Abstract
Nitrogen (N) is a major constituent of plant macromolecules, and nitrate (NO3 –) is the predominant form of inorganic N available to higher plants in aerobic soils. The concentration of NO3 – in soil solutions ranges from lower than 100 μM in natural ecosystems to higher than 10 mM in agricultural ecosystems. Nitrate, both from indigenous soil resources and from N inputs contributing to the plant-available soil N pool, varies greatly temporally and spatially. This N pool becomes the object of intense competition among plants and, in some environments, between plants and microorganisms. To cope with the heterogeneity of NO3 – concentration in the soil, to meet the energy requirements for its assimilation, and to secure a favorable carbon/nitrogen balance within the plant, plant roots have evolved diverse strategies for NO3 – acquisition. These strategies extend from (i) the ecosystem level, where there are associations with some microorganisms to acquire NO3 – and competition with others for NO3 –; (ii) the organism level, where plants optimize the allocation of resources between roots and shoots and the pattern of root system branching; and (iii) the molecular level, where the location (root vs. shoot) and time (diurnal cycles) of NO3 – uptake, transport, and assimilation must adapt to the prevailing environmental conditions. Understanding the diverse strategies that plant roots employ to convert external NO3 – to organic N will provide valuable information that can be used to improve plant N utilization efficiency and thereby enhance the sustainability of agricultural production under changing climates.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nitrogen is an essential element in biological molecules, such as nucleotides, amino acids, and proteins, and therefore fundamental for plant growth and development (Marschner 1995). In fact, plant performance, fitness, yield, nutrient efficiency, or susceptibility to biological and environmental stresses is highly dependent on nitrogen mineral nutrition (Epstein and Bloom 2005). Some plants can fix atmospheric nitrogen into organic forms through symbiotic relationships with soil microbes (not covered here), but most plants obtain their nitrogen directly from the soil via absorption of inorganic and organic forms of nitrogen. The concentrations of various forms of N (e.g., organic N, ammonium, nitrate, nitrite, nitrous oxide) in the soil depend on soil type, temperature, and the activities of microorganisms (Nasholm et al. 1998; Jackson et al. 2008).
Nitrate (NO3 –) is the major N source for higher plants in most agricultural and temperate zone soils (Epstein and Bloom 2005). Distribution and concentration of NO3 – in soils show substantial spatial and temporal heterogeneity (Jackson and Caldwell 1993), and NO3 – is the object of intense competition among neighboring plants (Cahill et al. 2010) and between plants and microorganisms (Jackson et al. 1989; Hodge et al. 2000; Miller et al. 2007a). Under conditions of limited NO3 – availability, the ability of a plant to acquire NO3 – from its surroundings largely depends on the amount of root area in contact with NO3 – in the soil solution and on the efficiency with which a root can transport NO3 – from its surroundings into the plant. Under conditions of unlimited soil NO3 – availability, roots absorb superfluous amounts, and root and shoot NO3 – concentrations can reach up to 100 mM, most of which is stored within vacuoles (Miller and Smith 1996). Despite considerable variation in the concentration of NO3 – in the soil solution, root cells keep cytosolic NO3 – at a controlled level, with values of approximately 3 mM in maize and 4–5 mM in barley (Miller and Smith 1996, 2008; Huang et al. 2012), possibly to minimize oxidative stress (Huang et al. 2012). Nitrate assimilation plays a central role in this homeostasis, along with NO3 – uptake, efflux, and xylem and vacuolar loading (Crawford and Glass 1998). Nitrate, in addition to being an important nutrient, may serve as an osmoticum for supporting root elongation and may act as signal molecule regulating nitrogen and carbon metabolism and coordinating whole-plant development (Redinbaugh and Campbell 1991; Crawford 1995; Miller et al. 2007b; Krouk et al. 2010a; Dechorgnat et al. 2011; Bloom et al. 2012a). Root strategies for NO3 – acquisition must cope with soil NO3 – heterogeneity and cytosolic NO3 – homeostasis as well as coordinate root growth with NO3 – sensing, uptake, translocation, and finally, assimilation to organic N.
The relationship between nitrogen acquisition and roots has long been of interest in plant biology and agriculture because of its influence in plant growth and food production (Oaks and Hirel 1985; Oaks 1992). The topic has been reviewed by Miller and Cramer (2005), with an emphasis in the molecular mechanisms that plants use in accessing N in the soil pools; by Jackson et al. (2008), with an emphasis on physiological and ecological functions that contribute to plant–microbe–soil N cycling; by Kraiser et al. (2011), focusing on the integration of nitrogen acquisition strategies from the ecosystem to molecular level; by Forde and Walch-Liu (2009) and Krouk et al. (2010a), with an emphasis in the role of NO3 – as a signal and its influence on root behavior and NO3 – regulation, respectively. Here, we review the different strategies that plant roots follow to acquire NO3 – from the soil.
2 Nitrate in the Soil
2.1 Origins and Fates
In natural ecosystems, NO3 – is the dominant form of nitrogen available to plants in all but very acidic and anaerobic soils, because of both the ability of particular soil microorganisms (e.g., Nitrosomonas, Nitrosospiras, and Nitrobacter species) to convert ammonium ion (NH4 +) to NO3 – and the ubiquitous distribution of these organisms (Oaks 1992; Hiorns et al. 1995). In agricultural systems, the baseline level of NO3 – is supplemented by the addition of N fertilizers to the soil. Soil NO3 – concentration averages 1 mM in natural ecosystems (Andrews 1986), whereas it averages 10 mM, ranging from 0 to 70 mM, in most agricultural systems (Reisenauer 1966). In both natural and agricultural ecosystems, NO3 – originates (i) through microbial decomposition of soil organic matter via intermediates (nitrification), (ii) through biological fixation, (iii) from atmospheric deposition, and (iv) only in agricultural ecosystems, from the incorporation of fertilizers. The major pathways of NO3 – losses from soil include (i) leaching to surface and ground water, (ii) microbial conversion to N2O and N2 (denitrification), (iii) microbes immobilization, and (iv) soil erosion.
