Abstract
During the last three decades, less than fifteen papers have described the results of scientific investigations in the field of gentian protoplast technology and somatic hybridization. Despite rather limited research already done on this subject, several important goals have been achieved. Protoplast-to-plant systems have been developed either for leading ornamental species or for specific medicinal plants. Two major protoplast sources were evaluated in gentians, namely differentiated leaf mesophyll cells and undifferentiated callus/cell suspensions. Plant regeneration proceeded by the two different pathways of shoot organogenesis or somatic embryogenesis. Some examples of somaclonal variation at the ploidy level were demonstrated within the pool of protoplast-derived regenerants. Totipotency exhibited by gentian protoplasts was exploited to create three different somatic hybrid combinations: intergeneric Swertia mussotii (+) Bupleurum scorzonerifolium , and interspecific Gentiana kurroo (+) G. cruciata and G. cruciata (+) G. tibetica.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
Plant cell totipotency as postulated by Schleiden and Schwann in the middle of the nineteenth century is the basis of modern plant biotechnology (Vasil 2008). Based on the ability of a living single plant cell to dedifferentiate and to convert into other cell types, it is possible to obtain a completely new plant from a cell and even from its protoplast. Consequently, plant regeneration from single protoplasts underlies the genetic manipulation technologies of somatic hybridization and direct genetic transformation by DNA uptake. However, the prerequisite for practical application of these technologies is the development of efficient and reproducible protoplast-to-plant systems for the species of interest.
Gentian plants are well known to be the source of valuable secondary metabolites of pharmaceutical use, while a range of genotypes attract attention because of the many ornamental attributes of such plants (Köhlein 1991). Unfortunately, the majority of species are rare and thus protected by law to avoid the risk of their extinction. All these gentian features have determined their introduction into tissue culture and exploitation as an object of biotechnological research. Investigations were focused mainly on the development of rapid and effective methods of micropropagation , conservation of biodiversity, and the production of efficient sources of pharmacologically active compounds. The high morphogenic potential of several species has facilitated some attempts at their genetic modification, mostly aimed at engineering new flower colors . Somatic hybridization, a protoplast-based technology, is an alternative to sexual hybridization involving distant crosses to generate interspecific and intergeneric hybrids in order to increase genetic variation and the creation of novel gentian genotypes with attractive traits.
7.2 Protoplast Culture of Gentians
Research on the culture of gentian protoplasts commenced in the 1980s, and it was Zhou et al. (1985) who first succeeded in obtaining callus from leaf mesophyll protoplasts of G. scabra Bunge. In the next decade, studies concentrated mainly on Japanese ornamental gentian species and cultivars such as G. scabra , G. triflora Pall., and their hybrids (Takahata and Jomori 1989; Jomori et al. 1995; Nakano et al. 1995) as well as on lisianthus, Eustoma grandiflorum (Griseb.) Schinners (O’Brien and Lindsay 1993; Kunitake et al. 1995). Most of these first achievements in the development of protoplast-to-plant systems for Gentiana species were discussed at length by Takahata et al. (1995). However, remarkable progress has been attained in recent years, especially in the area of plant regeneration from gentian protoplasts by the pathway of somatic embryogenesis and of evaluation of protoplast-derived regenerants (Meng et al. 1996; Fiuk and Rybczyński 2007; Tomiczak et al. 2015).
To date, within the family Gentianaceae, considerable effort has been invested in the development of protoplast-to-plant systems for 10 Gentiana species (including one line and 4 cultivars of G. triflora ), one interspecific Gentiana sexual hybrid (G. triflora × G. scabra WSP-3) and 14 cultivars of E. grandiflorum (Table 7.1). However, to the authors’ best knowledge, research concerning protoplast cultures of G. acaulis L., G. cruciata L., G. lutea L., and G. septemfida Pall. have not culminated, to date, in plant regeneration.
7.2.1 Source of Protoplasts
Theoretically, protoplasts can be isolated from various living tissues, sourced either from glasshouse-grown plants or from more uniform, axenic in vitro cultures (Power et al. 2004). In practical terms, the most popular plant materials that ensure large populations of released protoplasts are mesophyll tissue of expanded leaves excised from cultured shoots, seedling organs (including cotyledons, hypocotyls, and roots), and callus or cell suspensions of different origin (Davey et al. 2005b). In the case of gentians, the first and the last two sources have been the ones, exploited for protoplast isolation.
7.2.1.1 Leaf Mesophyll Tissue
The advantages of leaf mesophyll tissue include its convenience, availability, and usually higher cytogenetic uniformity in comparison with callus or suspension cells. However, highly differentiated mesophyll cells are typically less flexible plant material than embryogenic calli or cell suspensions from which to achieve efficient regeneration of shoots or somatic embryos. Within the family Gentianaceae, studies on protoplast isolation and culture from leaf mesophyll have focused on G. scabra (Zhou et al. 1985; Takahata and Jomori 1989), G. triflora (Jomori et al. 1995; Nakano et al. 1995), G. triflora × G. scabra (Nakano et al. 1995), G. acaulis (Jomori et al. 1995), G. kurroo Royle, G. cruciata , G. lutea , G. septemfida , G. tibetica King (Tomiczak 2011), and G. decumbens L.f. (Tomiczak et al. 2015). Cotyledon and leaf mesophyll cells of E. grandiflorum have also been evaluated as a protoplast source (O’Brien and Lindsay 1993; Kunitake et al. 1995). The average number of protoplasts obtained from 1 g of Gentiana mesophyll tissue ranged from 1 to 11 × 105, while in the case of Eustoma the yield reached 17 × 105 (Table 7.1).
