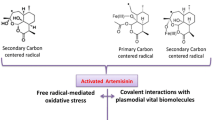
Abstract
Malaria is probably as old as mankind and continues to affect millions of people throughout the world. Today, some 500 million people in Africa, India, Southeast Asia, and South America are exposed to endemic malaria, and it is estimated to cause one and half million deaths annually, one million of which are children. As a consequence, effective therapeutic agents against malaria are continuously being sought, especially against those strains of Plasmodium falciparum, which have become resistant to nearly all antimalarial drugs, including chloroquine and quinine. In the absence of reports of artemisinin resistance in malaria parasite, WHO now recommends the use of artemisinin-based combination therapies (ACTs) with formulations containing an artemisinin derivative. Artemisinin, a sesquiterpene endoperoxide lactone, is isolated from the shoots of Artemisia annua L. plants. Apart from a novel and potent antimalarial drug, artemisinin and its derivatives are also used in therapies against hepatitis, leishmaniasis, and schistosomiasis. Artemisinin also possess lethal activities against cancerous cells, fungi, and bacteria. It has also shown to be immune-suppressant in mammals and a potent herbicide. Despite of its immense commercial value, the production of artemisinin is not cost-effective because of its low concentration (0.01–1.1 %) in the plant. Moreover, its de novo synthesis is complex, uneconomical and gives low yields. Further, classical breeding and selection techniques have failed to develop high-yielding strains of A. annua L. plants. Efforts are therefore, needed to elucidate the complex pathway of artemisinin biosynthesis and its biochemical and molecular regulation. Non-conventional approaches have to be developed to evolve novel strains of the plant to optimize and scale up the production of artemisinin in bulk and make it available to ACT manufacturers at a price much lower than their current cost in turn making an important contribution toward attaining the goals of global malaria eradication programs. The details of past and current status of both conventional and non-conventional approaches for enhancing artemisinin content in A. annua L. plants and its yield have been discussed in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Being the world’s most severe parasitic infection, malaria caused more than a million deaths and 500 million cased annually. Despite tremendous efforts for the control of malaria, the global morbidity and mortality have not been significantly changed in the 50 years (Reley 1995). The key problem is the failure to find effective medicines against malaria. Obtained from a Chinese medicinal plant Artemisia annua L., artemisinin, a sesquiterpene lactone containing an endoperoxide bridge, has become increasingly popular as an effective and safe alternative therapy against malaria (Luo and Shen 1987). Artemisinin and its derivative are effective against multidrug-resistant Plasmodium falciparum strain mainly in Southeast Asia and more recently in Africa, without any reported cases of resistance (Mohapatra et al. 1996; Krishana et al. 2004).
A. annua is a cosmopolitan species, growing wild in many countries, e.g., in China and Vietnam, the Balkan, the former Soviet Union, Argentina, and southern Europe (Van Geldre et al. 1997), and large differences exist in artemisinin content between different varieties of A. annua (Delabays et al. 1993; Woerdenbag et al. 1993). A substantial increase in the content of artemisinin would be required to make artemisinin available on a large scale also to the people in the Third World. Selection for high-producing lines and traditional breeding, and research on the effects of environmental conditions and cultural practices could perhaps lead to an improvement of artemisinin content (Delabays et al. 1993; Ferreira et al. 1995; Gupta et al. 1996).
Artemisinin is a sesquiterpene-lactone isolated from the aerial parts of A. annua L. plants. Besides being currently the best therapeutic agent against both drug-resistant and cerebral malaria causing strains of Plasmodium sp., (Newton and White 1999), it is also effective against other infectious diseases such as schistosomiasis, hepatitis B and leishmaniasis (Borrmann et al. 2001; Utzinger et al. 2001; Romero et al. 2005; Sen et al. 2007). More recently, it has also been shown to be effective against a variety of cancer cell lines including breast cancer, human leukemia, colon cancer, and small cell-lung carcinomas (Efferth et al. 2001; Singh and Lai 2001). Due to its current use in artemisinin-based combination therapy (ACT), its global demand continuously is increasing. The relatively low yield of artemisinin in A. annua L. plant leaves (0.01–1.1 %), however, a serious limitation to the commercialization of the drug Laughlin ( 1994; Van Agtmael et al. 1999; Abdin et al. 2003).
Artemisinin and its semisynthetic analogues have undergone clinical trials as new lifesaving antimalarials under the auspices of the World Health Organization and have been intensively studied due to their unique structure, with an endoperoxide (1,2,4-trioxane) linkage, their novel mechanism of action as the first non-nitrogenous antimalarial, and the worldwide resurgence of drug-resistant falciparum infections. The state-of-the-art of production of artemisinin by chemical and biotechnological methods and analytical aspects has recently been reviewed. Five various approaches have been employed to increase artemisinin content in the plant including conventional breeding, biochemical, physiological and molecular approaches, hairy root culture techniques, for the artemisinin biosynthetic pathway in A. annua L. (Smith et al. 1997; Liu et al. 1999; Chang et al. 2000; Wallaart et al. 2001; Wang et al. 2002; Abdin et al. 2003; Martin et al. 2003; Weathers et al. 2005; Picaud et al. 2005; Newman et al. 2006; Ro et al. 2006; Zeng et al. 2007). Chemical synthesis has also been attempted (Xu et al. 1986; Avery et al. 1992), but the yield of artemisinin is very low. Thus, it is economically not viable for the large-scale production of artemisinin. Four genes of artemisinin biosynthesis pathway (HMG-CoA reductase, fernesyl diphosphate synthase, amorpha-4,11-diene synthase (ADS), and CYP71AV1) were overexpressed (Han et al. 2006; Ping et al. 2008; Teoh et al. 2006; Aquil et al. 2009) and artemisinin content was increased by 34.4 %.
11.2 Biosynthetic Pathway of Artemisinin
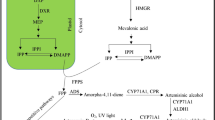
The biosynthetic pathway of artemisinin belongs to the isoprenoid metabolite pathway (Fig. 11.1). Based on the experimental evidences related to its biosynthesis, artemisinin is suggested to be derived from two common precursors, isopentenyl pyrophosphate (IPP) and its isomer, dimethylallyl diphosphate (DMAPP). It has been established that higher plants have two independent biosynthetic pathways leading to the formation of IPP: the cytosolic mevalonate pathway and the plastid-localized mevalonate-independent (MEP/Rohmer) pathway (Liu et al. 2005). As a result, mevalonate pathway has no more been considered as the sole route to the synthesis of artemisinin in A. annua L. It was further supported by isolation of two clones encoding deoxy-D-xylulose-5-phosphate synthase (DXPS) and deoxy-D-xylulose-5-phosphate reductoisomerase (DXPR) from transformed hairy roots of A. annua L. (Souret et al. 2002; Krushkal et al. 2003). The partial carbon supply to the synthesis of artemisinin was reported to be made by MEP pathway operating in plastids and DXR catalyzing the rate-limiting step (Towler and Weathers 2007). Recently, the relative contribution of these pathways toward carbon supply in artemisinin production was evaluated by Ram et al. (2010). They demonstrated that mevalonate pathway is the major contributor of carbon and supplies 80 % of the carbon to artemisinin biosynthesis, whereas MEP pathway supplies only 20 % of the carbon.
Proposed artemisinin biosynthesis pathway in A. annua L.CMK 4-(Cytidine 5′-diphospho)-2-C-methyl-D-erythritol kinase, CMS 2-C-methyl-D-erythritol 4-phosphate cytidyl transferase, DXR 1-deoxy-D-xylulose 5-phosphate reductoisomerase, DXS 1-deoxy-D-xylulose 5-phosphate synthase, FPPS farnesyl diphosphate synthetase, GPPS geranyl diphosphate synthase, HMGR 3-hydroxy-3-methylglutaryl coenzyme A(HMGCoA) reductase; HMGS HMG-CoA synthase; IDS isopentenyl diphosphate synthase, MCS 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase, MDD mevalonate diphosphate decarboxylase, MK mevalonate kinase, MPK mevalonate-5-phosphate kinase, SES sesquiterpene synthase, CYP71AV1, cytochrome P450 monooxygenase; Dbr2, artemisinic aldehyde reductase. Adapted from Liu et al. (2006)
In mevalonate pathway, three molecules of acetyl-coenzyme A condensed together to yield 3-hydroxy-3-methylglutaryl CoA (HMG-CoA), which is subsequently reduced by the enzyme HMG-CoA reductase (HMGR) to yield mevalonic acid (MVA). Then, under the catalysis of mevalonate kinase, mevalonate 5-diphosphate is formed which is subsequently decarboxylated to yield isopentenyl pyrophosphate (IPP) (Newman and Chappell 1999). The synthesis of IPP and DMAPP by either MVA or DXP pathways is followed by chain elongation. The carbonium ion is a potent alkylating agent that reacts with IPP, giving geranyl diphosphate (GPP). GPP has the active allylic phosphate group and further react with IPP to produce farnesyl pyrophosphate (FPP). FPP takes part in a cyclization reaction catalyzed by cyclases to produce various final products of isoprenoids including artemisinin (Barkovich and Liao 2001).
All the steps of mevalonate and MEP pathway have been fully characterized, but post-FPP production of artemisinin is not yet completely elucidated. Several enzymes involved in the early steps of artemisinin biosynthesis have been discovered which include HMGR, farnesyl pyrophosphate synthase (FPS), ADS, DXS, DXR, squalene synthase (SQS), and CYP71AV1. (Table 11.1).
The formation of the sesquiterpene carbon skeleton, amorpha-4, 11-diene is catalyzed by ADS (Bouwmeester et al. 1999) for which corresponding cDNAs have been cloned (Chang et al. 2000; Mercke et al. 2000; Wallaart et al. 2001). The non-descript arrangement of the amorphadiene product belies the unique structural features that ultimately allow for the formation of the 1, 2, 4-trioxane moiety (Sy and Brown 2002) (Fig. 11.2). Expression analysis of CYP71AV1 in A. annua L. tissues indicates that it is most highly expressed in secretory glandular trichomes (GSTs) (Teoh et al. 2006). The moderate expression observed in flower buds presumably reflects their high density of GSTs. Low but detectable levels of RT-PCR products could be observed in leaves. The role of CYP71AV1 in the hydroxylation of amorpha-4, 11-diene is undoubtedly important in artemisinin biosynthesis. The subsequent route to artemisinin is less clear. Most evidence implicates dihydroartemisinic acid as a late precursor to artemisinin biosynthesis, which is derived from artemisinic alcohol by oxidation at C12 and reduction in the C11–C13 double bond. This is based on in vitro biochemical evidence (Bertea et al. 2005), as well as the conversion of dihydroartemisinic acid to artemisinin both in vivo (Brown and Sy 2004) and in vitro in an oxygen-dependent non-enzymatic fashion (Sy and Brown 2002).
