Abstract
Plant phospholipase As (PLAs) are classified into two major types, PLA1 and PLA2, according to the hydrolysis sites of their membrane lipids. The lipid products released by PLAs have been suggested to act as bioactive molecules that mediate cellular signaling pathways functioning in plant growth and development, as well as responses to abiotic and biotic stimuli. The past few years have witnessed a wealth of new information regarding the function of these phospholipases in various biological processes. In this chapter, we discuss recent insights into lipid-based signaling mediated by PLAs and their lipid products, with particular emphasis on their emerging role as lipid mediators.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phospholipid-derived products generated by phospholipase A (PLA), such as free fatty acids (FFAs) and lysophospholipids, play critical roles in plants, as these products are the precursors of second messengers in signal transduction pathways. In addition, these signaling molecules function in important physiological processes such as cell elongation, gravitropism, anther dehiscence, biosynthesis of jasmonic acid (JA), and defense signaling (Chen et al. 2011; Ryu 2004; Wang et al. 2012). To understand the highly regulated production of these lipid mediators in response to diverse extracellular stimuli, it is important to study the functions and regulatory mechanisms of the diverse PLA enzymes. Recent developments in genetic and biochemical analysis have facilitated our understanding of the cellular functions of plant PLAs. The PLA superfamily has three subtypes, i.e., phospholipase A1 (PLA1), phospholipase A2 (PLA2), and patatin-like PLA (pPLA). PLA1 and PLA2 catalyze the hydrolysis of membrane glycerophospholipids at their sn-1 or sn-2 positions, respectively, while pPLA shows activity at both positions. In this chapter, we will describe the current classifications of PLA1s and PLA2s that have been identified in plants, especially in Arabidopsis, and recent advances in our understanding of how these PLAs are involved in various cellular signaling pathways.
2 Phospholipase A2
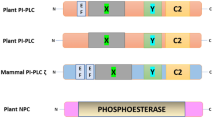
Based on the functional, structural, and catalytic properties of lipolytic enzymes, the PLA2 superfamily can generally be divided into five principal families in animals, categorized as secretory PLA2s (sPLA2), cytosolic PLA2s (cPLA2), Ca2+-independent PLA2s (iPLA2s), platelet-activating factor acetyl hydrolases (PAF-AHs), and lysosomal PLA2s (Schaloske and Dennis 2006). Only low molecular weight secretory PLA2s have been reported in plants, although patatin-like PLAs have been identified, which are similar to the iPLA2s or cPLA2 but exhibit both PLA1- and PLA2-like activity (Lee et al. 2005; Ryu 2004).
2.1 sPLA2 Grouping, Expression, and Localization
Four and three secretory PLA2 paralogs were identified in the Arabidopsis and rice genomes, respectively. Based on their primary structures, plant sPLA2s are equivalent to animal group XI sPLA2s, which are further divided into groups XIA and XIB. The former group includes AtsPLA2-α, OsPLA2-α, and OsPLA2-β, while the latter group includes AtsPLA2-β, AtsPLA2-γ, AtsPLA2-δ, and OsPLA2-γ (Lee et al. 2005; Schaloske and Dennis 2006; Singh et al. 2012).
AtsPLA2s exhibit different spatial and temporal expression patterns. AtsPLA 2 -α and AtsPLA2-β are expressed in all sporophytic tissues. Unlike AtsPLA 2 -α, AtsPLA 2 -β is strongly expressed in actively growing young tissues and pollen. AtsPLA 2 -γ and AtsPLA 2 -δ are exclusively expressed in pollen. While AtsPLA 2 -β is expressed during all stages of pollen development, AtsPLA 2 -γ is expressed at low levels during the early stage of pollen formation and is strongly expressed at the mature stage, and AtsPLA 2 -δ is expressed at the mature pollen stage (Bahn et al. 2003; Kim et al. 2011b; Lee et al. 2003).
