Abstract
In the twenty-first century, energy is considered as the most important issue for human sustainability. The world’s dependence on unsustainable fossil fuels (almost ), and the increasing population demand new sources of energy for sustainable human activities. Algae, and particularly microalgae have nowadays become of enormous importance as a new potential source of feedstock for renewable bioenergy production. As photosynthetic microorganisms, microalgae may potentially be produced as carbon neutral and can be produced on non-arable land and cultured in marine and wastewater effluents. Furthermore, microalgae can be used to produce a range of products such as protein-rich animal feed in aquaculture, high-value products, viz, polyunsaturated fatty acids, bioactive and functional pigments and natural dyes, health foods, cosmetics, and pharmaceuticals. In this chapter, an update of the advances in microalgal biotechnology is presented as a new biomass for the potential development of biofuels, and as a realistic source of highly valuable molecules of industrial interest. The potential to harness endogenous carbon storage compounds, triacylglyceride (GlossaryTerm
TAG
s) and starch, as products of photosynthesis, including the photoproduction of hydrogen, can contribute to diversify the sources and yields of feedstocks for biofuel production. Even if the production of microalgae for biofuels is highly promissory and clearly has potential for contributing to environmental, social, and economic sustainability, presently this alternative is unsustainable. Definitely, the combination of biofuel production by microalgal biotechnology with co-products may contribute to the sustainability of biofuels, a condition with null or less impact on natural resources and biodiversity. The integration of all the components of the uses of microalgae, i. e., high-value compounds, aquaculture, and bioremediation coupled to the production of biofuels will play an important role in the near future to make the production of biofuels from microalgae sustainable. The integration of genomics, metabolic engineering, nanotechnology, and other areas to the aforementioned issues shall lead to a wide range of benefits for the tasks demanded by the forthcoming bioenergy industries.Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Sustainable Biofuels from Marine Microalgae: Closer to Reality than Fiction
Even when terrestrial plants are employed for biofuel production, the strategies are recognized as functional at the small scale and highly controversial when scaled up by the conflict with food production, extended surface agricultural land, and uses of potable water, which make this and other hybrid technologies unsustainable to supplant a significant fraction of petroleum for the global demand for liquid fuels [1, 2]. Aquatic and marine biomass has been proposed as a very promising source for biofuel production; however, in order to realize this, a complete analysis of the full life cycle impact of algal biofuel production in the context of issues such as water resource management, energy balance, and the type of algae is very necessary [3]. According to [3], biofuel production will be sustainable only if it guarantees eco-friendly processes and respective interactions with other fields. In this eco-context, the reduction of the greenhouse gas (GlossaryTerm
GHG
) emission, can be achieved without effecting air, water, soil, or biodiversity, etc. These issues can be accomplished by using microalgae; however, other aspects such as social acceptability and economical viability still need to be overcome. Even though all these potentials can be met theoretically, in practice many scientific and technological aspects must still be applied in order to fully consider this biotechnology as sustainable. Nevertheless, in our concept and criteria, microalgae are one of the most promising renewable feedstocks for biofuel production and biorefineries.Microalgae can be grown almost anywhere, even in sewage, manure, salt water, ice, and in aerial conditions [4, 5] and do not require fertile land or food crops. Their processing requires less energy than the algae provides [6]. These indicators reflect the potential that renewable technologies have to supplement or replace liquid fossil fuels by biofuels and microalgae, which are still in their early developmental stages. According to specialized expertise, the expectative to expand will depend on whether underdeveloped petroleum fields are not sustainable, as reported by the International Energy Agency [7]. According to The National Research Council of the US National Academies, large-scale production of biofuels from algae is unsustainable using existing technologies.
The expectations and promises of the sustainable production of biofuels from microalgae are very high. However, present challenges of biofuels need to be overcome, mainly in issues related to the uses of industrial wastewater and flue gases, as well as sequestration, under carbon neutral conditions. It is expected that several major impacts will be able to influence the sustainability of biofuels, such as its contribution to the change in land use, their feedstock, and aspects of technology and scale [3].
The production of different biofuels has its own profits, uncertainties, and risks for sustainability, with many more still to come. It is only in a concerted action among governments, researchers, and companies that the benefits of biofuels from microalgae will become feasible for society. The production of sustainable liquid biofuels from microalgae will need to combine fuel production with co-products and bioprocesses, a strategy which could definitely contribute to the achievement of environmental and economic viability, as well as the adoption of this potential fuel source. In this chapter, we will address the issue of microalgal biotechnology and biofuel production, considering the aspects and conditions needed to achieve sustainability, viz, combining the production of compounds and processes of industrial interest with the production of biofuels. In all situations this alternative is closer to reality than to fiction when compared to other biotechnologies and sources of biomass.
2 Why Microalgae is Promissory for Biofuel Production
The autotrophic algae drive photosynthesis to harness sunlight and fix the inorganic carbon from . There are many algal species that are heterotrophic and able to take up small organic molecules and perform bioconversion into the building blocks of their own. Certain algae can perform mixotrophy by using either inorganic carbon from the atmosphere or organic carbon from the environment. Any of these processes confers to algae the ability to produce carbohydrates, lipids, and proteins that can be processed to produce biofuels. Microalgae are recognized as fast-growing photosynthetic organisms and have been reported to reach transformation of – of incoming light energy into biomass. Such figures, as well as its productivity place microalgae as the most promissory candidates for providing unlimited amounts of cheap biomass as food, fodder, or energy. It is recognized that microalgae have the potential to displace other feedstocks for biodiesel owing to their high vegetable oil content and biomass production rates. The potential of microalgae as a feedstock for biofuels production has led to bioprospection and bioscreening programs, hoping to find the ideal microalga. Figure 62.1 shows that in Chlamydomonas JSC4, nitrogen depletion can trigger the lipid content (from 15.3 to ), whereas the protein decreased from 45.4 to (after [7]). The following main advantages can be mentioned: uniqueness to thrive in seawater at growth rates five to ten times higher than that of higher plants and than any other source; efficient sunlight photo conversion for active growth and photosynthesis; and the massive accumulation of lipids and carbohydrate production, which do not compete with arable land and potable water. The property of microalgae in performing photosynthesis is considered the only process that uses sunlight as the energy source and carbon dioxide as the carbon source for the production of biomass.
Changes in biochemical composition of Chlamydomonas sp. JSC4 cultivated on nitrogen- rich (a) nitrogen-rich (70 to 80 % nitrogen consumed) and (b) in nitrate-depleted conditions (after [7]).