Proposed biosynthetic pathway of artemisinin starting from farnesyl diphosphate. On the left is the type of reaction; on the right is the enzyme for each known enzymatic action. Broken arrow indicates multiple steps. Adapted from Bertea et al. (2005)
Bertea et al. (2005) showed that A. annua L. leaf microsomes convert amorphadiene to artemisinic alcohol in the presence of NADPH. The route from artemisinic alcohol to artemisinin is still not entirely clear, which is evident from the published data reviewed by Li et al. (2006). In this regard, it is useful to consider the possible route(s) to artemisinin among the pathways shown in Fig. 11.2. These pathways are based on a few conversions whose order may vary. These conversions include the oxidation of C12 from alcohol to aldehyde as well as aldehyde to acid, the reduction of the double bond at C11, C13, and the formation of the 1,2,4-trioxane moiety. The later steps in artemisinin biosynthesis remain controversial and theories differ mainly in the identification of either artemisinic acid or dihydroartemisinic acid as the later precursor. The evidence for artemisinic acid has been reviewed by Li et al. (2006). This includes the suggestion that C11, C13 double-bond reductions occur at the level of an intermediate beyond artemisinic acid, such as arteannuin B or artemisitene. On the other hand, the co-occurrence of dihydroartemisinic acid with high artemisinin levels suggests that even if double-bond reduction could occur at a very late step, it also occurs in less oxidized precursors. The double-bond reduction at C11, C13 is of general interest biochemically, given the relative rarity of enzymes catalyzing double-bond reductions (Kasahara et al. 2006). The dihydroartemisinic acid is also being considered as a late precursor of artemisinin biosynthesis. Labeled dihydroartemisinic acid is incorporated into artemisinin in vivo, a sequence which can occur in the absence of enzymes (Brown and Sy 2004; Haynes et al. 2006; Sy and Brown 2002). Upstream of dihydroartemisinic acid, the order of oxidations, and reduction of artemisinic alcohol en route to dihydroartemisinic acid are still not settled. Bertea et al. (2005) provided biochemical evidence for the fate of artemisinic alcohol in A. annua L. using GST cell-free extracts.
11.3 Agrotechnology
A. annua (family Asteraceae), also known as qinghao (Chinese), annual or sweet wormwood, or sweet Annie, is an annual herb native to Asia, most probably China (McVaugh 1984). A. annua occurs naturally as part of the steppe vegetation in the northern parts of Chahar and Suiyuan provinces (408°N, 1098°E) in northern China (now incorporated into Inner Mongolia), at 1,000–1,500 m above sea level (Wang 1961). The plant now grows wild in many countries, such as Argentina, Bulgaria, France, Hungary, Romania (cultivated for its essential oil), Italy, Spain, USA, and former Yugoslavia (Klayman 1989; Klayman et al. 1993). In addition, it has been introduced into experimental cultivation in India (Singh et al. 1986), Vietnam, Thailand, Myanmar, Madagascar, Malaysia, USA, Brazil, Australia (Tasmania) and in Europe into the Netherlands, Switzerland, France, and as far north as Finland (Laughlin 2002).
A. annua is a xeromorphic temperate plant belonging to Asteraceae family that contains promising antimalarial drugs, the sesquiterpene lactone artemisinin and derivatives of this compound. An examination of the growth and flowering behavior of A. annua in the subtropical climate region of India demonstrated the plant grew normally and flowered profusely in the winter cropping season, late October to late April, at Lucknow, India. Considerable inter-plant variation was observed, however, in growth habit and flowering time. Plants could be grouped into four classes: early-maturing dwarf, early-maturing tall, late-maturing dwarf, and late-maturing tall. Early-maturing plants which flowered in February and March produced fertile achenes, completing the life cycle in 7–8 months. Late-flowering plants that flowered in May and June, when the maximum day temperature was over 40 °C, produced florets without seeds. The high-temperature conditions to which the late-flowering plants were exposed appeared to prematurely dry the stigma. Late-flowering plants sprouted branches from the vegetative and flowering parts of the plant during the rainy season.
A. annua appears to be the only Artemisia species that contain appreciable amounts of artemisinin. Chinese scientists have reported that extracts from 30 other different species of Artemisia did not show antimalarial activity (UNDP/World Bank/WHO special programme for Research and Training in Tropical Diseases Artemisia species, 1981), American scientists have failed to detect artemisinin in various species endemic to America (Klayman et al. 1984). In India, (Balachandran et al. 1987) also did not find artemisinin in various Artemisia species of Indian origin. Considering the importance of artemisinin which is tedious and difficult to synthesize chemically, an all out program was undertaken to develop A. annua plant varieties with high artemisinin content starting with development of agrotechnology for increase yield of these compounds, followed by improved extraction procedure. More particularly, the program focused on agrotechnology involving method of optimizing the planting time, transplanting scheduling, population density, number of harvests and harvesting schedule leading to enhanced yields of artemisinin and related metabolites which have pharmaceutical value of anti-infectives, particularly as antimalarial drug. In this direction, the inventors were successful in developing and releasing a variety named ‘Jeevan Raksha’ from an isolated population containing high artemisinin in the foliage (0.5–1.0 %) (Kumar et al. 1999). This plant ‘Jeevan Raksha’ not only produces high artemisinin but also maintains the synchronized conversion to higher level of artemisinin during May to October. As the content of artemisinin fluctuates from zero level at the time of planting to more than 0.4–1.00 % during May and June with subsequent functions of increase till October, it was necessary to scientifically develop cultivation methodology for the crop to maximize the vigor of the foliage and biosynthesis of artemisinin by systematic scheduling. For this purpose, the inventors carried out planned experiments with variation in planting times, population density, and number of harvest from the crop to increase the yield from limited area within optimum span of time. Until now, artemisinin production has depended on extraction from A. annua L. plants grown outdoors. There are two methods to enhance artemisinin production in intact A. annua plants. One method is to define the appropriate developmental stage at which to harvest the leaves of the plants. At this developmental stage, both the highest artemisinin content and leaves yielding can be obtained. The other method is to breed high-artemisinin-yielding strains.
11.4 Biochemical and Physiological Approach
Although artemisinin is an effective medicine for treating malaria, the application of this medicine is limited by the availability of the source. The artemisinin content in the leaves or florets of A. annua is very low, and the chemical method for the synthesis of this compound is difficult. These factors make the medicine expensive and hardly available on a global scale for patients (Van Geldre et al. 1997; Abdin et al. 2003). Despite the commercial value of artemisinin, exact biosynthetic pathway of artemisinin in A. annua, particularly about the early enzymatic steps leading to (dihydro) artemisinic acid are still unclear. Several authors have demonstrated that A. annua converts artemisinic acid and dihydroartemisinic acid to artemisinin (Sangwan et al. 1993; Wallaart et al. 1999b). Akhila et al. (1990) hypothesized a pathway in which the formation from farnesyl diphosphate (FDP) of an unidentified enzyme-bound sesquiterpene-like intermediate represents the first committed step in the biosynthesis of artemisinin. In addition, many authors have analyzed extracts of A. annua to search for possible intermediates in the biosynthesis of artemisinin. Artemisinic and dihydroartemisinic acid were reported by many authors, as well as many olefinic mono- and sesquiterpenes and putative intermediates en route from dihydroartemisinic acid to artemisinin (Brown 1994; Jung et al. 1990; Ranasinghe et al. 1993; Wallaart et al. 1999b; Woerdenbag et al. 1993). However, none of the reported olefinic sesquiterpenes seemed to fit in the biosynthetic pathway, nor was a possible intermediate between the sesquiterpene olefin and artemisinic acid ever detected, with the exception of artemisinic alcohol, which was tentatively identified in the roots of A. annua (Woerdenbag et al. 1993). It has been shown by several groups that the cyclization of the ubiquitous precursors GPP, FDP, and GPP to the respective olefinic mono-, sesqui- and diterpene skeletons represents the regulatory step in the biosynthesis of terpenoids (Gershenzon and Croteau 1990; Mc garry and Croteau 1995). The accumulation of artemisinic acid and dihydroartemisinic acid and the absence of any intermediates en route from FDP to these two compounds support that the first step(s) in the biosynthetic pathway of artemisinin [and again some step(s) from (dihydro) artemisinic acid to artemisinin] are indeed regulatory/rate limiting. Here, we describe the elucidation of the unknown four intermediates and the corresponding five enzymatic steps that constitute the first part of the artemisinin biosynthetic pathway. The implications for strategies to improve artemisinin production are discussed. Chemical synthesis of artemisinin is an expensive multistep process; the plant remains the only commercial source of the drug.
In our biosynthetic studies on artemisinin toward purification of endoperoxidase enzyme from A. annua, and determination of the source of the endoperoxide Oxygen Bridge by 18O-isotope labeling in plant cell-free and tissue culture, we required an experimental method suitable for direct detection, quantitation, and isotopomeric analysis since artemisinin is unstable and lacks a chromophore for UV detection in HPLC.
Alternatively, the C11-C13 double-bond reduction may occur in artemisinic alcohol or artemisinic aldehyde, yielding dihydroartemisinic alcohol or dihydroartemisinic aldehyde as intermediates, respectively. To study this unknown part of the pathway, we analyzed the presence of putative intermediates and enzymes involved in the conversion of these intermediates in leaves and glandular trichome extracts of A. annua. Hereto, first a number of reference compounds such as artemisinic alcohol, dihydroartemisinic alcohol, artemisinic aldehyde, and dihydroartemisinic aldehyde were synthesized using artemisinic acid and dihydroartemisinic acid as starting materials or isolated (artemisinic aldehyde). The structures of all isolated or synthesized compounds were confirmed using NMR and MS (Bertea et al. 2005). Subsequently, we looked for these compounds in extracts of A. annua leaves as well as in extracts of isolated trichomes. The chromatograms obtained with these two extracts were very similar, indicating that most (if not all) of A. annua terpenoids are present in the trichomes. In both cases, artemisinic alcohol, artemisinic aldehyde, artemisinic acid, dihydroartemisinic alcohol, dihydroartemisinic aldehyde, dihydroartemisinic acid, and a series of olefinic terpenes were detected (Bertea et al. 2005). Artemisinic acid, dihydroartemisinic acid, and the sesquiterpene olefins have been reported before as constituents of A. annua (Boumeester et al. 1999; Wallaart et al.1999b) but this was the first time artemisinic alcohol, artemisinic aldehyde, dihydroartemisinic alcohol, and dihydroartemisinic aldehyde have been identified in A. annua. In enzyme assays with microsomal pellets of A. annua leaf extracts, using amorpha-4, 11-diene as a substrate and in the presence of NADPH, we found a small, but consistent amorpha-4, 11-diene hydroxylase activity which was absent in the soluble protein fraction (150,000 g supernatant), confirming that a cytochrome-P450 enzyme catalyzes the formation of artemisinic alcohol from amorpha-4, 11-diene. The next putative enzymatic step was assayed by incubating a mixture of artemisinic alcohol and dihydroartemisinic alcohol with the 150,000 g young-leaf supernatant in the presence of NAD+/NADP+ at pH 9.0. In the presence of cofactors, the ratio between artemisinic alcohol and dihydroartemisinic alcohol strongly decreased showing that artemisinic alcohol was converted to artemisinic aldehyde, dihydroartemisinic aldehyde, and dihydroartemisinic acid (Bertea et al. 2005). None of these intermediates were formed in the absence of cofactors. Artemisinic acid was not detected in any of these experiments. To test whether the conversion of dihydroartemisinic aldehyde to dihydroartemisinic acid that was observed in leaf extracts also occurred in trichomes, we incubated the 150,000 g supernatant of the GSTs with dihydroartemisinic aldehyde in the presence of NAD+/NADP+. Under these conditions, we detected conversion of dihydroartemisinic aldehyde into dihydroartemisinic acid, whereas conversion did not occur in the absence of cofactors.
