Abstract
Screening for prostate cancer by use of serum prostate specific antigen (PSA) remains controversial. In the recent Cochrane analysis, an attempt is made to clarify the issue by conducting a meta analysis of available randomized screening trials. Two large trials are considered to provide data of similar and sufficient quality to conduct a separate meta analysis. However, in the view of this author, this analysis fails because standard Cochrand quality criteria are not observed. Details are given and the outcome suggests that one of the trials, the European Randomized Study of Screening for Prostate Cancer (ERSPC) should be considered superior to the Prostate, Lung, Colon, Ovary screening trial (PLCO) conducted in the USA.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The most recent review of randomized screening trials of prostate cancer identifies two studies, the European Randomized study of Screening for Prostate Cancer (ERSPC) and the Prostate, Lung, Colon and Ovarian screening trial (PLCO) as ‘posing a low risk of bias’. The review acknowledges that both studies show contradictory results but fails to present an explanation for this contradiction. In the present document an attempt is made to point out the differences between the two large screening trials which may explain why ERSPC shows a significant advantage in prostate cancer mortality while the PLCO study does not.
This recent Cochrane review (Ilic et al. 2013) is based on the Cochrane criteria for evaluating randomized controlled trials which are part of the ‘Cochrane handbook for systematic reviews of interventions’ (Higgins and Green 2008) and a more recent summary of the tools of the Cochrane collaboration for assessing risk of bias in randomized trials (Higgins et al. 2011). While the methodology mainly addresses the procedures around meta-analyses, it also provides criteria for including randomized controlled trials (RCT) into meta-analyses. One basic pre-requirement is phrased in (Higgins et al. 2011) as “to obtain reliable conclusions, review authors must carefully consider the potential limitations of the included studies”. The Cochrane methodology differentiates between risk assessment tools addressing seven different areas of interest and seven risk of bias tools which are clearly formulated in (Higgins et al. 2011). The cited references do not address the question whether the Cochrane tools are equally applicable to treatment trials and to RCTs of screening which essentially study the value of diagnostic tools in the application to define segments of the population (secondary screening).
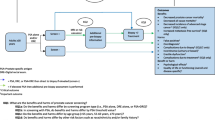
Figure 1 is derived from (Ilic et al. 2013) and shows the results of the application of six domains of the risk of bias tool to five pre-selected screening trials including ERSPC and PLCO. The authors conclusion that ERSPC and PLCO represent trials at a similar low level of bias is based on this evaluation using the six criteria indicated. Some of these criteria such as allocation concealment may not be applicable to randomized screening trials and other trials which do not allow the use of placebo. Also, while on the first page of (Ilic et al. 2013) the authors claim that the overall judgment on risk basis considers ‘the relative importance of domains’ such weighting is not found in the manuscript. Also, some of the most important differences between the two studies, such as the upfront use of PSA testing prior to randomization, the contamination by PSA use and the compliance with biopsy indications are included into ‘other bias’ without specification and weighting with respect to outcomes. In other words, in line with the Cochrane rules, it is not sufficient to state whether a bias is present or absent but also it is also necessary to quantify it and to quantify its possible impact with respect to the overall level of bias assigned. This procedure has not been followed in (Ilic et al. 2013). It can not be replaced by analyses of heterogeneity and sensitivity as carried out and reported in (Ilic et al. 2013). An attempt will therefore be made to compare the occurrence and possible effect of three important parameters between the ERSPC and PLCO study: use of testing prior to randomization, contamination and compliance with biopsy indication.
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study (Ilic et al. 2013, doi: 10.1002/14651858.CD004720.pub3). With permission of the Cochrane Collaboration and John Wiley and Sons
-
I.
Use of PSA testing prior to randomization
The PLCO study reports 53.1 and 54.8 % of PSA testing prior to randomization in the screening and control arm populations (Pinsky et al. 2012). Accurate data on the ERSPC study are not available. However, considering the period of randomization in most centers running from 1993 to 2000, the rate of PSA testing can be considered to be below the 20 % assumed in the power calculation. If the rate of screening had been similar in the ERSPC and PLCO studies, this should be visible in terms of the prostate cancer incidence figures and specifically the incidence of advanced disease, which is effectively classified as Gleason 8–10. Such cancers were found in 10.2 and 13 % in the screen and control arms of PLCO (Pinsky et al. 2012) and in 7.4 versus 12.5 % in the ERSPC study. Overall, the cancer detection rates in the PLCO study amounted to 26.4 versus 23.7 % for the age group 55–64 and to 58.9 and 58.6 % for the age group 65–74 between screening and control. The most recently reported detection rates for the core age group 55–69 years of the ERSPC study amounted to 9.6 and 6.0 % between the screen and control arms. The larger difference in overall detection and specifically in the detection of aggressive disease between the two arms of the ERSPC study is likely to be the result of a lower rate of screening prior to randomization.