The appearance and disappearance of soil NO3 – rapidly changes with rainfall and other factors influencing microbial activity such as pH, temperature, and oxygen concentrations, which in turn affect mineralization, nitrification, and denitrification (Haynes 1986). Nitrate with its negative charge is not adsorbed on negatively charged soil particle surfaces, allowing it to move relatively freely through the soil. For these reasons, NO3 – concentrations in natural and agricultural soils fluctuate greatly both temporally, even diurnally, and spatially even over short distances (Burger and Jackson 2004).
2.2 Nitrate in the Rhizosphere
In natural ecosystems, root acquisition of exogenous NO3 – is a function of its availability, whereas in croplands the addition of fertilizers containing NO3 – decreases the element of chance (Oaks 1992). NO3 – enters the rhizosphere because of NO3 – mobility in the soil (the NO3 – reaches the root) or activities of the root system itself (the root reaches the NO3 –). In the first case, NO3 – mobility in soils results in rapid diffusion to roots and thus easier plant access to the available NO3 – (Boudsocq et al. 2012). NO3 – can also reach the rhizosphere by mass flow, linked to transpiration and the depletion of solution near the root surface (Marschner 1995). In the second case, (i) the roots can directly intercept the NO3 – through their growth or (ii) the roots of some plants can change the rhizosphere’s conditions such as releasing oxygen (Kirk and Kronzucker 2005; Li et al. 2008) and exudates (Bais et al. 2006) that greatly influence the density and activity of microbial populations, which in turn can accelerate nitrification of NH4 + in the rhizosphere.
3 Root Strategies for Nitrate Acquisition
3.1 Ecosystem Level
3.1.1 Associations with Microorganisms
Roots form associations with microorganisms as a strategy to enhance resource capture (Hodge 2009; Kraiser et al. 2011). The majority of plants are capable of associating with arbuscular mycorrhizal fungi, which induce modifications in root system architecture (RSA) (Gutjahr et al. 2009). These modifications can increase plant NO3 – uptake and improve the nitrogen nutrition of plants, mainly those growing at low levels of nutrients (Cruz et al. 2004). Plant roots also associate with bacteria, which can increase nutrient accessibility, uptake, or both (Bertrand et al. 2000), improving plant growth. These bacteria are referred to as plant growth-promoting bacteria and can produce phytohormones affecting RSA (Persello-Cartieaux et al. 2001) or increase the activity of NO3 – uptake systems (Bertrand et al. 2000).
3.1.2 Competing for Nitrate
Nitrate in the soil is very dynamic and an “object of desire” to individuals of the same species (intraspecific competition) and among different species, regardless of taxonomic affiliation (interspecific competition) (Hodge et al. 2000; Schenk 2006; Cahill and McNickle 2011). The rapid diffusion of NO3 – through the soil allows different individual plants to be highly efficient at acquiring NO3 – even when they have restricted or simple RSA (Fitter et al. 2002). Some plants display pronounced proliferation in response to locally applied NO3 – (Drew 1975; Guo et al. 2002). Because of the mobility of NO3 – ions in soil, such proliferation increases NO3 – acquisition by plants when they are in competition with neighbors for a finite, spatially restricted, mixed N source (Robinson et al. 1999).
Not all plant species respond to the nutrient-rich zones in the same way (Campbell et al. 1991). Competitively dominant plants exploit nutrient-rich patches to a greater extent simply because they are larger and have higher growth rates rather than because they have greater flexibility within their root system. In contrast, competitively inferior plants, although smaller, allocate more of their new root growth to nutrient-rich areas; that is, they place their new roots with greater precision. Among plants and microorganism, Jackson et al. (1989) showed that in annual grasslands, the NO3 – pool is consumed as rapidly as it is produced, and microbial uptake is the major factor controlling NO3 – availability to plants. In the short term (hours), soil microorganisms do compete better than plants for NO3 – (Jackson et al. 1989), but after longer periods (days to weeks), plants absorb more NO3 – than microbes do because of microbial turnover (Inselsbacher et al. 2010). Recent work in which substantial microbes turnover was prevented, however, suggested that plants compete directly and more efficiently for NO3 – than microbes (Inselsbacher et al. 2010). Spatial differences in nitrogen availability, distribution of root and microorganisms, relative turnover times of roots and microorganisms, and changes in the soil C:N ratio are the key determinants of “success” in the competition for NO3 – (Hodge et al. 2000).
3.2 Organism Level
3.2.1 Resource Allocation to Roots
All plants face a basic economic decision: where best to invest their resources (Bloom et al. 1985). The costs associated with getting this wrong may lead to diminished nutrient capture; less resources for reproduction and hence reduced fitness; and at the extreme, competitive exclusion from the particular environment (Hodge 2009). When the roots of some plants encounter a NO3 –-rich patch, their growth becomes enhanced at the expense of the growth in poorer resource areas (Drew 1975). This mean that root development, and especially lateral root initiation, depends on the integrated effects of the local environment and the internal correlative relations between the roots (Gersani and Sachs 1992).