Artemisinin was first isolated from the aerial parts of A. annua by the Chinese scientists and later characterized by others. But, the details of isolation procedure were not published for long time (reviewed by Klayman 1985). The researchers at the Walter Reed Army Institute of Research, USA, spotted some A.annua growing in the neighborhood of Washington D.C. and extracted its various air-dried parts with a number of apolar organic solvents. The petroleum ether extraction proved most satisfactory for the isolation of artemisinin and its derivatives (Klayman 1985).
Artemisinin has been reported to accumulate in leaves, small green stems, buds, flowers, and seeds (Acton and Roth 1985; Ferreira et al. 1995, Liersch et al. 1986; Martinez and Staba 1988). Its content was found more in leaves and inflorescence, but neither artemisinin nor its precursors were detected in roots (Trigg 1990; Charles et al. 1991). Duke and Paul (1994) showed that artemisinin is sequestered in glandular trichome of A. annua. Artemisinin content in full-bloomed flowers was 4–5 times higher than in leaves (Ferreira et al. 1995). The artemisinin yield estimated at different steps of development reveals a possible correlation between plant age and artemisinin content. This is assumed to be due to both an increase in leaf yield and artemisinin content with the progressive increase in plant growth (Singh et al. 1988). Our own observations have revealed that the artemisinin content was highest at full vegetative stage. Some researchers reported that artemisinin content is highest just prior to flowering (Acton and Klayman 1985; ElSohly 1990; Liersch et al. 1986; Woerdenbag et al. 1991, 1993); others found an artemisinin peak at full-flowering stage (Morales et al. 1993; Pras et al. 1991; Singh et al. 1986).
Artemisinin yields reported from plants in China range from 0.01 to 0.5 % (w/w), varieties growing in Siachuan Province showing the highest content. Klayman et al. (1984) reported 0.06 % (W/W) yield from A. annua growing wild in Washington D.C. Other reports claim the yield to be 0.09–0.17 % (Liersch et al. 1986; Singh et al. 1986, UNDP/World bank/WHO special program for research/Training in Tropical Diseases 1986). The yields of the related sesquiterpenes, i.e., artemisinic acid and arteannuin B also show variation in their contents. In USA, artemisinic acid content is 8–10 times more than the artemisinin (Jung et al. 1990; Roth and Acton 1987) followed by arteannuin B (Klayman 1993). In India, the yield of arteannuin B (0.27 %) is relatively higher than the other two sesquiterpenes (Singh et al. 1986; Gulati et al. 1996).
A study on effect of levels of nitrogen (0, 50 and 100 kg ha−1) phosphorus (0 and 50 kg ha−1), and potassium (0 and 50 kg ha−1) on growth, oil and artemisinin yield revealed that application of 50 and 100 kgN/ha increased herbage, oil and artemisinin yield by 26.2 and 40.1 %, respectively, compared with control (no nitrogen) (Singh 2000; Jha et al. 2011). The influence of micro-nutrient imbalance on growth and artemisinin contents shows that A. annua was very sensitive to boron (B) deficiency. Boron-deficient plants did not show flowering and there was approximately 50 % reduction in artemisinin content. Similarly, artemisinin content declined by 25–30 % in Fe-, Mn-, Zn-, and Cu-deficient plants (Srivastava and Sharma 1990). Effect of plant growth regulators on yield, oil composition, and artemisinin content of A. annua under temperate condition was studied in 1998 by Yaseen. Foliar application of IAA at 100 ppm produced significantly higher herb and oil yields than the control, due to increase in plant height, leaf/stem ratio and oil content, and delayed leaf senescence. Although the artemisia ketone in the oil was highest following application of GA3 and IAA at 150 ppm, the artemisinin content was higher in the plants treated with six ppm triacontanol. Effect of bioregulators, chlormequat and triacontanol, was studied for artemisinin content, growth parameters, and leaf yield. Plants treated with chlormequat were found to have more herbage yield, but the effect of higher dose was not statistically significant (Shukla et al. 1992). Level of ABA in chlormequat-treated plants was higher than in control plants, whereas treatment with triacontanol lowered the abscisic acid level. On the contrary, application of triacontanol increased the level of endogenous GA3-like components while chlormequat caused reduction in their concentration (CIMAP 1988–1989). According to (Liersch et al. 1986), chlormequat was able to increase the artemisinin contents by 30 %. Local climatic conditions, season of harvesting as well as the post-harvest handling seems to play an important role in the levels of artemisinin content (Chen et al. 1987; Ferreira et al. 1995; Martinez and Staba 1988; Singh et al. 1986). The time of planting seems to play an important role on the yield of essential oils and artemisinin in A. annua. Plants planted between September and December produced significantly higher herbage yields [on the basis of fresh weight and dry weight (DW)] as compared to that of plants planted in February. Plants planted in September produced the highest amount of artemisinin. Plants established during pre-winter (August–September) and allowed to grow through the entire winter synthesized and accumulated more artemisinin than plants established during early (October–November) and late (February) winter periods. It was concluded that the artemisinin content was dependent on the weather conditions (Ram et al. 1997). Environmental stress, such as light, temperature, water, and salt significantly alter artemisinin yields (Weathers et al. 1994; Wallaart et al. 2000).
Genetic studies on A. annua have confirmed that the diploid plants are 2n = 18 (CIMAP India 1986–1987). The average artemisinin level in tetraploids was 38 % higher than that of the wild type (diploid) as measured over the whole vegetation period (Wallaart et al. 1999a). A hybrid form of A. annua was successfully cultivated in Central Africa. The aerial parts of the plants contained 0.63–0.7 % artemisinin on DW basis (Mueller et al. 2000).
11.4.1 Effect of Endophytic Bacteria and Fungus on the Growth of A. annua and Artemisinin Production
Plants constitute a vast and diverse niche for endophytic organisms resulting in the development of closer biological associations between the plant and the endophytes than epiphytes or other soil organisms (Strobel 2003). Evidence has also accumulated suggesting that secondary metabolites in plants actively take part in plant–microbe interactions. Endophytes colonize the internal tissue of the plant and are capable of triggering physiological plant responses (Hardoim et al. 2008; Van Wees et al. 2008) and influencing the production of secondary metabolites in the host plant (Yuan et al. 2007; Satheesan et al. 2012). Wang et al. (2001) purified a chemical elicitor from the extract of the endophytic fungus Colletotrichum sp., which could be used to stimulate the accumulation of artemisinin in A. annua hairy roots. Artemisinin biosynthesis has also been shown to be enhanced in A. annua after treatment with Piriformospora indica or arbuscular mycorrhizal fungi (Chaudhary et al. 2008; Kapoor et al. 2007; Varma et al. 2012). A large number of endophytic actinomycetes have been isolated from A. annua (Li et al. 2012a, b). However, A. annua-endophytic actinomycete interactions have only been rarely documented.
In a preliminary assay, 6-day-old geminated A. annua seedlings were inoculated with the endophytic strains YIM 63654, YIM 63673, YIM 63342 YIM 63538, and YIM 63111. The plants were incubated for 66 days, and all treated and untreated seedlings were harvested to determine the plant height and artemisinin content. The height of the seedlings that were inoculated with strains YIM 63654, YIM 63673, YIM 63342, and YIM 63538 were similar to the non-inoculated control plants, whereas the growth of A. annua seedlings inoculated with strain YIM 63111 was significantly inhibited when compared with the control plants. However, artemisinin production was significantly elevated upon inoculation with strain YIM 63111 (P, 0.05). The artemisinin content was 2.5760.18 mg g1 DW compared to 1.9960.09 mg g1 DW that was observed in the control samples (Li et al. 2012).
11.5 Biotechnological Production of Artemisinin
The commercial sources of most artemisinin are from field grown leaves and flowering tops of A. annua, which are subjected to seasonal and somatic variation and infestation of bacteria, fungi, and insects that can affect the functional medicinal content of this plant (Klayman 1985; Luo and Shen 1987). The total organic synthesis is very complicated with low yields, and economically unattractive (Avery et al. 1992; Xu et al. 1986). In view of these problems, artemisinin production from in vitro plant tissue culture has been considered as an attractive alternative. The biosynthesis of artemisinin was studied in the calli, suspension cells, shoots, and hairy roots of A. annua during their cultivation in vitro (He et al. 1983; Tawfiq et al. 1989; Weathers et al. 1994; Paniego and Giulietti 1996; Liu et al. 1997; Nair et al. 1986; Teo et al. 1995). A certain degree of differentiation of A. annua tissue cultures is a prerequisite for the synthesis of artemisinin. Paniego and Giulietti (1994) reported that no artemisinin was found in cell suspension cultures of A. annua, whereas trace amounts were found in the multiple shoot cultures. Woerdenbag et al. (1993) reported a high percentage of artemisinin content in A. annua shoots cultured on 1/2-MS medium supplemented with 0.05 mg/l naphthaleneacetic acid, 0.2 mg/l benzyladenine (BA), and 2 % sucrose. The flowering of A. annua was observed in vitro by supplementing with gibberellic acid (GA3) where artemisinin content reached 0.1 % in A. annua plantlets, and the highest artemisinin content in the plantlets was observed in full bloom (Gulati et al. 1996). Most groups did not find artemisinin in root part of A. annua plant. However, artemisinin content in the shoot part of cultured plantlet was higher than that in the cultured shoots without roots (Ferreira and Janick 1996; Martinez and Staba 1988). Attempts were also made to improve the artemisinin production by optimizing chemical and physical environmental factors. Wang and Tan (2002) reported the influence of the ratio of NO3/NH4 and total initial nitrogen concentration on the artemisinin yield in hairy roots. With the ratio of NO3/NH4 at 5:1(w/w), the optimum concentration of total nitrogen for artemisinin production was 20 mM. Under this concentration, artemisinin production was 57 % higher than that in the standard MS medium. Weathers’ research group investigated the effects of media sterilization method and types of sugar on growth and artemisinin accumulation of A. annua hairy roots. They found that biomass from filter-sterilized medium was greater than that from autoclaved medium, but artemisinin accumulation from filter-sterilized medium was less than that from autoclaved medium. Growth of hairy roots in the medium with sucrose (3.99 g DW/l) was equivalent to the growth in the medium with fructose (3.75 g DW/l) and significantly better than in the medium with glucose (2.16 g DW/l), while the roots that grew in glucose showed a dramatic stimulation in artemisinin content which is three- and twofold higher than that in medium with sucrose and fructose (Weathers et al. 2004). Casein hydrolysate, a source of amino acids and oligopeptides, at low concentration enhances artemisinin production in A. annua shoot cultures (Woerdenbag et al. 1993). A combination of BA and kinetin increased the yields of artemisinin in cultured shoots by 3.6- and 2.6-fold (Whipkey et al. 1992). GA3, a plant hormone that can induce blooming, has been reported to improve growth and artemisinin biosynthesis in shoot cultures, root cultures, and plantlets of A. annua (Fulzele et al. 1995; Charles et al. 1990; Smith et al. 1997; Weathers et al. 2005). The effects of light irradiation on growth and production of artemisinin were studied in hairy root cultures of A. annua L. by Liu et al. (2002). They found that when the hairy roots were cultured under illumination of 3,000 lx for 16 h using several cool-white fluorescent lamps, the DW and artemisinin concentration reached 13.8 g/l and 244.5 mg/l, respectively (Liu et al. 2002). Wang et al. (2001) investigated the dependence of biomass of hairy roots and artemisinin content on the light spectrum. They found that the highest biomass (5.73 g DW/l) and artemisinin content (31 mg/g) were obtained under red light at 660 nm which were 17 and 67 % higher than those obtained under white light, respectively. Temperature in the range of 15–35 °C also affected growth and artemisinin biosynthesis in the cultured A. annua hairy roots. The maximum hairy root growth was found at 25 °C. However, the highest artemisinin content in the root cultures was observed at 30 °C (Guo et al. 2004). Enhancing the artemisinin production by precursor feeding was also investigated. Addition of artemisinin precursors to the medium used for tissue cultures of A. annua resulted in a fourfold increase in artemisinin in the tissue and an 11-fold increase in artemisinin in the spent medium (Weathers et al. 1994). The feeding of MVA alone, however, did not induce an enhancement of artemisinin production (Woerdenbag et al. 1993). But the addition of some compounds such as naphtiphine (an inhibitor of the enzyme squalene epoxidase) to the medium improved the artemisinin production. Other additions, such as 5-azacytidine (a gene regulator), colchicine (a gene regulator), miconazole (an inhibitor of sterol demethylase), and terbinaphine (an inhibitor of the enzyme squalene epoxidase), were too toxic for the cultures to induce an enhancement of the artemisinin production (Woerdenbag et al. 1993). Kudakasseril et al. (1987), however, reported a concentration-dependent increase in the levels of artemisinin and growth of shoot cultures with miconazole. Other sterol inhibitors, such as chlorocholine chloride, 2-isopropyl-4-(trimethylammonium chloride)-5-methylphenylpiperidinecarboxylate, and 4-chloro-2-(2-diethylaminoethoxyphenyl)-2-(4-methyl-phenyl)-benzeneethanol, increased both the incorporation of 14C-IPP into artemisinin by cell-free extracts and the production of artemisinin in shoot culture of A. annua. Sterol inhibitors inhibited the enzyme in the mevalonate pathway, resulting in increased terpenoid production rather than sterol production (Fig. 11.3; Zhang et al. 2009). To develop more potent antimalarial agents with improved in vivo stability, tremendous efforts have been made toward structure modification of artemisinin and analogue synthesis. Due to the difficulties of structural modification by conventional chemical methods, microbial transformation serves as a valuable tool that comes to play an important role in the modification. To date, a number of oxidating products of artemisinin at different positions of artemisinin structure have been reported. These transformations include conversion to 3α-hydroxydeoxyartemisinin and deoxyartemisinin, conversion to 9β-hydroxy-artemisinin and 3α-hydroxy-artemisinin, and conversion to 10-hydroxy-artemisinin and 9β-hydroxy-11α-artemisinin. In addition, microbial transformations on some artemisinin analogue, such as artemether, arteether, artemisitene, and 12-deoxoartemisinin, have been reported to produce oxidative products by different microorganisms (Liu et al. 2006).