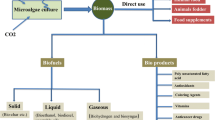
In general terms, it is recognized that sustainable production of microalgal biofuels still faces challenges to overcome. Its economic feasibility could be achieved if combined with bioprocesses and production of chemical compounds coupled to the use of low cost products and nutrients. According to [8], microalgae can be suitable feedstock for biofuels because certain species are among the most efficient biological producers of oil on the planet and a versatile biomass source. This is why some authors pointed out that microalgae may soon be one of the Earth’s most important renewable fuel crops [9]. Moreover, they are grown in variable climates and on non-arable land, including marginal areas unsuitable for agricultural purposes (e. g., desert and seashore lands), in non-potable water or even as for waste treatment purposes, use far less water than traditional crops, and do not displace food crop cultures; their production is not seasonal and can be harvested daily [10, 8]. Moreover, fixation by algae is also an important advantage for removing gases from power plants or other polluting activity and thus can be used to reduce greenhouse gases with a higher production of microalgal biomass and, consequently, a higher potential for biodiesel production [11]. A flow diagram of the carbon cycle pathway, biofixation, and the remarkable steps of algal biomass technologies for the production of biofuels is shown in Fig. 62.2.
Diagrame of the concept of culture of microalgae biomass integrated into a conversion system for synfuel production (after [12])
Several authors have emphasized that the key for large scale production of biofuels is to grow suitable biomass species in an integrated biomass production conversion system (GlossaryTerm
IBPCS
). In principle, a profit in the cost of operation of the overall system should be pursued. A conceptual model for integrated biomass production is shown in Fig. 62.2 which can be adopted for microalgal biodiesel production [13]. The design and implementation of such systems requires the integration and optimization of several components such as biomass culture, growth management, transport to conversion plants, drying, product separation, recycling, waste management, transport of saleable products and marketing. This model may has several options, for instance the conversion plants may be located in or near the biomass growth areas to minimize the cost of transporting biomass to the plants, of which all the non-fuel effluents are recycled to the growth areas (Fig. 62.2). Based on the versatility of that kind of models, the feasibility to simultaneously integrate microalgae to synfuel production is not so far from reality in the near future.3 Biodiesel Production by Microalgal Lipid Transesterification
In recent years considerable attention has been focused on methods to convert biomass to competitive liquid biofuels from various biomass materials, which may offer a promising alternative to petroleum based transportation fuels. In an epoch when fossil hydrocarbon are likely to become scarce and costly, two liquid transportation fuel at global scale from land plants are emerging. Among these emerging feedstocks, jatropha seems that can be converted to biodiesel with commercial processes, while processes capable of converting algae, are still at pre-commercial stages, but not far from outperform existing terrestrial plant sources into a comprehensive concept of sustainability. Most of bio-oils produced presently are derived from the following main sources: crop seed, animal feed, algal biomass, among others, through a transesterification process. Table 62.1 shows the yield of plant oils compared to microalgae, according to [10]. One of the undeniable advantage of microalgae species is it high content of oils in the cytosols and chloroplasts. Oil is extracted from microalgal biomass, which during the process of esterification is converted into biodiesel, a chemical reaction between triacylglycerols (GlossaryTerm
TAG
s) and alcohol in the presence of a catalyst. Finally, monoesters are produced, which are termed as biodiesel [3]. As a multiple step reaction, transesterification, is composed of three reversible steps in series, where triglycerides are converted to diglycerides, then diglycerides are converted to monoglycerides, and monoglycerides are then converted to esters (biodiesel) and glycerol (a by-product) [15]. The fact that microalgal or bio-oils are characterized by high viscosity, high molecular weight, a higher flash point (above ), and low volumetric heating values compared to diesel fuels [3] imposes challenges that need to be overcome. The associated problems with substituting triglycerides for diesel fuels are: high viscosities, low volatilities, and the polyunsaturated character [16]. It is generally accepted that the refinement of these molecules, is an essential step for turning bio-oils into quality fuel (biodiesel) [17]. Moreover, biochemical and engineering processes are applied in vegetable oil derivatives in order to approximate the properties and performance of hydrocarbon-based diesel fuels. As shown in Fig. 62.3, biomass conversion processes fall into three major categories: chemical, biological, and thermochemical, but the most efficient processes may be those that combine two or more processes and use the entire plant [14]. Present calculations on biofuels from algae reported that one hectare algae farm on wasteland can produce over – times of oil as compared to any other known source of oil-crops. While a crop cycle may take from three months to three years for production, algae can produce oil and can be harvested all year-round using sea water and non-potable water. These facts firmly reinforced and corroborates that algae for biofuels shall provide an ending point solution to food vs. fuel battle. An exemplification to that approach is conceptualized in Fig. 62.3, exhibiting the main steps of algal biomass technologies coupled to biofuel production [14].Flow diagram of main steps of bioconversion and processing of algal biomass for the production of biofuel (after [14])
4 Bioethanol from Microalgae: A Simpler Procedure
Algae have a high photon conversion efficiency and can synthesize and accumulate large quantities of carbohydrate biomass for bioethanol production, from inexpensive raw materials. This is an effective and promissory alternative to the low yield of lignocellulosic biomass materials as a feedstock and the high cost of the hydrolysis process based on current technologies [19]. Even when bioethanol can be produced from several different types biomass feedstock, the algal alternative has gained acceptation, as will be described in the following. The structural composition of microalgal cells (lacking hemicelluloses and lignins) confers advantages for the production of ethanol, by eliminating the chemical and enzymatic pre-treatment steps to transform these polymers in sugars [19]. Microalgae (Chlorella, Dunaliella, Chlamydomonas, Scenedesmus) are recognized as having a high content ( of the dry weight) of starch, cellulose, and glycogen, which are the basic components for ethanol production [20]. The starch biosynthesized by microalgae can be extracted from the cells with enzymes and then separated by extraction with water or an organic solvent and used for fermentation to yield bioethanol. Besides starch, several algae, especially green algae, can accumulate cellulose as the cell wall carbohydrate, which can also be used for ethanol production. The algal photosynthesis is mainly based on the Calvin cycle, in which ribulose-1,5-bisphosphate (GlossaryTerm
RuBP
) combines with to produce 3-phosphoglyceric acid (GlossaryTerm3-PGA
), which is utilized for the synthesis of glucose and other metabolites to make carbohydrates (GlossaryTermCH
). The GlossaryTermCH
molecules are vital source of storage of energy and provide the precursors to make the organic molecules and macromolecules of nearly all living cells. The process of ethanol from microalgae is more based on the carbohydrate conten of the biomass. The possibilities to utilize algal biomass for the production of biofuels, can follow different life cycle (GlossaryTermLCA
) as exemplified in Fig. 62.4 [6]. The assessment of the GlossaryTermLCA
options is based on indicators to compare alternative energy routes in terms of environmental impact and indirect natural resource costs towards different services and commodities. Among these, the life-cycle water and nutrients usage of microalgae-based biofuels production, which revealed that water footprint and nutrients usages during microalgae biodiesel production is an important aspect. From that, was calculated that water is required to generate microalgae biodiesel if freshwater is used without recycling [6]. When seawater and wastewater were used for algal culture, a reduction up to was reported in nitrogen usage, which eliminates the need of other ions such as potassium, magnesium, and sulfur. The geographic position of each country play a critical role when the life-cycle assessment is performed on the overall water footprint of microalgae-based biodiesel, which production gradually decreases from north to south as solar radiation and temperature increase. Nevertheless, the potential of microalgae as an energy source is confirmed, but emphasizes the need of decreasing the energy and fertilizer consumption. This aspect is actually considered in similar initiatives, and present models had reduced dependence on energy and fertilizers by using algae innate attributes such as sequestration and nutrients recovering from wastewater bioremediation initiatives. Hence, algal biomass can be utilized for the production of a range of different biofuels; their life cycles are presented in Fig. 62.4.Schemes of the life cycle stages of the production of biodiesel, bioethanol, and biomethane from algal biomass (a) exemplifies the production of biodiesel from microalgal glycerol; (b) shows the culturing process of microalgae to produce bioethanol; and (c) main steps to generate biomethane from cultured microalgae. In all three cases the produced water is recycled and bio-fertilizers are obtained (modified after [18, 3])
5 Microalgal Biohydrogen Production Through Sunlight and Seawater
The production of biohydrogen from microalgae is not new; this process has been known for more than years and was first observed in the green alga Scenedesmus obliquus [15, 3]. More recently, this mechanism was corroborated in many other prokaryotic photosynthetic species, including cyanobacteria [6]. The microalgal photoproduction of hydrogen from water is a promising mechanism and has been explored as a potentially emission-free fuel stream for the future. It is expected that the desired sustainability in biofuels from microalgae can be accomplished by the photoproduction of biohydrogen because of its natural feasibility of atmospheric -sequestration [15]. The green microalga Chlamydomonas reinhartii is a successful model that has been studied for algal hydrogen production [21].