Allocation of resources to above vs. belowground structures or to different parts of the root systems can make budgetary sense. In general, resource allocation to belowground structures relative to aboveground structures increases when N is limiting (Miller and Cramer 2005) (Fig. 12.1). This compensates for N deficiency by increasing the plant’s opportunity to obtain N for sustaining growth (Reynolds and Dantonio 1996; Sims et al. 2012). Such an acclimation response derives from metabolic changes in the shoot and an adjustment of carbohydrate transport to the root (Hermans et al. 2006). NO3 – in the shoot acts as a long-range signal molecule that regulates root growth (Adgo and Schulze 2002). Among the several changes that result in NO3 – accumulation in the shoot and could contribute to altered allocation patterns include an inhibition of starch synthesis and turnover in the leaves and a decrease of the transport of sucrose to the roots, resulting in an increase in the root-to-shoot ratio (Rufty et al. 1988). In Arabidopsis plants where tissue N is plentiful, NO3 – can specifically inhibit root system growth while having no effect on shoot system growth (Roycewicz and Malamy 2012). This suggests that plants regulate root-to-shoot ratio not specifically in response to nitrogen starvation but as a general mechanism to tailor their growth to environmental nitrogen supply.
3.2.2 Root Architecture
The root system is fundamentally important for plant growth and survival because of its role in water and nutrient uptake. The architecture of the root system is determined by the pattern of root branching, which in many species displays a high degree of plasticity to enable plant survival under variable environmental conditions (Sultan 2000; Hodge 2009). Soil NO3 – concentration and overall nutrient status of the plant in concert with the genetic makeup of the plant will determine the pattern of root branching and define the root strategies for NO3 – acquisition (Hodge 2004; Desnos 2008; Vidal et al. 2010).
First of all, when encountering a NO3 –-rich patch, a plant maximizes N capture through upregulating inflow and making a more competitive root system (Robinson et al. 1999). The strategies affecting RSA involve lateral root elongation, lateral root initiation, and primary root growth (Kraiser et al. 2011). A localized area of high NO3 – stimulates the elongation of lateral roots through a dual-affinity nitrate transporter, CHL1, that not only transports NO3 – but also senses external NO3 – concentrations (Ho et al. 2009) and activates the NITRATE REGULATED 1 (ANR1) (an MADS-box gene) that control changes in root architecture (Guo et al. 2002). ANR1 initiates a local-range signaling pathway and regulates NO3 –-stimulated lateral root elongation (Remans et al. 2006a). When plants are grown on high NO3 – or have high levels of N metabolites, the expression of ANR1 decreases and lateral root elongation is suppressed (Zhang et al. 1999; Gansel et al. 2001). The net result is that, if roots grown on low NO3 – are exposed to a localized region of high NO3 –, then lateral roots proliferate specifically in that region of the roots. The putative high-affinity NO3 – transporter NRT2.1, like NRT1.1, serves as a NO3 – sensor to coordinate the development of the root system. It acts directly on lateral root initiation under NO3 –-limiting conditions (Lejay et al. 1999; Little et al. 2005; Remans et al. 2006b).
It is interesting to note that root proliferation occurs in localized patches of NO3 –, which is a relatively mobile nutrient, but not in localized patches of NH4 +, which is a relatively immobile nutrient (Leyser and Fitter 1998). A possible explanation for this paradox might be the very mobility of the NO3 – ion, which makes it an ideal subterranean signal molecule (Forde and Zhang 1998). In unfertilized soils, a major source of nitrogen is from decaying organic matter, which under aerobic conditions releases both NH4 + and NO3 –. The relative immobility of NH4 + means that it is the NO3 – ion that will be the first to reach nearby roots through the soil solution. In this scenario, NO3 – provides the signal that allows roots to proliferate towards areas where less mobile forms of N are localized within the soil.
Finally, NO3 – itself stimulates primary root growth, both directly and by antagonizing the inhibitory effect of l-glutamate (Walch-Liu and Forde 2008). This highlights that relative abundance of inorganic nitrogen (e.g., NO3 –) and organic N (e.g., glutamate) influences RSA and therefore NO3 – acquisition. In several studies RSA adaptation to external NO3 – availability was observed whereby NO3 – was shown to interact with auxin, abscisic acid, and cytokinin signaling pathways (Signora et al. 2001; Garnett et al. 2009; Krouk et al. 2010b; Vidal et al. 2010; Ruffel et al. 2011; Wang et al. 2012).
There are other attributes of a root system in addition to its architecture that dictate its capacity and efficiency for NO3 – acquisition (Gastal and Lemaire 2002; Miller and Cramer 2005; Volder et al. 2005; Garnett et al. 2009; Hodge 2009). These include the proportion of active roots, root longevity, rooting depth, proportion of fine roots versus thick roots, and number, size, and location of the root hairs.
3.3 Molecular Level
3.3.1 Root Uptake Systems
To cope with the heterogeneity and dynamic variations of NO3 – concentrations, plants have evolved at least three NO3 – transport systems that function according to enzyme kinetics (Crawford and Glass 1998): (i) constitutive (active even when plants have not been previously supplied with NO3 –) high-affinity transport systems (CHATS) with low values of both K m (concentration of substrate that gives “half-maximum” absorption) and V max (maximum rate of absorption), typically 6–20 μM and 0.3–0.82 μmol g−1 h−1, respectively; (ii) NO3 –-inducible (hours to days of exposure to NO3 –) high-affinity transport systems (IHATS) with higher K m and V max values, typically 20–100 μM and 3–8 μmol g−1 h−1, respectively; and (iii) constitutive low-affinity transport systems (LATS), which become evident when NO3 – is plentiful (above 250 μM). Uptake by HATS and LATS is mediated by two families of NO3 – transporters, NRT1 and NRT2, respectively (Wang et al. 2012). The relative contribution of these transporters to NO3 – uptake is regulated by negative feedback, linking the expression and activity of NO3 – uptake to the C:N status of the plant (Lejay et al. 1999; Miller et al. 2007b): under low external NO3 – concentration, plants upregulate HATS, while under high external NO3 – concentration or when fertilizers are applied, plants change their dependence from the HATS pathway and root–microbe associations to the LATS pathway. Like RSA, local and long-range signaling pathways regulate the activity of NO3 – transport system in response to both external NO3 – and sugars transported from the shoot (Forde 2002).