-
II.
Contamination
PLCO reported an overall at least one time PSA use of 54.8 % (Pinsky et al. 2012). The estimated contamination rate for ERSPC is reported to be in the range of 30 % in the control arm (Roobol et al. 2009). This figure is the result of extrapolation from Dutch data to the rest of Europe. Large differences per country exist however, and have been documented by Ciatto et al. (2003). Still, even maximizing the contamination rate in ERSPC up to the year of 2005 to 30.7 % reveals a 22 % higher contamination rate in the PLCO study.
-
III.
Compliance with biopsy indication
Non-compliance with biopsy indication is another factor that may reduce the power of a screening trial by decreasing the incidence of cancers in the screen arm which may contribute to the mortality reduction by screening. The PLCO study reported 14–15 % positive tests in the screen arm and a biopsy rate of 40.2 % among these during the first round and 30.1 % during subsequent rounds of screening (Grubb et al. 2008). In the ERSPC trial 16.6 % of all men tested positive and of these at average of all centers 82.7 % were biopsied (Schröder et al. 2012). This very large difference in biopsy compliance is likely to contribute to the lower rate of cancer detection in the screen arm in PLCO and to the lack of a difference in the final outcome, prostate cancer mortality in comparison between the screen and control arms.
1 Conclusion
The author hopes to have shown that in applying the Cochrane criteria and specifically the group summarizing ‘other biases’ it is necessary to specify and quantify the criteria used. Omission of this procedure may result in misinterpretation of the available data and finally in wrong judgment on biases. Also, it is questionable whether the Cochrane criteria and tools for assessing the risk of bias in randomized trials are applicable without any change to randomized screening trials (Schröder et al. 2012). Quality requirements for screening trials, as the have been designed early during the ERSPC study, are clearly different from quality requirements for randomized treatment studies. A careful evaluation of the effect of the large differences seen with respect to the important diagnostic parameters upfront screening, contamination and compliance with biopsy indications on prostate cancer mortality is needed and is likely to explain why PLCO does not show an absolute and relative reduction of prostate cancer mortality by screening.
References
Ciatto S, Zappa M, Villers A, Paez A, Otto SJ, Auvinen A (2003) Contamination by opportunistic screening in the European randomized study of prostate cancer screening. BJU Int 92(2):97–100
Grubb RL 3rd, Pinsky PF, Greenlee RT, Izmirlian G et al (2008) Prostate cancer screening in the prostate, lung, colorectal and ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU Int 102(11):1524–1530
Higgins JPT, Green S (eds) (2008) Cochrane handbook for systematic reviews of interventions. Wiley, New York
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA (2011) Cochrane bias methods group; cochrane statistical methods group. BMJ 18(343):d5928
Ilic D, Neuberger MM, Djulbegovic M, Dahm P (2013) Screening for prostate cancer. Cochrane Database Syst Rev 1:CD004720
Pinsky PF, Black A, Parnes HL, Grubb R, Crawford DE, Miller A, Reding D, Andriole G (2012) Prostate cancer specific survival in the prostate, lung, colorectal, and ovarian (PLCO) cancer screening trial. Cancer Epidemiol 36(6):e401–e406
Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M, Stenman UH, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis L, Recker F, Berenguer A, Ruutu M, Kujala P, Bangma CH, Aus G, Tammela TL, Villers A, Rebillard X, Moss SM, de Koning HJ, Hugosson J, Auvinen A (2009) Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European randomized study of screening for prostate cancer (ERSPC). Eur Urol 56(4):584–591
Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ (2012) Prostate-cancer mortality at 11 years of follow-up. N Engl J Med 366:981–990
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Schröder, F.H. (2014). ERSPC, PLCO Studies and Critique of Cochrane Review 2013. In: Cuzick, J., Thorat, M. (eds) Prostate Cancer Prevention. Recent Results in Cancer Research, vol 202. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-45195-9_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-45195-9_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-45194-2
Online ISBN: 978-3-642-45195-9
eBook Packages: MedicineMedicine (R0)