7.2.1.2 Embryogenic Callus and Cell Suspensions
Established embryogenic calli and cell suspensions constitute a very efficient source of protoplasts. Under appropriate conditions, 1 g of hypocotyl-derived callus of G. crassicaulis provided a yield of 10–20 × 105 protoplasts (Meng et al. 1996). The productivity of protoplast isolation from cotyledon and hypocotyl-derived cell suspensions of G. kurroo reached 44.1 × 105 and 52.6 × 105 protoplasts per 1 g of fresh weight , respectively (Fiuk and Rybczyński 2007), whereas protoplast yield from leaf mesophyll tissue of the same species was approximately four times less (Tomiczak 2011). Also, the yield of protoplasts obtained from G. lutea cell suspensions was twice that isolated from leaf mesophyll cells (Takahata et al. 1995; Tomiczak 2011).
7.2.2 Factors Affecting Protoplast Isolation
Several factors influence protoplast release, including the cell wall degrading enzymes used, the nature and concentration of the osmoticum, temperature, and duration of enzyme incubation as well as gentle agitation of the plant tissue in the mixture of enzymes (Davey et al. 2005a).
Of the many commercially available cellulases and pectinases for protoplast release from gentian leaf mesophyll cells, mainly Cellulase Onozuka RS or R-10 at a concentration of 1–2 % (w/v) and 0.2–0.5 % (w/v) Macerozyme R-10 have been used (Table 7.1). In order to digest E. grandiflorum mesophyll tissues, O’Brien and Lindsay (1993) and Kunitake et al. (1995) supplemented the enzyme mixture with 0.05 % (w/v) Pectolyase Y-23. The exploitation of cell suspensions and callus as protoplast sources necessitated enrichment of the enzyme solution with 0.2–0.5 % (w/v) hemicellulase (Meng et al. 1996; Fiuk and Rybczyński 2007).
The osmoticum preventing “naked” cells from rupture is a significant constituent of the isolation solution besides the mixture of enzymes. For most of the Gentiana species, mannitol at 9–10 % (w/v) was found to be a suitable osmotic stabilizer (Table 7.1). However, in the case of G. lutea , 11 % (w/v) sorbitol was preferable (Takahata et al. 1995). Viable protoplasts of lisianthus were also isolated with an enzyme solution supplemented with 9–11 % (w/v) sorbitol (O’Brien and Lindsay 1993; Kunitake et al. 1995). Glucose at a concentration of 12 % or at 9 % (w/v), but in combination with 1.8 % (w/v) mannitol, was employed only by Zhou et al. (1985) and Meng et al. (1996), respectively.
Although most of the researchers applied overnight digestion of plant material (Zhou et al. 1985; O’Brien and Lindsay 1993; Takahata and Jomori 1989; Nakano et al. 1995; Meng et al. 1996; Fiuk and Rybczyński 2007), protoplasts were also obtained by a short duration (3–4 h) of enzyme treatment (Kunitake et al. 1995; Tomiczak et al. 2015). Gentle agitation (30–60 rpm) was employed occasionally to improve the release of protoplasts. The temperature of incubation varied from 20 °C (Nakano et al. 1995) to 30 °C (Zhou et al. 1985; Table 7.1).
7.2.3 Factors Influencing Protoplast and Callus Culture
Although protoplast isolation from gentian tissues has become almost routine, the culture techniques developed so far have not guaranteed callus development for all the species investigated. Besides plant genotype, the other most important factors influencing protoplast culture are the medium composition, type of culture and gelling agent, as well as the physical conditions of protoplast culture.
7.2.3.1 Protoplast Culture
Several media have been used to culture gentian protoplasts with the MS (Murashige and Skoog 1962) formulation being the most frequent (Table 7.2). As demonstrated earlier, the concentration of ammonium salts in MS medium is too high for protoplast survival and mitotic division (Bajaj 1989). Consequently, modification has been made to MS macronutrient composition, mainly the limitation of NH4NO3 to 400 mg/l (Takahata and Jomori 1989; Jomori et al. 1995) or its complete withdrawal (Kunitake et al. 1995) and replacement with glutamine (Fiuk and Rybczyński 2007; Tomiczak et al. 2015). Other media used successfully for gentian protoplast culture include nutrient-rich KM8P medium developed by Kao and Michayluk (1975) for cells and protoplasts cultured at a very low densities (Meng et al. 1996), V-KM medium reported by Bokelmann and Roest (1983) for potato protoplasts (O’Brien and Lindsay 1993), and B5 medium of Gamborg et al. (1968), as used by Takahata and Jomori (1989) and Nakano et al. (1995). Glucose and sucrose typically served as carbon sources, whereas mannitol ensured the correct osmotic pressure . The protoplasts were cultured mainly in darkness, at a density of 1 × 105 per 1 ml of medium at 20–28 °C (Table 7.2).