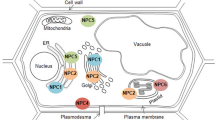
Studies of the subcellular localizations of plant sPLA2 have been performed using fused fluorescence proteins. These studies provide important insights into the functions of these proteins in plants. Early experiments involving transient expression analysis of green fluorescence protein (GFP) in onion epidermal cells showed that AtsPLA2-β and AtsPLA2-γ are secreted into the cell wall/extracellular space (Bahn et al. 2003; Lee et al. 2003), but this localization pattern is regarded as a result of the masking of the ER retention signal (KTEL) by that of the C terminal GFP fusion protein of AtsPLA2-β. More recently, Seo et al. (2008) reexamined the subcellular localization of AtsPLA2-β with N terminal GFP fusion protein of AtsPLA2-β and observed that it indeed localizes to the ER in Vicia faba guard cells, and the result was supported in AtsPLA2-β:YFP transgenic plants (Lee et al. 2010). Transient expression of AtsPLA 2 -γ:YFP and AtsPLA 2 -δ:YFP in tobacco leaf epidermal cells demonstrated that AtsPLA2-γ localizes to the ER and Golgi, while AtsPLA2-δ localizes to the ER and is found in the pollen of transgenic plants (Kim et al. 2011b). Subcellular localization of AtsPLA2-α revealed a rather complicated localization pattern unlike that of its paralogs. An analysis of AtsPLA 2 -α:DsRed2 transgenic Arabidopsis plants suggests that AtsPLA2-α localizes to the Golgi in root tissues (Lee et al. 2010). In another study, AtsPLA2-α exhibited different localizations in a time-dependent manner when this gene was introduced into Arabidopsis seedlings and tobacco leaves via Agrobacterium-mediated transient transformation (Froidure et al. 2010). The AtsPLA2-α:YFP signal was detected in cytoplasmic vesicles around the nucleus 36 h after inoculation and was detected at the extracellular spaces outside of the cells at 48 h after inoculation. AtsPLA2-α was partially localized to the cell nucleus when it was co-expressed with AtMYB30.
A more recent study shows that subcellular localization of AtsPLA2-α is dependent on the developmental stage of the leaf tissue. Fluorescence signals are present primarily at the Golgi apparatus in premature young leaves in transgenic AtsPLA2-α:RFP plants, while these signals are detected primarily in the apoplasts in mature leaves (Jung et al. 2012). Also, translocation of AtsPLA2-α to the apoplast is stimulated by bacterial infection in premature young leaves. In contrast, the signal of GFP:OssPLA2-α merges with that of an endoplasmic marker in onion epidermal cells, which indicates that the transiently expressed OssPLA2-α localizes to the ER (Singh et al. 2012). RFP fusion protein, but not GFP and YFP, is relatively stable in the apoplasts. Thus, it is desirable to use RFP fusion protein rather than GFP or YFP fusion proteins for apoplastic localization studies.
2.2 sPLA2 Functions
Plant PLA2s have been implicated in important physiological processes such as development, senescence, biotic and abiotic stress responses, and the induction of secondary metabolite accumulation (Lee et al. 2005; Mansfeld 2009; Ryu 2004; Wang 2001). Lysophosphatidylcholine (LPC) and linolenic acid, which are the products of PLA2, induce a decline in pH and accelerate the elongation of corn coleoptiles (Yi et al. 1996). Also, the PLA2 inhibitors aristolochic acid and manoalide inhibit the auxin-induced pH decrease and coleoptile elongation. These results suggest that PLA2 is activated by auxin, and its products induce acidification of the apoplast by activating the H+ pump through the signal transduction pathway of protein kinase, which in turn promotes corn coleoptile elongation (Yi et al. 1996). This hypothesis was supported by Lee et al. (2003) using transgenic plants. These authors reported that AtsPLA 2 -β transcripts are induced by auxin treatment. In addition, RNA interference-mediated silencing (RNAi) of AtsPLA 2 -β expression retards cell elongation, while overexpression of AtsPLA 2 -β promotes cell elongation. Moreover, AtsPLA 2 -β overexpressors exhibit faster stomatal opening than wild type, and AtsPLA2-β-RNAi plants exhibit delayed light-induced stomatal opening, which can be rescued by exogenous application of LPC or lysophosphatidylethanolamine (LPE). Also, exogenous applications of LPC or LPE enhance stomatal opening in wild-type plants (Seo et al. 2008). In contrast, AtsPLA2-β, AtsPLA2-γ, and AtsPLA2-δ are involved in pollen development and pollen germination (Kim et al. 2011b). AtsPLA 2 -β may play a more vital role in pollen development, while AtsPLA 2 -γ and -δ may function in pollen germination and pollen tube growth. Also, pollen germination is inhibited by the application of sPLA2 inhibitors and is recovered by exogenous application of LPE but not of LPC or lysophosphatidic acid (LPA). These results indicate that LPE in particular is a key signal molecule in pollen germination and tube growth (Kim et al. 2011b).