RNAi-mediated suppression of SQS gene (Zhang et al. 2009)
Many studies have been performed trying to enhance artemisinin content using bioengineering methods. Because A. annua is easily propagated in vitro, the production of artemisinin by cultures of cells or tissues (Nair et al. 1986; Kudakasseril et al. 1987; Martinez and Staba 1988; Tawfiq et al. 1989; Paniego et al. 1994), transformed hairy root (Qin et al. 1994; Jaziri et al. 1995; Wang et al. 2000; Xie et al. 2000; Kim et al. 2002, 2003; Souret et al. 2003), and shoot cluster (He et al. 1983; Woerdenbag et al. 1993) has been investigated widely. However, the yield of artemisinin remained low and undifferentiated cell or callus cultures, in particular, contained null or trace amounts of artemisinin. The production of artemisinin in shoot clusters and transformed hairy root is still disappointing at present.
It is envisaged to produce high-artemisinin-yielding transgenic strains of A. annua L. plants, which will ensure a constant high production of artemisinin by overexpressing the key enzymes in the terpene and artemisinin biosynthetic pathways, or by inhibiting enzyme(s) of another pathway competing for artemisinin precursors (Fig. 11.3).
In recent years, remarkable progress has been made in the understanding of molecular biology of artemisinin biosynthesis and its regulation (Bouwmeester et al. 1999; Weathers et al. 2006). The genes of the key enzymes involved in the biosynthesis of artemisinin, such as HMG-CoA reductase, FPS, ADS, and the genes of the enzymes involved in the pathway competing for artemisinin precursors, such as SQS involved in sterol biosynthesis, have been cloned from A. annua L. (Matsushita et al. 1996; Mercke et al. 2000; Wallaart et al. 2001; Liu et al. 2003; Abdin et al. 2003). On the other hand, (Weathers et al. 1994; Qin et al. 1994) induced hairy roots in A. annua L. employing Agrobacterium rhizogenes. Further, the factors influencing transformation efficiency of A. rhizogenes were explored to optimize the transformation system by Liu et al. (1998). Xie et al. (2001) induced hairy root in A. annua L. leaf blade pieces and petiole segments infected with A. rhizogenes strain 1,601 and obtained a clone with high content of artemisinin (1.195 mg/g DW).
To develop transgenic A. annua L. strains with high content of artemisinin by modulating the expression of above-mentioned genes, an efficient system of genetic transformation as well as regeneration of explants of A. annua L. should be in place. Vergauwe et al. (1996) developed an Agrobacterium tumefaciens-mediated transformation system for A. annua L. plants with high transformation rates (75 % regenerants harboring foreign gene). Artemisinin content in the leaves of regenerated plants was 0.17 %, a little bit higher than that present in the leaves of normally cultured plants (0.11 % DW). They further investigated the factors, viz. the age of explants, A. tumefaciens strain, and plant genotype influencing the transformation efficiency (Vergauwe et al. 1998). Later, (Han et al. 2005) established a high-efficiency genetic transformation and regeneration system for A. annua L. via A. tumefaciens.
Artemisinic acid is one of the precursors of biosynthesis of artemisinin, which has the cadinene structure. Chen et al. (1998) transformed a cotton cadinene synthase cDNA into the leaf explants of A. annua L. using A. rhizogenes. In the isoprenoid biosynthesis pathway, FPS catalyzes the two sequential 1-4 condensations of IPP with DMAPP to produce GPP and with GPP to give FPP, which is then utilized by isoprenoid pathway and artemisinin biosynthetic pathway to produce isoprenoids and artemisinin, respectively (Cane 1990). The cDNAs encoding FPS have been isolated from a number of plant species, including Arabidopsis thaliana (Delourme et al. 1994) and Lupinus albus (Attucci et al. 1995). Since 15-carbon FPP can be catalyzed by sesquiterpene cyclases, such as, ADS to form cyclic sesquiterpenoids (amorpha-4, 11-diene in A. annua L.), overexpressing foreign FPS gene into A. annua L. plants holds the possibility of affecting accumulation of artemisinin. A cDNA encoding cotton FPPS placed under a CaMV 35S promoter was, hence, transferred into A. annua L. Plants via A. tumefaciens strain LBA 4404 or A. rhizogenes strain ATCC 15834 mediated genetic transformation (Chen et al. 1999, 2000). In the transgenic plants, the concentration of artemisinin was approximately 8–10 mg/g DW, which was 2- to 3-fold higher than that in the control plants. Han et al. (2006) achieved about 34.4 % increase in artemisinin content by overexpressing FPS. We have overexpressed one of the key regulatory enzymes of MVA pathway (HMGR) in A. annua L. plants via A. tumefaciens-mediated transformation and achieved 39 % enhancement in artemisinin contents as compared to control plants (Tazyeen et al. 2010). Jing et al. (2008) simultaneously overexpressed cyp71av1 and cpr genes in A. annua L. and recorded 2.4-fold enhancement in artemisinin content. The cytokinin biosynthetic gene codes for the enzyme isopentenyl transferase (ipt), which catalyzes the condensation of isopentenyl pyrophosphate and adenosine monophosphate (AMP) to yield isopentenyl AMP, are believed to represent the rate-limiting step in cytokinin biosynthesis in tumorous plant tissue (Akiyoshi et al. 1983, 1984). The influence of overexpression of isopentenyl transferase gene on the physiological and biochemical characteristics of A. annua L. plant was studied by Geng et al. (2001). The transgenic A. annua L. plants were found to accumulate more cytokinins (2–3-fold), chlorophyll (20–60 %), and artemisinin (30–70 %), when compared with control plants (Geng et al. 2001). Previous studies indicated that capitate glands on the leaf surface (Duke and Paul 1994) and specialized chloroplasts of the capitate gland appeared to play very important role in artemisinin biosynthesis (Duke and Paul 1993). Light affects to terpene biosynthesis in general and artemisinin biosynthesis in particular by modulating carbon flux through regulation of HMG-CoA reductase, a key regulatory enzyme in mevalonate pathway. In case of potato, it has been reported that light regulates HMGR at both transcriptional and translational level (Korth et al. 2000). In A. annua L., β-pinene synthase was found to have a circadian pattern of gene expression, accompanied by a similar temporal pattern of β-pinene emission under light exerting a stimulatory effect (Lu et al. 2002). Analysis of root cultures of A. annua L. suggested that light also positively regulates artemisinin biosynthesis because the root cultures exhibited a substantial decrease in artemisinin content when moved from light to dark (Liu et al. 1997, 2002; Guo et al. 2004). Hong et al. (2009), hence, overexpressed Arabidopsis blue light receptor CRY1 in A. annua L. to evaluate its effect on artemisinin synthesis and accumulation. They found that overexpression of CYP1 gene had resulted in increased accumulation of both artemisinin (30–40 %) and anthocyanins (2-fold) as compared to control plants.
11.5.1 Combinatorial Biosynthesis of Artemisinin
Naturally occurring terpenoids are produced in small quantities, and thus, their purification results in low yields. Further, the complex structure of these molecules makes chemical synthesis challenging and often uneconomical due to poor yields. Metabolic engineering of these pathways in a common industrial biological host (Escherichia coli) offers an attractive alternative to extractions from plants or chemical syntheses for producing large quantities of these complex molecules. To accomplish this goal may require altering the MVA and the MEP pathways along with addition of very specialized enzymes, e.g., ADS. Based on preliminary work by others who described engineering of the MEP pathway to increase isoprenoid precursors for high-level production of carotenoids (Kajiwara et al. 1997; Farmer and Liao 2001; Kim and Keasling 2001; Abdin et al. 2003), Keasling’s group further developed a base technology for production of amorphadiene in E. coli (Martin et al. 2003). Bacteria already contain the MEP pathway for production of IPP/DMAPP, but they lack the MVA pathway. Keasling’s group posited that the MEP pathway is likely linked to unknown control elements in bacteria and that direct alteration might impair growth. Instead, they added a truncated MVA pathway from Saccharomyces cerevisiae that was coupled to ADS in E. Coli resulting in good bacterial growth and high-level production of amorphadiene estimated at 100 mg l 21 in 12 h. Keasling’s work is important because these engineered E. coli strains can serve as platform hosts for the production of essentially any terpenoid for which the biosynthetic genes are available because IPP and DMAPP produced by either arm of the terpenoid pathway are universal precursors to all terpenoids. More recently, (Teoh et al. 2006) have isolated the next enzyme in the artemisinin pathway, a cytochrome P450 enzyme (CYP71AV1); this enzyme appears to catalyze the next three steps in artemisinin biosynthesis, an enzymatic function also confirmed by Keasling’s group (J. Keasling, personal communication). Once cloned into a bacterial host and after optimization of the culture conditions, it should be possible to produce very large quantities of a close precursor to artemisinin in E. coli, thus making this important drug readily available in much larger quantities than previously thought possible. The concept of E. coli as a host cell producing sesquiterpenoids out of the endogenous pool of FDP has been investigated (Martin et al. 2001). This work resulted in the production of 10.3 μg of (+)-δ-cadinene, 0.24 μg of 5-epi-aristolochene, or 6.4 μg vetispiradiene per liter of bacterial culture. Furthermore, the authors concluded that the poor expression of the plant terpene cyclases was limiting for the synthesis of sesquiterpenes and not the endogenous supply of FDP. This has been confirmed in their further work by coexpressing the E. coli dxs gene, which did not result in an increase in sesquiterpenoids produced where it did result in an increase in lycopene production in E. coli (Harker and Bramley 1999; Kim and Keasling 2001). To overcome the low enzyme levels, the expression of amorphadiene synthase has been optimized by constructing a synthetic amorphadiene synthase gene completely optimized for the expression in the bacterial host. This strategy has been combined with engineering of genes from the mevalonate-dependent isoprenoid pathway (Fig. 11.4), which resulted in an E. coli strain producing 24 μg/ml amorpha-4,11-diene (calculated as caryophyllene equivalent) from acetyl-CoA after supplementation of 0.8 % glycerol (Martin et al. 2003).