Under optimal conditions, cultured protoplasts regenerate new cell walls early in culture and can remain viable even for several days in growth regulator-free medium. However, they require auxin and cytokinin for mitotic division (Pasternak et al. 2000). Plant growth regulators that sustained cell divisions in G. scabra protoplasts were 2,4-dichlorophenoxyacetic acid (2,4-D) , 1-naphthaleneacetic acid (NAA), and zeatin (Zhou et al. 1985). The most universal combination of growth regulators assuring cell colony formation in gentian leaf mesophyll protoplast cultures is NAA at a concentration of 2.0 mg/l and 6-benzylaminopurine (BAP) or thidiazuron (TDZ) at 1.0 and 0.1 mg/l, respectively. This enabled the development of visible microcalli of G. scabra (Takahata and Jomori 1989); G. triflora and G. triflora × G. scabra (Nakano et al. 1995); G. kurroo , G. decumbens , and G. tibetica (Tomiczak 2011; Tomiczak et al. 2015), as well as these of E. grandiflorum (Kunitake et al. 1995), within 6–8 weeks. However, in cultures of G. lutea cell suspension-derived protoplasts, such concentrations of these growth regulators were too high, because colony formation was inhibited when the concentrations of NAA and BAP exceeded 0.5 and 0.05 mg/l, respectively (Takahata et al. 1995). In contrast, protoplasts from G. crassicaulis Duthie ex Burk. embryogenic callus , or G. kurroo cell suspensions required similar concentrations of plant growth regulators to those used for induction and culture of initial plant material (Meng et al. 1996; Fiuk and Rybczyński 2007).
In addition to the composition of the culture medium, the type of culture is a crucial factor affecting cell wall regeneration by protoplasts and their further sustained mitotic division. Various approaches of protoplast culture, based on liquid or semisolid media and their combination, have been developed (Davey et al. 2005a). The first Gentiana leaf mesophyll protoplast cultures were carried out in simple liquid systems (Takahata and Jomori 1989; Jomori et al. 1995), resulting in a low plating efficiency, for example, 0.1 % as reported by Takahata and Jomori (1989) for G. scabra . Taking into consideration the many benefits of embedding protoplasts in semisolid media (Dons and Colijn-Hooymans 1989), Nakano et al. (1995) tested 3 different gelling agents for cultures of G. triflora and G. triflora × G. scabra , with 0.2 % gellan gum giving the highest percentage (25.6 %) of divisions of protoplast-derived cells. The advantage of an agarose-solidified dual layer culture compared to a liquid thin layer alone was shown by Meng et al. (1996) for G. crassicaulis callus protoplasts. Additionally, Fiuk and Rybczyński (2007) reported the best plating efficiency (up to 68.7 %) of G. kurroo cell suspension protoplasts when cultured in agarose beads in comparison with liquid medium and thin agarose layers. The usefulness of this type of culture was confirmed in subsequent studies (Tomiczak et al. 2015).
Browning with necrosis of protoplast cultures is a negative phenomenon observed for many species, caused primarily by the accumulation of phenol complexes resulting from the oxidation of mono- or di-phenols, which are released from plant cells into the surrounding medium (Saxena and Gill 1986; Zhu et al. 1997). In order to avoid this problem in E. grandiflorum cultures, Kunitake et al. (1995) implemented the addition of gellan gum blocks with 1 % activated charcoal to the liquid protoplast culture medium. The effect of activated charcoal on browning inhibition and colony formation was most significant when charcoal blocks were added at the early stage of culture (0–7 days). For other species, except G. cruciata and G. septemfida (Tomiczak 2011; Tomiczak et al. 2015), the addition of new medium or complete replacement of the existing medium (usually at weekly intervals) was generally sufficient to prevent cell death (Takahata and Jomori 1989; O’Brien and Linsday 1993; Nakano et al. 1995). A simultaneous gradual reduction of the osmotic pressure by application of media with a reduced osmoticum concentration also promoted sustained cell division. It is noteworthy that in cultures of callus or cell suspension-derived protoplasts, the reduction of osmotic pressure could be commenced just after the first or second round of cell divisions (Meng et al. 1996; Fiuk and Rybczyński 2007), whereas in the case of leaf mesophyll protoplasts, media with a reduced mannitol or sorbitol concentration were not added until after 3–4 weeks of culture (Takahata and Jomori 1989; Nakano et al. 1995; Tomiczak 2011).
7.2.3.2 Callus Proliferation
Of all the stages of gentian protoplast-to-plant systems, the callus proliferation phase is probably the least complicated. Visible microcalli of 0.5–2 mm in diameter, obtained usually within 2 months from protoplast isolation and transferred onto agar-solidified MS medium supplemented with plant growth regulators similar to those used in protoplast culture (Table 7.2), developed vigorously into callus tissue . As shown by O’Brien and Lindsay (1993) and by Fiuk and Rybczyński (2007), it was even possible for this step to be omitted, as gentian protoplast-derived microcalli could be placed directly onto plant regeneration medium.
7.2.4 Plant Regeneration from Protoplasts
Regeneration of plants from protoplast-derived tissues can proceed by two different pathways, namely shoot organogenesis (also known as caulogenesis) or somatic embryogenesis. Induction and sustained plant regeneration is dependent both on the culture medium and the inherent totipotency of the donor species. For more than 70 % of plant species capable of regenerating from protoplasts, organogenesis was the route reported, whereas somatic embryogenesis was predominant in the Cucurbitaceae, Gramineae, Fabaceae, Rutaceae, and Apiaceae (Power et al. 2004). Most of the results obtained in protoplast cultures of Gentianaceae have been indicated that somatic embryogenesis as a way of plant regeneration was possible only when undifferentiated embryogenic plant material constituted the source of protoplasts, whereas leaf mesophyll protoplasts could only regenerate into shoots via organogenesis.