Both AtsPLA2-α-RNAi Arabidopsis plants and Arabidopsis seedlings treated with the sPLA2 inhibitor ONO-RS-082 exhibit significantly disrupted plasma membrane (PM) localization of PINs in the root tissues, causing internal PIN compartments to form (Lee et al. 2010). Application of exogenous LPE restores the PM localization of PINs in an AtsPLA 2 -α mutant and in ONO-RS-082-treated seedling. These results indicate that AtsPLA2-α modulates PIN-FORMED protein trafficking to the PM in Arabidopsis roots, thereby revealing that sPLA2 also plays an important role in intracellular membrane trafficking in plants (Lee et al. 2010). AtsPLA2-α was also suggested to be as a negative regulator of the defense response through its interaction with AtMYB30, a transcription factor that functions in the hypersensitive response in short-day conditions (Froidure et al. 2010). Intriguingly, the regulation does not depend on the enzymatic activity of AtsPLA2-α but on the just physical binding of AtsPLA2-α to A+MYB30.
Aristolochic acid reduces root elongation and causes radial swelling of the root tip caused by microtubule disorganization (Gardiner et al. 2008). This indicates that PLA2 is involved in microtubule organization and anisotropic growth. Another study employing aristolochic acid suggests that PLA2 plays a critical role in programmed cell death induced by misexpression of fatty acid elongation, likely involving the exchange of very long chain fatty acids (VLCFAs) between phospholipids and the acyl-CoA pool (Reina-Pinto et al. 2009). PLA2 is involved in salt stress-induced LPA production in the unicellular green alga Chlamydomonas (Meijer et al. 2001). LPA accumulates in Chlamydomonas under conditions of salt and nonionic hyperosmotic stress. The fact that LPA is generated by PLA2 was recently confirmed using differential 32P-radiolabeling experiments (Arisz and Munnik 2011).
3 Phospholipase A1
Plant PLA1s are classified based on the presence of N terminal stretches, sequence similarities in the catalytic region, and substrate specificity. These PLA1s include group I, II, and III PLA1, phosphatidic acid-specific PLA1 (PA-PLA1), and lecithin:cholesterol acyltransferase-like PLA1 (LCAT-PLA1) in Arabidopsis and group I and II PLA1 and PA-PLA1 in rice (Chen et al. 2011; Singh et al. 2012).
3.1 Class I PLA1s
Class I PLA1s include seven PLA1 genes in Arabidopsis and eight in rice. Class I PLA1s are defined by the presence of a putative N terminal chloroplast-targeting signal (Chen et al. 2011; Seo et al. 2009). All class I PLA1s have a GXSXG motif in the lipase class 3 domain and catalytic triad (Ser, Asp and His residues). All GFP-class I PLA1 fusion proteins localize to the chloroplast (Ellinger et al. 2010; Grienenberger et al. 2010; Hyun et al. 2008; Ishiguro et al. 2001; Seo et al. 2009). However, a second opinion about the subcellular localization of AtPLA1-Iα1 has recently emerged (Ellinger et al. 2010). In this study, AtPLA1-Iα1 was co-localized with cytoplasmic lipid bodies instead of localizing to chloroplasts.
