463–466
Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M (1997) Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9:745–757
Ruzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Firml J, Benková E (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19:2197–2212
Ruzicka K, Simaskova M, Duclercq J, Petrasek J, Zazimalova E, Simon S, Friml J, Van Montagu MCE, Benková E (2009) Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc Natl Acad Sci U S A 106:4284–4289
Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy J, Benfey P, Leyser O, Bechtold N, Weisbeek P, Scheres B (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99:463–472
Santisree P, Nongmaithem S, Vasuki H, Sreelakshmi Y, Ivanchenko MG, Sharma R (2011) Tomato root penetration in soil requires a coaction between ethylene and auxin signaling. Plant Physiol 156:1424–1438
Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T (2007) Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446:811–814
Sarquis JI, Jordan WR, Morgan PW (1992) Effect of atmospheric pressure on maize root growth and ethylene production. Plant Physiol 100:2106–2108
Seago JL (1973) Developmental anatomy in roots of Ipomoea purpurea. II. Initiation and development of secondary roots. Am J Bot 60:607–618
Segura J (2000) Introducción al desarrollo. Concepto de hormona vegetal. In: Azcon-Bieto J, Talón M (eds) Fundamentos de Fisiología Vegetal. McGraw-Hill, Madrid, pp 285–303
Shkolnik-Inbar D, Bar-Zvi D (2010) ABI4 mediates abscisic acid and cytokinin inhibition of lateral root formation by reducing polar auxin transport in Arabidopsis. Plant Cell 22:3560–3573
Sievers A, Braun M, Monshausen GB (2002) The root cap: Structure and function. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Dekker, New York, pp 33–47
Silk WK (2002) The kinematic of primary growth. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half, 3rd edn. Dekker, New York, pp 113–116
Smalle J, Van der Straeten D (1997) Ethylene and vegetative development. Physiol Plant 100:593–605
Steinitz B, Barr N, Tabib Y, Vaknin Y, Bernstein N (2010) Control of in vitro rooting and plant development in Corymbia maculata by silver nitrate, silver thiosulfate and thiosulfate ion. Plant Cell Rep 29:1315–1323
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel Interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Strader LC, Beisner ER, Bartel B (2009) Silver ions increase auxin efflux independently of effects on ethylene response. Plant Cell 21:3585–3590
Sun P, Tian QY, Chen J, Zhang WH (2010) Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J Exp Bot 61:347–356
Sutcliffe JF, Sexton R (1968) β-Glycerophosphatase and lateral root development. Nature 217:1285
Sutherland J, McCully ME (1976) A note on the structural changes in the walls of pericycle cells initiating lateral root meristems in Zea mays. Can J Bot 54:2083–2087
Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GTS, Sandberg G, Bhalerao R, Ljung K, Bennett M (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19:2186–2196
Taiz L (1994) Expansins: Proteins that promote cell wall loosening in plants. Proc Natl Acad Sci U S A 91:7387–7389
Tanimoto M, Roberts K, Dolan L (1995) Ethylene is a positive regulator of root hair development in Arabidopsis thaliana. Plant J 8:943–948
Thimann KV (1936) Auxins and the growth of roots. Am J Bot 23:561–569
Torrey JG (1962) Auxin and purine interactions in lateral root initiation in isolated pea root segments. Physiol Plant 45:177–185
Tsurumi S, Ohwaki Y (1978) Transport of 14C-labeled indoleacetic acid in Vicia faba root segments. Plant Cell Physiol 19:1195–1206
Van Staden J, Davey JE (1979) The synthesis, transport and metabolism of endogenous cytokinins. Plant Cell Environ 2:93–106
Van Staden J, Ntingane BM (1996) The effect of a combination of decapitation treatments, zeatin and benzyladenine on the initiation and emergence of lateral roots in Pisum sativum. S Afr J Bot 62:11–16
Van Tieghem P, Douliot H (1888) Recherches comparatives sur l'origine des membres endogènes dans les plantes vasculaires. Ann Sci Nat (Botanique), 7ième série 8:1–660
Vuylsteker C, Dewaele E, Rambour S (1998) Auxin induced lateral root formation in chicory. Ann Bot 81:449–454
Wang L, Hua DP, He JN, Duan Y, Chen ZZ, Hong XH, Gong ZZ (2011) Auxin response factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet 7:e1002172
Webster BD, Radin JW (1972) Growth and development of cultured radish roots. Am J Bot 59:744–751
Wen TJ, Schnable PS (1994) Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. Am J Bot 81:833–842
Wightman F, Schneider EA, Thimann KV (1980) Hormonal factors controlling the initiation and development of lateral roots. II. Effects of exogenous growth factors on lateral root formation in pea roots. Physiol Plant 49:304–314
Yang RL, Evans ML, Moore R (1990) Microsurgical removal of epidermal and cortical cells evidence that the gravitropic signal moves through the outer cell layers in primary roots of maize. Planta 180:530–536
Yin CX, Wu QR, Zeng HL, Xia K, Xu JW, Li RW (2011) Endogenous Auxin is Required but Supraoptimal for Rapid Growth of Rice (Oryza sativa L.) Seminal Roots, and Auxin Inhibition of Rice Seminal Root Growth is Not Caused by Ethylene. J Plant Growth Regul 30:20–29
Zacarias L, Reid MS (1992) Inhibition of ethylene action prevents root penetration through compressed media in tomato (Lycopersicon esculentum) seedlings. Physiol Plant 86:301–307
Zeadan SM, MacLeod RD (1984) Some effects of indol-3-yl-acetic acid on lateral root development in attached and excised roots of Pisum sativum L. Ann Bot 54:759–766
Zeier J, Ruel K, Ryser U, Schreiber L (1999) Chemical analysis and immunolocalisation of lignin and suberin in endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta 209:1–12
Zhang NG, Hasenstein KH (1999) Initiation and elongation of lateral roots in Lactuca sativa. Int J Plant Sci 160:511–519
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Alarcón, M.V., Lloret, P.G., Salguero, J. (2014). The Development of the Maize Root System: Role of Auxin and Ethylene. In: Morte, A., Varma, A. (eds) Root Engineering. Soil Biology, vol 40. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54276-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-54276-3_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54275-6
Online ISBN: 978-3-642-54276-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)