7.2.4.1 Organogenesis
Caulogenesis in gentian protoplast cultures was reported for the first time by Takahata and Jomori (1989). Using agar-solidified MS medium supplemented with 1.0 mg/l indole-3-acetic acid (IAA) and 6.0 mg/l BAP, they induced organogenesis on greenish callus obtained from G. scabra leaf mesophyll protoplasts. However, the frequency of plant regeneration was low at about 1 %. More effective caulogenesis was reported by Nakano et al. (1995) in protoplast cultures of G. triflora and G. triflora × G. scabra . The application of a high concentration (10.0 mg/l) of TDZ in combination with 0.1 mg/l NAA in the regeneration medium enabled 13.3 % of G. triflora calli to regenerate shoots . This percentage was twofold higher in cultures of the interspecific hybrid, G. triflora × G. scabra. Efficient shoot organogenesis was also induced in protoplast cultures of lisianthus on MS regeneration medium containing 0.02 mg/l indole-3-butyric acid (IBA) and 1.0 mg/l BAP (O’Brien and Lindsay 1993), or on half-strength MS medium with only 1.0–2.0 mg/l BAP (Kunitake et al. 1995).
7.2.4.2 Somatic Embryogenesis
Somatic embryogenesis as a pathway of plant regeneration from gentian protoplasts was reported by Meng et al. (1996) during the culture of G. crassicaulis protoplasts isolated from hypocotyl-derived embryogenic callus . Microcalli derived from these protoplasts turned into yellow granular embryogenic calli during a 3-week-long culture on MS medium containing 2.0 mg/l BAP , 3.0 mg/l zeatin , 1.0 mg/l NAA, 1.0 mg/l gibberellic acid (GA3), and 500 mg/l lactalbumin hydrolysate (LH) . Embryoids and somatic embryos that converted into whole plantlets were obtained as a result of further callus culture on hormone-free MS medium .
An outstanding example of expression of the totipotency of gentian protoplasts by their development into plants via somatic embryogenesis was described by Fiuk and Rybczyński (2007). Protoplasts isolated from highly embryogenic cell suspensions of G. kurroo expressed their morphogenic potential through abundant indirect and direct somatic embryogenesis on both induction (MS + 0.5 mg/l 2,4-D + 1.0 mg/l kinetin) and regeneration medium (MS + 1.0 mg/l kinetin + 0.5 mg/l GA3 + 80 mg/l adenine sulfate—AS). The number of somatic embryos in each agarose bead (100 µl of agarose medium) reached 65.3 with the conversion rate to plants of up to 62.5 %.
Even though it seemed that only protoplasts derived from undifferentiated plant material expressing high morphogenic potential are able to regenerate plants by somatic embryogenesis, the induction of indirect somatic embryogenesis from G. kurroo , G. decumbens , and G. tibetica protoplasts isolated from strongly differentiated leaf mesophyll cells has been reported recently (Tomiczak 2011; Tomiczak et al. 2015). However, considerable attention should be paid to improve the frequency of embryo formation, since the number of obtained embryos was no more than 2.5 per agarose bead (Tomiczak et al. 2015).
Hormone-free MS or half-strength MS medium was used for further growth of all Gentiana regenerants. Protoplast-derived shoots of E. grandiflorum were rooted by culture for 1 week on MS medium supplemented with 1.0 mg/l IAA (O’Brien and Lindsay 1993). Regenerated plants were cultured subsequently on MS medium with the addition of 0.06 mg/l IBA, 0.3 mg/l BAP and 0.1 mg/l GA3 (O’Brien and Lindsay 1993), or on half-strength MS with 0.5 mg/l IBA alone (Kunitake et al. 1995).
7.2.5 Evaluation of Regenerants
Since Larkin and Scowcroft (1981) summarized various reports on genetic variability originating in plant cell cultures which they defined as somaclonal variation , considerable attention has been paid to the evaluation of plants regenerated from tissue cultures. The process of protoplast isolation and indirect plant regeneration , usually with a long-term callus phase, can induce somaclonal variation, seen in altered morphology and DNA content , as well as in changes in chromosome number (Karp et al. 1982; Ramulu et al. 1989; Nyman and Wallin 1992).
The occurrence of somaclonal variation in E. grandiflorum protoplast cultures was reported by Lindsay et al. (1994). Of 5 protoplast-derived plants which survived 18 months in a glasshouse, all were tetraploids, as revealed by leaf and flower characteristics and by flow cytometry. In contrast, lisianthus plants obtained from protoplasts by Kunitake et al. (1995) exhibited no differences either in flower and leaf characters, or in pollen fertility compared with controls. Also, the regenerants of G. triflora and G. triflora × G. scabra showed no visible symptoms of somaclonal variation and all tested plants (at least 10 of each genotype) possessed 26 chromosomes, typical of control cultivars (Nakano et al. 1995).
Recently, a high percentage of polyploids (30–90 %) has been detected among G. kurroo plants regenerated from cell suspensions and leaf mesophyll-derived protoplasts (Fiuk and Rybczyński 2007; Tomiczak 2011). Also, all G. decumbens and 14.3 % G. tibetica regenerants from leaf mesophyll protoplasts possessed a twofold greater DNA content and chromosome number than control plants of these species (Tomiczak 2011; Tomiczak et al. 2015). It cannot be excluded that the high proportion of polyploids was also a result of spontaneous protoplast fusion occurring during the isolation process, especially when actively dividing cells and tissues were used as source material (Bhojwani and Razdan 1996).