Defective in Anther Dehiscence1 (DAD1, AtPLA 1 -Iβ1) was the first reported PLA1 enzyme in Arabidopsis. This enzyme preferentially hydrolyzes phosphatidylcholine (PC) at the sn-1 position and regulates anther dehiscence in flowers by releasing linolenic acid for the initial step of JA biosynthesis (Ishiguro et al. 2001). DONGLE (DGL, AtPLA 1 -Iα1) and DAD1 are necessary and sufficient for JA production (Hyun et al. 2008). DGL plays a specific role in maintaining basal JA content under normal conditions. During wounding, DGL was shown to be required for the rapid JA burst in the early phase, and DAD1 was shown to play a role in the late phase of JA production. However, the initial step in the biosynthesis of wound- and pathogen-induced JA production remains controversial. Ellinger et al. (2010) suggest that AtPLA 1 -Iγ1 is a novel target gene for the manipulation of jasmonate biosynthesis and that, in addition to DAD1 and AtPLA 1 -Iγ1, as yet unidentified enzymes with sn-1 and sn-2 hydrolase activity are involved in stress-induced JA formation, indicating that there is functional redundancy within the lipase family.
3.2 Class II PLA1s
Class II PLA1s comprise four PLA1 genes in Arabidopsis and three in rice (Chen et al. 2011; Singh et al. 2012). Based on their sequence homology, PLA1s from other species are also included in class II, including Dclipase from Dianthus caryophyllus (carnation) (Hong et al. 2000), LeLID1 from Lycopersicon esculentum (tomato) (Matsui et al. 2004) and CaPLA 1 from Capsicum annuum (hot pepper) (Seo et al. 2007). Class II PLA1s lack N terminal signal peptides and are predicted to localize to the cytosol. Cytosolic localization of AtPLA1-IIδ and DAD1-like Seedling Establishment-related Lipase (AtDSEL, AtPLA1-IIγ) were confirmed through GFP fusion protein studies (Kim et al. 2011a; Lo et al. 2004). AtPLA 1 -IIδ expression is induced by treatment with sublethal levels of UV-B. Plants with suppressed AtPLA 1 -IIδ expression via antisense technology exhibit increased tolerance to sublethal levels of UV-B stress and are unable to upregulate the expression of pathogenesis-related protein 1 (PR-1) in response to UV-B treatment. These results indicate that AtPLA1-IIδ is capable of deesterifying membrane phospholipids and is induced in response to UV-B irradiation (Lo et al. 2004). Recombinant AtDSEL expressed in Escherichia coli (E. coli) shows a preference for 1,3-diacylglycerol and 1-monoacylglycerol over PC, which suggests that AtDSEL is a sn-1-specific lipase.
AtDSEL overexpressors are defective in post-germinative seedling growth on medium lacking an exogenous carbon source; this phenotype is rescued by the addition of sucrose to the growth medium. By contrast, atdsel-1 and atdsel-2 exhibit a mildly fast-growing phenotype in the absence of an exogenous carbon source. AtDSEL-overexpressors retained numerous peroxisomes and oil bodies in their 5-day-old cotyledons, while these organelles are exhausted in wild-type or mutant cotyledons. These results suggest that AtDSEL is involved in the negative regulation of seedling establishment by inhibiting the breakdown of storage oils (Kim et al. 2011a). Dclipase transcript levels increase just as carnation flowers begin to senesce, and the expression of this gene is also induced by ethylene treatment. Southern blot analysis confirmed that these flowers contain a single copy of Dclipase. Dclipase is predicted to be involved in mediating the onset of senescence (Hong et al. 2000). Recombinant LeLID1 protein exhibits high activity against triacylglycerols (TAGs) with long acyl chains but little activity against PC or monogalactosyldiacylglycerol. Transcript levels of LeLID1 increase rapidly in seeds during germination, reaching a maximum level just before cotyledon opening, followed by a rapid decrease. Low levels of LeLID1 expression are detected in flowers and fruits, while none can be detected in roots. LeLID1 is thought to function as a lipase during lipid mobilization to liberate fatty acids from TAG’s stored in oil bodies (Matsui et al. 2004).
