Abstract
TRPV5 is one of the two channels in the TRPV family that exhibit high selectivity to Ca2+ ions. TRPV5 mediates Ca2+ influx into cells as the first step to transport Ca2+ across epithelia. The specialized distribution in the distal tubule of the kidney positions TRPV5 as a key player in Ca2+ reabsorption. The responsiveness in expression and/or activity of TRPV5 to hormones such as 1,25-dihydroxyvitamin D3, parathyroid hormone, estrogen, and testosterone makes TRPV5 suitable for its role in the fine-tuning of Ca2+ reabsorption. This role is further optimized by the modulation of TRPV5 trafficking and activity via its binding partners; co-expressed proteins; tubular factors such as calbindin-D28k, calmodulin, klotho, uromodulin, and plasmin; extracellular and intracellular factors such as proton, Mg2+, Ca2+, and phosphatidylinositol-4,5-bisphosphate; and fluid flow. These regulations allow TRPV5 to adjust its overall activity in response to the body’s demand for Ca2+ and to prevent kidney stone formation. A point mutation in mouse Trpv5 gene leads to hypercalciuria similar to Trpv5 knockout mice, suggesting a possible role of TRPV5 in hypercalciuric disorders in humans. In addition, the single nucleotide polymorphisms in Trpv5 gene prevalently present in African descents may contribute to the efficient renal Ca2+ reabsorption among African descendants. TRPV5 represents a potential therapeutic target for disorders with altered Ca2+ homeostasis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- TRPV5
- TRPV6
- Gene duplication
- Calcium channel
- Calcium transport
- Calcium reabsorption
- Distal convoluted tubule
- Hypercalciuria
- Vitamin D
- Protein–protein interaction
- Single nucleotide polymorphisms
- African American
1 Introduction
Ca2+ serves as an important intracellular and extracellular messenger, and it is indispensable for many physiological activities, such as muscle contraction, neuron excitability, and blood coagulation (Brown et al. 1995; Clapham 1995). For this reason, the plasma Ca2+ concentration is monitored by a Ca2+-sensing receptor, which controls the secretion of parathyroid hormone (PTH) (Brown 1991). PTH regulates the synthesis of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Henry 1985; Yang et al. 1999). PTH and 1,25(OH)2D3 regulate Ca2+ homeostasis via intestinal absorption and renal reabsorption, as well as deposition into and mobilization from the bone. In the intestine and kidney, Ca2+ is transported across epithelia through the tight junction between cells (paracellular pathway), or across the apical and basolateral membranes of epithelial cells (transcellular pathway) (Bronner 1998; Wasserman and Fullmer 1995). Only the transcellular pathway allows Ca2+ to be transported against an electrical and concentration gradient. The Ca2+-transporting epithelial cells in the intestinal and the kidney express proteins critical in the transcellular Ca2+ transport pathway. Ca2+ channels TRPV5 and TRPV6 in the apical membrane mediate Ca2+ entering into the cell (Hoenderop et al. 1999; Peng et al. 1999; Zhuang et al. 2002). The steep Ca2+ concentration gradient (approximately 1 mM extracellular vs. 0.1 μM intracellular) and the transmembrane potential (typically −60 mV inside the cell) allow Ca2+ to enter the cells via channels. Ca2+ exits the cell via high-affinity Ca2+–ATPase (PMCA) and/or Na+–Ca2+ exchanger 1 (NCX1) at the expense of energy stored in ATP or in Na+ concentration gradient (Wasserman et al. 1992). Ca2+-binding proteins (e.g., calbindins) are also expressed to keep the intracellular Ca2+ concentration ([Ca2+] i ) low so that the driving force for Ca2+ entry is maintained and [Ca2+] i will not rise to a level which is toxic to cells (Christakos et al. 1992; Lambers et al. 2006; Zheng et al. 2004). Although TRPV5 and TRPV6 were identified at a much later date compared to calbindins, PMCA, and NCX, their specific roles in the transcellular Ca2+ transport pathway energized the studies of Ca2+ absorption and reabsorption at molecular level since the cloning of TRPV5 and TRPV6. Compared to the broader distribution of TRPV6, TRPV5 is relatively confined to the kidney, where it is responsive to physiological signals to regulate Ca2+excretion into the urine.
2 Gene
TRPV5 was cloned as a result of searching for genes involved in Ca2+ reabsorption in kidney cells (Hoenderop et al. 1999). Using an expression cloning approach, Hoenderop and Bindels identified a cDNA from primary cells of rabbit connecting tubule (CNT) and cortical collecting duct (CCD) that stimulates 45Ca2+ uptake in Xenopus laevis oocytes. The cDNA encodes a protein of 730 amino acid residues and was initially named as epithelial Ca2+ channel (ECaC) (Hoenderop et al. 1999). At the time of cloning, the only mammalian protein similar to TRPV5 was rat vanilloid receptor VR1 (TRPV1). Human, rat, and mouse TRPV5 share high levels of amino acid identity to rabbit TRPV5 (Peng et al. 2000a, 2001). The protein most similar to TRPV5 is TRPV6 (75 % amino acid identity), which was independently identified from rat duodenum using similar approach (Peng et al. 1999). TRPV5 shares approximately 40–45 % amino acid identity with other members of the TRPV family, including TRPV1 to TRPV4.
Trpv5 gene is located in human chromosome 7q35 side by side with TRPV6 gene in 7q33–34 (Muller et al. 2000; Peng et al. 2001). The human Trpv5 gene comprises 15 exons. Except for the first and last exons, exons 2–14 of Trpv5 and Trpv6 genes are identical in size (Peng et al. 2001). In fact, all genes of the TRPV family share a conserved gene structure (Peng et al. 2001). In addition to Trpv5 and Trpv6 in 7q34–35, genes for Trpv1, Trpv2, and Trpv3 are located in 17q11.2 to 17q13.2, suggesting recent gene duplication events in this subfamily. Fish and birds have only one Trpv6-like gene (Qiu and Hogstrand 2004; Yang et al. 2011), whereas mammalians have both Trpv5 and Trpv6 genes. Based on the Joint Genome Institute (JGI; http://genome.jgi-psf.org/) genome database for Xenopus tropicalis (Western clawed frog), five Trpv6-like genes are present. This is not restricted to Trpv6, as 6 Trpv4-like genes are present in X. tropicalis (Saito and Shingai 2006). Thus, it is likely that duplication events occurred in Trpv6 gene when amphibians and mammals were evolved, respectively (Fig. 1). The physiological roles of the Trpv6-like genes in X. tropicalis are unclear. In mammals, the duplicated gene became Trpv5 as it gained the ability for specialized expression in the distal tubule of kidney and properties for a finer regulation of channel function and trafficking.
Trpv5 gene was likely produced by the duplication of Trpv6 gene during the process of evolution. Gene duplication events are indicated on the respective branches. WC frog, Western clawed frog (Xenopus tropicalis). Plus symbol indicates present, minus symbol indicates absent, and asterisk indicates that there are five genes similar to TRPV6 in Western clawed frogs
3 Tissue Distribution
In contrast to the broader tissue distribution of TRPV6, TRPV5 is rather specifically distributed to the kidney. Due to the high sequence similarity between TRPV5 and TRPV6, initial studies showed strong signals in Northern blot analyses with TRPV5 cDNA probe in rabbit and rat duodenum (Peng et al. 2000a; Hoenderop et al. 1999), but this is likely due to cross hybridization with TRPV6 mRNA, which is highly expressed in the duodenum (Peng et al. 2000a). Similarly, although TRPV5 is also expressed in the placenta, the mRNA abundance of TRPV5 is much lower than that of TRPV6 in human placenta (Peng et al. 2001).
Based on Expressed Sequence Tag (EST) database from the National Center for Biotechnology Information (NCBI), among 45 human organs listed, TRPV5 is detected in only two of them (numbers in brackets are tag/million): blood [8] and lung [2]. In contrast, TRPV6 is present in 13 organs/tissues: bladder [33], blood [16], brain [4], cervix [20], eye [9], intestine [8], lung [2], mammary gland [6], pancreas [4], placenta [215], prostate [47], testis [6], and trachea [19]. Both TRPV5 and TRPV6 are not detected in human kidney, indicating their number of EST is lower than 1/million. In mouse, among 47 organs/tissues listed, TRPV5 is present in 2, including embryonic tissue [1] and kidney [24], whereas TRPV6 is present in 9 of them including brain [10], embryonic tissue [2], extraembryonic tissue [120], lung [30], mammary gland [69], pancreas [37], salivary gland [103], skin [8], and thymus [8]. These EST profiles may not be accurate due to various reasons; however, they provide an unbiased overview of the gene expression. The expression profile of TRPV5 in mouse suggests a specific role of TRPV5 in the kidney.
In rabbit kidney, TRPV5 protein is expressed in the apical membrane of distal convoluted tubule (DCT), CNT, and CCD, where it co-localizes with calbindin-D28k (Hoenderop et al. 1999). In rats, TRPV5 mRNA exhibits similar expression pattern as NCX1 and calbindin-D28k (Peng et al. 2000a). In mouse kidney, TRPV5 is expressed in the late segment of DCT and CNT (Loffing et al. 2001).
In mice and rats, TRPV5 mRNA is much more abundant than that of TRPV6 in the kidney (Song et al. 2003; Van Cromphaut et al. 2007). TRPV5 mRNA level is approximately 10–20 times higher than that of TRPV6 in mouse kidney (Song et al. 2003). In contrast, we have observed much higher mRNA level of TRPV6 than TRPV5 in the human kidney (Peng et al. 2001). Our unpublished observations also indicated a higher mRNA level of TRPV6 in outer medulla than cortex of human kidney, opposite to that of TRPV5. Similarly, TRPV6 is also much abundant than TRPV5 in horse kidney (Rourke et al. 2010). Thus, TRPV6 may play more significant roles in some species such as humans and horses.
Even though TRPV5 is mainly expressed in the kidney, TRPV5 mRNA or protein has also been detected in other organs/tissues. TRPV5 is detected in mouse testis (Jang et al. 2012) and inner ear (Takumida et al. 2009) and rat testis, spermatogenic cells, sperm (Li et al. 2010), cochlea (Yamauchi et al. 2010), and osteoclast-like cells (Yan et al. 2011). In horse, TRPV5 mRNA is expressed in the duodenum and proximal jejunum (Rourke et al. 2010), and TRPV5 protein is detected in chondrocytes from the superficial zone of articular cartilage (Hdud et al. 2012). In human, TRPV5 protein and mRNA are present in placenta (Bernucci et al. 2006), osteoclasts (van der Eerden et al. 2005), retinal pigment epithelium (Kennedy et al. 2010), lymphocytes, Jurkat leukemia T cells (Vassilieva et al. 2013), and leukemia K562 cells (Semenova et al. 2009).
4 Structural Aspects
The mammalian TRPV5 proteins comprise around 730 amino acid residues (729, 731, 730, and 723 amino acids in human, horse, rabbit, rat, and mouse, respectively). Mammalian TRPV5 proteins consist of an intracellular N- and C-termini, six transmembrane domains, and a pore region between the last two transmembrane domains. In the intracellular portions of TRPV5, six ankyrin repeats are present in the N-terminal region, and a PDZ-binding motif is located in the C terminus (Phelps et al. 2008; de Groot et al. 2011b; Palmada et al. 2005; Jing et al. 2011). An N-linked glycosylation site is located in the first extracellular loop, and asparagine 358 (N358) is indispensable for the N-linked glycosylation of TRPV5 (Chang et al. 2005; Jiang et al. 2008). Putative protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites and calmodulin-binding sites are present within the N- and C-terminal regions (Hoenderop et al. 1999; Kovalevskaya et al. 2012). Some of these sites have already been shown to be important in regulating TRPV5.
Four TRPV5 molecules form a homotetramer with a single pore in the middle (Nilius et al. 2001b; Hoenderop et al. 2003b). Two critical motifs at positions 64–77 (part of the first ankyrin repeat) in the N terminus and at 596–601 in the C terminus of TRPV5 are needed for the formation of the functional channel complex (Chang et al. 2004). The N-terminal ankyrin repeats appear to be important for channel assembly. The first ankyrin repeat does not appear to be critical to the formation of TRPV5 tetramer; however, it is likely essential for correct folding of TRPV5 into a functional channel complex (de Groot et al. 2011b). In contrast, the third ankyrin repeat initiates a zippering process through the fifth ankyrin repeat, and they form an anchor for channel assembly (Erler et al. 2004). The tetramerization of TRPV5 allows the four aspartate 542 (D542) residues in pore-forming loop between transmembrane domain 5 and 6 to form a Ca2+-selective ring, which is also involved in Mg2+ blockade (Nilius et al. 2001b; Voets et al. 2004; Lee et al. 2005).
TRPV5 and TRPV6 appeared to form heterotetramers when four pieces of TRPV5 and TRPV6 were linked together and heterologously expressed in X. laevis oocytes (Hoenderop et al. 2003b). The more TRPV6 subunits presented in the heterotetramer, the more TRPV6 characteristics were exhibited (Hoenderop et al. 2003b). Other TRPV members preferentially form homomeric assembly; however, TRPV5 and TRPV6 can form heteromeric complex in HEK293 cells (Hellwig et al. 2005). This is likely due to the high degree of similarity between TRPV5 and TRPV6. Unlike in transfected cells, TRPV5 is likely outnumbered by TRPV6 in most cells. It is unclear whether heteromeric TRPV5 and TRPV6 complexes play a significant physiological role if they exist in vivo. In addition, TRPV5 and TRPV6 share certain structure–function similarities with TRPML3 (Grimm et al. 2007). They can form heteromers which display different features than the respective homomers (Guo et al. 2013). TRPML3 and TRPV5 are expressed in the kidney and inner ear (Takumida et al. 2009; Castiglioni et al. 2011); however, it is yet to be determined whether TRPML3 and TRPV5 form heteromeric complexes in native cells.