3.3.2 Vacuolar Loading and Long-Distance Transport
Once inside the root cell cytoplast, NO3 – can be translocated across the tonoplast and stored in the vacuoles, be loaded into the xylem vessels and subsequently unloaded in plant aerial tissues, or enter the amino acid biosynthesis pathway (Wang et al. 2012). Nitrate may be an important osmoticum solute in the vacuole of root cells (Zhen et al. 1991). Also in the base of the root growth zone, NO3 – can be considered a significant component of the osmotic pool supporting its expansion (Bloom et al. 2012a). Long-distance NO3 – transport (e.g., root-to-shoot NO3 – transport) is finely tuned in response to various environmental conditions (Smirnoff and Stewart 1985). The influence of the environment on long-distance NO3 – transport (e.g., transport from root to shoot) is evident in the different shoot and root NO3 – concentrations found during different times of the day and between plant species (Fig. 12.2). AtNRT1.5 mediates the first step in loading of NO3 – into xylem vessels and facilitates root-to-shoot xylem NO3 – transport (Lin et al. 2008). AtNRT1.8 and AtNRT1.9 regulate root-to-shoot NO3 – translocation; in specific, AtNRT1.8 mediates NO3 – removal from xylem and may also diminish root-to-shoot NO3 – transport (Li et al. 2010), whereas AtNRT1.9 facilitates the loading of NO3 – into the root phloem and enhances downward NO3 – transport in roots (Wang and Tsay 2011). AtNRT1.9 prevents excess amounts of NO3 – being transported to the shoot. For example, in different ecotypes of Arabidopsis, the capacity to maintain NO3 – reserves under low NO3 – supply confers higher tolerance to low NO3 – environments (North et al. 2009). All together these studies show that roots play a key step in regulating NO3 – distribution in the plant and again highlight that NO3 – is a key component in the regulation of plant development and growth.
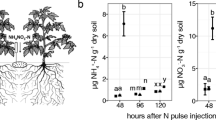
Shoot and root nitrate concentration of wheat (14 days old) and Arabidopsis (40 days old) plants after the light and dark period. Wheat and Arabidopsis plant growth in nutrient solution containing NO3 – (0.2 mM) as solely N source and in a light/dark cycle of 16/8 and 9/15 h, respectively (Bloom et al. unpublished data)
3.3.3 Assimilation: Where and When?
NO3 – that is not transported to the shoot or vacuoles is reduced to nitrite (NO2 –) in the cytosol via NO3 – reductase and then further to NH4 + by NO2 – reductase in the plastids (Crawford et al. 2000). NH4 + is then added to C skeletons to produced glutamine and glutamate through the sequential actions of glutamine synthetase and glutamate synthase, which are located in the root plastids (Lam et al. 1996). In the root, carbohydrate oxidation provides the approximate 10 mol ATP and reductants needed for NO3 – assimilation (Neuhaus and Emes 2000). The biochemical reactions responsible for root NO3 – uptake and assimilation are so energy intensive that this process largely determines the carbon balance of a plant as well as its nitrogen budget (Bloom et al. 1992). Despite considerable research effort, the relative proportion of NO3 – that is reduced in the shoot and root is still a matter of considerable debate (Nunes-Nesi et al. 2010). It is known that NO3 – assimilation may vary between the root and the shoot tissue depending on the species and the growth conditions (Miller and Cramer 2005). Generally, species native to temperate regions rely more heavily on root NO3 – assimilation than do species of tropical or subtropical origins (Andrews 1986). Roots also appear to be the predominant site of NO3 – assimilation when plants are grown under low external NO3 – availability (Gojon et al. 1991; Scheurwater et al. 2002).
Timing, as well as location, of NO3 – assimilation will have significant implications for the plant energy budget and therefore for plant performance and adaptation (Nunes-Nesi et al. 2010). During the day, shoots can use surplus light to assimilate NO3 – and divert relatively little energy away from photosynthetic carbon assimilation and thus detract little from plant growth (McDermitt and Loomis 1981; Bloom et al. 1989). Under these conditions, NO3 – assimilation will have lower energy cost in the leaf than in the root, because the reducing equivalents and ATP for NO3 – assimilation are obtained without any decrease in the rate of CO2 fixation. When light limits photosynthesis or during the night, however, no advantage will be gained in assimilating NO3 – in the shoot, because NO3 – assimilation and CO2 fixation will directly compete for ATP and reductant generated by photosynthetic electron transport (Canvin and Atkins 1974), leading to a decrease in CO2 fixation. Another disadvantage of leaf NO3 – assimilation, independent of the light level, is that hydroxyl ions generated in the leaf during this process must be neutralized by the synthesis of organic acids (in the root, the pH balance may be maintained via decreased proton excretion or increased bicarbonate excretion) (Smirnoff and Stewart 1985). In vitro studies showed that day and night cycles do not influence NO3 – reductase activities in roots, suggesting that rates of NO3 – assimilation in the root are similar day and night (Stohr and Mack 2001). Still, the in vivo rates of root NO3 − assimilation in comparison with shoot NO3 − assimilation and the influence of light and dark cycle away its clarification. In addition, root NO3 − assimilation will gain in both physiological and ecological importance because the elevated atmospheric CO2 concentrations anticipated during the next few decades strongly inhibit shoot NO3 − assimilation in C3 plants (Bloom et al. 2010, 2012b).