3.3 Class III PLA1
There is only one class III PLA1 in the Arabidopsis genome and none in the rice genome (Chen et al. 2011; Singh et al. 2012). Recombinant DAD1-like acylhydrolase (AtDLAH, AtPLA1-III) displays a stronger preference for 1-lysophosphatidylcholine, 1-monoacylglycerol, and phosphatidic acid than for PC, which indicates that AtDLAH is an sn-1-specific acylhydrolase. AtDLAH exclusively localizes to the mitochondria. Seeds of Arabidopsis AtDLAH overexpressors are more tolerant to accelerated-aging treatment than wild type, and thus these seeds have higher germination rate than wild-type seeds. By contrast, atdlah knockout mutant seeds are susceptible to accelerated-aging conditions. These results suggest that AtDLAH plays an important role in Arabidopsis seed viability (Seo et al. 2011).
4 PA-PLA1
SGR2 in Arabidopsis is classified as a PA-PLA1 based on the similarity of its domain structures to that of a mammalian PA-PLA1. One gene of PA-PLA1 was found to be present in the Arabidopsis and rice genome. The fusion protein of AtPA-PLA1 and GFP localizes to the membranes of vacuoles and small organelles. However, the localization of OsPA-PLA1 was predicted to be nuclear using in silico tools. AtPA-PLA1 mutants exhibit abnormal gravitropism in inflorescence stems and hypocotyls and irregularly shaped seeds. Mutant analysis suggests that AtPA-PLA1 is involved in a vacuolar membrane system that affects the early step of shoot gravitropism. Microarray data and quantitative RT-PCR in rice show that OsPA-PLA 1 transcripts are upregulated in response to salt and drought stress (Kato et al. 2002; Morita et al. 2002; Singh et al. 2012). In animals, LPA is a phospholipid mediator with multiple biological roles, functions via interactions with G protein-coupled seven-transmembrane receptors (GPCRs), and is implicated in various human diseases. While two pathways for LPA production have been identified in animal cells, little is known about the function of LPA at the molecular level. It was demonstrated that LPA produced by hair follicle-specific PA-PLA1 is an important signaling molecule for hair follicle development, which functions by modulating epidermal growth factor receptor signaling (Aoki et al. 2008; Chen et al. 2011; Inoue et al. 2011). However, PLA1 activity was not detected with recombinant AtPA-PLA1 proteins expressed in E. coli. Moreover, GPCRs are not found in plants. Elucidating the mechanisms of PA-PLA1 function in plants will be challenging.
5 LCAT-PLA1
During a search for plant genes encoding enzymes involved in sterol esterification by free acids, At3g03310 was identified as lecithin:cholesterol acyltransferase (LCAT) (Noiriel et al. 2004). This gene has sequence homology with the recently discovered gene encoding phosphatidylcholine:diacylglycerol acyltransferase from Saccharomyces cerevisiae (ScPDAT). PLA1 activity was first revealed by LCAT or PDAT assays, and the gene encoding PLA1 was designated AtLCAT-PLA 1 (Chen et al. 2011; Noiriel et al. 2004). Yeast expressing AtLCAT-PLA 1 accumulates TAG (Noiriel et al. 2004). Thus, AtLCAT-PLA1 may play a role in acyl-editing during TAG formation (Bates et al. 2009; Lu et al. 2009) but not in signaling.
6 Conclusions
Recent discoveries have significantly advanced our understanding of the biochemical and genetic requirements of distinct phospholipid signaling in plants. However, several unanswered questions still remain. For example, which isoforms of the PLA families are activated in response to specific external stimuli? What are the downstream targets of the lipid signals that are generated by PLA? These targets may be lysophospholipid-specific receptors, protein kinases, mitogen-activated protein kinase, or other signaling enzymes. And what are the upstream regulators of PLA activation? These regulators may take the form of receptors, activators, inhibitors, or hormones. The next coming years will likely produce significant advances towards the elucidation of PLA-mediated membrane phospholipid signaling in plants at both the biochemical and genetic levels. Moreover, the construction of knockout mutants and activation tagging lines, as well as the analysis of rapid protein activation without involving gene expression, will help clarify the phospholipid-derived signaling cascades.