5 Biophysical Properties
5.1 Ca2+ Selectivity
TRPV5 is a Ca2+ permeant ion channel as it was identified as a result of increasing 45Ca2+ uptake activity when expressed in Xenopus oocytes (Hoenderop et al. 1999). The most distinctive feature of TRPV5 is its Ca2+ selectivity. The permeability ratio between Ca2+ and Na+ (P Ca:P Na) is over 100 (Vennekens et al. 2000). The divalent cation selectivity profile of TRPV5 is Ca2+ > Mn2+ > Ba2+ ≈ Sr2+ (Vennekens et al. 2000). In the absence of divalent cation in the extracellular solution, TRPV5 allows monovalent cations to pass through. The permeation sequence for monovalent cations through TRPV5 is Na+ > Li+ > K+ > Cs+ > NMDG+ (Nilius et al. 2000). The Na+ current of TRPV5 correlates with the amplitude of Ca2+ uptake and is often used as a measure of TRPV5 channel activity. Some trivalent and divalent cations block current through TRPV5, and the sequence of block is Pb2+ = Cu2+ = Gd3+ > Cd2+ > Zn2+ > La3+ > Co2+ > Fe2+ > Fe3+ (Nilius et al. 2001a). TRPV5 is also sensitive to ruthenium red and econazole (Nilius et al. 2001a). TRPV5 and TRPV6 show difference in ruthenium red sensitivity: the IC50 of ruthenium red for TRPV5 is around 121 nM, which is nearly100-fold lower than that for TRPV6 (Hoenderop et al. 2001b). Extracellular and intracellular Mg2+ blocks TRPV5 voltage dependently (Voets et al. 2001; Lee et al. 2005; Hoenderop et al. 2001b). D542 is the key residue to form the selectivity filter of TRPV5 and determines Ca2+ permeation and Mg2+ blockade (Nilius et al. 2001b; Lee et al. 2005; Dodier et al. 2007). The nonsynonymous single nucleotide polymorphism (SNP) A563T variation, close to D542, increases TRPV5 sensitivity to extracellular Mg2+, resulting in suppressed Na+ permeation through TRPV5 (Na et al. 2009). In clinical trials, urinary Ca2+ excretion is proportional to changes in magnesium excretion, and this is likely related to the blockade of TRPV5 by Mg2+ (Bonny et al. 2008).
TRPV5 currents exhibit strong inward rectification (Vennekens et al. 2000). Using TRPV6 as a model, Voets et al. demonstrated that intracellular Mg2+ acts as a permeant pore blocker and contributes to strong inward rectification of the channel (Voets et al. 2003). In addition, TRPV6 also exhibits intrinsic Mg2+-independent inward rectification for which the mechanism is not fully understood (Voets et al. 2003). Similar mechanisms are likely applicable to TRPV5 due to the high degree of similarity in structure and function between TRPV5 and TRPV6. Unitary channel activity of TRPV5 could be detected in the absence of extracellular divalent cations (Nilius et al. 2000). The single-channel conductance is 77.5 pS for rabbit TRPV5 using Na+ as a charge carrier (Nilius et al. 2000). Single-channel activity of TRPV5 was also detected using K+ as a charge carrier (Vassilev et al. 2001). In addition, subconductance state (partial channel opening) of TRPV5 was also observed (subconductance at 29 pS vs. full conductance at 59 pS at intracellular pH 7.4) (Cha et al. 2007).
5.2 pH Sensitivity
Another feature of TRPV5 is its pH sensitivity. In the initial characterization, TRPV5-mediated Ca2+ uptake was significantly inhibited when the pH value in the extracellular medium was lowered to 5.9 (Hoenderop et al. 1999). At single-channel level, both full and subconductance and their open probabilities were reduced by lower intracellular pH (Cha et al. 2007). On the other hand, high pH stimulates TRPV5-mediated Ca2+ uptake (Peng et al. 2000a). Low pH increases the blockage of monovalent cation current by extracellular Mg2+ (Vennekens et al. 2001). Glutamate 522 (E522) of rabbit TRPV5 acts as the extracellular pH sensor, and extracellular protons decrease the estimated diameter of channel pore to inhibit TRPV5 (Yeh et al. 2003, 2005). Glutamate 535 (E535) is also involved; however, its effect depends on E522 (Yeh et al. 2006). Meanwhile, intracellular protons also regulate TRPV5 (Yeh et al. 2005). Intracellular acidification promotes proton binding to lysine 607, intracellular pH sensor, and induces rotation of the pore helix, leading to decreases in the pore diameter, open probability, and single-channel conductance (Yeh et al. 2005; Cha et al. 2007).
In addition to channel property, pH may also affect the level of TRPV5 protein at the cell surface. Extracellular alkalization induces the recruitment of TRPV5 proteins to the cell surface from TRPV5-containing vesicles, and extracellular acidification leads to the retrieval of TRPV5 from plasma membrane (Lambers et al. 2007). Thus, TRPV5 could be regulated by pH in both channel activity and plasma membrane expression. Metabolic acidosis and alkalosis affect Ca2+ transport in the kidney (Sutton et al. 1979). Clinical trial data indicate that changes in urinary Ca2+ excretion and urine pH are inversely related (Bonny et al. 2008). The pH sensitivity of TRPV5 may contribute to the dysregulation of Ca2+ reabsorption under disturbance in acid–base balance.
5.3 Ca2+-Dependent Inactivation
High level of free Ca2+ is toxic to cells. The Ca2+-dependent inactivation limits the amount of Ca2+ ions that enter the cell through TRPV5 and prevents the toxic impact of Ca2+ overload in the cell. Calbindin-D28k binds Ca2+ beneath the plasma membrane and therefore relieves the Ca2+-dependent inactivation of TRPV5 to some extent; it may also buffer free Ca2+ to limit the toxic effect (Lambers et al. 2006). TRPV5 exhibits Ca2+-dependent autoregulatory mechanisms, including fast inactivation and slow rundown (Vennekens et al. 2000). The C-terminal sequences in rabbit TRPV5 701–730 and 650–653 are critical determinants of Ca2+-dependent inactivation (Nilius et al. 2003). Compared to TRPV6, TRPV5 exhibit a much slower initial inactivation phase (Nilius et al. 2002). The first intracellular loop is critical to the kinetics of the initial phase in the inactivation process; three residues at positions 409, 411, and 412 in TRPV5 are critical for the delayed inactivation (Nilius et al. 2002). In addition, Q579 following the last transmembrane domain of TRPV5 is also important to the fast Ca2+-dependent inactivation kinetics of TRPV5 (Suzuki et al. 2002). The first intracellular loop of TRPV5 is capable of binding to CaM (Kovalevskaya et al. 2012), so is the C terminus of TRPV5 (de Groot et al. 2011a; Kovalevskaya et al. 2012). TRPV5 mutant deficient in interacting with CaM at C terminus (residues 696–729) exhibits diminished Ca2+-dependent inactivation (de Groot et al. 2011a). However, it is yet to be clarified what roles the other CaM-binding sites of TRPV5 play in the Ca2+-dependent inactivation mechanism. In addition, a helix-breaking mutation (M490P) in transmembrane domain 5 reduces Ca2+-dependent inactivation of TRPV5 and induces apoptosis due to Ca2+ overload in cells expressing TRPV5M490P (Lee et al. 2010). Corresponding mutation in TRPML3 (A419P) leads to constitutive channel activity, which likely results in hair cell death in the inner ear and profound deafness and other abnormalities in mice homozygous in TRPML3A419P (Grimm et al. 2007).
Although TRPV5 works constitutively in the kidney, its activity depends on the availability of phosphatidylinositol-4,5-bisphosphate (PIP2). As the level of PIP2 decreases, so does the activity of TRPV5 (Lee et al. 2005). PIP2 activates TRPV5 in part by reducing conformational change-induced Mg2+ binding (Lee et al. 2005). Arginine 599 in mouse or rat TRPV5 (corresponding to R606 in rabbit and human TRPV5) in the “TRP domain” is likely the PIP2-binding site (Rohacs et al. 2005). Elevation of [Ca2+] i due to Ca2+ influx through TRPV5 may activate phospholipase C (PLC), which depletes PIP2. Thus, removal of PIP2 is an important Ca2+-dependent inactivation mechanism for TRPV5 as well as TRPV6 (Thyagarajan et al. 2008).
5.4 Responsiveness to Fluid Flow
Flow-stimulated K+ secretion in the distal nephron involves Ca2+-activated K+ channels. TRPV5 is expressed in the apical membrane in the distal nephron and therefore may provide a Ca2+ influx pathway to activate K+ channels. Recent study indicates that both TRPV5 and TRPV6 expressed in HEK cells are activated by shear force generated by fluid flow in physiological range (Cha et al. 2013). Flow-induced surge in TRPV5 or TRPV6 activity leads to the activation of co-expressed Slo1 maxi-K+ channel without affecting ROMK channel (Cha et al. 2013). Activation of maxi-K+ channel may counteract the depolarization due to Ca2+ influx and cause hyperpolarization which in turn would improve Ca2+ influx through TRPV5. N-linked glycosylation of the channels is involved in the responsiveness to fluid flow (Cha et al. 2013). The responsiveness of TRPV5 and TRPV6 to flow may have implications not only in flow-stimulated K+ secretion but also in thiazide-induced Ca2+ reabsorption.
6 Regulation by Interacting, Co-expressed, and Tubular Proteins
A number of proteins have been found to regulate TRPV5. These include TRPV5-interacting proteins, proteins co-expressed with TRPV5, and proteins in the tubular fluid (Table 1). Regulation of TRPV5 by some of the proteins is briefly summarized in the following.
6.1 Regulation by Interacting Proteins
Some TRPV5-interacting proteins, such as S100A10, Rab11a, and NHERF2, regulate the trafficking and plasma membrane abundance of TRPV5. On the other hand, some TRPV5-interacting proteins, such as calbindin-D28k, CaM, 80H-K, BSPRY, and FKBP52, regulate TRPV5 activity. It is worth noting that the C terminus of TRPV5 interacts with most of its binding partners, and the region from 598 to 608 in TRPV5 within the conserved “TRP domain” binds to S100A10, Rab11a, 80K-H, NHERF4, and CaM (Fig. 2). This region also contains the PIP2-binding site (R606) and a pH sensor (K607). It is also not far away from histidine 711 (corresponding to H712 in rabbit TRPV5), a critical residue involved in the constitutive internalization of TRPV5 (de Groot et al. 2010). Thus, it is plausible that TRPV5-binding partners may affect TRPV5 function, membrane stability, and trafficking. However, it is not likely that these TRPV5-binding proteins regulate TRPV5 at the same time. It is possible that some of the proteins only regulate TRPV5 at a certain cell type, at a specific developmental stage, or at a state being stimulated by a hormone or other physiological cues. Most TRPV5-interacting proteins also interact with TRPV6 due to the conserved binding sites in the two channels. Thus, it is likely some of the TRPV5-interacting proteins may be more involved in regulating TRPV6 than TRPV5. Lastly, although overlapping expression with TRPV5 was found for most of the TRPV5-interacting proteins, they do express in cells that do not express TRPV5; they all have functions other than regulating TRPV5.
Functional domains/sites in human TRPV5 (GenBank #: AF304464.1). Key residues, domains, and interacting motifs/sites for partners are indicated in the predicted structure of TRPV5. Factors with positive and negative effects on TRPV5 are labeled with green and red rectangles, respectively. See text for details
6.1.1 S100A10
S100A10 binds to a conserved motif of five residues (598VATTV602) in the C terminus of TRPV5 (van de Graaf et al. 2003). S100A10 is a member of the EF-hand containing S100 protein family, and it forms heterotetramer with annexin 2, a Ca2+, and phospholipid-binding protein in association with cytoskeleton underneath cell membrane (Gerke and Moss 2002). S100A10–annexin 2 complex is important for the plasma membrane localization of TRPV5 (van de Graaf et al. 2003). In addition, this complex may be involved in 1,25(OH)2D3-induced Ca2+ reabsorption as S100A10 is upregulated by 1,25(OH)2D3 (van de Graaf et al. 2003).
6.1.2 Rab11a
The small GTPase Rab11a is another TRPV5-interacting protein that regulates its trafficking to the plasma membrane (van de Graaf et al. 2006a). It binds to 596MLERK600 of mouse TRPV5 (603–607 in human TRPV5), following the S100A10-binding motif (van de Graaf et al. 2006a). Glycine substitution in the MLERK motif disrupts the binding of Rab11a to TRPV5 and the plasma membrane expression of TRPV5 (van de Graaf et al. 2006a). TRPV5 is one of a few cargo proteins that bind directly to the GDP-bound Rab proteins.