One of the most attractive characteristics of the bio- process is that it uses sunlight to convert water to hydrogen and oxygen, which are released in a two-phase process occurring in all oxygenic photosynthetic organisms, such as marine microalgae. A second reaction by special iron-containing chloroplast-hydrogenase enzymes also occurs in a specific group of microalgae. Cyanobacteria also exhibit a natural production of from water in a different and alternative photo-biochemical process, involving a water-splitting reaction under light and aerobic conditions. In this process, hydrogen and electrons from the water-splitting reaction of photosynthesis are used for the synthesis of adenosine triphosphate (GlossaryTerm
ATP
) and nicotine adenine disphosphonucleotide (GlossaryTermNADPH
), reduced). In the absence of , both GlossaryTermATP
production and the formation of GlossaryTermNADH
/GlossaryTermNADPH
are inhibited, and the stored energy in carbohydrates (starch, glycerol) is re-oriented [22] to the chloroplast hydrogenase to facilitate GlossaryTermATP
production via photophosphorylation. Thus, under fermentative conditions hydrogenase plays the role of a releasing valve of protons/electrons, a condition in which protons from the medium and e- from reduced ferredoxin are balanced to produce hydrogen gas that is excreted from the cell [23]. C. reinhardtii is one of the microalgae most studied for solar-driven bio- production from water and can also use other fermentative processes. The fact that hydrogen production does not accumulate in the culture but instead is quickly released into the gas phase, makes photo-production of hydrogen one of the most preferred biofuels.6 Genomics and Metabolic Engineering of Microalgae for Biofuels Production
The gene reservoir of microalgae is only beginning to be explored. In general, microalgal genomes are structurally complex, the reported size ranges from 12.6 GlossaryTerm
Mbp
for Chlorophyte Ostreococcus tauri while 10.000 GlossaryTermMbp
for Karenia brevis. The present development in genetic manipulation in microalgae has been expanded in algal biofuels which is limited a very small number of alga models. The significant advances achieved until now include the efficient expression of transgenes, the mechanisms of gene regulation, and the action of inducible nuclear promoters as well as inducible chloroplast gene expression. The nuclear genomes of a number of algae have been transformed, with a variety of reporter genes, as well as drug-resistance genes; however, extensive analysis of transgene expression has only been performed in C. reinhardtii [1]. C. reinhardtii has been extensively studied, and presently, abundant genomic biological and physiological data are available from this alga [24], which is probably not the best species for biofuel production, but as a model of study has potential for application in other suitable algal species. Microorganisms are especially attractive for biofuel production because the content in lipids is higher, – times more lipids, than in any plant [25]. Presently, more than complete genomes have been sequenced in lipid producing cyanobacteria. In eukaryotic microalgae having significant amounts of lipids, the nuclear genome of only 10 species has been sequenced, viz, Chlamydomonas reinhardtii, Volvox carteri, Ostreococcus marinus, Phaeodactylum tricornutum and Thalasiosira pseudonana [24].The growing interest in biofuels has led to the overexpression of acetyl-GlossaryTerm
CoA
carboxylase (GlossaryTermACCase
) in the diatom Cyclotella cryptica to improve lipid content by increasing the first enzyme in the lipid biogenesis pathway. The result of these studies evidenced discrete results as the alga did not yield increased lipid production. The first successful transformation of microalgal strains with potential for biodiesel production was achieved in 1994, C. triptica and Navicula saprophila [26, 27]. Currently there are only a few species of microalgae that fulfill even some of these crucial requirements. Apart from C. reinhardtii and P. tricornutum, only Chlorella kessleri, Porphyridium, and fairly recently Nannochloropsis and Dunaliella salina have been successfully transformed [28]. Acetyl GlossaryTermCoA
carboxylase and other enzymes of the lipid biosynthesis pathway have been used as targets for improving oil production. Lipid metabolism and the biosynthesis of fatty acids, glycerolipids, sterols, hydrocarbons, and ether lipids in eukaryotic algae were recently reviewed in the context of optimization for biodiesel production. Enhancement of the algal potential for the genetic modification of their lipid pathways can be followed either by up-regulation of fatty acid biosynthesis or by down-regulation of β-oxidation. By knocking out or modifying the enzymes responsible for the synthesis of polyunsaturated lipids in the cell, it should be possible to enhance the proportion of monounsaturated lipids. In this aspect, the specificity of algal species will drive its own biosynthetic pathway of lipids, which is why it is highly recommended to utilize species that have a suitable lipid profile for biodiesel production. At present, the mechanisms involved in the fatty acid biosynthetic pathway in microalgae have not been extensively studied [29]. Genes encoding the key enzymes involved in fatty acid biosynthesis have been identified in Ostreococcus tauri, T. pseudonana, C. reindhardtii, and in P. tricornutum. Recently [26] studied lipid metabolism and glycerol biosynthesis in the diatom Phaedactylum tricornutum (genome sequence available at: http://genome.jgipsf.org/Phatr2/Phatr2.home.html). Overexpression of Glycerol-3-phosphate dehydrogenase (GlossaryTermGPDH
) involved in lipid metabolism and glycerol biosynthesis led to the successful conversion of dihydroxyacetone phosphate to glycerol-3-phosphate and indicates that the GlossaryTermGPDH
transgene was incorporated into the transgenic diatom cells. The transcriptional expression of the introduced transgene consequently increased the GlossaryTermGPDH
transcript abundance in the transgenic diatom cells. The obtained results reported glycerol content, fold in GlossaryTermGPDH
-overexpressing cells of P. tricornutum, compared with the wild type (Fig. 62.5). Moreover, genomics, transcriptomics, proteomics, and metabolomics are elucidating aspects of metabolic pathway regulation and integration linked to targets to optimize biofuel production. The recent progress in metabolic engineering toward biofuel production has incorporated random insertional mutagenesis and targeted gene disruption in microalgae. However, further research is required to better understand the partitioning of fixed carbon toward the increased production of starch for subsequent fermentation into or ethanol, or the redirection of photosynthate from starch into lipids for conversion to diesel fuels. New developments in metabolic engineering toward enhanced carbon storage in unicellular microalgae capable of synthesizing a range of biofuels are actually under development, as in the case of lipids and carbohydrates (the main energy storage molecules in algae). These approaches are basic for the understanding of primary metabolism to manipulate electron flux toward these products or for bioenergy applications. But at the level of organelles, the situation becomes more complex, such in the case of isoforms of phosphoenolpyruvate carboxylase (GlossaryTermPEP-C