Recently, attempts to use S. cerevisiae for the production of artemisinin precursors have been described. The expression of the amorphadiene synthase gene in yeast using plasmids and chromosomal integration led to the production of, respectively, 600 and 100 μg/amorpha-4, 11-diene after 16 days batch cultivation (Lindahl et al. 2006). Using a S. cerevisiae strain containing an engineered MVA pathway coupled with the genes encoding amorphadiene synthase and CYP71AV1, the production of artemisinic acid up to 100 mg/l has been reported (Ro et al. 2006). This strain transported the artemisinin precursor outside the yeast cell, which makes purification of the product less complex. Artemisinic acid can be used for the semisynthesis of artemisinin, but to lower the costs for production of the drug, bioprocessing must be optimized (Liu et al. 1998).
Dafra Pharma International NV and Plant Research International (PRI) have initiated new research to produce artemisinin via genetically modified chicory plants. In studies carried out at Wageningen, the complete biosynthetic pathway of artemisinin was resolved (de Kraker et al. 2003; Bertea et al. 2005; Fig. 11.5).
In addition, the Wageningen group, headed by Prof. Harro Bouwmeester and Dr. Maurice Franssen, demonstrated that chicory enzyme(s) normally involved in the biosynthesis of the bitter sesquiterpene lactones in chicory, were capable of performing reactions required for the biosynthesis of artemisinin (de Kraker et al. 2003). The group of Prof. Bouwmeester has tried to produce the chemical precursor for artemisinin (dihydroartemisinic acid) in the roots of chicory via a diversion of the biosynthesis of bitter compounds. On the other hand, the group of Prof. Bouwmeester has shown in a wide range of plant species that diversion of the biosynthesis of terpenes can be carried out very efficiently (Kappers et al. 2005). Moreover, they also demonstrated that up to 40 kg ha−1 dihydroartemisinic acid can be produced using genetically modified chicory.
11.5.2 Breeding and Marker-Assisted Breeding
Because the A. annua is a hybrid species, these transformed strains can only be preserved in flasks in the laboratory and the characteristic of high artemisinin content will be lost through sexual propagation. Although the transgenic strains can be multiplied on a large scale using micropropagation methods, the cost is high.
By bringing herbs into cultivation, traditional and biotechnological plant-breeding techniques can be applied at the genetic level to improve yield and uniformity, and to modify potency or toxicity. The high heritability and useful range of variation for artemisinin suggests that the development of molecular tags for the trait and their exploitation in a marker-assisted breeding program are feasible (Delabays et al. 2001). Although the impact to date has been minimal, it is certain that the ‘-omics’ revolution, as it spreads out from model species to those with more complex genomes (so-called muddle species) will influence research and exploitation of medicinal species as it will plant in general.
Increasing the production of active phytochemical constituents is a well-established target for genetic manipulation but presents some severe challenges. In particular, the metabolic pathways by which active compounds are biosynthesized are mostly poorly understood, and relatively few genes for key enzymatic or regulatory steps have been isolated. Nevertheless, there are examples of pathway engineering leading to improvements of potential value in the breeding of medicinal plants (Ferreira and Duke 1997; Charlwood and Pletsch 2002).
The artemisinin content is distinctly different in A. annua of diverse origins. Although the artemisinin content in leaves is influenced by environmental factors and the developmental situation of the herb, the primary factor contributing to variations in the artemisinin content is genetic (Delabays et al. 2001). So, it is important to analyze genetic variations of different strains of annua that have different artemisinin contents. To evaluate the availability of the genetic variability of strains of A. annua, (Zhang et al. 2006) performed RAPD analysis of selected chemotypes. The data clearly supported the conclusion of distinct variation of heredity among these chemotypes (Sangwan et al. 1999). Studying the variation of heredity between high- and low-yielding strains by RAPD techniques is more accessible for the selection and breeding of high-yielding strains of A. annua. In the present study, the OPA151000 band could be used as a marker to predict the strains with high artemisinin content.
A recent project in our department includes the study of the production of an antimalarial compound, artemisinin in A. annua. Artemisinin is currently still extracted from the plant itself, but due to the low amount of artemisinin in the plant, there is a shortage in the production (Cyranoski 2004). In the case of A. annua, it has been shown that the genetic variability linked to artemisinin content can be used to generate improved high-yielding varieties (Delabays et al. 2001); thus, it is likely that genetic factors will be identified that are involved in the low or high artemisinin trait. A biosynthetic pathway of artemisinin has been proposed, but most of the genes underlying the synthesis and the control of it, are not yet identified (Bertea et al. 2005; (Bouwmeester et al. 1999) in press). In our department, the biosynthesis of artemisinin will be studied using cDNA–AFLP gene profiling. The knowledge of genes activated upon artemisinin biosynthesis, combined with metabolome data from the same time points as used for the transcript profiling, will allow us to identify key genes, encoding enzymes and/or transcription factors involved in the biosynthesis of artemisinin and the regulation of this pathway. This information will be the basis for a more detailed study, which will ideally give the information needed to engineer a high-artemisinin-producing plant through metabolic engineering.
11.5.3 Current Status and Future Prospective
Knowledge of the exact biosynthesis of artemisinin should enable us to influence its formation in a direct way, for example by metabolic engineering. As an alternative to targeting an individual rate-limiting enzyme reaction, exploiting transcription factors that turn whole secondary pathways on or off shows great promise as a metabolic engineering strategy. New genomic approaches and efficient gene isolation methods applied to difficult secondary pathways in medicinal plant metabolism will undoubtedly expand the range and precision of manipulations via transgenesis, providing potentially superior material for the breeder. Plant natural products have been a very productive source in drug development. The study of plant secondary metabolism is a fully expanding and challenging field in molecular biology and biotechnology, with many opportunities ahead. New tools of functional genomics combined with metabolomics and proteomics will revolutionize our knowledge on the pathways and enzymes involved in the synthesis of natural products and thus allow a more focused approach for their production. With the increasing need for novel drugs for newly identified molecular targets, this field will likely become increasingly relevant. The appealing economic aspects of large-scale production of pharmaceuticals in plants could attract increasing investments and create new opportunities in this promising research field. It would be interesting to develop transgenic plants of A. annua to ensure a constant high production of artemisinin after the introduction of genes encoding enzyme(s) regulating the biosynthesis pathway of artemisinin.
11.5.4 Conclusion and Summary
A. annua is the main source of artemisinin, the potent and efficacious antimalarial after quinine. Recently, artemisinin has also been proved to be a selective anticancer drug (Moore et al. 1995; Efferth et al. 2001). Currently, the limited availability of artemisinin and the lack of real competition among producers of raw material seem to be major barriers to scaling-up production and are partially responsible for its high price (World Bank 2003). Also, the lack of affordable certified seeds hampers the extension of A. annua cultivation around the world. Breeding high-yielding, late-flowering cultivars of A. annual adapted to the tropics, where malaria is endemic, is a desirable approach that needs to be pursued. Scientists are trying to understand the intricate and self-regulated biosynthetic pathway of artemisinin, its potential increase by the manipulation of terpene cyclase genes, although commercially feasible results are still to be seen. Currently, the hope to curb malaria rests on hampering the spread of the disease by mosquito vectors, on the availability of an effective and affordable vaccine, on the widespread use of insecticide-treated nets, on new antimalarial drugs effective against multidrug-resistant Plasmodium, and on meeting the world demand for artemisinin-combination treatments. Of course this last factor depends on a steady production of artemisinin, at affordable prices, to meet global demand. Although field production of A. annua is presently the most commercially feasible approach to produce artemisinin and related compounds, farmers must have access to good-quality seed generated from high-artemisinin parents. Although these seeds do not constitute ‘true hybrids’ because the parents are not homozygous, artemisinin content found currently in seeds available for research is approximately twice as high as it was 10 years ago (1.0 % compared to less than 0.5 %). Also, the agricultural aspects of artemisinin production such as soil fertility and pH, plant density, water availability, latitude and altitude, hormones, harvesting and drying protocols must be fine-tuned for each geographic area where artemisinin is to be produced as a raw material.
In addition, factors that affect temporal (when artemisinin reaches its maximum) or spatial (tissue localization) accumulation must not be ignored when evaluating the commercial potential of A. annua as a new crop for tropical or temperate regions. Artemisinin-based combination therapies (ACTs) have been long considered more effective than the existing drugs. ACTs are much more expensive than other drugs because of the relatively low yields of artemisinin in A. annua. Therefore, there have been many efforts to enhance the production of artemisinin in vivo and in vitro by biotechnology. Even though viable methods of increasing artemisinin content, e.g., A. annua organ culture, hormone medium, and metabolic manipulation, have been investigated and show potential for future development, the improvements delivered by these methods have not yet met the demand. To increase the yield of artemisinin by biotechnology, it is necessary to study the enzymatic pathway. Enzymes and precursors involved in the artemisinin biosynthesis have to be isolated and characterized. In recent years, many researchers have focused their efforts on investigating the molecular regulation of artemisinin biosynthesis and the genes coding for the key enzymes involved in the artemisinin biosynthesis. The high efficiency of genetic transformation and regeneration procedure developed by (Han et al. 2006) allows the manipulation of artemisinin biosynthesis by genetic methods. By genetic engineering, we can overexpress the key enzymes involved in biosynthesis of artemisinin or inhibit the enzymes involved in other pathways competing for its precursors to obtain transgenic high-yielding. A. annua. Although greatly improved yields were obtained by combining the expression of a synthetic sesquiterpene synthase with a recombinant mevalonate pathway, the data suggest that a maximum yield was not attained. Furthermore, in vitro evolution and combinatorial biosynthesis of sesquiterpene biochemical pathways in microbes may lead to artemisinin derivatives or even new sesquiterpene compounds. Efforts, therefore, are being made to enhance the production of artemisinin both in vivo and in vitro. Chemical synthesis of artemisinin is very complex and uneconomical. Breeding of high-artemisinin-yielding plants as well as the manipulation of culture conditions, growth media, and hormone levels to increase the yield of artemisinin in tissue and cell culture have not been successful. It is, therefore, essential to look for non-conventional, alternate strategies, which are economically viable for commercial production of artemisinin. Two approaches can be used to achieve this goal. The first approach could be the use of a semisynthetic route for the synthesis of artemisinin from its simple precursors such as artemisinic acid and arteannuin B. The second approach could involve the use of genetic engineering to overexpress enzyme(s) catalyzing the rate-limiting steps of artemisinin biosynthesis or by using antisense RNA technology to inhibit the enzyme(s) of other pathway competing for its precursors.