6.1.3 PDZ Proteins
The last four amino acids of TRPV5 and TRPV6 are PDZ (postsynaptic density-95, Drosophila discs-large protein, zonula occludens protein 1)-binding motifs. TRPV5 interacts with Na+/H+ exchanger regulating factors 2 (NHERF2) (Palmada et al. 2005). Removal of the last three amino acids of TRPV5 abolishes the interaction (van de Graaf et al. 2006b). TRPV5 also interacts with NHERF4; however, the interacting motif is located between residues 596 and 617 of mouse TRPV5 (603–624 of human TRPV5) (van de Graaf et al. 2006b). In contrast, NHERF4 interact with the last three amino acid residues of TRPV6 (Kim et al. 2007), suggesting it is likely TRPV6 is the real binding partner of NHERF4. The 4th PDZ domain of NHERF4 and the second PDZ domain of NHERF2 are essential for their interactions with TRPV5 (Kim et al. 2007; Palmada et al. 2005). NHERF2 is important to the effects of SGK1 and WNK4 on TRPV5 (Embark et al. 2004; Jing et al. 2011).
6.1.4 Calbindin-D28k
Calbindins are Ca2+-binding proteins that are well known for their responsiveness to vitamin D and possible roles in Ca2+ absorption and reabsorption (Christakos et al. 1992). Calbindin-D28k is co-expressed with TRPV5 in DCT and CNT (Loffing et al. 2001). Both N- and C-termini of TRPV5 interact with calbindin-D28k in the absence but not in the presence of Ca2+ ions (Lambers et al. 2006). When [Ca2+] i is low, calbindin-D28k translocates toward the plasma membrane and associates with TRPV5. Calbindin-D28k buffers Ca2+ close to the vicinity of the channel opening, thereby reducing local accumulation of free Ca2+ ions. After binding to Ca2+, calbindin-D28k disassociates from TRPV5 and facilitates the diffusion of Ca2+ to the basolateral membrane (Lambers et al. 2006). Calbindin-D28k expression in the kidney is greatly reduced in mice lacking TRPV5 (Hoenderop et al. 2003a). Mice lacking calbindin-D28k do not show hypercalciuria, and mice lacking both TRPV5 and calbindin-D28k do not exhibit more severe phenotype in Ca2+ homeostasis than mice lacking TRPV5 alone (Gkika et al. 2006a). These results indicate TRPV5 but not calbindin-D28k is critical to Ca2+ reabsorption. Calbindin-D28k acts as a dynamic Ca2+ buffer to avoid a sudden elevation of [Ca2+] i ; however, it appears that its role could be compensated by other vitamin D-regulated Ca2+-binding proteins. The role of calbindin-D28k in Ca2+ homeostasis could only be observed in the absence of the effects of vitamin D: mice lacking vitamin D receptor (VDR) and calbindin-D28k display more severe hypercalciuria and secondary hyperparathyroidism than mice lacking VDR alone (Zheng et al. 2004).
6.1.5 Calmodulin
Being an intracellular Ca2+ sensor, calmodulin (CaM) is expected to play a role in modulating TRPV5 function. However, CaM antagonists calmidazolium R24571 and trifluoperazine only modestly inhibit TRPV5 at high concentrations (Nilius et al. 2001a). In addition, Ca2+-insensitive CaM mutants significantly reduced Na+ and Ca2+ currents of TRPV6 but not those of TRPV5 (Lambers et al. 2004). Yet, CaM is capable of binding to TRPV5. So far five CaM-binding sites in TRPV5 have been identified, including residues 696–712 (de Groot et al. 2011a; Kovalevskaya et al. 2012) and 591–612 (Kovalevskaya et al. 2012; Holakovska et al. 2011) in the C terminus, 401–428 in the first intracellular loop (Kovalevskaya et al. 2012), and 310–330 and 133–154 in the N-terminal region (Kovalevskaya et al. 2012). These CaM-binding sites display diversity in binding mode, stoichiometry, and affinity in interaction with CaM in vitro (Kovalevskaya et al. 2012). The first intracellular loop of TRPV5/6 plays a critical role in the fast and slow inactivation kinetics (Nilius et al. 2002), but it is unclear to what extent Ca2+/CaM is involved in this process. CaM negatively modulates TRPV5 activity by binding to the residues 696–729, and PTH-mediated PKA phosphorylation of the CaM-binding site reverses this action (de Groot et al. 2009, 2011a). In addition, phosphorylation of TRPV5 by PKC at serine 144 (S144) in the N-terminal CaM-binding site (133–154) results in decreased pore size and open probability in TRPV5 (Tudpor et al. 2012). This regulation is important in mediating the action of plasmin on TRPV5 (Tudpor et al. 2012).
6.1.6 80K-H
80K-H is a Ca2+-binding protein and a PKC substrate (Sakai et al. 1989). It was identified as a potential TRPV5-interacting protein because it was downregulated in 1,25(OH)2D3-deficient mice and was upregulated by dietary Ca2+ (Gkika et al. 2004). 80K-H-binding site in TRPV5 is located between residues 598 and 608 (Gkika et al. 2004). Co-expression of 80K-H did not alter the level of TRPV5 in the cell surface, but increased the sensitivity of TRPV5 to [Ca2+] i (Gkika et al. 2004). Thus, 80K-H acts as a Ca2+ sensor to control the activity of TRPV5 in addition to CaM.
6.1.7 BSPRY
BSPRY (B-box and SPRY-domain-containing protein), a protein with unknown function, interacts with TRPV5 C terminus (van de Graaf et al. 2006c). It inhibits Ca2+ transport activity of TRPV5 without altering its surface level. BSPRY co-localized with TRPV5 in the DCT and CNT, and it is inversely regulated by 1,25(OH)2D3 (van de Graaf et al. 2006c). Thus, BSPRY adds a layer of regulation of TRPV5 by 1,25(OH)2D3.
6.1.8 FKBP52
Immunophilin FKBP52, one of the downstream targets of FK-506, co-localizes with TRPV5 in the DCT and CNT and inhibits TRPV5 activity in vitro (Gkika et al. 2006c). The peptidyl-propyl cis-trans isomerase activity of FKBP52 is essential to its inhibitory effect on TRPV5, and the inhibitory effect is reversed by the administration of FK-506. FKBP52 interacts with full-length TRPV5, but not its N- or C terminus (Gkika et al. 2006c). FKBP52 expression is decreased in Trpv5 KO mice, indicating a link between the two proteins (Gkika et al. 2006c). Since FK-506 induces hypercalciuria and causes a reduction in calbindin-D28k and TRPV5 expression in the kidney (Nijenhuis et al. 2004; Lee et al. 2011; Aicher et al. 1997), further investigation is necessary to clarify the role of interaction between FKBP52 and TRPV5 in FK-506-induced hypercalciuria.
6.2 Regulation of TRPV5 by Co-expressed Proteins
In addition to the proteins that interact directly with TRPV5, proteins co-expressed with TRPV5 may also regulate TRPV5. These TRPV5-regulating proteins were studied because of physiological relevance, not as results of searching for TRPV5-binding partners. Transient protein interaction may or may not be involved in these regulations.
6.2.1 Klotho
Klotho functions to suppress aging process (Kuro-o et al. 1997). Klotho exists in membrane bound and circulating forms; it converts fibroblast growth factor (FGF) receptor FGFR1(IIIc) into a specific receptor for FGF 23 by binding with it (Urakawa et al. 2006). In the kidney, klotho is expressed in the DCT where it co-localizes with TRPV5 and increases TRPV5 activity in vitro (Chang et al. 2005; Lu et al. 2008; Cha et al. 2008a). The mechanism by which klotho regulates TRPV5 is not well understood. Because klotho exhibits β-glucuronidase activity (Tohyama et al. 2004), it was thought that klotho modifies TRPV5 glycan through this activity (Chang et al. 2005). Like klotho, β-glucuronidase also activates TRPV5 and TRPV6 but not related TRP channels TRPV4 and TRPM6 (Lu et al. 2008). Alternatively, klotho removes terminal sialic acids from their glycan chains of TRPV5 and exposes disaccharide galactose-N-acetylglucosamine, which binds to galactoside-binding lectin galectin-1 (Cha et al. 2008a). The galectin-1-linked TRPV5 proteins are likely resistant to endocytosis. However, complete removal of N-glycan by endoglycosidase-F also increases TRPV5 activity (Lu et al. 2008). However, galectin-3 but not galactin-1 co-localizes with TRPV5 in the DCT (Leunissen et al. 2013). Sialidase appears to increase TRPV5 activity by inhibiting lipid-raft-mediated endocytosis, and it does not discriminate N-glycan-deficient N358Q mutant and wild-type TRPV5 (Leunissen et al. 2013). Thus, klotho regulates TRPV5 in an N-glycan-dependent manner, whereas sialidase regulates TRPV5 in an N-glycan-independent and lipid-raft-mediated endocytosis-dependent manner (Leunissen et al. 2013). Although the mechanisms are still to be clarified, the regulation of TRPV5 by klotho represents a novel area of ion channel regulation. The dysregulation of TRPV5 may be responsible for the increased excretion of Ca2+ in Kl (klotho gene)-deficient mice, in which PTH-stimulated Ca2+ reabsorption in the CNT is impaired (Tsuruoka et al. 2006). Lower plasma klotho concentration is associated with older age and lower serum Ca2+ level and is an independent risk factor for mortality in community-dwelling adults of 65 years or older (Semba et al. 2011). Presumably reduced TRPV5 activity due to the lower klotho level likely contributes to the lower serum Ca2+ and may play a role in the aging process. Although klotho is considered a hormone, its action on TRPV5 from the luminal side is distinct from its hormonal action from interstitium. However, it could be considered as a factor in the tubular fluid similar to plasmin and uromodulin.
6.2.2 Tissue Transglutaminase
Tissue transglutaminase (tTG) catalyzes Ca2+-dependent covalent cross-linking of specific lysine and glutamine residues of substrate proteins (Lorand and Graham 2003). Calbindin-D28K and S100A10, which regulate TRPV5, are substrates of tTG (Vig et al. 2007; van de Graaf et al. 2003; Ruse et al. 2001; Lambers et al. 2006). In addition, activity of TRPV5 is inhibited by extracellular tTG treatment in HEK-293 and in rabbit CNT/CCD cells (Boros et al. 2012). This is caused by the reduction in channel pore diameter in aggregated TRPV5 in the plasma membrane as a result of tTG activity. N-glycosylation-deficient TRPV5 mutant is insensitive to tTG (Boros et al. 2012). Klotho and tTG both require the presence of N-glycan of TRPV5 for their action, but they direct TRPV5 activity to opposite directions.
6.2.3 Tissue Kallikrein
Tissue kallikrein (TK) is a serine protease expressed in CNT (Figueroa et al. 1988). TK knockout (KO) mice exhibit hypercalciuria (Picard et al. 2005). Consistent with this, TRPV5 was shown to be regulated by TK via PLC/diacylglycerol/PKC pathway (Gkika et al. 2006b). TK enhances TRPV5-mediated Ca2+ influx by delaying its retrieval from the plasma membrane (Gkika et al. 2006b). This effect was abolished by S299A and S654A mutations, which may disrupt phosphorylation of TRPV5 by PKC (Gkika et al. 2006b), which increases TRPV5 activity by inhibiting its endocytosis from plasma membrane (Cha et al. 2008b).
6.2.4 SGK1/3
Co-expression of NHERF2 and serum/glucocorticoid regulated kinase 1 or 3 (SGK1/3) with TRPV5 enhances TRPV5 activity in X. laevis oocytes (Embark et al. 2004). However, NHERF2 or SGK1/3 alone does not alter TRPV5 activity (Embark et al. 2004). The second PDZ domain in NHERF2 is required for the stimulatory effect of SGK1/NHERF2 on TRPV5 (Palmada et al. 2005). In the SGK1 KO mice, TRPV5 protein abundance in the CNT was reduced; however, urinary Ca2+ excretion was also reduced (Sandulache et al. 2006). The increased Ca2+ reabsorption is likely due to a compensatory increase in the function of thick ascending limb in response to the salt loss in the aldosterone-sensitive distal nephron. The reduced expression of TRPV5 in the CNT of SGK1 KO mice is possibly a compensatory effect due to increased Ca2+ reabsorption in the thick ascending limb, not necessarily a result due to the lack of positive regulation of TRPV5 by SGK1 (Sandulache et al. 2006).
6.2.5 WNK Kinases
Point mutations in with-no-lysine (K) kinase 4 (WNK4) result in pseudohypoaldosteronism type II (PHAII, also known as familial hyperkalemia and hypertension or Gordon’s syndrome) (Wilson et al. 2001). Hypercalciuria was observed in PHAII patients with WNK4Q565E mutation, leaving the possibility that WNK4 may regulate a Ca2+ channel or transporter in the kidney (Mayan et al. 2004). We found WNK4 increases the activity of TRPV5 channel by increasing the forward trafficking of the channel to the plasma membrane via the secretory pathway in X. laevis oocytes (Jiang et al. 2007, 2008). The positive effect of WNK4 on TRPV5 was greatly reduced when NCC was co-expressed (Jiang et al. 2007). Similar to WNK4, WNK3 also increases Ca2+ influx mediated by TRPV5 via a kinase-dependent pathway (Zhang et al. 2008). The effect of WNK4 on TRPV5 is enhanced and stabilized by NHERF2 (Jing et al. 2011). When the last two amino acid residues of TRPV5 were replaced by those of TRPV6, the effect of NHERF2 on TRPV5 was abolished (Jing et al. 2011). Thus, WNK4 and NHERF2 increase TRPV5 forward trafficking and membrane stability synergistically, leading to additive enhancement in TRPV5-mediated Ca2+ transport. On the other hand, Cha et al. found that WNK4 kinase enhances the endocytosis and decreases the plasma membrane abundance of TRPV5 in HEK293 cells (Cha and Huang 2010). The reason for the difference in WNK4-mediated regulation on TRPV5 is unclear.