References
Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781:513–518
Arisz SA, Munnik T (2011) The salt stress-induced LPA response in Chlamydomonas is produced via PLA2 hydrolysis of DGK-generated phosphatidic acid. J Lipid Res 52:2012–2020
Bahn SC, Lee HY, Kim HJ, Ryu SB, Shin JS (2003) Characterization of Arabidopsis secretory phospholipase A2-γ cDNA and its enzymatic properties. FEBS Lett 553:113–118
Bates PD, Durrett TP, Ohlrogge JB, Pollard M (2009) Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol 150:55–72
Chen G, Snyder CL, Greer MS, Weselake RJ (2011) Biology and biochemistry of plant phospholipases. Crit Rev Plant Sci 30:239–258
Ellinger D, Stingl N, Kubigsteltig II, Bals T, Juenger M, Pollmann S, Berger S, Schuenemann D, Mueller MJ (2010) DONGLE and DEFECTIVE IN ANTHER DEHISCENCE1 lipases are not essential for wound-and pathogen-induced jasmonate biosynthesis: redundant lipases contribute to jasmonate formation. Plant Physiol 153:114–127
Froidure S, Canonne J, Daniel X, Jauneau A, Brière C, Roby D, Rivas S (2010) AtsPLA2-α nuclear relocalization by the Arabidopsis transcription factor AtMYB30 leads to repression of the plant defense response. Proc Natl Acad Sci U S A 107:15281–15286
Gardiner J, Andreeva Z, Barton D, Ritchie A, Overall R, Marc J (2008) The phospholipase A2 inhibitor, aristolochic acid, disrupts cortical microtubule arrays and root growth in Arabidopsis. Plant Biol 10:725–731
Grienenberger E, Geoffroy P, Mutterer J, Legrand M, Heitz T (2010) The interplay of lipid acyl hydrolases in inducible plant defense. Plant Signal Behav 5:1181–1186
Hong Y, Wang T-W, Hudak KA, Schade F, Froese CD, Thompson JE (2000) An ethylene-induced cDNA encoding a lipase expressed at the onset of senescence. Proc Natl Acad Sci U S A 97:8717–8722
Hyun Y, Choi S, Hwang H-J, Yu J, Nam S-J, Ko J, Park J-Y, Seo YS, Kim EY, Ryu SB (2008) Cooperation and functional diversification of two closely related galactolipase genes for jasmonate biosynthesis. Dev Cell 14:183–192
Inoue A, Arima N, Ishiguro J, Prestwich GD, Arai H, Aoki J (2011) LPA-producing enzyme PA-PLA1a regulates hair follicle development by modulating EGFR signalling. EMBO J 30:4248–4260
Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001) The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13:2191–2209
Jung J, Kumar K, Lee HY, Park Y-I, Cho H-T, Ryu SB (2012) Translocation of phospholipase A2α to apoplasts is modulated by developmental stages and bacterial infection in Arabidopsis. Front Plant Sci 3:126
Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14:33–46
Kim EY, Seo YS, Kim WT (2011a) AtDSEL, an Arabidopsis cytosolic DAD1-like acylhydrolase, is involved in negative regulation of storage oil mobilization during seedling establishment. J Plant Physiol 168:1705–1709
Kim HJ, Ok SH, Bahn SC, Jang J, Oh SA, Park SK, Twell D, Ryu SB, Shin JS (2011b) Endoplasmic reticulum–and golgi-localized phospholipase A2 plays critical roles in Arabidopsis pollen development and germination. Plant Cell 23:94–110
Lee HY, Bahn SC, Kang YM, Lee KH, Kim HJ, Noh EK, Palta JP, Shin JS, Ryu SB (2003) Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. Plant Cell 15:1990–2002
Lee HY, Bahn SC, Shin JS, Hwang I, Back K, Doelling JH, Ryu SB (2005) Multiple forms of secretory phospholipase A2 in plants. Prog Lipid Res 44:52–67
Lee O, Kim S, Kim H, Hong J, Ryu S, Lee S, Ganguly A, Cho H (2010) Phospholipase A2 is required for PIN-FORMED protein trafficking to the plasma membrane in the Arabidopsis root. Plant Cell 22:1812–1825