References
Abdin MZ, Israr M, Rehman RU, Jain SK (2003) Artemisinin, a novel antimalarial drug: biochemical and molecular approaches for enhanced production. Planta Med 69:289–299
Acton N, Klayman DL, Rollman IJ (1985) Reductive electrochemical HPLC assay for artemisinin (qinghaosu). Planta Med 5:445--446
Acton N, Roth RS (1985) On the conversion of dihydroartemisinic acid into artemisinin. J Org Chem 57:3610–3614
Akhila A, Kumkum R, Thakur RS (1990) Biosynthesis of artemisinin in Artemisia annua. Phytochemistry 29:2129–2132
Akiyoshi DE, Klee H, Amasino R, Nester EW, Gordon MP (1984) T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci USA 81:5994–5998
Aquil S, Husaini AM, Abdin MZ, Rather GN (2009) Overexpression of HMG-CoA reductase gene leads to enhanced artemisinin biosynthesis in transgenic A. annua L. plants. Planta Med 75:1–6
Attucci S, Aitken SM, Ibrahim RK, Gulick PJA (1995) cDNA encoding farnesyl pyrophosphate synthesis in white lupine. Plant Physiol 108:835–836
Avery MA, Chong WKM, Jennings-White C (1992) Stereoselective total synthesis of (+)-artemisinin the antimalarial constituent of Artemisia annua L. J Am Chem Soc 114:974–979
Balachandran S, Vishwakarma RA, Popli SP (1987) Chemical investigation of some Artemisia annua Species: search for artemisinin or other related sesquiterpene lactones with a peroxides bridge. Ind J Pharma Sci 49:152--154
Barkovich R, Liao JC (2001) Metabolic engineering of isoprenoids. Metab Eng 3:27--39
Bertea CM, Freije JR, van der Woude H, Verstappen FW, Perk L, Marquez V, de Kraker JW, Posthumus MA, Jansen BJ, de Groot A, Franssen MC, Bouwmeester HJ (2005) Identification of intermediates and enzymes involved in the early steps of artemisinin biosynthesis in Artemisia annua. Planta Med 71:40–47
Borrmann S, Szlezak N, Faucher JF, Matsiegui PB, Neubauer R, Biner RK, Lell B, Kremsner PG (2001) Artesunate and praziquantel for the treatment of Shistosoma haematobium infections: a doubleblind, randomized, placebo-controlled study. J Infect Dis 184:1363–1366
Bouwmeester HJ, Wallaart TE, Janssen MH, van Loo B, Jansen BJ, Posthumus MA, Schmidt CO, de Kraker JW, Knig WA, Franssen MC (1999) Amorpha-4,11-diene synthase catalyze the first probable step in artemisinin biosynthesis. Phytochemistry 52:843–854
Brown GD (1994) Secondary metabolism in tissue culture of Artemisia annua. J Nat Prod 57(7):975–977
Brown GD, Sy LK (2004) In vivo transformations of dihydroartemisinic acid in Artemisia annua plants. Tetrahedron 60:1139–1159
Cai Y, Jia JW, Crock J, Lin ZX, Chen XY, Croteau RA (2002) cDNA clone for bcaryophyllene synthase from Artemisia annua. Phytochem 61:523--529
Cane DE (1990) Enzymatic formation of sesquiterpenes. Chem Rev 90:1089–1103
Chang YJ, Song SH, Park SH, Kim SU (2000) Amorpha-4, 11-diene synthase of Artemisia annua: cDNA isolation and bacterial expression of a terpene synthase involved in artemisinin biosynthesis. Arch Biochem Biophys 383:178–184
Charels DJ, Cebert E, Simon JE (1991) Characterization of essential oils of Artemisia annua L. J Ess Oil Res 3:33--39
Charles DJ, Simon JE, Wood KV, Heinsten P (1990) Germplasm variation in artemisinin content of Artemisinin annua using an alternative method of artemisinin analysis from crude plant extracts. J Nat Prod 53:157–160
Charlwood BV, Pletsch M (2002) Manipulation of natural product accumulation in plants through genetic engineering. J Herbs Spices Med Plants 9:139–151
Chaudhary V, Kapoor R, Bhatnagar AK (2008) Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua L. Appl Soil Ecol 40:174–181
Chen DH, Meng Y, Ye HC, Li GF, Chen XY (1998) Cultures of transgenic Artemisia annua hairy root with cadinene synthase gene. Acta Bot Sin 40:711–714
Chen D, Ye H, Li G (2000) Expression of a chimeric farnesyl diphosphate synthase gene in Artemisia annua L. transgenic plants via Agrobacterium tumefaciens-mediated transformation. Plant Sci 155:179–185
Chen D, Liu C, Ye H, Li G, Liu B, Meng Y, Chen X (1999) Ri-mediated transformation of Artemisia annua with a recombinant farnesyl diphosphate synthase gene for artemisinin production. Plant Cell, Tissue Organ Cult 57:157–162
Chen FT, Zang GH (1987) Studies on several physiological factors on artemisinin synthesis in Artemisia annua L. Plant Physiol 5:26--30
CIMAP (India) Annual Project Report (1988--1989) Development of Agrotechnologies for Artemisia annua for antimalarial drug, artemisinin
CIMAP (India) Annual Project Report (1986--1987) Development of Agrotechnologies for Artemisia annua for antimalarial drug, artemisinin
Cyranoski D (2004) Campaign to fight malaria hit by surge in demand for medicine. Nature 432(7015):259
de Kraker J, Schurink M, Franssen MCR, König WA, Groot A, Bouwmeester HJ (2003) Hydroxylation of sesquiterpenes by enzymes from chicory (Cychorium intybus L.) roots. Tetrahedron 59:409–418
Delabays N, Benakis A, Collet G (1993) Selection and breeding for high artemisinin (qinghaosu) yielding strains of Artemisia annua. Acta Hort 330:203–206
Delourme D, Lacroute F, Karst F (1994) Cloning of an Arabidopsis thaliana cDNA coding for farnesyl diphosphate synthase by function complementation in yeast. Plant Mol Biol 26:1867–1873
Delabays N, Simonnet X, Gaudin M (2001) The genetics of artemisinin content in Artemisia annua and the breeding of high yielding cultivars. Curr Med Chem 8:1795–1801
Duke SO, Paul RN (1993) Development and fine structure of the glandular trichomes of Artemisia annua L. Int J Plant Sci 154:107–118
Duke MV, Paul RN (1994) Localization of artemisinin and artemisitene in foliar tissue of glanded and glandless biotypes of Artemisia annua. Int J Plant Sci 155:365–372
Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18:767–773
ElSohly HN (1990) A large-scale extraction technique of artemisinin from Artemisia annua. J Nat Prod 53:1560–1564
Farmer WR, Liao JC (2001) Precursor balancing for metabolic engineering of lycopene production in Escherichia coli. Biotechnol Prog 17:57--61
Ferreira JFS, Simon JE, Janick J (1995) Developmental studies of Artemisia annua: flowering and artemisinin production under greenhouse and field conditions. Planta Med 61:167–170
Ferreira JFS, Janick J (1996) Distribution of artemisinin in Artemisia annua. In: Janick J (ed) Progress in new crops. ASHS Press, Arlington, VA, pp 579–584
Ferreira JFS, Duke SO (1997) Approaches for maximizing biosynthesis of medicinal plant secondary metabolites. AgBiotech News Inf 9:309N–316N
Fulzele DP, Heble MR, Rao PS (1995) Production of terpenoid from Artemisia annua L. plantlet cultures in bioreactor. J Biotechnol 40:139–143
Van Geldre E, Vergauwe A, Eecdkhout EVD (1997) State of the art of the production of the antimalarial compound artemisinin in plants. Plant Mol Biol 33:199–209
Geng S, Ye HC, Li GF (2001) Effects of ipt gene expression on the physiological and chemical characteristics of Artemisia annua L. Plant Sci 160:691–698
Gershenzon and Croteau (1990) Gershenzon and Croteau regulation of monoterpene biosynthesis in higher plants. Recent Adv Phytochem 24:99–160
Gulati A, Bharel S, Jain SK, Abdin MZ, Srivastava PS (1996) In vitro micropropagation and flowering in Artemisia annua. J Plant Biochem Biotechnol 5:31–35
Guo C, Liu CZ, Ye HC, Li GF (2004) Effect of temperature on growth and artemisinin biosynthesis in hairy root cultures of Artemisia annua. Acta Bot Boreal-Occident Sin 24:1828–1831
Gupta MM, Jain DC, Mathur AK, Singh AK, Verma RK (1996) Planta Med 62:280
Han JL, Wang H, Ye HC, Liu Y, Li ZQ, Zhang Y, Zhang YS, Yan F, Li GF (2005) High efficiency of genetic transformation and regeneration of Artemisia annua L. via Agrobacterium tumefaciens-mediated procedure. Plant Sci 168:73–80
Han JL, Liu BY, Ye HC, Wang H, Li ZQ, Li GF (2006) Effects of overexpression of the endogenous farnesyl diphosphate synthesis on the artemisinin content in Artemisia annua L. J Integr Palnt Biol 48(4):482–487
Hardoim PR, van Overbeek LS, van Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Harker M, Bramley PM (1999) Expression of prokaryotic 1-deoxy-D-xylulose-5-phosphatases in Escherichia coli increases carotenoid and ubiquinone biosynthesis. FEBS Lett 448:115–119
Haynes KA, Caudy AA, Collins L, Elgin SCR (2006) Element 1360 and RNAi components contribute to HP1-dependent silencing of a pericentric reporter. Curr Biol 16(22):2222--2227
He XC, Zeng MY, Li GF, Liang Z (1983) Callus induction and regeneration of plantlets from Artemisia annua and changes of qinghaosu contents. Acta Bot Sin 25:87–90
Hong GJ, Hu WL, Li JX, Chen XY, Wang LJ (2009) Increased accumulation of artemisinin and anthocyanins in Artemisia annua expressing the Arabidopsis blue light receptor CRY1. Plant Mol Biol Rep. doi:10.1007/s11105-008-0088-6
Jaziri M, Shimonurec K, Yoshimatsu K, Fauconmiez ML, Marlier M, Homes J (1995) Establishment of normal and transformed root cultures of Artemisia annua L. for artemisinin production. J Plant Physiol 145:175--177
Jha P, Ram M, Khan MA, Zhaffar M, Abdin MZ (2011) Impact of organic manure and chemical fertilizers on artemisinin content and yield in Artemisia annua L. Ind Crops Prod 33(2):296–301
Jing F, Zhang L, Li M, Tang K (2008) Over-expressing cyp71av1 and cpr genes enhances artemisinin content in Artemisia annua L. J Agric Sci Technol 10:64–70
Jung M, ElSohly HN, Mc Chesney JD (1990) Artemisinic acid: a versatile chiral synthon and bioprecursor to natural products. Planta Med 56:624
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421--426
Kasahara H, jiao Y, Bedgar DL, Kim SJ, Patten AM, Xia ZQ, Davin LB, Lewis NG (2006) Pinus taeda phenylpropenal double-bond reductase: purification, cDNA cloning, heterologous expression in Escheria Ccoli, and subcellular localization in P. taeda. Phytochem 67:1765--1780