6.2.6 Ubiquitin E3 Ligases
Nedd4-2 is an archetypal member of the ubiquitin E3 ligase family regulating cell surface stability of membrane proteins (Staub and Rotin 2006). Nedd4-2 is expressed in the DCT (Verrey et al. 2003) and CCD (Flores et al. 2005) in the kidney where TRPV5 is functionally expressed. When expressed in Xenopus oocytes, TRPV5/6-mediated Ca2+ uptake and Na+ current were decreased by Nedd4-2 and Nedd4 due to the reduction in TRPV5 protein level (Zhang et al. 2010). In all cases, Nedd4-2 exhibited stronger inhibitory effects than Nedd4 on both TRPV5 and TRPV6. WW1 and WW2 domains of Nedd4-2 may serve as a molecular switch to limit the ubiquitination of TRPV6/5 by the HECT domain (Zhang et al. 2010). Although Nedd4 and Nedd4-2 mediate TRPV5 degradation in vitro, it is unclear to what extent they do so in vivo. In addition, the degradation of TRPV5 is reduced by knocking down of Ubiquitin recoginition 4 (UBR4), another E3 ubiquitin ligase (Radhakrishnan et al. 2013). The physiological significance of these TRPV5 degradation pathways warrants further studies.
6.2.7 Ca2+-Sensing Receptor
The mRNA and protein of Ca2+-sensing receptor (CaR) are expressed in the DCT/CNT (Riccardi et al. 1996, 1998; Hoenderop et al. 1999). Activation of CaR increases TRPV5-mediated currents and elevates [Ca2+] i in cells co-expressing TRPV5 and CaR (Topala et al. 2009). Phorbol-12-myristate-13-acetate (PMA)-insensitive PKC isoforms are likely involved in the signal pathway by which CaR stimulates TRPV5 (Topala et al. 2009). The stimulatory effect was abolished by mutation of two putative PKC phosphorylation sites, S299 and S654 in TRPV5, or by a dominant-negative CaR (R185Q) (Topala et al. 2009).
6.3 Regulation by Proteins in the Tubular Fluid
6.3.1 Plasmin
Plasminogen, which is elevated and can be filtered into the urine in nephrotic syndrome, is converted into active plasmin in the tubular fluid. When incubated with plasmin, either from commercial source or purified from nephrotic urine, TRPV5-mediated Ca2+ uptake in HEK-293 cells was inhibited (Tudpor et al. 2012). As a serine protease, plasmin does not cleave TRPV5 or alter TRPV5 surface abundance; instead, it affects TRPV5 by binding to protease-activated receptor-1 (PAR-1) (Tudpor et al. 2012). This activates the PAR-1/PLC/PKC pathway, which likely leads to phosphorylation of S144 within a CaM-binding site in the N terminus of TRPV5. The phosphorylation of S144 results in an alteration of CaM binding to TRPV5 and, in turn, a reduction in pore size and open probability (Tudpor et al. 2012). Suppression of TRPV5 by plasmin likely takes effect in nephrotic patients.
6.3.2 Uromodulin
Uromodulin (also known as Tamm–Horsfall glycoprotein/THP) is a urinary glycoprotein secreted by the thick ascending loop of Henle (Bachmann et al. 1990). Co-expression with uromodulin increases TRPV5 current density and surface abundance in HEK293 cells (Wolf et al. 2013). Acting from the tubular luminal side, uromodulin decreases caveolin-mediated endocytosis of TRPV5, and the level of TRPV5 is lower in uromodulin KO mice (Wolf et al. 2013). This result suggests that uromodulin may act as a physiological regulator of TRPV5 to prevent kidney stone formation.
7 Regulation by Hormones
Trpv5 expression is regulated under physiological conditions. Trpv5 expression in the kidney is upregulated under dietary Ca2+ restriction in mice (Song et al. 2003; Van Cromphaut et al. 2001); this regulation appears to be VDR dependent (Van Cromphaut et al. 2001). TRPV6 and TRPV5 in rat small intestine were decreased by immobilization and induced by endurance swimming, and a 1,25(OH)2D3-dependent pathway is likely involved in these changes (Sato et al. 2006; Teerapornpuntakit et al. 2009). TRPV5 was upregulated by high-salt intake and downregulated by low-salt intake or dehydration in rats; this was likely caused by the increase or decrease in the delivery of Ca2+ to the distal tubule in the kidney (Lee et al. 2012). Aging is associated with alterations in Ca2+ homeostasis, such as decreased Ca2+ absorption and increased urinary Ca2+ excretion. Duodenal TRPV5/6 mRNA level in adult (12-month old) rats was less than half of that in young (2-month old) rats (Brown et al. 2005). Trpv5 KO mice develop age-related hyperparathyroidism and osteoporotic characteristics earlier than wild-type mice, possibly due to the age-related vitamin D resistance and less robust compensatory expression of intestinal TRPV6 in older mice (van Abel et al. 2006). Thus, as Trpv5 KO mice age, vitamin D resistance prevents the animals to compensate the renal loss of Ca2+ through intestinal absorption, leading to a negative Ca2+ balance.
Hormonal regulation is at least in part behind the adaptation of TRPV5 expression in response to physiological conditions. Indeed, a number of hormones, including 1,25(OH)2D3, PTH, sex hormones, and vasopressin, have been found to regulate TRPV5 (Table 2). The effects of these hormones on TRPV5 are summarized briefly below.
7.1 Vitamin D
In 1991, Bindels and colleagues reported that 1,25(OH)2D3 increases transcellular Ca2+ absorption in primary culture of rabbit kidney CCD (Bindels et al. 1991) where TRPV5 was later cloned (Hoenderop et al. 1999). After the identification, TRPV5 mRNA was shown to be upregulated by 1,25(OH)2D3 in the kidney of vitamin D-depleted rats (Hoenderop et al. 2001a). Moreover, a single injection of 1,25(OH)2D3 in vitamin D-deficient mice induced ~three- to fourfold increase in TRPV5 mRNA in the kidney that peaked at 12 h after injection following the peak of duodenal TRPV6 (6 h after injection) (Song et al. 2003). This suggests that the increase in TRPV5 in the kidney might be a secondary event due to the increased Ca2+ absorption. In mice deficient in 25-hydroxyvitamin D3-1α-hydroxylase with undetectable level of 1,25(OH)2D3, the reduction of renal TRPV5 and hypocalcemia were normalized by high Ca2+ diet, suggesting that TRPV5 is upregulated by increased Ca2+ load to the distal tubule as a result of 1,25(OH)2D3-induced Ca2+ absorption (Hoenderop et al. 2002). Although potential vitamin D response elements (VDREs) have been identified in the promoter region of Trpv5 gene (Muller et al. 2000; Weber et al. 2001), their function has not been examined as those bona fide VDREs in Trpv6 gene promoter (Meyer et al. 2006). At least in mice duodenum, FGF-23 could reduce circulating 1,25(OH)2D3 and diminish 1,25(OH)2D3-induced surge of TRPV6 and TRPV5 (Khuituan et al. 2012).
7.2 Parathyroid Hormone
PTH regulates both the expression and activity of TRPV5. In parathyroidectomized rats and calcimimetic compound NPS R-467-infused mice that had a lower PTH level, renal TRPV5, calbindin-D28k, and NCX1 levels were decreased; and they were restored by PTH supplementation (van Abel et al. 2005). The decrease in other Ca2+ transport proteins by PTH is likely secondary to the reduction of TRPV5-mediated Ca2+ influx (van Abel et al. 2005).
PTH is also capable of acutely increasing Ca2+ transport in the distal tubule (Bacskai and Friedman 1990). Both PKA and PKC are involved in this regulation (Friedman et al. 1996). Indeed, activation of TRPV5 by PTH via both PKA and PKC has been reported (de Groot et al. 2009; Cha et al. 2008b). PTH activates the cAMP–PKA signaling cascade and increases TRPV5 open probability via phosphorylation of threonine 709 (T709) in TRPV5 (de Groot et al. 2009). This regulation requires a strong buffering of intracellular Ca2+. However, the PKA phosphorylation site T709 is not conserved in human TRPV5. In addition to PKA, the increase of TRPV5 activity by heterogeneously expressed PTH receptor was prevented by PKC inhibitor (Cha et al. 2008b). Mutation of PKC phosphorylation sites S299/S654 in TRPV5 abolished the regulation. Caveolae-mediated endocytosis of TRPV5 appears to be inhibited by PTH via a PKC-dependent pathway (Cha et al. 2008b).
Besides vitamin D and PTH, calcitonin is a hormone that regulates Ca2+ homeostasis. However, calcitonin affects renal Ca2+ reabsorption mainly through the thick ascending limb (Elalouf et al. 1984; Di Stefano et al. 1990). TRPV5, which is mostly expressed in the DCT and CNT, is not involved in the effects of calcitonin on urinary excretion of Ca2+, Na+, and K+ as no difference was observed between wild-type and Trpv5 KO mice in this regard (Hsu et al. 2009).
7.3 Estrogen and Testosterone
As the most potent estrogen, 17β-estradiol increases TRPV5 mRNA and protein expression in the kidney (van Abel et al. 2002, 2003). Estrogen deficiency in aromatase-deficient mice results in decreased mRNA levels of renal TRPV5 and other Ca2+ transporters and Ca2+ wasting (Oz et al. 2007). In addition, 17β-estradiol (20–50 nM) rapidly increases TRPV5 current and [Ca2+] i in rat CCD cells (Irnaten et al. 2009). Thus, estrogen acutely elevates TRPV5 activity and chronically increases its expression.
In contrast to the positive effects of estrogen, male hormone testosterone appears to have a negative effect on TRPV5. Male mice have higher urinary Ca2+ excretion than female mice (Hsu et al. 2010). Androgen deficiency increases renal TRPV5 mRNA and protein and decreases urinary Ca2+ excretion; these were normalized by testosterone treatment (Hsu et al. 2010). In addition, the negative effect of dihydrotestosterone on transcellular Ca2+ transport was demonstrated in primary rabbit CNT/CCD cells (Hsu et al. 2010).
7.4 Vasopressin
Arginine vasopressin stimulates transepithelial Ca2+ transport in primary cultures of rabbit CCD cells (van Baal et al. 1996). Vasopressin induces an increase in Ca2+ uptake and [Ca2+] i in mpkDCT cells and freshly isolated late DCT and CNT cells, respectively, indicating that TRPV5 is a target of vasopressin (Diepens et al. 2004; Hofmeister et al. 2009).
8 Physiological Functions in Native Cells, Organs, Organ Systems
8.1 Ca2+ Reabsorption in the Kidney
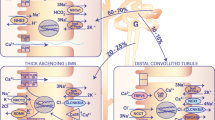
TRPV5 is mainly distributed in the apical membrane of tubular cells in the DCT and CNT where it mediates the final reabsorption of Ca2+ via a transcellular pathway. TRPV5, calbindin-D28k, NCX1, and PMCA are the major components of the pathway. Lines of evidence suggest that the expression of other Ca2+ transporters depends on TRPV5-mediated Ca2+ influx (van Abel et al. 2005; Hoenderop et al. 2003a). In this sense, TRPV5 is the rate-limiting component in the transcellular pathway of Ca2+ reabsorption.
TRPV5 is likely constitutively active in the apical membrane of tubular cells. At macroscopic level, TRPV5 acts as a facilitative transporter exhibiting saturable kinetics with apparent K m values at sub-millimolar range, which are well suited for the luminal Ca2+ level in the distal tubule (Hoenderop et al. 1999; Peng et al. 1999, 2000a, b). The responsiveness of TRPV5 to vitamin D, PTH, Ca2+ load, pH, and tubular factors such as klotho, uromodulin, and plasmin makes TRPV5 well suited for the fine-tuning of Ca2+ reabsorption in the kidney.
8.2 Bone Resorption by Osteoclasts
TRPV5 also expresses in the ruffled border membrane of mouse osteoclasts (van der Eerden et al. 2005). Osteoclast numbers and area are increased, and paradoxically urinary bone resorption marker deoxypyridinoline was reduced in mice lacking TRPV5 (Hoenderop et al. 2003a). In vitro bone marrow culture system indicates that bone resorption by osteoclasts was impaired in Trpv5 KO mice (van der Eerden et al. 2005). These results suggest a malfunction of osteoclast to some degree in the absence of TRPV5. However, bone resorption inhibitor alendronate normalizes the reduced bone thickness in Trpv5 KO mice, even though it specifically increases bone TRPV5 expression (Nijenhuis et al. 2008). Furthermore, vitamin D analog ZK191784 partially restores the decreased bone matrix mineralization in Trpv5 KO mice, suggesting the bone phenotype of Trpv5 KO mice is secondary to the elevated 1,25(OH)2D3 (van der Eerden et al. 2013). Thus, TRPV5 plays a role in osteoclast function, but it is not absolutely required for bone reabsorption. The role of TRPV5 in osteoclast function is not well understood, but TRPV5 is involved in receptor activator of NF-κB ligand (RANKL)-induced rise in [Ca2+] i in human osteoclasts (Chamoux et al. 2010). This process is likely a part of the negative feedback loop to terminate RANKL-induced bone resorption (Chamoux et al. 2010).