Because leaf NR is a highly regulated enzyme (Lillo et al. 2004), NO3 – assimilation is central for achieving cytosolic NO3 – homeostasis (Cookson et al. 2005; Huang et al. 2012). Roots also maintain cytosolic NO3 – homeostasis under deprivation and resupply of NO3 – (Zhen et al. 1991; van der Leij et al. 1998), but the role of NO3 – assimilation in keeping cytosolic NO3 – homeostasis in the root has received less attention probably because the physiological and/or environmental events that lead to changes in root NR activity are less obvious.
4 Nitrate Use Efficiency
To achieve the doubling in global food production anticipated during the next 50 years will require a threefold increase in nitrogen fertilization rate (Frink et al. 1999). Nitrogen fertilizer will play a key role in this expansion and intensification of agriculture. Unfortunately, excess N compounds released from agricultural systems are detrimental to the environment, threatening the quality of air, water, and soil (Canfield et al. 2010). In addition, such releases are a waste of valuable resources and may cause human health problems. Today, intensification of agriculture must be done through nitrogen management strategies that do not compromise the environment (Matson et al. 1997; Godfray et al. 2010). To this end, improving the efficiency with which plants obtain nitrogen from the environment is of critical importance (Xu et al. 2012), and nitrogen use efficiency (NUE) of individual plants plays a key role. NUE has two components, nitrogen uptake efficiency (NUpE) and nitrogen utilization efficiency (NUtE) (Epstein and Bloom 2005). Roots are the key plant organ for improvement of both NUpE and NUtE (Xu et al. 2012) because they determined NO3 – uptake and have a critical role in NO3 – assimilation and transport to other parts of the plant. Root traits can be selected using traditional breeding and marker-assisted selection (Good et al. 2004). Natural variation of NUE in genetic resources can help to select root traits (Chardon et al. 2010). Some important root traits seem to be controlled by a single dominant gene (Werner et al. 2010); for example, overexpressing cytokinin-degrading cytokinin oxidase/dehydrogenase (CKX) genes resulted in an elongation of primary root and an increase in root branching and root biomass. NUpE can be improved by targeting root morphology, root-to-shoot ratios, and root NO3 – transporters (Garnett et al. 2009; Werner et al. 2010). NUtE can be improved by targeting NO3 – assimilation enzymes (Andrews et al. 2004) and mitochondria metabolism (Foyer et al. 2011). Nevertheless, a combination of traditional breeding and transgenic approaches will be needed to make significant improvements in NUE because of the multiple interacting genetic and environmental factors that govern NUE.
5 Conclusions
Nitrogen (N) is a major constituent of plant macromolecules, and nitrate (NO3 –) is the predominant form of inorganic N available to higher plants in aerobic soils. The concentration of NO3 – in soil solutions ranges from lower than 100 μM in natural ecosystems to higher than 10 mM in agricultural ecosystems. Nitrate, both from indigenous soil resources and from N inputs contributing to the plant-available soil N pool, varies greatly temporally and spatially. This N pool becomes the object of intense competition among plants and, in some environments, between plants and microorganisms. To cope with the heterogeneity of NO3 – concentration in the soil, to meet the energy requirements for its assimilation, and to secure a favorable carbon/nitrogen balance within the plant, plant roots have evolved diverse strategies for NO3 – acquisition. These strategies extend from (i) the ecosystem level, where there are associations with some microorganisms to acquire NO3 – and competition with others for NO3 –; (ii) the organism level, where plants optimize the allocation of resources between roots and shoots and the pattern of root system branching; and (iii) the molecular level, where the location (root vs. shoot) and time (diurnal cycles) of NO3 – uptake, transport, and assimilation must adapt to the prevailing environmental conditions. Understanding the diverse strategies that plant roots employ to convert external NO3 – to organic N will provide valuable information that can be used to improve plant N utilization efficiency and thereby enhance the sustainability of agricultural production under changing climates.
As sessile organisms, plants have evolved developmental and metabolic patterns that acclimate to the prevailing environmental conditions. In particular, plant roots adopt different strategies to acquire NO3 – from the soil:
-
Some plant roots establish associations with diverse microorganisms to ensure NO3 – accessibility and uptake.
-
Flexibility, precision, and relative turnover times of roots are important characteristics in the competition with other plants and microbes for NO3 –.
-
Plant roots sense NO3 – as a signal. Local (external NO3 –) and long-range (internal N status) signaling pathways adjust root system architecture and resource allocation, respectively, to the physiological state of the plant and the distribution of NO3 – in the environment.
-
Plant roots can modify their capacity to acquire NO3 – in a range of concentrations by modulating the expression and function of genes in different NO3 – uptake systems.
-
The transport of NO3 – between the root and shoot will determine the partition of NO3 – assimilation between root and shoot.
-
Location and timing of NO3 – assimilation will be adjusted according to the energy budget and C metabolism.
-
Recent progress in the understanding of the molecular basis of the root responses to external supply of NO3 – suggests that root responses are largely regulated by hormone homeostasis and signaling pathways.