Kapoor R, Chaudhary V, Bhatnagar AK (2007) Effects of arbuscular mycorrhiza and phosphorus application on artemisinin concentration in Artemisia annua L. Mycorrhiza 17:581–587
Kappers IF, Aharoni A, Vanherpen TWJM, Luckerhoff LLP, Dick M, Bouwmeester HJ (2005) Genetic engineering of terpenoid metabolism attracts bodyguards to Arabidopsis. Science 309:2070–2072
Kim YJ, Wyslouzil BE, Weathers PJ (2002) Secondary metabolism of hairy root cultures in bioreactors. In Vitro Cell Dev Biol Plant 38:1–10
Kim SW, Keasling JD (2001) Metabolic engineering of the nonmevalonate isopentenyl diphosphate synthesis pathway in Escherichia coli enhances lycopene production. Biotechnol Bioeng 72: 408--415
Kim YJ, Weathers PJ, Wyslouzil BE (2003) Growth dynamics of Artemisia annua hairy roots in three culture systems. Biotechnol Bioeng 83:428–443
Klayman DL (1989) Weeding out malaria. Nat Hist October:18–26
Klayman DL (1993) Artemisia annua: from weed to respectable antimalarial plant. In: Kinghorn AD, Balandri MF (eds) Human medicinal agents from plants. American Chemical Society, Washington, DC, pp 242–255
Klayman DL (1985) Qinghaosu (Artemisinin): an antimalarial drug from China. Science 228:1049–1055
Klayman DL, Lin AJ, Acton N, Scovill JP, Hock JM, Milhous WK, Theoharides AD (1984) Isolation of artemisinin (qinghaosu) from Artemisia annua growing in the United States. J Nat Prod 47:715–717
Korth KL, Jaggard DAW, Dixon RA (2000) Development and light regulated post translational control of 3-hydroxy 3-methylglutary coenzyme A reductase levels in potato. The Plant J 23:507--516
Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci USA 102:8369--8374
Krishna S, Uhlemann AC, Haynes RK (2004) Artemisinins: mechanisms of action and potential for resistance. Drug Resist Updat 7:233–244
Krushkal J, Pistilli M, Ferrell KM, Souret FF, Weathers PJ (2003) Computational analysis of the evolution of the structure and function of 1-deoxy-D-xylulose-5-phosphate synthase, a key regulation of the mevalonate-independent pathway in plants. Gene 313:127--138
Kudakasseril GJ, Lukem L, Stabam EJ (1987) Effect of sterol inhibitors on incorporation of 14C-isopentenyl pyrophosphate into artemisinin by a cell free system from Artemisia annua tissue culture and plants. Planta Med 53:280–284
Kumar S, Khanuja SPS, Shasany AK, Darokar MP (1999) “Jeevan Raksha” from an isolated population containing high artemisinin in foliage (0.5–1.0%). J Med Arom Plant Sci 21:47–48
Laughlin JC (2002) Post-harvest drying treatment effects on antimalarial constituents of Artemisia annua L. Acta Hortic 576:315–320
Laughlin JC (l994) Agricultural production of artemisinin: a review. Trans Royal Soc Trop Med Hyg 88 (Suppl.1):21–22
Liersch R, Soicke H, Stehr C (1986) Formation of artemisinin in Artemisia annua during one vegetation period. Planta Med 52:387–388
Li J, Zhao G-Z, Huang H-Y, Qin S, Zhu W-Y et al (2012a) Isolation and characterization of culturable endophytic actinobacteria associated with Artemisia annua L. Antonie Van Leeuwenhoek 101:515–527
Li J, Zhao G-Z, Varma A, Qin S, Xiong Z, Huang H-Y, Zhu W-Y, Zhao L-X, Xu L-H, Zhang S, Li W-J (2012b) An endophytic pseudonocardia species induces the production of artemisinin in Artemisia annua. PLoS ONE 7:e51410
Li Y, Huang H, Wu YL (2006) Qinghaosu artemisinin a fantastic antimalarial drug from a traditional Chines medicine. In: Liang X, Fang WS (ed), Medicinal Chemistry of Bioactive Natural Products. John Wiley & Sons, Inc, pp. 183--256
Lindahl AL, Olsson ME, Mercke P, Tollbom O, Schelin J, Brodelius M, Brodelius PE (2006) Production of the artemisinin precursor amorpha- 4,11-diene by engineered Saccharomyces cerevisiae. Biotechnol Lett 28:571--580
Liu Y, Wang H, Ye HC, Li GF (2005) Advances in the plant isoprenoid biosynthesis pathway and its metabolic engineering. J Integr Plant Biol 47:769--782
Liu Y, Ye HC, Wang H, Li GF (2003) Molecular cloning, Escherichia coli expression and genomic organization of squalene synthase gene from Artemisia annua. Acta Bot Sin 45:608--613
Liu C, Zhao Y, Wang Y (2006) Artemisinin: current state and perspectives for biotechnological production of an antimalarial drug. Appl Microbiol Biotechnol 72:11–20
Liu CZ, Guo C, Wang YC, Ouyang F (2002) Effect of light irradiation on hairy root growth and artemisinin biosynthesis of Artemisia annua L. Process Biochem 38:581–585
Liu CZ, Wang YC, Ouyang F, Ye HC, Li GF (1999) Improvement of artemisinin accumulation in hairy root culture of Artemisia annua L. by fungal elicitor. Bioprocess Eng 20:161–164
Liu CZ, Wang YC, Ouyang F, Ye HC, Li GF (1997) Production of artemisinin by hairy root culture of Artemisia annua L. Biotechnol Lett 19:927–929
Liu BY, Ye HC, Li GF (1998) Studies on dynamic of growth and artemisinin biosynthesis of hairy root of Artemisia annua L. Chin J Biotechnol 14:401–404
Lu S, Xu R, Jia J, Pang J, Matsuda SPT (2002) Chen, X. Cloning and functional characterization of a b-pinene synthase from Artemisia annua that shows a circadian pattern of expression. Plant Physiol 130:477-486
Luo XD, Shen CC (1987) The chemistry, pharmacology and clinical application of (qinghaosu) artemisinin and its derivatives. Med Res Rev 7:29–52
Mc garry and Croteau (1995) Terpenoid metabolism. Plant Cell 7(7):1015–1026
McVaugh R (1984) A descriptive account of the vascular plants of Western Mexico. In: Andersohn WR (ed.) Flora Novo-Galiciana, vol 12. Compositae. University of Michigan Press, Ann Arbor
Martinez BC, Staba EJ (1988) The production of artemisinin in Artemisia annua L. tissue cultures. Adv Cell Cult 6:69–87
Martin VJ, Yoshikuni Y, Keasling JD (2001) The in vivo synthesis of plant sesquiterpenes by E. coli. Biotechnol Bioeng 75:497--503
Martin VJJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802
Mercke P, Crock J, Croteau R, Brodelius PE (1999) Cloning, expression, and characterization of epi-cedrol synthase, a sesquiterpene cyclase from Artemisia annua L. Arch Biochem Biophys 369:213--222
Mercke P, Bengtsson M, Bouwmeester HJ, Posthumus MA, Brodelius PE (2000) Molecular cloning, expression and characterization of amorpha-4, 11-diene synthase, a key enzyme of artemisinin biosynthesis in Artemisia annua L. Arch Biochem Biophys 381:173–180
Mohapatra PK, Khan AM, Prakash A, Mahanta J, Srivastava VK (1996) Effect of arteether alpha/beta on uncomplicated falciparum malaria cases in Upper Assam. Ind J Med Res 104:284–287
Matsushita Y, Kang WY, Charlwood BV (1996) Cloning and analysis of a cDNA encoding farnesyl diphosphate synthase from Artemisia annua. Gene 172:207–209
Moore JC, Lai H, Li J-R, Ren R-L, McDougall JA, Singh NP, Chou C-K (1995) Oral administration of dihydroartemisinin and ferrous sulfate retarded implanted fibrosarcoma growth in the rat. Cancer Lett 98:83–87
Morales MR, Charles DJ, Simon JE (1993) Seasonal accumulation of artemisinin in Artemisia annua L. Acta Hort 344:416–420
Mueller MS, Karhagombc IB, Hirt HM, Wemakor E (2000) The potential of Artemisia annua L. as locally produced remedy for malaria in the tropics: agricultural, chemical and clinical aspects. Ethanopharmacol 73:487–493
Nair MS, Acton N, Klayman DL, Kendrick K, Basile DV, Mante S (1986) Production of artemisinin in tissue cultures of Artemisia annua. J Nat Prod 49(3):504–507
Newman JD, Chappell J (1999) Isoprenoid biosynthesis in plants: Carbon partitioning within the cytoplasmic pathway. Crit Rev Biochem Mol Biol 34:95--106
Newman JD, Marshall J, Chang MCY, Nowroozi F, Paradise E, Pitera D, Newman KL, Keasling JD (2006) High-level production of amorpha-4,11-diene in a two phase partitioning bioreactor of metabolically engineered Escherichia coli. Biotechnol Bioeng 95:684–691
Newton P, White N (1999) Malaria: new development in treatment and prevention. Ann Rev Med 50:179–192
Paniego NB, Giulietti AM (1996) Artemisinin production by Artemisia annua L.-transformed organ cultures. Enzyme Microb Technol 18:526–530
Paniego NB, Giulietti AM (1994) Artemisia annua L.: dedifferentiated and differentiated cultures. Plant Cell Tiss Organ Cult 36:163–168
Picaud S, Olifsson L, Brodelius PE (2005) Expression, purification and characterization of recombinant amorpha-4,11-diene synthase from Artemisia annua L. Arch Biochem Biophys 436:215–226
Ping ZQ, Chang Z, Lulu Y, Yi YR, Mei ZX, Ying H, Ling FL, Qiu YX (2008) Cloning of artemisinin biosynthetic cDNA and novel gene overexpression. Sci China Ser C-Life Sci 51:232–244
Pras N, Visser JF, Batterman S, Woerdenbag HJ, Maligre TM, Lugt CB (1991) Laboratory selection of Artemisia annua L. for high artemisinin yielding types. Phytochem Anal 2:80–83
Qin MB, Li GZ, Yun Y, Ye HC, Li GF (1994) Induction of hairy root from Artemisia annua with Agrobacterium rhizogenes and its culture in vitro. Acta Bot Sin 36(Suppl):165–170
Ram M, Gupta MM, Dwivedi S, Kumar S (1997) Effect of plant density on the yields of artemisinin and essential oil in Artemisia annua cropped under low input cost management in North-Central India. Planta Med 63:372–374
Ram M, Khan MA, Jha P, Khan S, Kiran U, Ahmad MM, Javed S, Abdin MZ (2010) HMG-CoA reductase limits artemisinin biosynthesis and accumulation in Artemisia annua L. Plants. Acta Physiol Plant 32:859–866
Ranasinghe A, Sweatlock JD, Cooks RG (1993) A rapid screening method for artemisinin and its congeners using MS/MS: search for new analogues in Artemisia annua. J Nat Prod 56:552–563
Riley EM (1995) The London School of Tropical Medicine: a new century of malarial research. Mem Inst Oswaldo Cruz 95:25–32
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Romero MR, Effeth T, Serrano MA, Castano B, Macias RI, Briz O, Martin JJ (2005) Effect of artemisinin/artesunate as inhibitors of hepatitis B virus production in an ‘in vitro’ system. Antiviral Res 68:75–83