7.3 Somatic Hybridization of Gentians
Somatic hybridization has enabled the mixing of both nuclear and cytoplasmic genomes of protoplasts from two distantly related, to closely related plants through cell fusion, and opened up several possibilities for the parasexual manipulation of plants. In the Gentianaceae, somatic hybrids representing different nucleocytoplasmic combinations would be very useful as new ornamental varieties and valuable sources of secondary metabolites . However, only two reports concerning protoplast fusion within this family have been published so far. In order to improve the ornamental attributes of gentians, mesophyll protoplasts of E. grandiflorum and G. scabra were fused with cell suspension-derived protoplasts of G. lutea (Takahata et al. 1995), but no further information was reported of heterokaryon culture and somatic hybrid regeneration. In 2011, Wang et al. described the fusion between callus protoplasts of Swertia mussotii Franch. and Bupleurum scorzonerifolium Willd. aimed at introgression of secondary metabolites and related genes from a species facing the risk of extinction (S. mussotii) into the genome of a less endangered species (B. scorzonerifolium).
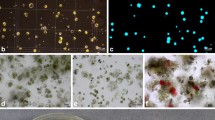
In order to verify the feasibility of somatic hybridization for transfer of morphogenic potential , symmetric fusion has been carried out between cell suspension (“white”) protoplasts of G. kurroo and G. cruciata (Fig. 7.1a, b) with “green” leaf mesophyll protoplasts of G. cruciata and G. tibetica (Fig. 7.1c, d; Tomiczak 2011).
Fusion of gentian protoplasts. a Embryogenic cell suspension of G. cruciata and (b) its “white” protoplasts, c shoot culture of G. tibetica and (d) “green” leaf mesophyll protoplasts, e “pearl chain” formation in AC electric field mixture of “white” and “green” protoplasts after DC pulse, f arrows indicate newly formed heterokaryons, g a single heterokaryon before and h after cytoplasmic mixing, i an increase in heterokaryon cell volume 1 week after fusion, j asymmetric heterokaryon division after 2 weeks in culture, k a multicellular hybrid aggregate after 3 weeks of culture, l granular embryogenic callus, m regenerating somatic embryo, n embryo conversion into plantlets, o acclimatized somatic hybrid plant
7.3.1 Conditions of Protoplast Fusion
Currently, two different procedures of protoplast fusion are in common use. Chemical fusion involves protoplast aggregation by treatment with polyethylene glycol (PEG) and protoplast fusion induced by a high-pH Ca2+ solution (Kao and Saleem 1986). During electrofusion (Senda et al. 1979; Zimmermann and Scheurich 1981), protoplasts are aligned to form “pearl chains” by a high frequency alternating current (AC) field (Fig. 7.1e). Fusion is then induced by a rectangular short, direct current (DC) pulse(s) (Fig. 7.1f). Both methods were applied for gentian somatic hybridization. Takahata et al. (1995) first described the procedure of protoplast electrofusion, with the optimum conditions for protoplasts of G. scabra and E. grandiflorum of 1 MHz, 75 V/cm, and 15 s in an AC field and 533 V/cm, 40 µs as a DC pulse. Fusion of G. lutea and E. grandiflorum protoplasts was achieved by an AC field of 1 MHz, 100 V/cm and 10 s, and a DC pulse of 900 V/cm and 60 µs. The percentage of heterokaryons obtained varied from 2.1 to 4.1 % (Table 7.3).
Our investigations showed that the most appropriate electrofusion conditions for G. kurroo + G. cruciata and G. kurroo + G. tibetica species combinations were an AC field strength of 67 V/cm and two DC pulses of 1330 V/cm. The combination G. cruciata + G. tibetica species required a weaker AC field and DC pulse strengths (60 and 1170 V/cm, respectively). These conditions guaranteed the percentage of heterokaryons from 4.3 to 6.7 % (Table 7.3) with 45–50 % of viable protoplasts 24 h after fusion. Higher values of current strength theoretically enabled a 2–3 times higher percentage of heterokaryons after fusion, but 24 h later, the number of burst protoplasts exceeded 80 % (Tomiczak 2011).
Chemical fusion can sometimes be more effective than electrofusion (Assani et al. 2005). However, the frequency of heterokaryons obtained with the use of PEG for the same 3 gentian species combinations ranged from 2.42 % to only 3.65 % (Table 7.3; Ładyżynski et al. 2006). Chemical protoplast fusion was successful for production of S. mussotii (+) B. scorzonerifolium somatic hybrids (Wang et al. 2011). The procedure of fragmentation and partial elimination of S. mussotii nuclear DNA by irradiation of protoplasts with UV light leading to the production of asymmetric hybrids with only a small amount of genome introgression from the donor species was also implemented in this work.