References
Adgo E, Schulze J (2002) Nitrogen fixation and assimilation efficiency in Ethiopian and German pea varieties. Plant Soil 239:291–299
Andrews M (1986) The partitioning of nitrate assimilation between root and shoot of higher plants. Plant Cell Environ 9:511–519
Andrews M, Lea PJ, Raven JA, Lindsey K (2004) Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Ann Appl Biol 145:25–40
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol 57:233–266
Bertrand H, Plassard C, Pinochet X, Touraine B, Normand P, Cleyet-Marel JC (2000) Stimulation of the ionic transport system in Brassica napus by a plant growth-promoting rhizobacterium (Achromobacter sp.). Can J Microbiol 46:229–236
Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants – an economic analogy. Annu Rev Ecol Syst 16:363–392
Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J (1989) Oxygen and carbon-dioxide fluxes from barley shoots depend of nitrate assimilation. Plant Physiol 91:352–356
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99:1294–1301
Bloom AJ, Burger M, Rubio-Asensio JS, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903
Bloom AJ, Randall L, Taylor AR, Silk WK (2012a) Deposition of ammonium and nitrate in the roots of maize seedlings supplied with different nitrogen salts. J Exp Bot 63:1997–2006
Bloom AJ, Rubio-Asensio JS, Randall L, Rachmilevitch S, Cousins AB, Carlisle EA (2012b) CO2 enrichment inhibits shoot nitrate assimilation in C-3 but not C-4 plants and slows growth under nitrate in C-3 plants. Ecology 93:355–367
Boudsocq S, Niboyet A, Lata JC, Raynaud X, Loeuille N, Mathieu J, Blouin M, Abbadie L, Barot S (2012) Plant preference for ammonium versus nitrate: a neglected determinant of ecosystem functioning? Am Nat 180:60–69
Burger M, Jackson LE (2004) Plant and microbial nitrogen use and turnover: rapid conversion of nitrate to ammonium in soil with roots. Plant Soil 266:289–301
Cahill JF Jr, McNickle GG (2011) The behavioral ecology of nutrient foraging by plants. In: Futuyma DJ, Shaffer HB, Simberloff D (eds) Annu Rev Ecol Evol Syst 42:289–311
Cahill JF Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, Clair CCS (2010) Plants integrate information about nutrients and neighbors. Science 328:1657–1657
Campbell BD, Grime JP, Mackey JML (1991) A trade-off between scale and precision in foraging. Oecologia 87:532–538
Canfield DE, Glazer AN, Falkowski PG (2010) The evolution and future of earth’s nitrogen cycle. Science 330:192–196
Canvin DT, Atkins CA (1974) Nitrate, nitrite and ammonia assimilation by leaves – effect of fight, carbon-dioxide and oxygen. Planta 116:207–224
Chardon F, Barthelemy J, Daniel-Vedele F, Masclaux-Daubresse C (2010) Natural variation of nitrate uptake and nitrogen use efficiency in Arabidopsis thaliana cultivated with limiting and ample nitrogen supply. J Exp Bot 61:2293–2302
Cookson SJ, Williams LE, Miller AJ (2005) Light–dark changes in cytosolic nitrate pools depend on nitrate reductase activity in Arabidopsis leaf cells. Plant Physiol 138:1097–1105
Crawford NM (1995) Nitrate-nutrient and signal for plant-growth. Plant Cell 7:859–868
Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3:389–395
Crawford NM, Kahn M, Leustrek T, Long S (2000) Nitrogen and sulphur. In: Buchanan R, Gruissem W, Jones R (eds) Biochemistry and molecular biology of plants. The American Society of Plant Physiology, Waldorf, pp 786–849
Cruz C, Green JJ, Watson CA, Wilson F, Martins-Loucao MA (2004) Functional aspects of root architecture and mycorrhizal inoculation with respect to nutrient uptake capacity. Mycorrhiza 14:177–184
Dechorgnat J, Nguyen CT, Armengaud P, Jossier M, Diatloff E, Filleur S, Daniel-Vedele F (2011) From the soil to the seeds: the long journey of nitrate in plants. J Exp Bot 62:1349–1359
Desnos T (2008) Root branching responses to phosphate and nitrate. Curr Opin Plant Biol 11:82–87
Drew MC (1975) Comparison of effects of a localized supply of phosphate, nitrate, ammonium and potassium on growth of seminal root system, and shoot, in barley. New Phytol 75:479–490
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives. Sinauer Associates, Sunderland, MA
Fitter A, Williamson L, Linkohr B, Leyser O (2002) Root system architecture determines fitness in an Arabidopsis mutant in competition for immobile phosphate ions but not for nitrate ions. Proc R Soc B Biol Sci 269:2017–2022
Forde BG (2002) Local and long-range signaling pathways regulating plant responses to nitrate. Annu Rev Plant Biol 53:203–224
Forde BG, Walch-Liu P (2009) Nitrate and glutamate as environmental cues for a responses in plant roots. Plant Cell Environ 32:682–693
Forde BG, Zhang H (1998) …response: nitrate and root branching. Trends Plant Sci 3:204–205
Foyer CH, Noctor G, Hodges M (2011) Respiration and nitrogen assimilation: targeting mitochondria-associated metabolism as a means to enhance nitrogen use efficiency. J Exp Bot 62:1467–1482
Frink CR, Waggoner PE, Ausubel JH (1999) Nitrogen fertilizer: retrospect and prospect. Proc Natl Acad Sci USA 96:1175–1180
Gansel X, Munos S, Tillard P, Gojon A (2001) Differential regulation of the NO3- and NH4+ transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: relation with long-distance and local controls by N status of the plant. Plant J 26:143–155
Garnett T, Conn V, Kaiser BN (2009) Root based approaches to improving nitrogen use efficiency in plants. Plant Cell Environ 32:1272–1283
Gastal F, Lemaire G (2002) N uptake and distribution in crops: an agronomical and ecophysiological perspective. J Exp Bot 53:789–799