8.3 Function in the Inner Ear
The low Ca2+ concentration of mammalian endolymph in the inner ear is required for normal hearing and balance. Marcus and colleagues showed that TRPV5 and TRPV6 may play roles in the function of inner ear (Yamauchi et al. 2005, 2010; Wangemann et al. 2007; Nakaya et al. 2007). TRPV5 was detected in native semicircular canal duct (SCCD) epithelial cells, cochlear lateral wall, and stria vascularis of adult rats along with other Ca2+ transport proteins (Yamauchi et al. 2010). TRPV5 protein was localized close to the apical membrane of strial marginal cells and in outer and inner sulcus cells of the cochlea (Yamauchi et al. 2010). TRPV5 transcript was responsive to 1,25(OH)2D3 (Yamauchi et al. 2005); however, the protein level was not upregulated (Yamauchi et al. 2010). The levels of TRPV5 were decreased in the inner ear of older mice (Takumida et al. 2009). Mutations in pendrin (SLC26A4, an anion exchanger) cause the most common form of syndromic deafness. Reduced pH and utricular endolymphatic potential and increased Ca2+ concentration were found in pendrin KO mice (Wangemann et al. 2007; Nakaya et al. 2007). The reduced pH likely blocks the activity of TRPV5 and TRPV6, whose Ca2+ transport activity is reduced at low pH, similar to what was observed in primary SCCD cells (Nakaya et al. 2007). The elevation of endolymphatic Ca2+ level in pendrin KO mice may inhibit sensory transduction necessary for hearing and promote the degeneration of the sensory hair cells, which is necessary for the development of normal hearing (Wangemann et al. 2007).
8.4 Intestinal and Placental Ca2+ Transport
The levels of TRPV5 mRNA in intestine and placenta are much lower than TRPV6 (Peng et al. 2001). TRPV5 is regulated in the same direction with TRPV6 in the intestine in most cases, such as immobilization and exercise-induced alteration of gene expression (Teerapornpuntakit et al. 2009; Sato et al. 2006). In the placenta, Ca2+ transport in human syncytiotrophoblasts is insensitive to voltage and L-type Ca2+ channel modulators but is sensitive to TRPV5/6 blocker Mg2+ and ruthenium red (Moreau et al. 2002a). In cultured human trophoblasts isolated from term placenta, TRPV5 and TRPV6 expression correlated with the Ca2+ uptake potential along the differentiation of the trophoblasts (Moreau et al. 2002b). In contrast to the demonstrated role of TRPV6 in placental Ca2+ transport (Suzuki et al. 2008), the role of TRPV5 is unclear. TRPV5 likely plays a minor role in intestinal and placental Ca2+ transport due to its low levels of expression in these organs.
9 Lesson from TRPV5 Knockout Mice
The most distinctive features of Trpv5 KO mice include 2.9-fold elevation in serum 1,25(OH)2D3, sixfold increase in Ca2+ excretion, moderate reduced bone thickness in the femoral head, and normal level of plasma Ca2+ (Hoenderop et al. 2003a). In addition, polyuria and urine acidification were observed in Trpv5 KO mice (Hoenderop et al. 2003a). These alterations in renal function are caused by the activation of CaR by the increased tubular Ca2+ in the collecting duct and subsequently upregulated proton secretion by the H+–ATPase and reduced water reabsorption due to the downregulation of aquaporin 2 (Renkema et al. 2009b). These physiological adaptations reduce the risk of Ca2+ precipitations and stone formation. Intestinal Ca2+ absorption is increased in Trpv5 KO mice as duodenal TRPV6 and calbindin-D9k mRNAs are increased due to the elevated 1,25(OH)2D3 level (Hoenderop et al. 2003a). However, the serum Ca2+ levels are largely normal, even though the PTH level is elevated in older Trpv5 KO mice (van Abel et al. 2006). The major phenotypes of Trpv5 KO mice are listed in Table 3.
Studies with Trpv5 KO mice reveal some important points in Ca2+ transport physiology: (1) the transcellular Ca2+ transport in the DCT and CNT, as represented by TRPV5, plays an important role in Ca2+ reabsorption. This is supported by the fact that removal of TRPV5 results in significant hypercalciuria. (2) TRPV5 is not indispensable to maintain a Ca2+ balance as long as vitamin D and related systems work normally. The body reset Ca2+ homeostasis by elevating the level of 1,25(OH)2D3, which increases intestinal Ca2+ absorption to compensate the renal loss of Ca2+ (Renkema et al. 2005). A new Ca2+ balance is achieved at higher intestinal absorption and higher urinary Ca2+ excretion in the absence of TRPV5. (3) The TRPV5 KO model shows an example that polyuria and reduced urinary pH are natural responses to increased Ca2+ excretion in order to prevent kidney stone formation. In addition, Trpv5 KO mouse is a useful model to clarify some basic physiological mechanisms. For example, with this model it has been shown that calcitonin or thiazide-induced hypocalciuria occurs independent of TRPV5; thus, the responsible mechanisms likely reside in more proximal segments of the nephron (Hsu et al. 2009; Nijenhuis et al. 2005). All the studies indicate that TRPV5 does not alter the bulk Ca2+ reabsorption; rather, it provides a key mechanism for the fine-tuning of Ca2+ reabsorption.
10 Role in Hereditary and Acquired Diseases
As we learned from Trpv5 KO mice, the lack of TRPV5 could be compensated through an elevation of 1,25(OH)2D3, which regulates other proteins (e.g., TRPV6) in maintaining Ca2+ homeostasis. Thus, humans with defect in TRPV5 may have altered Ca2+ homeostasis, but could be clinically asymptomatic except for hypercalciuria. However, no mutation in the exons of Trpv5 gene was found to be associated with autosomal dominant hypercalciuria (Muller et al. 2002). In another study involving 20 renal hypercalciuria patients, nonsynonymous variation of TRPV5 (A8V, R154H, and A561T) and synonymous variations were identified; however, these variants apparently do not alter the property of TRPV5 (Renkema et al. 2009a). Recently, S682P mutation of TRPV5 causes autosomal dominant hypercalciuria in mouse model (Loh et al. 2013). Interestingly, no significant functional differences were found between S682P and wild-type TRPV5 when expressed in Xenopus oocytes or HEK293 cells; the only difference was that S682P produced a lower baseline [Ca2+] i than wild-type TRPV5 in HEK293 cells (Loh et al. 2013). However, mice with homozygous TRPV5S682P did show reduced TRPV5 immunostaining in the kidney and phenotype resembling that of Trpv5 KO mice (Loh et al. 2013). This study suggests that mutations in TRPV5 could result in hypercalciuria; however, the functional changes of the TRPV5 mutations may not be detected in vitro. Thus, it remains to be further examined that mutations in Trpv5 gene cause hypercalciuric disorders in humans.
In addition to a possible role of TRPV5 in hypercalciuria, TRPV5 expression and potentially activity are altered in response to a number of diseases and therapeutic conditions (Table 4). Mechanisms for the change in TRPV5 expression are often not fully understood. TRPV5 expression could be regulated by hormones, chemicals, and likely intracellular or extracellular Ca2+. A change in Trpv5 expression likely leads to alteration in urinary Ca2+ excretion; on the other hand, the alteration of filtered Ca2+ load or reabsorption of Ca2+ elsewhere in the nephron leads to increased or decreased delivery of Ca2+ to the DCT and CNT and in turn causes an elevation or reduction in Trpv5 expression. If specific modulators for TRPV5 and TRPV6 could be developed, they could be useful to correct abnormality in Ca2+ homeostasis under disease or therapeutic conditions. For example, under disease conditions caused by vitamin D signaling defect, such as in vitamin D-dependent rickets, type 2a (Online Mendelian Inheritance in Man #277440), intestinal and renal TRPV5 and TRPV6 expressions are likely disrupted as demonstrated in the Vdr KO mice (Van Cromphaut et al. 2001). Enhancing TRPV5- and TRPV6-mediated Ca2+ absorption would be helpful to achieve a positive Ca2+ under this condition. Similarly, enhancers for TRPV5 and TRPV6 are desirable to prevent osteoporosis in women after the menopause due to reduced estrogen level (Oz et al. 2007; van Abel et al. 2002; Irnaten et al. 2009). On the other hand, TRPV6 inhibitors would be needed in preventing absorptive hypercalciuria and kidney stone disease, and TRPV5 stimulator would be useful in preventing kidney stone formation in patients with renal Ca2+ leak. Stimulators of TRPV5 would be also helpful in tacrolimus-induced hypercalciuria (Nijenhuis et al. 2004) or other conditions associated with reduced expression of TRPV5 in Table 4.
11 Single Nucleotide Polymorphisms of TRPV5 in African Populations
TRPV5 and TRPV6 have been shown to have high frequency of SNPs in African populations. By analyzing SNP data from 24 African Americans and 23 European Americans in genes sequenced in SeattleSNPs, a 115-kb region in chromosome 7q34-35 of 4 contiguous genes, including EPHB6, TRPV6, TRPV5, and KEL4, was identified with features of a recent demographic selection (Stajich and Hahn 2005; Akey et al. 2004). TRPV6 SNPs defined by three nonsynonymous SNPs (C157R, M378V, and M681T) exhibit most striking footprint of positive selection (Akey et al. 2006; Hughes et al. 2008). In addition, four nonsynonymous SNPs in TRPV5 were identified by SeattleSNPs: three of them (A8V, A563T, and L712F) were only present in African Americans, not in European Americans; R154H is common in both populations. The nonsynonymous SNP variations in TRPV6 ancestral haplotype are conserved in other species; in contrast, the variations in TRPV5 are newly derived as they are not commonly present in other species surveyed including chimpanzee, dog, rat, and mouse, with the exceptions of 563T in dog and 8V in rat (Na et al. 2009). In addition, the nonsynonymous SNPs of TRPV5 are not associated with each other as are those in TRPV6. By expression in Xenopus oocytes, we found that two of the SNPs, A563T and L712F, significantly increased TRPV5-mediated Ca2+ uptake by approx. 50 % and 25 %, respectively (Na et al. 2009). For A563T variant, the increased Ca2+ uptake activity was not associated with increased protein abundance in the plasma membrane; rather it was associated with increased apparent K m for Ca2+ and increased sensitivity to extracellular Mg2+, suggesting increased permeation of Ca2+ in the cation translocation pathway of the channel (Na et al. 2009). The A563 residue in the last transmembrane domain is 20 residues away from D542 residue in the Ca2+ filter in the pore. It is likely in the cation translocation path of the channel (Fig. 2).
African Americans exhibit lower urinary Ca2+ excretion than Caucasians (Braun et al. 2007; Pratt et al. 1996; Taylor and Curhan 2007), and the risk of kidney stone in African Americans is lower than that in Caucasians (Sarmina et al. 1987; Stamatelou et al. 2003). In addition, African Americans have higher bone mass (Bell et al. 1991) and lower incidence of osteoporosis-related fractures than whites (Bohannon 1999). Because of the high allele frequencies of TRPV5 and TRPV6 SNPs in African populations, these SNPs may contribute to the Ca2+ conservation mechanisms in African populations. Further population studies are necessary to clarify the relationship of SNPs in TRPV5 and TRPV6 and Ca2+ homeostasis in African populations.