). This enzyme that carboxylates PEP, have been identified in C. reinhardtii, both of which are responsive to inorganic carbon and nitrogen levels. Additionally, six pyruvate kinase and five malate dehydrogenase homologs are present in the C. reinhardtii genome exhibiting different level of expression. These results emphasizes the complexity of algal metabolism and the need to characterize the systems on an enzymatic level.Analysis of glycerol-3-phosphate dehydrogenase transgenic P. tricornutum cells. (a) transgenic diatom cells testes by genomic polymerase chain reaction (PCR). Lane M: plus DNA ladder; lane 1: PCR of wild type with primers on the transformation vector; lane 2: PCR of transgenic line with primers on the transformation vector flanking GPDH; lane 3: PCR of transgenic line with primers on both ends of GPDH. (b) GPDH mRNA expression in the diatom cells determined by quantitative PCR. (c) Protein expression of introduced GPDH detected by western blotting with anti-myc antibody (Invitrogen, USA). M: Protein molecular weight marker; lane 1: transgenic P. tricornutum overexpressing GPDH tagged with myc; lane 2: wild type (after [26])
7 Microalgal Culture Systems: A Contribution to the Sustainability of Biofuels
7.1 Culture Conditions and Better Yields of Biomass for Biofuels
It is recognized that the optimization of the microalgal production system is an important factor for the achievement of sustainability of microalgal-based biofuels. Successful histories of high-value products from microalgae have been reported with the use of conventional open-pond algal production systems, although closed algal bioreactors have already achieved economic viability in the case when the production of high-value products such as astaxanthin and nutraceuticals are aimed for human consumption. Moreover, many interrelated factors affecting strains can each be limiting in the final yield of biomass, and hence in the outcome of biofuels.
Another critical and important factor, is media formulation, which is important to ensure a sufficient and stable supply of nutrients to attain maximal growth acceleration and cell density, and ultimately to produce biofuels at higher efficiencies [30]. There are many different recipes and formulations, depending on the species and particularities of the objective being sought. In the process of biomass optimization, the culture mode plays a preponderant role in the yield and biofuel production. It has been recognized that batch feeding of heterotrophic algal cultures and enrichment of photoautotrophic algal cultures can significantly increase the biomass. On the other hand, the proper selection and optimization of mineral nutrients increases culture productivity. Two important nutrients that are constitutive of the microalgal metabolism are nitrogen and phosphorous, mostly at early stages in the mineral optimization of the media formulations. Other equally important components of the growth media are minerals, which are vital elements for support of the structural and metabolic biochemistry of the cell and in modulating the molecular configuration of photosynthetic complexes and enzymatic reactions. Unless otherwise stated, vitamins are generally omitted in massive cultures because of the susceptibility of the large-scale level, which in general are more fragile and hard to control, apart from economic reasons.
The use of organic substances is a common task for the improvement of the growth and strain preferences. Organic nutrition can sometimes be supplied with the use of domestic waste water, after secondary treatment. The dilemma is how to conciliate sanitation with water shortage due to the increasing scarcity of freshwater resources in many countries. In such situation, the use of wastewater bioremediated is an attractive presently explored for biofuel production. Maintenance of an acceptable pH range throughout culturing is of utmost importance as it impacts all aspects of media biochemistry and culture progression. Both ionic absorption from the media and the metabolic biochemistry of the cell exert significant pressure upon the pH. In high-performance cultures their effect is powerful enough to overcome the neutralizing capacity of exogenous buffering agents. Currently, microinjection of strong acids and alkalis, metabolic balancing in heterotrophic cultures, and regulated dissolution in both photoautotrophic and heterotrophic cultures are the most practical and economical strategies for pH control.
7.2 Open Ponds and Raceways: Low-Cost Production Systems
Most of the microalgae produced presently are cultured in open ponds. Open ponds and raceways are built in whatever shape best suits the location. According to the needs and objectives, these are usually not mechanically mixed but driven by gravity flow or provided with paddle wheels. Traditional open ponds are highly diverse in shape, size, and technology, but the most commonly used design is the raceway pond. In these, the whole area is sectioned into a rectangular grid, with each divided rectangle containing a channel in the shape of an oval. The water flow is driven continuously by a paddle wheel all around the circuit. The reported functional and operational water depth of – are preferred for obtaining biomass concentrations of dry weight per liter productivities of – (i. e., –) [31]. However, differences and year round productivities cannot be maintained. Raceway ponds are more expensive to construct due to the extra technologies required (paddle wheel) and the need to keep pond integrity and cell properties, a condition imposed by the flow rates. Probably, open ponds are the simplest facilities to construct, since no transparent material is required in their construction and they are relatively easy to maintain considering the large open access, which facilitates the cleaning of overgrowth and biofouling of unwanted microorganisms that builds up on surfaces. The conditions of an open culture system to the atmosphere are one of the main disadvantages that faces this system, because it is a constant exposition to unwanted species and the loss of water by evaporation, and the consequent increase of salinity. These kinds of ponds are suitable for the growth of extremophile microalgal species that tolerate and outcompete other species in conditions imposed by its particular requirements (e. g., high/low pH or salinity, or alkalinity). Examples of these conditions can be found in strains of Arthrospira platensis/maxima and Dunaliella salina/bardawil. Figure 62.6 shows the different culture systems of the massive production of microalgae worldwide.