7.3.2 Culture of Fusion Products and Plant Regeneration
Normally, to establish an efficient protocol of post-fusion protoplast culture , the procedures developed previously as protoplast-to-plant regeneration systems of parental species must be exploited. Thus, the media effective for plant regeneration from B. scorzonerifolium protoplasts were used to obtain S. mussotii (+) B. scorzonerifolium somatic hybrids (Wang et al. 2011). Conditions that were optimal for leaf mesophyll protoplast culture of G. tibetica , as well as for culture of cell suspension protoplasts of G. kurroo , were also tested for the culture of Gentiana protoplasts after electrofusion (Tomiczak 2011). Despite this, most of the heterokaryons formed during electrofusion (Fig. 7.1g, h) after introduction into agarose bead culture only increased in volume (Fig. 7.1i) and finally burst. Cell divisions were observed sporadically (Fig. 7.1j). Protoplast cultures established for G. kurroo + G. cruciata were more prone to browning than these of other combinations, probably because of the recalcitrance in culture of G. cruciata leaf mesophyll protoplasts. Among all media tested, MS lacking NH4NO3 and supplemented with 3 g/l glutamine, 3 % glucose, 9 % mannitol, 2 mg/l NAA, and 0.1 mg/l TDZ provided the best survival of protoplasts and the highest percentage of cell divisions leading to the formation of multicellular aggregates (Fig. 7.1k; Table 7.3).
The callus proliferation stage, without difficulty in gentian protoplast-to-plant systems, seemed to be more complicated after protoplast fusion. Even though 253 individual post-fusion Gentiana calli lines were obtained, more than 30 % did not survive the first 8 weeks in culture and the majority of those remaining grew very slowly. Agar-solidified MS medium containing 2 mg/l NAA and 0.2 mg/l TDZ or 1 mg/l dicamba, 0.1 mg/l NAA, 2 mg/l BAP, and 80 mg/l AS was the most appropriate for callus proliferation (Fig. 7.1l; Table 7.3).
The regeneration of viable plants is often the main bottleneck in somatic hybridization. Of a total of 194 calli obtained from Swertia + Bupleurum fusion and a total of 174 calli from all three Gentiana combinations, only 3 and 9 were able to regenerate green plants, respectively (Fig. 7.1m, n; Table 7.3). In the case of S. mussotii (+) B. scorzonerifolium , much of the problem appeared to be related to the hybrid incompatibility of the parental species, which could be alleviated only if S. mussotii chromosomes were almost completely eliminated (Wang et al. 2011). Low regeneration efficiency of Gentiana calli could also derive from high genetic instability and genomic imbalance of hybrid cells (Tomiczak 2011). The influence of the composition of particular regeneration media cannot be omitted, since 73 % of all regenerated Gentiana plants have been obtained on MS medium supplemented with 1 mg/l kinetin, 0.5 mg/l GA3, and 80 mg/l AS (Table 7.3).
7.3.3 Identification of Somatic Hybrids
For the preliminary confirmation of hybridity, the morphological characters of regenerated plants are usually intermediate between those of the two parents and can be a convenient indicator. However, unequivocal identification of true somatic hybrids necessitates demonstration of the presence of DNA from both fusion partners in hybrid cells. Molecular markers , especially those based on the polymerase chain reaction (PCR), superseded the isoenzyme technique used commonly in the 1970s and 1980s. Randomly amplified polymorphic DNA (RAPD), amplified fragment length polymorphism (AFLP), simple sequence repeats (SSR), and inter-simple sequence repeats (ISSR) are currently among the most popular markers used for hybrid verification.
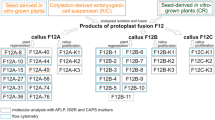
Since codominant microsatellite markers have been developed for only a limited number of gentian species (Li et al. 2007; Sato-Ushiku et al. 2011), Wang et al. (2011) applied dominant but quick and universal RAPD markers for the identification of S. mussotii (+) B. scorzonerifolium somatic hybrids. Besides fragments specific for both parents, fragments not present in either of the parents were found in all the clones tested, indicating putative advanced genome recombination of parental species. In order to identify the somatic hybrids between G. kurroo and G. cruciata , G. kurroo and G. tibetica , and G. cruciata and G. tibetica (Fig. 7.1o), AFLP markers were used since these are more reproducible than RAPD and amplify a greater number of fragments (Agarwal et al. 2008). Eventually, the hybrid character was confirmed of 3 calli and 87 regenerants from G. kurroo + G. cruciata and of 6 calli and 82 plants from G. cruciata + G. tibetica (Fig. 7.2a, b). Unfortunately, no G. kurroo (+) G. tibetica somatic hybrids were obtained (Tomiczak 2011).
Identification and description of gentian somatic hybrids. AFLP electrophoretic patterns obtained for species combinations: G. kurroo + G. cruciata (a) and G. cruciata + G. tibetica (b), c exemplary histogram of the flow cytometry analysis of a G. kurroo (+) G. cruciata F12A-7 somatic hybrid having a significantly greater DNA content than parental species, d root-tip metaphase plates of G. kurroo (+) G. cruciata F12A-10 and (e) G. cruciata (+) G. tibetica F30B1 somatic hybrids possessing more chromosomes than the parental species. Abbreviations: K—G. kurroo, C—G. cruciata , T—G. tibetica. Black letters from A to Z are the symbols of particular calli, numbers from 1 to 6 are the numbers of individual regenerants. Yellow arrows indicate bands specific for “suspension” fusion partners; green arrows indicate bands specific for “mesophyll” fusion partners; red arrows indicate electrophoretic profiles of true somatic hybrids
7.3.4 Characteristics of Somatic Hybrids
Since protoplast fusion leads to novel configurations of both nuclear and organellar genomes, analysis of the inheritance of mitochondria and chloroplasts is a vital part of somatic hybrid description. Restriction fragment length polymorphism (RFLP) analysis combined with southern hybridization of mitochondrial DNA (mtDNA) and chloroplast DNA (cpDNA) probes was exploited by Wang et al. (2011) to demonstrate that either mtDNA or cpDNA of both parents, S. mussotii and B. scorzonerifolium , coexisted in hybrid cell lines. Evidence was also found for mtDNA and cpDNA recombination.