References
Aicher L, Meier G, Norcross AJ, Jakubowski J, Varela MC, Cordier A, Steiner S (1997) Decrease in kidney calbindin-D 28 kDa as a possible mechanism mediating cyclosporine A- and FK-506-induced calciuria and tubular mineralization. Biochem Pharmacol 53:723–731
Akey JM, Eberle MA, Rieder MJ, Carlson CS, Shriver MD, Nickerson DA, Kruglyak L (2004) Population history and natural selection shape patterns of genetic variation in 132 genes. PLoS Biol 2:e286
Akey JM, Swanson WJ, Madeoy J, Eberle M, Shriver MD (2006) TRPV6 exhibits unusual patterns of polymorphism and divergence in worldwide populations. Hum Mol Genet 15:2106–2113
Bachmann S, Metzger R, Bunnemann B (1990) Tamm-Horsfall protein-mRNA synthesis is localized to the thick ascending limb of Henle’s loop in rat kidney. Histochemistry 94:517–523
Bacskai BJ, Friedman PA (1990) Activation of latent Ca2+ channels in renal epithelial cells by parathyroid hormone. Nature 347:388–391
Bell NH, Shary J, Stevens J, Garza M, Gordon L, Edwards J (1991) Demonstration that bone mass is greater in black than in white children. J Bone Miner Res 6:719–723
Bernucci L, Henriquez M, Diaz P, Riquelme G (2006) Diverse calcium channel types are present in the human placental syncytiotrophoblast basal membrane. Placenta 27:1082–1095
Bindels RJ, Hartog A, Timmermans J, van Os CH (1991) Active Ca2+ transport in primary cultures of rabbit kidney CCD: stimulation by 1,25-dihydroxyvitamin D3 and PTH. Am J Physiol 261:F799–F807
Bohannon AD (1999) Osteoporosis and African American women. J Womens Health Gend Based Med 8:609–615
Bonny O, Rubin A, Huang CL, Frawley WH, Pak CY, Moe OW (2008) Mechanism of urinary calcium regulation by urinary magnesium and pH. J Am Soc Nephrol 19:1530–1537
Boros S, Xi Q, Dimke H, van der Kemp AW, Tudpor K, Verkaart S, Lee KP, Bindels RJ, Hoenderop JG (2012) Tissue transglutaminase inhibits the TRPV5-dependent calcium transport in an N-glycosylation-dependent manner. Cell Mol Life Sci 69:981–992
Braun M, Palacios C, Wigertz K, Jackman LA, Bryant RJ, McCabe LD, Martin BR, McCabe GP, Peacock M, Weaver CM (2007) Racial differences in skeletal calcium retention in adolescent girls with varied controlled calcium intakes. Am J Clin Nutr 85:1657–1663
Bronner F (1998) Calcium absorption–a paradigm for mineral absorption. J Nutr 128:917–920
Brown EM (1991) Extracellular Ca2+ sensing, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol Rev 71:371–411
Brown EM, Vassilev PM, Hebert SC (1995) Calcium ions as extracellular messengers. Cell 83:679–682
Brown AJ, Krits I, Armbrecht HJ (2005) Effect of age, vitamin D, and calcium on the regulation of rat intestinal epithelial calcium channels. Arch Biochem Biophys 437:51–58
Castiglioni AJ, Remis NN, Flores EN, Garcia-Anoveros J (2011) Expression and vesicular localization of mouse Trpml3 in stria vascularis, hair cells, and vomeronasal and olfactory receptor neurons. J Comp Neurol 519:1095–1114
Cha SK, Huang CL (2010) WNK4 kinase stimulates caveola-mediated endocytosis of TRPV5 amplifying the dynamic range of regulation of the channel by protein kinase C. J Biol Chem 285:6604–6611
Cha SK, Jabbar W, Xie J, Huang CL (2007) Regulation of TRPV5 single-channel activity by intracellular pH. J Membr Biol 220:79–85
Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro O, Huang CL (2008a) Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci USA 105:9805–9810
Cha SK, Wu T, Huang CL (2008b) Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294:F1212–F1221
Cha SK, Kim JH, Huang CL (2013) Flow-induced activation of TRPV5 and TRPV6 channel stimulates Ca-activated K channel causing membrane hyperpolarization. Biochim Biophys Acta 1833(12):3046–3053
Chamoux E, Bisson M, Payet MD, Roux S (2010) TRPV-5 mediates a receptor activator of NF-kappaB (RANK) ligand-induced increase in cytosolic Ca2+ in human osteoclasts and down-regulates bone resorption. J Biol Chem 285:25354–25362
Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG (2004) Molecular determinants in TRPV5 channel assembly. J Biol Chem 279:54304–54311
Chang Q, Hoefs S, van der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG (2005) The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science 310:490–493
Christakos S, Gill R, Lee S, Li H (1992) Molecular aspects of the calbindins. J Nutr 122:678–682
Clapham DE (1995) Calcium signaling. Cell 80:259–268
de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG (2009) Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20:1693–1704
de Groot T, Verkaart S, Xi Q, Bindels RJ, Hoenderop JG (2010) The identification of Histidine 712 as a critical residue for constitutive TRPV5 internalization. J Biol Chem 285:28481–28487
de Groot T, Kovalevskaya NV, Verkaart S, Schilderink N, Felici M, van der Hagen EA, Bindels RJ, Vuister GW, Hoenderop JG (2011a) Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol Cell Biol 31:2845–2853
de Groot T, van der Hagen EA, Verkaart S, te Boekhorst VA, Bindels RJ, Hoenderop JG (2011b) Role of the transient receptor potential vanilloid 5 (TRPV5) protein N terminus in channel activity, tetramerization, and trafficking. J Biol Chem 286:32132–32139
Di Stefano A, Wittner M, Nitschke R, Braitsch R, Greger R, Bailly C, Amiel C, Roinel N, de Rouffignac C (1990) Effects of parathyroid hormone and calcitonin on Na+, Cl-, K+, Mg2+ and Ca2+ transport in cortical and medullary thick ascending limbs of mouse kidney. Pflugers Arch 417:161–167
Diepens RJ, den DE, Bens M, Weidema AF, Vandewalle A, Bindels RJ, Hoenderop JG (2004) Characterization of a murine renal distal convoluted tubule cell line for the study of transcellular calcium transport. Am J Physiol Renal Physiol 286:F483–F489
Dodier Y, Dionne F, Raybaud A, Sauve R, Parent L (2007) Topology of the selectivity filter of a TRPV channel: rapid accessibility of contiguous residues from the external medium. Am J Physiol Cell Physiol 293:C1962–C1970
Elalouf JM, Roinel N, de Rouffignac C (1984) ADH-like effects of calcitonin on electrolyte transport by Henle’s loop of rat kidney. Am J Physiol 246:F213–F220
Embark HM, Setiawan I, Poppendieck S, van de Graaf SF, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJ, Lang F (2004) Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem 14:203–212
Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA (2004) Ca2+-selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 279:34456–34463
Figueroa CD, MacIver AG, Mackenzie JC, Bhoola KD (1988) Localisation of immunoreactive kininogen and tissue kallikrein in the human nephron. Histochemistry 89:437–442
Flores SY, Loffing-Cueni D, Kamynina E, Daidie D, Gerbex C, Chabanel S, Dudler J, Loffing J, Staub O (2005) Aldosterone-induced serum and glucocorticoid-induced kinase 1 expression is accompanied by Nedd4-2 phosphorylation and increased Na + transport in cortical collecting duct cells. J Am Soc Nephrol 16:2279–2287
Friedman PA, Coutermarsh BA, Kennedy SM, Gesek FA (1996) Parathyroid hormone stimulation of calcium transport is mediated by dual signaling mechanisms involving protein kinase A and protein kinase C. Endocrinology 137:13–20
Gerke V, Moss SE (2002) Annexins: from structure to function. Physiol Rev 82:331–371
Gkika D, Mahieu F, Nilius B, Hoenderop JG, Bindels RJ (2004) 80K-H as a new Ca2+ sensor regulating the activity of the epithelial Ca2+ channel transient receptor potential cation channel V5 (TRPV5). J Biol Chem 279:26351–26357
Gkika D, Hsu YJ, van der Kemp AW, Christakos S, Bindels RJ, Hoenderop JG (2006a) Critical role of the epithelial Ca2+ channel TRPV5 in active Ca2+ reabsorption as revealed by TRPV5/calbindin-D28K knockout mice. J Am Soc Nephrol 17:3020–3027
Gkika D, Topala CN, Chang Q, Picard N, Thebault S, Houillier P, Hoenderop JG, Bindels RJ (2006b) Tissue kallikrein stimulates Ca2+ reabsorption via PKC-dependent plasma membrane accumulation of TRPV5. EMBO J 25:4707–4716
Gkika D, Topala CN, Hoenderop JG, Bindels RJ (2006c) The immunophilin FKBP52 inhibits the activity of the epithelial Ca2+ channel TRPV5. Am J Physiol Renal Physiol 290:F1253–F1259
Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jors S, Kros CJ, Ricci AJ, Heller S (2007) A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitint-waddler mouse. Proc Natl Acad Sci USA 104:19583–19588
Guo Z, Grimm C, Becker L, Ricci AJ, Heller S (2013) A novel ion channel formed by interaction of TRPML3 with TRPV5. PLoS One 8:e58174
Hache S, Takser L, Lebellego F, Weiler H, Leduc L, Forest JC, Giguere Y, Masse A, Barbeau B, Lafond J (2011) Alteration of calcium homeostasis in primary preeclamptic syncytiotrophoblasts: effect on calcium exchange in placenta. J Cell Mol Med 15(3):654–667
Hdud IM, El-Shafei AA, Loughna P, Barrett-Jolley R, Mobasheri A (2012) Expression of Transient Receptor Potential Vanilloid (TRPV) Channels in Different Passages of Articular Chondrocytes. Int J Mol Sci 13:4433–4445
Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M (2005) Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 118:917–928
Henry HL (1985) Parathyroid hormone modulation of 25-hydroxyvitamin D3 metabolism by cultured chick kidney cells is mimicked and enhanced by forskolin. Endocrinology 116:503–510
Hoenderop JG, van der Kemp AW, Hartog A, van de Graaf SF, van Os CH, Willems PH, Bindels RJ (1999) Molecular identification of the apical Ca2+ channel in 1, 25-dihydroxyvitamin D3-responsive epithelia. J Biol Chem 274:8375–8378
Hoenderop JG, Muller D, van der Kemp AW, Hartog A, Suzuki M, Ishibashi K, Imai M, Sweep F, Willems PH, van Os CH, Bindels RJ (2001a) Calcitriol controls the epithelial calcium channel in kidney. J Am Soc Nephrol 12:1342–1349
Hoenderop JG, Vennekens R, Muller D, Prenen J, Droogmans G, Bindels RJ, Nilius B (2001b) Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J Physiol 537:747–761
Hoenderop JG, Dardenne O, van Abel M, van der Kemp AW, van Os CH, St Arnaud R, Bindels RJ (2002) Modulation of renal Ca2+ transport protein genes by dietary Ca2+ and 1,25-dihydroxyvitamin D3 in 25-hydroxyvitamin D3-1alpha-hydroxylase knockout mice. FASEB J 16:1398–1406
Hoenderop JG, van Leeuwen JP, van der Eerden BC, Kersten FF, van der Kemp AW, Merillat AM, Waarsing JH, Rossier BC, Vallon V, Hummler E, Bindels RJ (2003a) Renal Ca2+ wasting, hyperabsorption, and reduced bone thickness in mice lacking TRPV5. J Clin Invest 112:1906–1914
Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ (2003b) Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J 22:776–785
Hofmeister MV, Fenton RA, Praetorius J (2009) Fluorescence isolation of mouse late distal convoluted tubules and connecting tubules: effects of vasopressin and vitamin D3 on Ca2+ signaling. Am J Physiol Renal Physiol 296:F194–F203
Holakovska B, Grycova L, Bily J, Teisinger J (2011) Characterization of calmodulin binding domains in TRPV2 and TRPV5 C-tails. Amino Acids 40:741–748
Hsu YJ, Dimke H, Hoenderop JG, Bindels RJ (2009) Calcitonin-stimulated renal Ca2+ reabsorption occurs independently of TRPV5. Nephrol Dial Transplant 25(5):1428–1435
Hsu YJ, Dimke H, Schoeber JP, Hsu SC, Lin SH, Chu P, Hoenderop JG, Bindels RJ (2010) Testosterone increases urinary calcium excretion and inhibits expression of renal calcium transport proteins. Kidney Int 77:601–608
Hughes DA, Tang K, Strotmann R, Schoneberg T, Prenen J, Nilius B, Stoneking M (2008) Parallel selection on TRPV6 in human populations. PLoS One 3:e1686
Ignat M, Teletin M, Tisserand J, Khetchoumian K, Dennefeld C, Chambon P, Losson R, Mark M (2008) Arterial calcifications and increased expression of vitamin D receptor targets in mice lacking TIF1alpha. Proc Natl Acad Sci USA 105:2598–2603
Irnaten M, Blanchard-Gutton N, Praetorius J, Harvey BJ (2009) Rapid effects of 17beta-estradiol on TRPV5 epithelial Ca2+ channels in rat renal cells. Steroids 74:642–649
Jang HR, Kim S, Heo NJ, Lee JH, Kim HS, Nielsen S, Jeon US, Oh YK, Na KY, Joo KW, Han JS (2009) Effects of thiazide on the expression of TRPV5, calbindin-D28K, and sodium transporters in hypercalciuric rats. J Korean Med Sci 24(Suppl):S161–S169
Jang Y, Lee Y, Kim SM, Yang YD, Jung J, Oh U (2012) Quantitative analysis of TRP channel genes in mouse organs. Arch Pharm Res 35:1823–1830
Jiang Y, Ferguson WB, Peng JB (2007) WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol 292:F545–F554
Jiang Y, Cong P, Williams SR, Zhang W, Na T, Ma HP, Peng JB (2008) WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem Biophys Res Commun 375:225–229