Different culture systems for the industrial production of microalgae. (a) raceway facilities (NBT, Eliat Israel); (b) closed bioreactors ; (c) alveolar panels (Beer Sheva, Israel); (d) cascade systems (University of Liege, Belgica); (e,f) open ponds (Dunaliella salina plant at Hutt Lagoon Western Australia, Cognis)
7.3 Closed Bioreactors: Improving Feedstocks for Biofuels
There are different and unlimited designs of closed photobioreactors for the production of high yields of biomass. The most common shapes and designs are tubular reactors, plate reactors, or bubble column reactors. These different bioreactors have several advantages, such as saving water, energy and chemicals, and many other advantages that are increasingly making them the reactor of choice for biofuel production. Probably the most important advantages are the high control and quality of products that can be obtained [32].
It is to be mentioned that the most important among these aspects is that they support up to fivefold higher productivity with respect to reactor volume and consequently have a smaller footprint on a yield basis. The latter advantage is reinforced by the fact that they can collect as much solar energy as possible per square meter. The higher yields per square meter obtained in photobioreactors contributes to compensating for, or in some cases amortizing, the cost of photo-bioreactors [33]. Nevertheless, there are structures and operations of bioreactors that need to be optimized in order to design a performing reactor according to the needs of the algal cells at the lowest cost, ensuring the economic viability of the process [32]. According to the current energy cost and productivity, bioreactor costs should not exceed US$ per m [32]. Sunlight energy is another important parameter to control when dealing with photobioreactors, since most microalgae exhibit growth/light kinetics at which light saturation occurs. Sunlight may exert photoinhibition or even photobleaching in microalgae, and as a consequence, low figures in productivity and final biomass yields.
Another important factor in bioreactors is mixing, which prevents sedimentation of the cells and allows efficient homogenization of and . Recently, the use of more economical material for bioreactor construction, viz, polyethylene bags on annular reactors or plate reactors, has contributed to refine the challenges of productivity by footprint bioreactors. The algal productivity registered based on the theoretical maximum average accounts for a yield of . According to the present expectative for the development of second-generation microalgal biofuel systems, higher microalgal yields and photobioreactor optimization can lead to optimal and economically viable systems [33]. It is emphasized that the yield of terrestrial crops for the production of biodiesel cannot compete with that of algae, as exemplified from the case of soybean: of oil/ha/year, while microalgae, depending on their oil composition, can produce up to [33, 34].
8 Products of Industrial Interest from Microalgae
Microalgae are an important source of food and chemicals for different industries (Table 62.2).
The demand for shellfish feed is increasing for human consumption, because of the environmental concerns over open-ocean fishing and because of the high demand for aquaculture markets. Microalgal biomass is rich in proteins, fatty acids, oils, and carbohydrates. These biomass and respective primary and secondary metabolites are of paramount importance for biotechnology and are among the most promising sources for new products and applications. With the development of sophisticated culture and screening techniques, microalgal biotechnology can already meet the high demands of both the food and pharmaceutical industries [31].
In the last years, most of the relevant developments in microalgal biotechnology were performed in four microalgae, viz, the green algae (Chlorophycea) Chlorella vulgaris, Haematococcus pluvialis, and Dunaliella salina, and the cyanobacteria Arthrospira (Spirulina) maxima or platensis, which have been commercialized worldwide and applied as ingredients and supplements for humans and animal feed additives. The characteristics and attributes of each of these microalgal species, emphasizing their functional pigments and respective industrial applications, are described in the following.
Spirulina (Arthrospira) under the tribal name of tecuitlatl [35] was part of the diet of the Mexican Aztec population. This Cyanophyte has attracted special attention due to its importance as a nutritious supplement and its demonstrated (in vitro and in vivo) functional properties. The high protein content of this microalga, up to dry weight, and its amino acid composition is of great interest, not only because S. platensis possesses all of the essential amino acids, but also because these amino acids have a great bioavailability [36, 37]. The phycobiliproteins (allophycocyanin and c-phycocyanin) are one of the major compounds in Arthrospira [37]. Important medical and pharmacological attributes such as hepatoprotective, anti-inflammatory, and antioxidant properties have been described and are thought to be basically related to the presence of phycobilins. Besides, phycobiliproteins might have an important role in different photodynamic therapies of various cancer tumors and leukemia treatment [38]. Another equally important microalga, the green unicellular D. salina, is an extremophilic microalga well-known for being the most abundant natural source of β-carotene (up to of its dry weight) [39]. This microalgae is undoubtedly one of most studied [40, 41] because β-carotene has important applications in the food, pharmaceutical, and cosmetics industries. Studies on carotenogenic D. salina samples carried-out by Olmos-Soto et al. [42], successfully differentiated carotenogenic from non-carotenogenic species based on introns within 18S GlossaryTerm
rDNA
.Haematococcus pluvialis is especially important due to its ability to accumulate, under stress conditions, large quantities of astaxanthin, up to – on a dry weight basis [43]. The carotenogenic process in Haematococcus induces different changes in the cell physiology and morphology of this alga, resulting in large red palmelloid cells. Astaxanthin is present in lipid globules outside the chloroplast, although the signal for induction to the massive accumulation of astaxanthin has been reported as originating in cytosol [44]. The pigment arsenal in Haematococcus possesses powerful biological activities, including antioxidant capacity [45], ulcer prevention, immunomodulation, and cancer prevention [46]. Experimental studies carried out on Chlorella demonstrated its antitumor effect, hepatoprotective and antioxidant properties [46, 47], antibacterial effects [48], or even the immunostimulant activity of enzymatic protein hydrolysates from this microalgae. Chlorella contains many dietary antioxidants, which may be responsible for some of its functional activities.
8.1 Health Foods and High-Value Added Substances from Microalgae
During the past decades, microalgal biomass was predominately utilized in the health food market, with more than of the annual microalgal biomass. Currently, most products launched to serve the health food market are supplied as tablets or in powder or liquid formulations. However, algal extracts in various product forms are creating a new market sector for each algal species (Table 62.2). The market of functional foods is believed to be the most dynamic sector in the food industry and could constitute up to of the entire food market within the next few years. Food supplemented with microalgal biomass might have other positive influences, e. g., prebiotic effects or mineral fortification.