Lo M, Taylor C, Wang L, Nowack L, Wang T-W, Thompson J (2004) Characterization of an ultraviolet B-induced lipase in Arabidopsis. Plant Physiol 135:947–958
Lu C, Xin Z, Ren Z, Miquel M (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci U S A 106:18837–18842
Mansfeld J (2009) Plant phospholipases A2: perspectives on biotechnological applications. Biotechnol Lett 31:1373–1380
Matsui K, Fukutomi S, Ishii M, Kajiwara T (2004) A tomato lipase homologous to DAD1(LeLID1) is induced in post-germinative growing stage and encodes a triacylglycerol lipase. FEBS Lett 569:195–200
Meijer HJ, Arisz SA, Van Himbergen JA, Musgrave A, Munnik T (2001) Hyperosmotic stress rapidly generates lyso‐phosphatidic acid in Chlamydomonas. Plant J 25:541–548
Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M (2002) Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell 14:47–56
Noiriel A, Benveniste P, Banas A, Stymne S, Bouvier-Nave P (2004) Expression in yeast of a novel phospholipase A1 cDNA from Arabidopsis thaliana. Eur J Biochem 271:3752–3764
Reina-Pinto JJ, Voisin D, Kurdyukov S, Faust A, Haslam RP, Michaelson LV, Efremova N, Franke B, Schreiber L, Napier JA (2009) Misexpression of FATTY ACID ELONGATION1 in the Arabidopsis epidermis induces cell death and suggests a critical role for phospholipase A2 in this process. Plant Cell 21:1252–1272
Ryu SB (2004) Phospholipid-derived signaling mediated by phospholipase A in plants. Trends Plant Sci 9:229–235
Schaloske R, Dennis E (2006) The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761:1246–1259
Seo YS, Kim EY, Mang HG, Kim WT (2007) Heterologous expression, and biochemical and cellular characterization of CaPLA1 encoding a hot pepper phospholipase A1 homolog. Plant J 53:895–908
Seo J, Lee HY, Choi H, Choi Y, Lee Y, Kim YW, Ryu SB, Lee Y (2008) Phospholipase A2-b mediates light-induced stomatal opening in Arabidopsis. J Exp Bot 59:3587–3594
Seo YS, Kim EY, Kim JH, Kim WT (2009) Enzymatic characterization of class I DAD1-like acylhydrolase members targeted to chloroplast in Arabidopsis. FEBS Lett 583:2301–2307
Seo YS, Kim EY, Kim WT (2011) The Arabidopsis sn-1-specific mitochondrial acylhydrolase AtDLAH is positively correlated with seed viability. J Exp Bot 62:5683–5698
Singh A, Baranwal V, Shankar A, Kanwar P, Ranjan R, Yadav S, Pandey A, Kapoor S, Pandey GK (2012) Rice phospholipase A superfamily: organization, phylogenetic and expression analysis during abiotic stresses and development. PLoS One 7:e30947
Wang X (2001) Plant phospholipases. Annu Rev Plant Physiol Plant Mol Biol 52:211–231
Wang G, Ryu S, Wang X (2012) Plant phospholipases: an overview. Methods Mol Biol 861:123–137
Yi HJ, Park D, Lee Y (1996) In vivo evidence for the involvement of phospholipase A and protein kinase in the signal transduction pathway for auxin-induced corn coleoptile elongation. Physiol Plant 96:359–368
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kim, H.J., Ryu, S.B. (2014). sPLA2 and PLA1: Secretory Phospholipase A2 and Phospholipase A1 in Plants. In: Wang, X. (eds) Phospholipases in Plant Signaling. Signaling and Communication in Plants, vol 20. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-42011-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-42011-5_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-42010-8
Online ISBN: 978-3-642-42011-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)