Jing H, Na T, Zhang W, Wu G, Liu C, Peng JB (2011) Concerted actions of NHERF2 and WNK4 in regulating TRPV5. Biochem Biophys Res Commun 404:979–984
Kennedy BG, Torabi AJ, Kurzawa R, Echtenkamp SF, Mangini NJ (2010) Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in retinal pigment epithelium. Mol Vis 16:665–675
Khuituan P, Teerapornpuntakit J, Wongdee K, Suntornsaratoon P, Konthapakdee N, Sangsaksri J, Sripong C, Krishnamra N, Charoenphandhu N (2012) Fibroblast growth factor-23 abolishes 1,25-dihydroxyvitamin D3-enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab 302:E903–E913
Kim HJ, Yang DK, So I (2007) PDZ domain-containing protein as a physiological modulator of TRPV6. Biochem Biophys Res Commun 361:433–438
Kim MH, Lee GS, Jung EM, Choi KC, Jeung EB (2009a) The negative effect of dexamethasone on calcium-processing gene expressions is associated with a glucocorticoid-induced calcium-absorbing disorder. Life Sci 85:146–152
Kim MH, Lee GS, Jung EM, Choi KC, Oh GT, Jeung EB (2009b) Dexamethasone differentially regulates renal and duodenal calcium-processing genes in calbindin-D9k and -D28k knockout mice. Exp Physiol 94:138–151
Kovalevskaya NV, Bokhovchuk FM, Vuister GW (2012) The TRPV5/6 calcium channels contain multiple calmodulin binding sites with differential binding properties. J Struct Funct Genomics 13:91–100
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390:45–51
Lambers TT, Weidema AF, Nilius B, Hoenderop JG, Bindels RJ (2004) Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+-sensor calmodulin. J Biol Chem 279:28855–28861
Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ (2006) Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 25:2978–2988
Lambers TT, Oancea E, de Groot T, Topala CN, Hoenderop JG, Bindels RJ (2007) Extracellular pH dynamically controls cell surface delivery of functional TRPV5 channels. Mol Cell Biol 27:1486–1494
Lee CT, Shang S, Lai LW, Yong KC, Lien YH (2004) Effect of thiazide on renal gene expression of apical calcium channels and calbindins. Am J Physiol Renal Physiol 287:F1164–F1170
Lee J, Cha SK, Sun TJ, Huang CL (2005) PIP2 activates TRPV5 and releases its inhibition by intracellular Mg2+. J Gen Physiol 126:439–451
Lee CT, Lien YH, Lai LW, Chen JB, Lin CR, Chen HC (2006) Increased renal calcium and magnesium transporter abundance in streptozotocin-induced diabetes mellitus. Kidney Int 69:1786–1791
Lee CT, Chen HC, Lai LW, Yong KC, Lien YH (2007) Effects of furosemide on renal calcium handling. Am J Physiol Renal Physiol 293:F1231–F1237
Lee KP, Nair AV, Grimm C, van Zeeland F, Heller S, Bindels RJ, Hoenderop JG (2010) A helix-breaking mutation in the epithelial Ca2+ channel TRPV5 leads to reduced Ca2+-dependent inactivation. Cell Calcium 48:275–287
Lee CT, Ng HY, Lien YH, Lai LW, Wu MS, Lin CR, Chen HC (2011) Effects of cyclosporine, tacrolimus and rapamycin on renal calcium transport and vitamin D metabolism. Am J Nephrol 34:87–94
Lee CT, Lien YH, Lai LW, Ng HY, Chiou TT, Chen HC (2012) Variations of dietary salt and fluid modulate calcium and magnesium transport in the renal distal tubule. Nephron Physiol 122:19–27
Leunissen EH, Nair AV, Bull C, Lefeber DJ, van Delft FL, Bindels RJ, Hoenderop JG (2013) The epithelial calcium channel TRPV5 is regulated differentially by klotho and sialidase. J Biol Chem 288(41):29238–29246
Lewit-Bentley A, Rety S, Sopkova-de Oliveira SJ, Gerke V (2000) S100-annexin complexes: some insights from structural studies. Cell Biol Int 24:799–802
Li S, Wang X, Ye H, Gao W, Pu X, Yang Z (2010) Distribution profiles of transient receptor potential melastatin- and vanilloid-related channels in rat spermatogenic cells and sperm. Mol Biol Rep 37:1287–1293
Loffing J, Loffing-Cueni D, Valderrabano V, Klausli L, Hebert SC, Rossier BC, Hoenderop JG, Bindels RJ, Kaissling B (2001) Distribution of transcellular calcium and sodium transport pathways along mouse distal nephron. Am J Physiol Renal Physiol 281:F1021–F1027
Loh NY, Bentley L, Dimke H, Verkaart S, Tammaro P, Gorvin CM, Stechman MJ, Ahmad BN, Hannan FM, Piret SE, Evans H, Bellantuono I, Hough TA, Fraser WD, Hoenderop JG, Ashcroft FM, Brown SD, Bindels RJ, Cox RD, Thakker RV (2013) Autosomal dominant hypercalciuria in a mouse model due to a mutation of the epithelial calcium channel, TRPV5. PLoS One 8:e55412
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4:140–156
Lu P, Boros S, Chang Q, Bindels RJ, Hoenderop JG (2008) The beta-glucuronidase klotho exclusively activates the epithelial Ca2+ channels TRPV5 and TRPV6. Nephrol Dial Transplant 23:3397–3402
Mayan H, Munter G, Shaharabany M, Mouallem M, Pauzner R, Holtzman EJ, Farfel Z (2004) Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J Clin Endocrinol Metab 89:4025–4030
Meyer MB, Watanuki M, Kim S, Shevde NK, Pike JW (2006) The human transient receptor potential vanilloid type 6 distal promoter contains multiple vitamin D receptor binding sites that mediate activation by 1,25-dihydroxyvitamin D3 in intestinal cells. Mol Endocrinol 20:1447–1461
Moreau R, Daoud G, Bernatchez R, Simoneau L, Masse A, Lafond J (2002a) Calcium uptake and calcium transporter expression by trophoblast cells from human term placenta. Biochem Biophys Acta 1564:325–332
Moreau R, Hamel A, Daoud G, Simoneau L, Lafond J (2002b) Expression of calcium channels along the differentiation of cultured trophoblast cells from human term placenta. Biol Reprod 67:1473–1479
Muller D, Hoenderop JG, Merkx GF, van Os CH, Bindels RJ (2000) Gene structure and chromosomal mapping of human epithelial calcium channel. Biochem Biophys Res Commun 275:47–52
Muller D, Hoenderop JG, Vennekens R, Eggert P, Harangi F, Mehes K, Garcia-Nieto V, Claverie-Martin F, Os CH, Nilius B, Bindels JM (2002) Epithelial Ca2+ channel (ECAC1) in autosomal dominant idiopathic hypercalciuria. Nephrol Dial Transplant 17:1614–1620
Na T, Zhang W, Jiang Y, Liang Y, Ma HP, Warnock DG, Peng JB (2009) The A563T variation of the renal epithelial calcium channel TRPV5 among African Americans enhances calcium influx. Am J Physiol Renal Physiol 296:F1042–F1051
Nakaya K, Harbidge DG, Wangemann P, Schultz BD, Green ED, Wall SM, Marcus DC (2007) Lack of pendrin. Am J Physiol Renal Physiol 292:F1314–F1321
Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ (2003) Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int 64:555–564
Nijenhuis T, Hoenderop JG, Bindels RJ (2004) Downregulation of Ca2+ and Mg2+ transport proteins in the kidney explains tacrolimus (FK506)-induced hypercalciuria and hypomagnesemia. J Am Soc Nephrol 15:549–557
Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ (2005) Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115:1651–1658
Nijenhuis T, van der Eerden BC, Hoenderop JG, Weinans H, van Leeuwen JP, Bindels RJ (2008) Bone resorption inhibitor alendronate normalizes the reduced bone thickness of TRPV5-/- mice. J Bone Miner Res 23:1815–1824
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G (2000) Whole-cell and single channel monovalent cation currents through the novel rabbit epithelial Ca2+ channel ECaC. J Physiol 527(Pt 2):239–248
Nilius B, Prenen J, Vennekens R, Hoenderop JG, Bindels RJ, Droogmans G (2001a) Pharmacological modulation of monovalent cation currents through the epithelial Ca2+ channel ECaC1. Br J Pharmacol 134:453–462
Nilius B, Vennekens R, Prenen J, Hoenderop JG, Droogmans G, Bindels RJ (2001b) The single pore residue Asp542 determines Ca2+ permeation and Mg2+ block of the epithelial Ca2+ channel. J Biol Chem 276:1020–1025
Nilius B, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Weidema AF, Droogmans G, Bindels RJ (2002) Fast and slow inactivation kinetics of the Ca2+ channels ECaC1 and ECaC2 (TRPV5 and TRPV6). Role of the intracellular loop located between transmembrane segments 2 and 3. J Biol Chem 277:30852–30858
Nilius B, Weidema F, Prenen J, Hoenderop JG, Vennekens R, Hoefs S, Droogmans G, Bindels RJ (2003) The carboxyl terminus of the epithelial Ca2+ channel ECaC1 is involved in Ca2+-dependent inactivation. Pflugers Arch 445:584–588
Oz OK, Hajibeigi A, Howard K, Cummins CL, van Abel M, Bindels RJ, Word RA, Kuro-o M, Pak CY, Zerwekh JE (2007) Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice *. J Bone Miner Res 22:1893–1902
Palmada M, Poppendieck S, Embark HM, van de Graaf SF, Boehmer C, Bindels RJ, Lang F (2005) Requirement of PDZ domains for the stimulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase SGK1. Cell Physiol Biochem 15:175–182
Peng JB, Chen XZ, Berger UV, Vassilev PM, Tsukaguchi H, Brown EM, Hediger MA (1999) Molecular cloning and characterization of a channel-like transporter mediating intestinal calcium absorption. J Biol Chem 274:22739–22746
Peng JB, Chen XZ, Berger UV, Vassilev PM, Brown EM, Hediger MA (2000a) A rat kidney-specific calcium transporter in the distal nephron. J Biol Chem 275:28186–28194
Peng JB, Chen XZ, Berger UV, Weremowicz S, Morton CC, Vassilev PM, Brown EM, Hediger MA (2000b) Human calcium transport protein CaT1. Biochem Biophys Res Commun 278:326–332
Peng JB, Brown EM, Hediger MA (2001) Structural conservation of the genes encoding CaT1, CaT2, and related cation channels. Genomics 76:99–109
Phelps CB, Huang RJ, Lishko PV, Wang RR, Gaudet R (2008) Structural analyses of the ankyrin repeat domain of TRPV6 and related TRPV ion channels. Biochemistry 47:2476–2484
Picard N, van Abel M, Campone C, Seiler M, Bloch-Faure M, Hoenderop JG, Loffing J, Meneton P, Bindels RJ, Paillard M, Alhenc-Gelas F, Houillier P (2005) Tissue kallikrein-deficient mice display a defect in renal tubular calcium absorption. J Am Soc Nephrol 16:3602–3610
Pratt JH, Manatunga AK, Peacock M (1996) A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med 127:67–70
Qiu A, Hogstrand C (2004) Functional characterisation and genomic analysis of an epithelial calcium channel (ECaC) from pufferfish, Fugu rubripes. Gene 342:113–123
Radhakrishnan VM, Ramalingam R, Larmonier CB, Thurston RD, Laubitz D, Midura-Kiela MT, McFadden RM, Kuro O, Kiela PR, Ghishan FK (2013) Post-translational loss of renal TRPV5 calcium channel expression, Ca2+ wasting, and bone loss in experimental colitis. Gastroenterology 145(3):613–624
Renkema KY, Nijenhuis T, van der Eerden BC, van der Kemp AW, Weinans H, van Leeuwen JP, Bindels RJ, Hoenderop JG (2005) Hypervitaminosis D mediates compensatory Ca2+ hyperabsorption in TRPV5 knockout mice. J Am Soc Nephrol 16:3188–3195
Renkema KY, Lee K, Topala CN, Goossens M, Houillier P, Bindels RJ, Hoenderop JG (2009a) TRPV5 gene polymorphisms in renal hypercalciuria. Nephrol Dial Transplant 24:1919–1924
Renkema KY, Velic A, Dijkman HB, Verkaart S, van der Kemp AW, Nowik M, Timmermans K, Doucet A, Wagner CA, Bindels RJ, Hoenderop JG (2009b) The calcium-sensing receptor promotes urinary acidification to prevent nephrolithiasis. J Am Soc Nephrol 20:1705–1713
Riccardi D, Lee WS, Lee K, Segre GV, Brown EM, Hebert SC (1996) Localization of the extracellular Ca2+-sensing receptor and PTH/PTHrP receptor in rat kidney. Am J Physiol 271:F951–F956
Riccardi D, Hall AE, Chattopadhyay N, Xu JZ, Brown EM, Hebert SC (1998) Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am J Physiol 274:F611–F622
Rohacs T, Lopes CM, Michailidis I, Logothetis DE (2005) PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci 8:626–634
Rourke KM, Coe S, Kohn CW, Rosol TJ, Mendoza FJ, Toribio RE (2010) Cloning, comparative sequence analysis and mRNA expression of calcium transporting genes in horses. Gen Comp Endocrinol 167(1):6–10
Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL (2001) S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry 40:3167–3173
Saito S, Shingai R (2006) Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 27:219–230
Sakai K, Hirai M, Minoshima S, Kudoh J, Fukuyama R, Shimizu N (1989) Isolation of cDNAs encoding a substrate for protein kinase C: nucleotide sequence and chromosomal mapping of the gene for a human 80K protein. Genomics 5:309–315
Sandulache D, Grahammer F, Artunc F, Henke G, Hussain A, Nasir O, Mack A, Friedrich B, Vallon V, Wulff P, Kuhl D, Palmada M, Lang F (2006) Renal Ca2+ handling in sgk1 knockout mice. Pflugers Arch 452:444–452
Sarmina I, Spirnak JP, Resnick MI (1987) Urinary lithiasis in the black population: an epidemiological study and review of the literature. J Urol 138:14–17
Sato T, Yamamoto H, Sawada N, Nashiki K, Tsuji M, Nikawa T, Arai H, Morita K, Taketani Y, Takeda E (2006) Immobilization decreases duodenal calcium absorption through a 1,25-dihydroxyvitamin D-dependent pathway. J Bone Miner Metab 24:291–299
Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM, Ferrucci L (2011) Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci 66:794–800
Semenova SB, Vassilieva IO, Fomina AF, Runov AL, Negulyaev YA (2009) Endogenous expression of TRPV5 and TRPV6 calcium channels in human leukemia K562 cells. Am J Physiol Cell Physiol 296:C1098–C1104
Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S (2003) Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology 144:3885–3894
Stajich JE, Hahn MW (2005) Disentangling the effects of demography and selection in human history. Mol Biol Evol 22:63–73
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC (2003) Time trends in reported prevalence of kidney stones in the United States: 1976-1994. Kidney Int 63:1817–1823
Staub O, Rotin D (2006) Role of ubiquitylation in cellular membrane transport. Physiol Rev 86:669–707
Sutton RA, Wong NL, Dirks JH (1979) Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int 15:520–533
Suzuki M, Ohki G, Ishibashi K, Imai M (2002) A single amino acid mutation results in a rapid inactivation of epithelial calcium channels. Biochem Biophys Res Commun 291:278–285
Suzuki Y, Kovacs CS, Takanaga H, Peng JB, Landowski CP, Hediger MA (2008) Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J Bone Miner Res 23:1249–1256
Takumida M, Ishibashi T, Hamamoto T, Hirakawa K, Anniko M (2009) Age-dependent changes in the expression of klotho protein, TRPV5 and TRPV6 in mouse inner ear. Acta Otolaryngol 129:1340–1350
Taylor EN, Curhan GC (2007) Differences in 24-hour urine composition between black and white women. J Am Soc Nephrol 18:654–659
Teerapornpuntakit J, Dorkkam N, Wongdee K, Krishnamra N, Charoenphandhu N (2009) Endurance swimming stimulates transepithelial calcium transport and alters the expression of genes related to calcium absorption in the intestine of rats. Am J Physiol Endocrinol Metab 296:E775–E786
Thyagarajan B, Lukacs V, Rohacs T (2008) Hydrolysis of phosphatidylinositol 4,5-bisphosphate mediates calcium-induced inactivation of TRPV6 channels. J Biol Chem 283:14980–14987
Tohyama O, Imura A, Iwano A, Freund JN, Henrissat B, Fujimori T, Nabeshima Y (2004) Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem 279:9777–9784
Topala CN, Schoeber JP, Searchfield LE, Riccardi D, Hoenderop JG, Bindels RJ (2009) Activation of the Ca2+-sensing receptor stimulates the activity of the epithelial Ca2+ channel TRPV5. Cell Calcium 45:331–339
Tsuruoka S, Nishiki K, Ioka T, Ando H, Saito Y, Kurabayashi M, Nagai R, Fujimura A (2006) Defect in parathyroid-hormone-induced luminal calcium absorption in connecting tubules of Klotho mice. Nephrol Dial Transplant 21:2762–2767
Tudpor K, Lainez S, Kwakernaak AJ, Kovalevskaya NV, Verkaart S, van Genesan S, van der Kemp A, Navis G, Bindels RJ, Hoenderop JG (2012) Urinary plasmin inhibits TRPV5 in nephrotic-range proteinuria. J Am Soc Nephrol 23:1824–1834
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T (2006) Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–774
van Abel M, Hoenderop JG, Dardenne O, St Arnaud R, van Os CH, Van Leeuwen HJ, Bindels RJ (2002) 1,25-dihydroxyvitamin D3-independent stimulatory effect of estrogen on the expression of ECaC1 in the kidney. J Am Soc Nephrol 13:2102–2109
van Abel M, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ (2003) Regulation of the epithelial Ca2+ channels in small intestine as studied by quantitative mRNA detection. Am J Physiol Gastrointest Liver Physiol 285:G78–G85
van Abel M, Hoenderop JG, van der Kemp AW, Friedlaender MM, van Leeuwen JP, Bindels RJ (2005) Coordinated control of renal Ca2+ transport proteins by parathyroid hormone. Kidney Int 68:1708–1721
van Abel M, Huybers S, Hoenderop JG, van der Kemp AW, van Leeuwen JP, Bindels RJ (2006) Age-dependent alterations in Ca2+ homeostasis: role of TRPV5 and TRPV6. Am J Physiol Renal Physiol 291:F1177–F1183
van Baal J, Raber G, de Slegte J, Pieters R, Bindels RJ, Willems PH (1996) Vasopressin-stimulated Ca2+ reabsorption in rabbit cortical collecting system: effects on cAMP and cytosolic Ca2+. Pflugers Arch 433:109–115
Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van HE, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G (2001) Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA 98:13324–13329
Van Cromphaut SJ, Stockmans I, Torrekens S, Van HE, Carmeliet G, Bouillon R (2007) Duodenal calcium absorption in dexamethasone-treated mice: functional and molecular aspects. Arch Biochem Biophys 460:300–305
van de Graaf SF, Hoenderop JG, Gkika D, Lamers D, Prenen J, Rescher U, Gerke V, Staub O, Nilius B, Bindels RJ (2003) Functional expression of the epithelial Ca2+ channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex. EMBO J 22:1478–1487
van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ (2006a) Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Mol Cell Biol 26:303–312
van de Graaf SF, Hoenderop JG, van der Kemp AW, Gisler SM, Bindels RJ (2006b) Interaction of the epithelial Ca2+ channels TRPV5 and TRPV6 with the intestine- and kidney-enriched PDZ protein NHERF4. Pflugers Arch 452:407–417
van de Graaf SF, van der Kemp AW, van den Berg D, van Oorschot M, Hoenderop JG, Bindels RJ (2006c) Identification of BSPRY as a novel auxiliary protein inhibiting TRPV5 activity. J Am Soc Nephrol 17:26–30
van der Eerden BC, Hoenderop JG, de Vries TJ, Schoenmaker T, Buurman CJ, Uitterlinden AG, Pols HA, Bindels RJ, van Leeuwen JP (2005) The epithelial Ca2+ channel TRPV5 is essential for proper osteoclastic bone resorption. Proc Natl Acad Sci USA 102:17507–17512
van der Eerden BC, Fratzl-Zelman N, Nijenhuis T, Roschger P, Zugel U, Steinmeyer A, Hoenderop JG, Bindels RJ, Klaushofer K, van Leeuwen JP (2013) The vitamin D analog ZK191784 normalizes decreased bone matrix mineralization in mice lacking the calcium channel TRPV5. J Cell Physiol 228:402–407
Vassilev PM, Peng JB, Hediger MA, Brown EM (2001) Single-channel activities of the human epithelial Ca2+ transport proteins CaT1 and CaT2. J Membr Biol 184:113–120
Vassilieva IO, Tomilin VN, Marakhova II, Shatrova AN, Negulyaev YA, Semenova SB (2013) Expression of transient receptor potential vanilloid channels TRPV5 and TRPV6 in human blood lymphocytes and Jurkat leukemia T cells. J Membr Biol 246:131–140
Vennekens R, Hoenderop JG, Prenen J, Stuiver M, Willems PH, Droogmans G, Nilius B, Bindels RJ (2000) Permeation and gating properties of the novel epithelial Ca2+ channel. J Biol Chem 275:3963–3969
Vennekens R, Prenen J, Hoenderop JG, Bindels RJ, Droogmans G, Nilius B (2001) Modulation of the epithelial Ca2+ channel ECaC by extracellular pH. Pflugers Arch 442:237–242
Verrey F, Loffing J, Zecevic M, Heitzmann D, Staub O (2003) SGK1: aldosterone-induced relay of Na + transport regulation in distal kidney nephron cells. Cell Physiol Biochem 13:21–28
Vig PJ, Wei J, Shao Q, Hebert MD, Subramony SH, Sutton LT (2007) Role of tissue transglutaminase type 2 in calbindin-D28k interaction with ataxin-1. Neurosci Lett 420:53–57
Voets T, Prenen J, Fleig A, Vennekens R, Watanabe H, Hoenderop JG, Bindels RJ, Droogmans G, Penner R, Nilius B (2001) CaT1 and the calcium release-activated calcium channel manifest distinct pore properties. J Biol Chem 276:47767–47770
Voets T, Janssens A, Prenen J, Droogmans G, Nilius B (2003) Mg2+-dependent gating and strong inward rectification of the cation channel TRPV6. J Gen Physiol 121:245–260
Voets T, Janssens A, Droogmans G, Nilius B (2004) Outer pore architecture of a Ca2+-selective TRP channel. J Biol Chem 279:15223–15230
Wade-Gueye NM, Delissen O, Gourmelon P, Aigueperse J, Dublineau I, Souidi M (2012) Chronic exposure to natural uranium via drinking water affects bone in growing rats. Biochim Biophys Acta 1820:1121–1127
Wang S, Hu D, Xi Q, Su S, Bai J, Liu J, Ye Z (2008) The expression and implication of TRPV5, Calbindin-D28k and NCX1 in idiopathic hypercalciuria. J Huazhong Univ Sci Technol Med Sci 28:580–583
Wangemann P, Nakaya K, Wu T, Maganti RJ, Itza EM, Sanneman JD, Harbidge DG, Billings S, Marcus DC (2007) Loss of cochlear. Am J Physiol Renal Physiol 292:F1345–F1353
Wasserman RH, Fullmer CS (1995) Vitamin D and intestinal calcium transport: facts, speculations and hypotheses. J Nutr 125:1971S–1979S
Wasserman RH, Chandler JS, Meyer SA, Smith CA, Brindak ME, Fullmer CS, Penniston JT, Kumar R (1992) Intestinal calcium transport and calcium extrusion processes at the basolateral membrane. J Nutr 122:662–671
Weber K, Erben RG, Rump A, Adamski J (2001) Gene structure and regulation of the murine epithelial calcium channels ECaC1 and 2. Biochem Biophys Res Commun 289:1287–1294
Will C, Breiderhoff T, Thumfart J, Stuiver M, Kopplin K, Sommer K, Gunzel D, Querfeld U, Meij IC, Shan Q, Bleich M, Willnow TE, Muller D (2010) Targeted deletion of murine Cldn16 identifies extra- and intrarenal compensatory mechanisms of Ca2+ and Mg2+ wasting. Am J Physiol Renal Physiol 298(5):F1152–F1161
Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP (2001) Human hypertension caused by mutations in WNK kinases. Science 293:1107–1112
Wolf MT, Wu XR, Huang CL (2013) Uromodulin upregulates TRPV5 by impairing caveolin-mediated endocytosis. Kidney Int 84(1):130–137
Yamauchi D, Raveendran NN, Pondugula SR, Kampalli SB, Sanneman JD, Harbidge DG, Marcus DC (2005) Vitamin D upregulates expression of ECaC1 mRNA in semicircular canal. Biochem Biophys Res Commun 331:1353–1357
Yamauchi D, Nakaya K, Raveendran NN, Harbidge DG, Singh R, Wangemann P, Marcus DC (2010) Expression of epithelial calcium transport system in rat cochlea and vestibular labyrinth. BMC Physiol 10:1
Yan P, Li T, Bo M, Die L, Xing L (2011) Inhibition of bone resorption by econazole in rat osteoclast-like cells through suppressing TRPV5. Arch Pharm Res 34:1007–1013
Yang W, Friedman PA, Kumar R, Omdahl JL, May BK, Siu-Caldera ML, Reddy GS, Christakos S (1999) Expression of 25(OH)D3 24-hydroxylase in distal nephron: coordinate regulation by 1,25(OH)2D3 and cAMP or PTH. Am J Physiol 276:E793–E805
Yang SS, Lo YF, Yu IS, Lin SW, Chang TH, Hsu YJ, Chao TK, Sytwu HK, Uchida S, Sasaki S, Lin SH (2010) Generation and analysis of the thiazide-sensitive Na+ -Cl- cotransporter (Ncc/Slc12a3) Ser707X knockin mouse as a model of Gitelman syndrome. Hum Mutat 31:1304–1315
Yang JH, Hou JF, Farquharson C, Zhou ZL, Deng YF, Wang L, Yu Y (2011) Localisation and expression of TRPV6 in all intestinal segments and kidney of laying hens. Br Poult Sci 52:507–516
Yeh BI, Sun TJ, Lee JZ, Chen HH, Huang CL (2003) Mechanism and molecular determinant for regulation of rabbit transient receptor potential type 5 (TRPV5) channel by extracellular pH. J Biol Chem 278:51044–51052
Yeh BI, Kim YK, Jabbar W, Huang CL (2005) Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J 24:3224–3234
Yeh BI, Yoon J, Huang CL (2006) On the role of pore helix in regulation of TRPV5 by extracellular protons. J Membr Biol 212:191–198
Zhang W, Na T, Peng JB (2008) WNK3 positively regulates epithelial calcium channels TRPV5 and TRPV6 via a kinase-dependent pathway. Am J Physiol Renal Physiol 295:F1472–F1484
Zhang W, Na T, Wu G, Jing H, Peng JB (2010) Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4-2. J Biol Chem 285:36586–36596
Zheng W, Xie Y, Li G, Kong J, Feng JQ, Li YC (2004) Critical role of calbindin-D28k in calcium homeostasis revealed by mice lacking both vitamin D receptor and calbindin-D28k. J Biol Chem 279:52406–52413
Zhuang L, Peng JB, Tou L, Takanaga H, Adam RM, Hediger MA, Freeman MR (2002) Calcium-selective ion channel, CaT1, is apically localized in gastrointestinal tract epithelia and is aberrantly expressed in human malignancies. Lab Invest 82:1755–1764
Acknowledgments
We thank our colleagues Yi Jiang, Wei Zhang, Guojin Wu, and Haiyan Jiang for their contribution to our understanding of TRPV5 and TRPV6 through research and discussion. The research in our laboratory was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072154) and the American Heart Association (0430125N, 09GRNT2160024, and 09POST2160010).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Na, T., Peng, JB. (2014). TRPV5: A Ca2+ Channel for the Fine-Tuning of Ca2+ Reabsorption. In: Nilius, B., Flockerzi, V. (eds) Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, vol 222. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54215-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-54215-2_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54214-5
Online ISBN: 978-3-642-54215-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)