There is an increasing demand for products from microalgae, which after special biotechnological processes acquire a higher value in the market considering the uniqueness of microalgae as specialty products. Among these products, polyunsaturated fatty acids occupy a preponderant position, because according to [49], only plants and algae are able to synthesize omega-3 fatty acids (GlossaryTerm
ω-3
) (Fig. 62.7). Polyunsaturated fatty acids (GlossaryTermPUFA
s) are essential components for the growth of higher eukaryotes and provide significant health benefits, reducing cardiac diseases and having other beneficial effects for depression, rheumatoid arthritis, and asthma [29]. Currently, the main source of these fatty acids for human consumption is marine fish. Actually, the relevant advantages from microalgae over fish oils, such as the lack of unpleasant odor, the reduced risk of chemical contamination, and a better purification potential, place microalgae as an excellent and preferred source of GlossaryTermPUFA
s. Microalgal GlossaryTermPUFA
s are established on the market both for food and feed, e. g., health-promoting purified GlossaryTermPUFA
s are added to infant milk formulas in Europe, and hens are fed with special microalgae to produce OMEGA eggs. The production of docosahexaenoic acid (GlossaryTermDHA
) and eicosapentaenoic acid (GlossaryTermEPA
) products from microalgal biotechnology for human and other applications has already been released by biotechnological companies.Some microalgae have also developed protective mechanisms to prevent the accumulation of free radicals and reactive oxygen species, and thus counteract cell-damaging activities. These attributes confers the properties of antioxidants to some microalgae. For functional food/nutraceuticals, the radical-scavenging capacity of microalgal products is of growing interest, especially in the beverage market segment and in pharmaceutical applications for the therapy of oxidation-associated diseases like inflammation. Microalgae, including cyanobacteria, are overcharged with a multitude of photosynthetic and accessory pigments, which improve the efficiency of light energy utilization (phycobiliproteins) and protect them against solar radiation (carotenoids). The structure and function of these pigments have acquired anthropogenic importance due to their bioactivity, especially as health promoting compounds in human health and as therapies for functional disorders, neurodegenerative diseases, and cancer.
8.2 Microalgae in Aquaculture: A Successful Living History
Aquaculture is a fast growing industrial food sector. Aquaculture plays an important and critical function in the food system, because supply nearly people with at least of their animal protein intake. In many developing countries, aquaculture has accomplished its role and has been sufficiently profitable which has enhanced and harnessed strong growth in different world regions. Microalgae as live food is undeniably one of the most important components in aquacultural enterprises. In fact, no activity is conceived in aquacultural production systems if lacking in live microalgae. The main applications of microalgae for aquaculture are directly related with nutrition, being used fresh or as food additive of health and coloring feeds or for inducing other biological functions. Worldwide, two trends dominate the field of microalgal applications in aquaculture: (1) the production of microalgal species, as live feeds to meet the feeding requirements of invertebrate larvae, and (2) the selection and production of specific strains of microalgae into shellfish feed to increase yields at industrial scale. As the basis of the natural food chain, microalgae play a key role in aquaculture, especially mariculture, being the food source for larvae of many species of mollusks, crustaceans, and fish. In addition, microalgae serve as a food source for live zooplankton production (Artemia, rotifers, copepods), which in turn are reused as feed for rearing fish and mollusk larvae [50]. More than species of microalgae are used in aquaculture worldwide, depending on the special requirements of local seafood production.
Concerning the microalgal species used in aquaculture, a remarkable feature is the fact that presently, almost years after the initial efforts, hatcheries are still using essentially the same strains for their production. The attributes of microalgae for filter feeder invertebrates reflect their importance in production nurseries and hatcheries of aquaculture species of commercial importance. Microalgae are required for larva nutrition, directly or indirectly at least in a short period of their life cycle of most of cultured shellfish species. Microalgal strains to be used in aquaculture have to meet various criteria of efficiency and functionality. In first term, it has to be easily cultured and nontoxic and needs to be of the correct size and shape to be ingested. Moreover, microalgae for aquaculture must have high nutritional qualities and a digestible cell wall to make nutrients available [51]. Moreover, they should undergo fast growth rates, and be scalable to mass culture, and a high nutritional value, including absence of toxins. Among these properties, protein and highly unsaturated fatty acid (e.g., eicosapentaenoic acid (GlossaryTerm
EPA
), arachidonic acid (GlossaryTermAA
) and docosahexaenoic acid (GlossaryTermDHA
)) content are major factor determining the nutritional value of microalgae. Recently, the role of ratios of GlossaryTermDHA
, GlossaryTermEPA
and GlossaryTermAA
has be elucidated as more important than their absolute levels as well as microalgal vitamin content. The following microalgae genera are usually supplied in aquaculture facilities: Chaetoceros, Thalassiosira, Tetraselmis, Isochrysis, Nannochloropsis, Pavlova, and Skeletonema. However, the use and time period of uses of each can vary depending on the cultured shellfish species. For practical reasons, we have chosen the representative group of shellfish species, in which these microalgal strains are an unavoidable source of feed. Bivalve mollusks are probably the most dependent and representative group of aquaculture species, as in the case of oysters, clams, and abalones. The intensive rearing of these bivalves relies on live algae and they represent the largest biomass in the hatchery and demand the highest weight-specific rations. It is well known that the suitable algal species: C. calcitrans, T. pseudonana (3H), I. galbana, and T. suecica (for larvae in length) are preferred for the larvae of bivalves, either as single monoculture (unialgal diets) or in combinations with different other microalgal strains (flagellates and diatoms).A different scheme of intensive feeding in hatcheries is exhibited by penaeid shrimp. The penaeid shrimp species, which is cultured worldwide, usually refers to the white shrimp Penaeus vannamei, the blue shrimp P. stilirrostris, or P. monodon. These crustaceans are fed with algae added during the non-feeding nauplius stage in order to have algae available upon molting into the protozoa stage. The most often used algal species are Tetraselmis chui, Chaetoceros gracilis, and Skeletonema costatum. As feeding preferences change from primarily herbivorous to carnivorous during the mysis stages, the quantity of algae is reduced. Nevertheless, a background level of algae is maintained as this may stabilize water quality. In spite of all efforts to replace live algae by inert feeds, aquaculturists are still dependent on the production and use of microalgae and microinvertebrates (Zooplankton, Artemia and Brachionus sp.) as live food for commercially important fish, molluscs and crustaceans, during at least part of their life cycle. However, it is expected that technological advances in the integration among complementary approaches such as synbiotics, probiotics, bioengineered and functional feeds as well as microalgae of bioremediation activities shall lead to a more sustainable production and efficient use of microalgae. But in any situation for the purposes of industrial enterprises of microalgae, the main factor to consider is to reduce production costs while maintaining reliability in microalgae feed [51].
9 Future Needs: Making Sustainable the Unsustainable Lightness of Biofuels
The promising and clear potential of algal biofuels for contributing to environmental, social, and economic sustainability need to be transformed into a sustainable reality. Which biofuel will win: biodiesel, bioethanol, or biohydrogen? There is no doubt that an important volume of research, bioengineering, and technology is needed in order to answer these questions and to confer the sustainability tag. Microalgae are presently the most promissory renewable resource to achieve sustainable production of biofuels , considering the following aspects and uniquenesss:
-
a)
Low impacts on the environment and in the world’s food supply
-
b)
High photon bioconversion efficiency and biomass production from photosynthesis
-
c)
Supply energy, food and nutraceuticals for the human population
-
d)
Can be used in bioremediation processes and simultaneously coupling -neutral fuel production with sequestration
-
e)
Can be cultured and harvested all-year-round
-
f)
Can be grown on non-arable land, and in seawater and wastewater streams (avoiding the use of freshwater and recycling nutrients).