Gersani M, Sachs T (1992) Development correlations between roots in heterogeneous environments. Plant Cell Environ 15:463–469
Godfray HCJ, Beddington JR, Crute IR, Haddad L, Lawrence D, Muir JF, Pretty J, Robinson S, Thomas SM, Toulmin C (2010) Food security: the challenge of feeding 9 billion people. Science 327:812–818
Gojon A, Bussi C, Grignon C, Salsac L (1991) Distribution of NO3-reduction between roots and shoots of peach-tree seedlings as affected by NO3 uptake rate. Physiol Plant 82:505–512
Good AG, Shrawat AK, Muench DG (2004) Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production? Trends Plant Sci 9:597–605
Guo S, Bruck H, Sattelmacher B (2002) Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants – Nitrogen form and water uptake. Plant Soil 239:267–275
Gutjahr C, Casieri L, Paszkowski U (2009) Glomus intraradices induces changes in root system architecture of rice independently of common symbiosis signaling. New Phytol 182:829–837
Haynes RJ (1986) Nitrification. In: Haynes RJ (ed) Mineral nitrogen in the plant-soil system. Academic, London, pp 127–165
Hermans C, Hammond JP, White PJ, Verbruggen N (2006) How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci 11:610–617
Hiorns WD, Hastings RC, Head IM, McCarthy AJ, Saunders JR, Pickup RW, Hall GH (1995) Amplification of 16 s Ribosomal-RNA genes of autotrophic ammonia-oxidizing bacteria demonstrates the ubiquity of nitrosospira in the environment. Microbiology 141:2793–2800
Ho C-H, Lin S-H, Hu H-C, Tsay Y-F (2009) CHL1 functions as a nitrate sensor in plants. Cell 138:1184–1194
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Hodge A (2009) Root decisions. Plant Cell Environ 32:628–640
Hodge A, Robinson D, Fitter A (2000) Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Huang Y, Drengstig T, Ruoff P (2012) Integrating fluctuating nitrate uptake and assimilation to robust homeostasis. Plant Cell Environ 35:917–928
Inselsbacher E, Hinko-Najera Umana N, Stange FC, Gorfer M, Schueller E, Ripka K, Zechmeister-Boltenstern S, Hood-Novotny R, Strauss J, Wanek W (2010) Short-term competition between crop plants and soil microbes for inorganic N fertilizer. Soil Biol Biochem 42:360–372
Jackson RB, Caldwell MM (1993) The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74:612–614
Jackson LE, Schimel JP, Firestone MK (1989) Short-term partitioning of ammonium and nitrate between plants and microbes in an annual grassland. Soil Biol Biochem 21:409–415
Jackson LE, Burger M, Cavagnaro TR (2008) Roots nitrogen transformations, and ecosystem services. Annu Rev Plant Biol 59:341–363
Kirk GJD, Kronzucker HJ (2005) The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: A modelling study. Ann Bot 96:639–646
Kraiser T, Gras DE, Gutierrez AG, Gonzalez B, Gutierrez RA (2011) A holistic view of nitrogen acquisition in plants. J Exp Bot 62:1455–1466
Krouk G, Crawford NM, Coruzzi GM, Tsay Y-F (2010a) Nitrate signaling: adaptation to fluctuating environments. Curr Opin Plant Biol 13:266–273
Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nacry P, Gojon A (2010b) Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18:927–937
Lam HM, Coschigano KT, Oliveira IC, MeloOliveira R, Coruzzi GM (1996) The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:569–593
Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel-Vedele F, Gojon A (1999) Molecular and functional regulation of two NO3- uptake systems by N- and C-status of Arabidopsis plants. Plant J 18:509–519
Leyser O, Fitter A (1998) Roots are branching out in patches. Trends Plant Sci 3:203–204
Li YL, Fan XR, Shen QR (2008) The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Environ 31:73–85
Li J-Y, Fu Y-L, Pike SM, Bao J, Tian W, Zhang Y, Chen C-Z, Zhang Y, Li H-M, Huang J, Li L-G, Schroeder JI, Gassmann W, Gonga J-M (2010) The arabidopsis nitrate transporter NRT1.8 functions in nitrate removal from the xylem sap and mediates cadmium tolerance. Plant Cell 22:1633–1646
Lillo C, Meyer C, Lea US, Provan F, Oltedal S (2004) Mechanism and importance of post-translational regulation of nitrate reductase. J Exp Bot 55:1275–1282
Lin S-H, Kuo H-F, Canivenc G, Lin C-S, Lepetit M, Hsu P-K, Tillard P, Lin H-L, Wang Y-Y, Tsai C-B, Gojon A, Tsay Y-F (2008) Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20:2514–2528
Little DY, Rao HY, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102:13693–13698
Marschner H (1995) Mineral nutrition of higher plants. Academic, London
Matson PA, Parton WJ, Power AG, Swift MJ (1997) Agricultural intensification and ecosystem properties. Science 277:504–509
McDermitt DK, Loomis RS (1981) Elemental composition of biomass and its relation to energy content, growth efficiency, and growth-yield. Ann Bot 48:275–290
Miller AJ, Cramer MD (2005) Root nitrogen acquisition and assimilation. Plant Soil 274:1–36
Miller AJ, Smith SJ (1996) Nitrate transport and compartmentation in cereal root cells. J Exp Bot 47:843–854
Miller AJ, Smith SJ (2008) Cytosolic nitrate ion homeostasis: could it have a role in sensing nitrogen status? Ann Bot 101:485–489
Miller AE, Bowman WD, Suding KN (2007a) Plant uptake of inorganic and organic nitrogen: neighbor identity matters. Ecology 88:1832–1840
Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM (2007b) Nitrate transport and signalling. J Exp Bot 58:2297–2306
Nasholm T, Ekblad A, Nordin A, Giesler R, Hogberg M, Hogberg P (1998) Boreal forest plants take up organic nitrogen. Nature 392:914–916
Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51:111–140