In addition to molecular markers for detailed description of somatic hybrids, flow cytometry and methods of molecular cytogenetic analysis are commonly used, particularly genomic in situ hybridization (GISH) enabling identification of chromosomes of parental species, For example, Wang et al. (2011) proved that S. musssotii (+) B. scorzonerifolium hybrids possessed a chromosome number approximate to the sum of that of the parental species or intermediate between them. The majority of cells carried 11–13 intact B. scorzonerifolium chromosomes, none intact chromosomes of S. mussotii , but several recombined chromosomes. In contrast, all Gentiana somatic hybrids possessed a significantly higher DNA content (Fig. 7.2c) and chromosome number than parental species (Fig. 7.2d; Tomiczak 2011).
An important part of hybrid description is the analysis of traits of interest such as cytoplasmic male sterility (CMS), resistance to pests and diseases, tolerance to abiotic stresses, or synthesis of valuable secondary metabolites. Using high-performance liquid chromatography (HPLC), S. mussotii (+) B. scorzonerifolium hybrids were tested for accumulation of gentiopicoside, swertiamarin, and mangiferin , and the content of volatile compounds was assessed by gas chromatography–mass spectrometry (GC-MC). Additionally, the accumulation of swertiamarin was correlated with up-regulation of the expression of the gene encoding the enzyme geraniol 10-hydroxylase (SmG10H; Wang et al. 2011). Detailed analysis of secondary metabolites of Gentiana somatic hybrids is also planned.
7.4 Conclusions
Somatic hybridization can serve as a tool for the production of genetically novel plants with a modified secondary metabolite profile. Protoplast fusion also enables the transfer of morphogenic ability from highly embryogenic gentian protoplasts to their hybrids. These two examples of research in the field of somatic hybridization show that gentian protoplast-based technologies have considerable potential. However, from the practical point of view somatic hybridization is not fully exploited. Some limitations of this technique, especially lack of accurate control over interactions between nuclear and organellar genomes deriving from two different parental species, as well as difficulties in hybrid plant regeneration, mean that somatic hybridization is often displaced by more precise methods of genetic transformation.
References
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep 27:617–631
Assani A, Chabane D, Haïcour R, Bakry F, Wenzel G, Foroughi-Wehr B (2005) Protoplast fusion in banana (Musa spp.): comparison of chemical (PEG: polyethylene glycol) and electrical procedure. Plant Cell Tiss Org Cult 83:145–151
Bajaj YPS (ed) (1989). Biotechnology in agriculture and forestry. Plant protoplasts and genetic engineering I, vol. 8. Springer, Berlin, pp 1–444
Bhojwani SS, Razdan MK (1996) Plant tissue culture: theory and practice, a revised edition. Elsevier Science BV, Amsterdam Lausanne New York Oxford Shannon Tokyo, pp 1–767
Bokelmann GS, Roest S (1983) Plant regeneration from protoplasts of potato (Solanum tuberosum cv. Bintje). Z Pflanzenphysiol 109:259–265
Davey MR, Anthony P, Power JB, Lowe KC (2005a) Plant protoplasts: status and biotechnological perspectives. Biotechnol Adv 23:131–171
Davey MR, Anthony P, Power JB, Lowe KC (2005b) Plant protoplast technology: current status. Acta Physiol Plant 27:117–129
Dons JJM, Colijn-Hooymans CM (1989) Agarose plating of protoplasts and its applications. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Plant protoplasts and genetic engineering I, vol. 8. Springer, Berlin, pp 50–62
Fiuk A, Rybczyński JJ (2007) The effect of several factors on somatic embryogenesis and plant regeneration in protoplast cultures of Gentiana kurroo (Royle). Plant Cell Tiss Org Cult 91:263–271
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension culture of soyabean root cells. Exp Cell Res 50:151–158
Jomori H, Takahata Y, Kaizuma N (1995) Plant regeneration from leaf-derived calli of gentians and their protoplast culture. Acta Hort 392:81–86
Kao KN, Michayluk MR (1975) Nutritional requirements for growth of Vicia hajastana cell and protoplasts at a very low population density in liquid media. Planta 126:105–110
Kao KN, Saleem M (1986) Improved fusion of mesophyll and cotyledon protoplasts with PEG and high pH-Ca2+ solutions. J Plant Physiol 122:217–225
Karp A, Nelson RS, Thomas E, Bright SWJ (1982) Chromosome variation in protoplast-derived potato plants. Theor Appl Genet 63:265–272
Köhlein F (1991) Gentians. Timber Press, Portland
Kunitake H, Nakashima T, Mori K, Tanaka M, Mii M (1995) Plant regeneration from mesophyll protoplasts of lisianthus (Eustoma grandiflorum) by adding activated charcoal into protoplast culture medium. Plant Cell Tiss Org Cult 43:59–65