However, an assessment of the life cycle, energy and carbon balance, biofuels yield per unit area, and high efficiency production, are important issues and current limitations needed to overcome in order to make sustainable the biofuels production from microalgae.
An important strategy is the combination of fuel production with co-products, which could contribute to sustainable biofuels and reinforced by eco-friendly initiatives with minimum impact on the present reserves of air, water, and biodiversity. Moreover, it must be socially acceptable and economically viable. There is no doubt that integration of all the components of uses of microalgae: high-value compounds, aquaculture, bioremediation coupled to the production of biofuels, will play an important role in the near future to make sustainable the unsustainable lightness of biofuels from microalgae. Even with these potentialities and strategies, we believe that biofuel production from microalgae will achieve cost competitiveness in the next couple of decades, only if the aforementioned issues are considered, as well as aspects of engineering to the particular tasks demanded by bioenergy industries that are now under development. However, present innovations of new ways to make the process economically feasible shall provide many improvements and technologies contributing to the sustainability of the biofuels production from microalgae.
Abbreviations
- 3-PGA:
-
3-phosphoglyceric acid
- AA:
-
arachidonic acid
- ACCase:
-
acetyl-CoA carboxylase
- ATP:
-
adenosine triphosphate
- CH:
-
carbohydrate
- CoA:
-
coenzyme A
- DHA:
-
docosahexaenoic acid
- DNA:
-
deoxyribonucleic acid
- EPA:
-
eicosapentaenoic acid
- GHG:
-
greenhouse gas
- GPDH:
-
glycerol-3-phosphate dehydrogenase
- IBPCS:
-
integrated biomass production conversion system
- LCA:
-
life cycle
- Mbp:
-
million base pairs
- NADH:
-
nicotiamide adenine dinucleotide
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- PCR:
-
polymerase chain reaction
- PEP-C:
-
phosphoenolpyruvate carboxylase
- PUFA:
-
polyunsaturated fatty acid
- RuBP:
-
ribulose-1,5-bisphosphate
- TAG:
-
triacylglyceride
triacylglycerol
- mRNA:
-
messenger RNA
- rDNA:
-
ribosomal DNA
- ω-3:
-
Omega-3 fatty acid
References
M. Hannon, J. Gimpel, M. Tran, B. Rasala, S. Mayfield: Biofuels from algae: Challenges and potential, Biofuels 1(5), 763–784 (2010)
M. Gravislescu, Y. Christi: Biotechnology – a sustainable alternative for chemical industry, Biotechnol. Adv. 23, 471–499 (2005)
A. Singh, D. Pant, S.I. Olsen, P.S. Nigam: Key issues to consider in microalgae based biodiesel production, Energ. Educ. Sci. Technol. A Energ. Sci. Res. 29(1), 687–700 (2012)
J. Paniagua-Michel, B.C. Farfán, L.F. Buckle Ramirez: Culture of marine microalgae with natural biodigested resources, Aquaculture 64, 249–256 (1987)
J.E. Zamora Castro, J. Paniagua-Michel, C. Lezama-Cervantes: A novel approach for bioremediation of a coastal marine wastewater effluent based on artificial microbial mats, Mar. Biotechnol. 10, 181–189 (2008)
A. Singh, S.I. Ohlsen: A critical review of biochemical conversion, sustainability and life cycle assessment of algal biofuels, Appl. Energ. 88, 3548–3555 (2011)
S.H. Ho, A.N. Ye, J.S. Chang, K. Hara, T. Hasunuma, A. Kondo: Optimizing biodiesel production in marine Chlamydomonas sp. JSC4 through metabolic profiling and an innovative salinity-gradient strategy, Biotechnol. Biofuels 7, 1–16 (2014)
L. Gouveia, A.C. Oliveira: Microalgae as a raw material for biofuels production, J. Ind. Microbiol. Biotechnol. 36, 269–274 (2009)
C.J. Campbell: The Coming Oil Crisis (Multi-science Publ. Co., Brentwood 1997)
Y. Chisti: Biodiesel from microalgae beats bioethanol, Trends Biotechnol. 26, 126–131 (2008)
K.G. Zeiler, D.A. Heacox, S.T. Toon, K.L. Kadam, L.M. Brown: The use of microalgae for assimilation and utilization of carbon dioxide from fossil fuel-red power plant flue gas, Energ. Convers. Manag. 36, 707–712 (1995)
P. Lavens, P. Sorgeloos: Manual on the production and use of live food for aquaculture (FAO, Rome 1996)
V. Patil, K.Q. Tran, H.R. Gilselrod: Towards sustainable production of biofuels from microalgae, Int. J. Mol. Sci. 9, 1188–1195 (2008)
M.F. Demirbas: Biofuels from algae for sustainable development, Appl. Energ. 88, 3473–3480 (2011)
T.M. Mata, A.A. Martins, N.S. Caetano: Microalgae for biodiesel production and other applications: A review, Renew. Sustain. Energ. Rev. 14, 217–232 (2010)
M. Balat: Challenges and opportunities for large-scale production of biodiesel, Energ. Educ. Sci. Technol. A Energ. Sci. Res. 27, 427–434 (2011)
L. Lin, Z. Cunshan, S. Vittayapadung, S. Xiangqian, D. Mingdong: Opportunities and challenges for biodiesel fuel, Appl. Energ. 88, 1020–1031 (2011)
T.A. Milne, R.J. Evans, N. Nagle: Catalytic conversion of microalgae and vegetable oils to premium gasoline, with shape selective zeolites, Biomass 21, 219–232 (1990)
R.P. John, G.S. Anisha, K.M. Nampoothiri, A. Pandey: Micro and macroalgal biomass: A renewable source for bioethanol, Bioresour. Technol. 102, 186–193 (2011)
R. Ueda, S. Hirayama, K. Sugata, H. Nakayama: Process for the production of ethanol from microalgae, US 5578472 A (1996)
E.H. Harris: The Chlamydomonas sourcebook: A comprehensive guide to biology and laboratory use (Academic, San Diego 1989)
O. Kruse, J. Rupprecht, K.P. Bader, S.T. Hall, P.M. Schenk, G. Finzazzio, B. Hankamer: Improved photobiological H${}_{2}$ production in engineered green algal cells, J. Biol. Chem. 280, 34170–34177 (2005)
D.A. Graves, C.V. Tevault, E. Greenbaum: Control of photosynthetic reductant — the role of light and temperature on sustained hydrogen photoevolution by Chlamydomonas sp. in an anoxic, carbon dioxide-containing atmosphere, Photochem. Photobiol. 50, 571–576 (1989)