North KA, Ehlting B, Koprivova A, Rennenberg H, Kopriva S (2009) Natural variation in Arabidopsis adaptation to growth at low nitrogen conditions. Plant Physiol Biochem 47:912–918
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996
Oaks A (1992) A reevaluation of nitrogen assimilation in roots. Bioscience 42:103–111
Oaks A, Hirel B (1985) Nitrogen-metabolism in roots. Annu Rev Plant Physiol Plant Mol Biol 36:345–365
Persello-Cartieaux F, David P, Sarrobert C, Thibaud MC, Achouak W, Robaglia C, Nussaume L (2001) Utilization of mutants to analyze the interaction between Arabidopsis thaliana and its naturally root-associated Pseudomonas. Planta 212:190–198
Redinbaugh MG, Campbell WH (1991) Higher-plant responses to environmental nitrate. Physiol Plant 82:640–650
Reisenauer HM (1966) Mineral nutrients in soil solution. In: Altman PL, Dittmer DS (eds) Environmental biology. Federation of American Societies for Experimental Biology, Bethesda, MD
Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, Tillard P, Forde BG, Gojon A (2006a) The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci USA 103:19206–19211
Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A (2006b) A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 140:909–921
Reynolds HL, Dantonio C (1996) The ecological significance of plasticity in root weight ratio in response to nitrogen: Opinion. Plant Soil 185:75–97
Robinson D, Hodge A, Griffiths BS, Fitter AH (1999) Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc R Soc B Biol Sci 266:431–435
Roycewicz P, Malamy JE (2012) Dissecting the effects of nitrate, sucrose and osmotic potential on Arabidopsis root and shoot system growth in laboratory assays. Philos Trans R Soc B Biol Sci 367:1489–1500
Ruffel S, Krouk G, Ristova D, Shasha D, Birnbaum KD, Coruzzi GM (2011) Nitrogen economics of root foraging: transitive closure of the nitrate-cytokinin relay and distinct systemic signaling for N supply vs. demand. Proc Natl Acad Sci USA 108:18524–18529
Rufty TW, Huber SC, Volk RJ (1988) Alterations in leaf carbohydrate-metabolism in response to nitrogen stress. Plant Physiol 88:725–730
Schenk HJ (2006) Root competition: beyond resource depletion. J Ecol 94:725–739
Scheurwater I, Koren M, Lambers H, Atkin OK (2002) The contribution of roots and shoots to whole plant nitrate reduction in fast- and slow-growing grass species. J Exp Bot 53:1635–1642
Signora L, De Smet I, Foyer CH, Zhang HM (2001) ABA plays a central role in mediating them regulatory effects of nitrate on root branching in Arabidopsis. Plant J 28:655–662
Sims L, Pastor J, Lee T, Dewey B (2012) Nitrogen, phosphorus and light effects on growth and allocation of biomass and nutrients in wild rice. Oecologia 170:65–76
Smirnoff N, Stewart GR (1985) Nitrate assimilation and translocation by higher plants: Comparative physiology and ecological consequences. Physiol Plant 64:133–140
Stohr C, Mack G (2001) Diurnal changes in nitrogen assimilation of tobacco roots. J Exp Bot 52:1283–1289
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends Plant Sci 5:537–542
van der Leij M, Smith SJ, Miller AJ (1998) Remobilisation of vacuolar stored nitrate in barley root cells. Planta 205:64–72
Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutierrez RA (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA 107:4477–4482
Volder A, Smart DR, Bloom AJ, Eissenstat DM (2005) Rapid decline in nitrate uptake and respiration with age in fine lateral roots of grape: implications for root efficiency and competitive effectiveness. New Phytol 165:493–501
Walch-Liu P, Forde BG (2008) Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J 54:820–828
Wang Y-Y, Tsay Y-F (2011) Arabidopsis Nitrate Transporter NRT1.9 Is Important in Phloem Nitrate Transport. Plant Cell 23:1945–1957
Wang Y-Y, Hsu P-K, Tsay Y-F (2012) Uptake, allocation and signaling of nitrate. Trends Plant Sci 17:458–467
Werner T, Nehnevajova E, Koellmer I, Novak O, Strnad M, Kraemer U, Schmuelling T (2010) Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in arabidopsis and tobacco. Plant Cell 22:3905–3920
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182
Zhang HM, Jennings A, Barlow PW, Forde BG (1999) Dual pathways for regulation of root branching by nitrate. Proc Natl Acad Sci USA 96:6529–6534
Zhen RG, Koyro HW, Leigh RA, Tomos AD, Miller AJ (1991) Compartmental nitrate concentrations in barley root-cells measured with nitrate-selective microelectrodes and by single-cell sap sampling. Planta 185:356–361
Acknowledgements
JS Rubio-Asensio and C Lopez-Berenguer are supported by a postdoctoral junior grant “Juan de la Cierva,” and J Garcia-de la Garma is supported by a JAE-Predoc Fellowship, all funding by the Ministerio de Economía y Competitividad (Spain). This work was supported in part by NSF-IOS-08-18435 and the National Research Initiative Competitive Grant 2008–35100 04459 from the USDA National Institute of Food and Agriculture to AJ Bloom.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rubio-Asensio, J.S., López-Berenguer, C., García-de la Garma, J., Burger, M., Bloom, A.J. (2014). Root Strategies for Nitrate Assimilation. In: Morte, A., Varma, A. (eds) Root Engineering. Soil Biology, vol 40. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54276-3_12
Download citation
DOI: https://doi.org/10.1007/978-3-642-54276-3_12
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54275-6
Online ISBN: 978-3-642-54276-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)