Ładyżyński M, Fiuk A, Rybczyński JJ (2006) Protoplast fusion by polyethylene glycol within Gentiana spp. XI all-polish conference of plant in vitro culture and biotechnology. Międzyzdroje, Poland, 6–9 Sept 2006. (http://gentiana.pl/posters/ladyzynski.pdf)
Larkin PJ, Scowcroft WR (1981) Somaclonal variation—a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214
Li Y, Li L-F, Chen G-Q, Ge X-J (2007) Development of ten microsatellite loci for Gentiana crassicaulis (Gentianaceae). Conserv Genet 8:1499–1501
Lindsay GC, Hopping ME, O’Brien IEW (1994) Detection of protoplast-derived DNA tetraploid lisianthus (Eustoma grandiflorum) by leaf and flower characteristics and by flow cytometry. Plant Cell Tiss Org Cult 38:53–55
Meng Y, Gao Y, Jia J (1996) Plant regeneration from protoplasts isolated from callus of Gentiana crassicaulis. Plant Cell Rep 16:88–91
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakano M, Hosokawa K, Oomiya T, Yamamura S (1995) Plant regeneration from protoplasts of Gentiana by embedding protoplasts in gellan gum. Plant Cell Tiss Org Cult 41:221–227
Nyman M, Wallin A (1992) Improved culture technique for strawberry (Fragaria × ananassa Duch.) protoplasts and the determination of DNA content in protoplast derived plants. Plant Cell Tiss Org Cult 30:127–133
O’Brien IEW, Lindsay GC (1993) Protoplasts to plants of gentianaceae. Regeneration of lisanthus (Eustoma grandiflorum) is affected by calcium ion preconditioning, osmolarity and pH of the culture media. Plant Cell Tiss Org Cult 33:31–37
Pasternak T, Miskolczi P, Ayaydin F, Mészáros T, Dudits D, Fehér A (2000) Exogenous auxin and cytokinin dependent activation of CDKs and cell division in leaf protoplast-derived cells of alfalfa. Plant Growth Regul 32:129–141
Power JB, Davey MR, Anthony P, Lowe KC (2004) Protoplast culture and regeneration. In: Goodman RM (ed) Encyclopedia of Plant and Crop Science. Marcel Dekker Inc., New York, pp 1065–1068
Ramulu KS, Dijkhuis P, Roest S (1989) Patterns of phenotypic and chromosome variation in plants derived from protoplast cultures of monohaploid, dihaploid and diploid genotypes and in somatic hybrids of potato. Plant Sci 60:101–110
Sato-Ushiku Y, Shimada N, Saito M, Yamada E, Hikage T, Nakatsuka T, Nishihara M (2011) Development of simple sequence repeat markers for identification of Japanese gentian cultivars. J Japan Soc Hort Sci 80:475–485
Saxena PK, Gill R (1986) Removal of browning and growth enhancement by polyvinylpolypyrrolidone in protoplast cultures of Cyamopsis tetragonoloba L. Biol Plant 28:313–315
Senda M, Takeda J, Abe S, Nakamura T (1979) Induction of cell fusion of plant protoplasts by electrical stimulation. Plant Cell Physiol 20:1441–1443
Takahata Y, Jomori H (1989) Plant regeneration from mesophyll protoplasts of gentiana (Gentiana scabra Bungei). Plant Tiss Cult Lett 6:19–21
Takahata Y, Jomori H, Miyano S, Kunitake H, Mii M (1995) Regeneration of plants from protoplasts of Gentiana species (Gentian). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Plant Protoplasts and genetic engineering VI, vol 34. Springer, Berlin, pp 57–62
Tomiczak K (2011) Application of morphogenic potential of somatic cell protoplasts in gentian interspecific hybrids formation. Ph.D. thesis. Library of PAS Botanical Garden-CBDC in Powsin WarsawPoland, pp 1–208
Tomiczak K, Mikuła A, Sliwinska E, Rybczynski JJ (2015) Autotetraploid plant regeneration by indirect somatic embryogenesis from leaf mesophyll protoplasts of diploid Gentiana decumbens L.f. In Vitro Cell Dev Biol Plant. doi:10.1007/s11627-015-9674-0
Vasil IK (2008) A short history of plant biotechnology. Phytochem Rev 7:387–394
Wang J, Zhao C, Liu C, Xia G, Xiang F (2011) Introgression of Swertia mussotii gene into Bupleurum scorzonerifolium via somatic hybridization. BMC Plant Biol 11:71
Zhou Y, Qian Y, Cai Q, Zhang Z, Yan X (1985) Studies on the callus formation of mesophyll protoplast from Gentiana scabra Bunge. Acta Bot Sin 27:148–151
Zhu YM, Hoshino Y, Nakano M, Takahashi E, Mii M (1997) Highly efficient system of plant regeneration from protoplasts of grapevine (Vitis vinifera L.) through somatic embryogenesis by using embryogenic callus and activated charcoal. Plant Sci 123:151–157
Zimmermann U, Scheurich P (1981) High frequency of fusion of plant protoplasts by electric fields. Planta 151:26–32
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Tomiczak, K., Mikuła, A., Rybczyński, J.J. (2015). Protoplast Culture and Somatic Cell Hybridization of Gentians. In: Rybczyński, J., Davey, M., Mikuła, A. (eds) The Gentianaceae - Volume 2: Biotechnology and Applications. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-54102-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-54102-5_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-54101-8
Online ISBN: 978-3-642-54102-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)