F. Mus, A. Dubini, M. Seibert, M.I. Posewitz, A.R. Grossman: Anaerobic adaptation in C. reinhardtii anoxic gene expression, hydrogenase induction and metabolic pathways, J. Biol. Chem. 282, 25475–25486 (2007)
J. Sheehan, T. Dunahay, J. Benemann, P.G. Roesleer: A Look Back at the U.S. Department of Energy's Aquatic Species Program: Biodiesel from Algae (National Renewable Energy Laboratory, Golden 1998)
Y. Yao, Y. Lu, K. Tao Peng, T. Huang, Y.F. Niu, W.H. Xie, W.D. Yang, J.S. Liu, H.Y. Li: Glycerol and neutral lipid production in the oleaginous marine diatom Phaeodactylum tricornutum promoted by overexpression of glycerol-3-phosphate dehydrogenase, Biotechnol. Biofuels 7, 110–119 (2014)
Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert, A. Darzins: Microalgaltriacylglycerols as feedstocks for biofuel production: Perspectives and advances, Plant J. 54, 621–639 (2008)
W.D.A. Larkum, L.I. Ross, O. Kruse, B. Hankamer: Selection, breeding and engineering of microalgae for bioenergy and biofuel production, Trends Biotechnol. 30, 4 (2012)
C. Adame-Vega, D.K. Lim, M. Timmins, F. Vernen, Y. Li, P.M. Schenk: Microalgalbiofactories: A promising approach towards sustainable omega-3 fatty acid production, Microbiol. Cell Fact. 11, 1–11 (2012)
C. Dayananda, R. Sarada, S. Bhattacharya, G.A. Ravishankar: Effect of media and culture conditions on growth and hydrocarbon production by Botryococcus braunii, Process Biochem. 40, 3125–3131 (2005)
O. Pulz: Photobioreactors: Production systems for phototrophic microorganisms, Appl. Microbiol. Biotechnol. 57, 287–293 (2001)
P.M. Schenk, S.R. Thomas-Hall, E. Stephens, U.C. Marx, J.H. Mussgnug, C. Posten, O. Krus, B. Hankamer: Second generation biofuels: High-Efficiency microalgae for biodiesel production, Bioenerg. Res. 1, 20–43 (2008)
Y. Chisti: Biodiesel from microalgae, Biotech. Adv. 25, 294–306 (2007)
G.I. Anemaet, M. Bekker, J.K. Hellingwerf: Algal photosynthesis as the primary driver for a sustainable development in energy, feed, and food production, Mar. Biotechnol. 12, 619–629 (2010)
J. Paniagua-Michel, E. Dujardin, C. Sironval: Le tecuitlatl, concentré de spirulines, source de proteines comestibles chez les Azteques, Bull. Sci. Acad. Roy. Belg. 6(3), 253–263 (1992)
A. Richmond: Spirulina. In: Micro-Algal Biotechnology, ed. by M.A. Borowitzka, L.J. Borowitzka (Cambridge Univ. Press, Cambridge 1988) p. 86
N.T. Eriksen: Production of phycocyanin – a pigment with applications in biology, biotechnology, foods and medicine, Appl. Microbiol. Biotechnol. 80, 1–14 (2008)
J. Subhashini, S.V.K. Mahipal, M.C. Reddy, M.M. Reddy, A. Rachamallu, P. Reddanna: Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562, Biochem. Pharmacol. 68, 453–462 (2004)
F.B. Metting: Biodiversity and application of microalgae, J. Ind. Microbiol. 17, 477–489 (1996)
A. Ben-Amotz, M. Avron: On the factors which determine massive b-carotene accumulation in the halotolerant alga Dunaliella bardawil, Plant Physiol. 72, 593–597 (1983)
J.D.J. Paniagua Michel, J. Olmos Soto, M.D.J. Acosta Ruiz: Pathways of carotenoid biosynthesis in bacteria and microalgae. In: Microbial Carotenoids from Bacteria and Microalgae: Methods and Protocols, ed. by J.-L. Barredo (Springer, Berlin, Heidelberg 2012) pp. 1–12
J. Olmos-Soto, J.D.J. Paniagua-Michel, R. Contreras, L. Ochoa: DNA fingerprinting intron-sizing method to accomplish a specific, rapid, and sensitive identification of carotenogenic Dunaliella species. In: Microbial Carotenoids from Bacteria and Microalgae: Methods and Protocols, ed. by J.L. Barredo (Springer, Berlin, Heidelberg 2012) pp. 269–281
M.A. Borowitzka, L.J. Borowitzka (Eds.): Micro-Algal Biotechnology (Cambridge Univ. Press, Cambridge 1988)
S. Boussiba: Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response, Physiol. Plant 108, 111–117 (2000)
Y. Lemoine, S. Benoît: Secondary ketocarotenoidastaxanthin biosynthesis in algae: A multifunctional response to stress, Photosynth. Res. 106, 155–177 (2010)
M.C. Ceron, M.C. García-Malea, J. Rivas, F.G. Acien, J.M. Fernandez, E. Del Río, M.G. Guerrero, E. Molina: Antioxidant activity of Haematococcus pluviales cells grown in continuous culture as a function of their carotenoid and fatty acid content, Appl. Microbiol. Biotechnol. 74, 1112–1119 (2007)
Y. Okai, K. Higashi-Okai: Possible immunomodulating activities of carotenoids in in vitro cell culture experiments, Int. J. Immunopharmacol. 18, 753–758 (1996)
I. Rodriguez-García, J.L. Guil-Guerrero: Evaluation of the antioxidant activity of the three microalgal species for use as dietary supplements and in the preservation of foods, Food Chem. 108, 1023–1026 (2008)
K. Vijayavel, C. Anbuselvam, M.P. Balasubramanian: Antioxidant effect of the marine Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats, Mol. Cell. Biochem. 303, 39–44 (2007)
O. Pulz, W. Gross: Valuable products from biotechnology of microalgae, Appl. Microbiol. Biotechnol. 65, 635–648 (2004)
S. Hemaiswarya, R. Raja, R. Kumar, V. Ganesan, C. Anbazhagan: Microalgae: A sustainable feed source for aquaculture, World J. Microbiol. Biotechnol. 27, 1737–17467 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Paniagua-Michel, J.d.J., Morales-Guerrero, E., Olmos Soto, J. (2015). Microalgal Biotechnology: Biofuels and Bioproducts. In: Kim, SK. (eds) Springer Handbook of Marine Biotechnology. Springer Handbooks. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-53971-8_62
Download citation
DOI: https://doi.org/10.1007/978-3-642-53971-8_62
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-53970-1
Online ISBN: 978-3-642-53971-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)