Roth RJ, Acton N (1987) A simple conversion of artemisinic acid into artemisinin. Planta Med 53:501
Sa G, Mi M, He-chun Y, Ben-ye L, Guo-feng L, Kang C (2001) Effect of ipt gene expression on the physiological and chemical characteristices of Artemisia annua L. Plant Sci 160:691--698
Sangwan RS, Agarwal K, Luthra R, Thakur RS, Sangwan NS (1993) Biotransformation of arteannuic acid into arteannuin B and artemisinin in Artemisia annua. Phytochemistry 34:1301–1302
Sangwan RS, Sangwan NS, Jain DC, Kumar S, Ranade SA (1999) RAPD profile based genetic characterization of chemotypic variants of Artemisia annua L. Biochem Mol Biol Int 47(6):935–944
Satheesan J, Narayanan AK, Sakunthala M (2012) Induction of root colonization by Piriformospora indica leads to enhanced asiaticoside production in Centella asiatica. Mycorrhiza 22:195–202
Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P (2007) Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania dovani promastigotes. J Med Microbiol 56:1213–1218
Singh A, Kaul VK, Mahajan VP, Sing A, Misra LN, Thakur RS, Husain A (1986) Introduction of Artemisia annua in India and isolation of artemisinin, a promising antimalarial drug. Indian J Pharmaceut Sci 48:137–138
Singh A, Vishwakarma RA, Husain A (1988) Evaluation of Artemisia annua strain for higher artemisinin production. Planta Med 54:475–477
Singh M (2000) Effect of nitrogen, phosphorus and potassium nutrition on herb, oil and artemisinin yield of Artemsia annua under semi-arid tropical condition. J Med Aro Plant Sci 22/4A:368–369
Singh NP, Lai H (2001) Selective toxicity of dehydroartemisinin and holotransferrin on human breast cancer cells. Life Sci 70:49–56
Smith TC, Weathers PJ, Cheetham RC (1997) Effect of gibberellic acid on hair root cultures of Artemisia annua growth and artemisinin production. In vitro Cell Dev Biol Plant 33:75–79
Srivastava NK, Sharma S (1990) Influence of micronutrient imbalance on growth and artemisinin content in Artemisia annua. Ind J Pharm Sci 52:225
Shukla A, Abad Farooqi AH, Shukla YN, Sharma S (1992) Effect of tricontanol and chlormequat on growth, plant hormones and artemisinin yield in Artemisia annua L. Plant Growth Regul 11:165–171
Souret FF, Weathers PJ, Wobbe KK (2002) The mevalonateindependent pathway is expressed in transformed roots of Artemisia annua and regulated by light and culture age. In Vitro Cell Dev Biol Plant 38:581–588
Souret FF, Kim Y, Wyslouzil BE, Wobbe KK, Weathers PJ (2003) Scale-up of Artemisia annua L. hairy root cultures produces complex patterns of terpenoid gene expression. Biotechnol Bioeng 83:653–667
Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5:535–544
Sy LK, Brown GD (2002) The mechanism of the spontaneous autoxidation of dihydroartemisinic acid. Tetrahedron 58:897--908
Tawfiq NK, Anderson LA, Roberts MF, Phillipson JD, Bray DH, Warhurst DC (1989) Antiplasmodial activity of Artemisia annua plant cell cultures. Plant Cell Rep 8:425–428
Tazyeen N, Akmal M, Ram M, Alam P, Ahlawat S, Anis M, Abdin MZ (2010) Enhancement of artemisinin content by constitutive expression of HMG-CoA reductase gene in high yielding strain of Artemisia annua L. doi:10.1007/s11816-010-0156-x
Teo CKH, Yap AW, Chan KL, Gan EK, Tanaka M (1995) Artemisinin production from callus of Artemisia annua cultured in fluorocarbon polymer film culture bag. Asia-Pac J Mol Biol Biotechnol 3:317–321
Teoh KH, Polichuk DR, Reed DW, Nowak G, Covello PS (2006) Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett 580:1411–1416
Towler MJ, Weathers PJ (2007) Evidence of artemisinin production from IPP stemming from both mevalonate and nonmevalanate pathways. Plant Cell Rep 26:2129--2136
Trigg PL (1990) Qinghaosu as an antimalarial drug. Econ Med Plant Res 3:20–25
Utzinger J, Xiao S, N’Goran EK, Berquist R, Tanner M (2001) The potential of artemether for the control of shistosomiasis. Int J Parasitol 31:1549–1562
Van Agtmael MA, Eggetle TA, Van Boxtel CJ (1999) Artemisinin drugs in the treatment of malaria: from medicinal herb to registered medication. Trends Pharmacol Sci 20:199–204
van Geldre E, De Pauw I, Inze D, Van Montagu M, Van den Eeckhout E (2000) Cloning and molecular analysis of two new sesquiterpene cyclises from Artemisia annua L. Plant Sci 158:163--171
Van Wees SCM, Van der Ent S, Pieterse CMJ (2008) Plant immune responses triggered by beneficial microbes. Curr Opin Plant Biol 11:443–448
Varma A, Bakshi M, Lou B, Hartmann A, Oelmueller R (2012) Piriformospora indica: a novel plant growth-promoting Mycorrhizal Fungus. Agric Res 1:117–131
Vergauwe A, Cammaert R, Vandenberghe D, Genetello C, Van Montagu M, Vanden Eeckhout E (1996) Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. and regeneration of transgenic plant. Plant Cell Rep 15:929–937
Vergauwe A, Van GE, Inze D, Van DEE (1998) Factor influencing Agrobacterium tumefaciens-mediated transformation of Artemisia annua L. Plant Cell Rep 18:105–110
Wallaart TE, Van Uden W, Lubberink HG, Woerdenbag HJ, Pras N, Quax WJ (1999a) Isolation and identification of dihydroartemisinic acid from Artemisia annua and its possible role in the biosynthesis of artemisinin. J Nat Prod 62:430–433
Wallaart TE, Van Uden W, Lubberink HG, Woerdenbag HJ, Pras N, Quax WJ (1999b) Isolation and identification of dihydroartemisinic acid from Artemisia annua: a novel biosynthetic precursor of artemisinin. J Nat Prod 62:1160–1162
Wallaart TE, Pras N, Beekman AC, Quax WJ (2000) Seasonal variation of artemisinin and its biosynthetic precursors in plants of Artemisia annua of different geographical origin: proof for the existence of chemotypes. Plant Med 66:57–62
Wallaart TE, Boumeester HJ, Hille J, Poppinga L, Maijers NCA (2001) Amorpha-4,11-diene synthase: cloning and functional expression of a key enzyme in the biosynthetic pathway of the novel antimalarial drug artemisinin. Planta 212:460–465
Wang CW (1961) The forests of China, with a survey of grassland and desert vegetations. In: Harvard University Maria Moors Cabot Foundation No. 5. Harvard University Press, Cambridge, MA, pp 171–187
Wang JW, Tan RX (2002) Artemisinin production in Artemisia annua hairy root cultures with improved growth by altering the nitrogen source of the medium. Biotechnol Lett 24:1153–1156
Wang JW, Zhang Z, Tan X (2001) Stimulation of artemisinin production in Artemisia annua hairy roots by the elicitor from the endophytic Colletotrichum sp. Biotechnol Lett 23:857–860
Wang H, Ye HC, Li GF, Liu BY, Chong K (2000) Effects of fungal elicitors on cell growth and artemisinin accumulation in hairy root cultures of Artemisia annua. Acta Bot Sinica 42:905–909
Weathers P, Elkholy S, Wobbe KK (2006) Invited review: Artemisinin: The biosynthetic pathway and its regulation in Artemisia annua, a terpenoid-rich species. In Vitro Cell Dev Biol—Plant 42:309--317
Weathers PJ, Cheetham RD, Follansbee E, Tesh K (1994) Artemisinin production by transformed roots of Artemisia annua. Biotech Lett 16:1281–1286
Weathers PJ, Bunk G, Mccoy MC (2005) The effect of phytohormones on growth and artemisinin production in Artemisia annua hairy roots. In Vitro Cell Dev Biol Plant 41:47–53
Weathers PJ, DeJesus-Gonzalez L, Kim YJ (2004) Alteration of biomass and artemisinin production in Artemisia annua hairy roots by media sterilization method and sugars. Plant Cell Rep 23:414–418
Whipkey A, Simon JE, Charles DJ, Janick J (1992) In vitro production of artemisinin from Artemisia annua L. Phytother Res 1:15–25
Woerdenbag HJ, Lüers JFJ, van Uden W, Pras N, Malingré TM, Alfermann AW (1993) Production of the new antimalarial drug artemisinin in shoot cultures of Artemisia annua L. Plant Cell Tiss Org Cult 32:247–257
World Bank (2003) Expert Consultation on the Procurement & Financing of Antimalarial Drugs. Meeting Report, Draft 3. World Bank, Washington, DC, 7 Nov 2003
Xie DY, Wang LH, Ye HC, Li GF (2000) Isolation and production of artemisinin and stigmasterol in hairy root cultures of Artemisia annua. Plant Cell Tiss Org Cult 63:161–166
Xie DY, Zou ZR, Ye HC, Li GF, Guo ZC (2001) Selection of hairy root clones of Artemisia annua L. for artemisinin production. Isr J Plant Sci 49:129–134
Xu XX, Zhu J, Huang DZ, Zhou WS (1986) Total synthesis of arteannuin and deoxyarteannuin. Tetrahedron 42:819–828
Yaseen M, Tajuddin (1998) Effect of plant growth regulators on yield, oil composition and artemisinin from Artemisia annua under temperate condition. J Med Aromat Plant Sci 20:1038–1041
Yuan ZL, Dai CC, Chen LQ (2007) Regulation and accumulation of secondary metabolites in plant-fungus symbiotic system. Afr J Biotechnol 6:1266–1271
Zeng QP, Qiu F, Yuan L (2007) Production of artemisinin by genetically modified microbes. Biotechnol Lett. doi:10.1007/s10529-007-9596-y
Zhang L, Ye H, Li G (2006) Effect of development stage on the artemisinin content and the sequence characterized amplified region (SCAR) markers of high-artemisinin yielding strains of Artemisia annua L. J Intg Plant Biol 48:1054–1062
Zhang L, Jing F, Li F, Li M, Wang Y, Wang G, Sun X, Tang K (2009) Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA mediated gene silencing. Biotechnol Appl Biochem 52:199–207
Zhang YS, Liu B Y, Li ZQ, Ye HC, Wang HL, Guo-Feng HJL (2004) Molecular cloning of a classical plant peroxidase from Artemisia annua and its effect on the biosynthesis of artemisinin in vitro. Acta Bot Sin 46:1338--1346
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Ram, M., Jain, D.C., Mishra, H., Mandal, S., Abdin, M.Z. (2014). Recent Advances to Enhance Yield of Artemisinin: A Novel Antimalarial Compound, in Artemisia annua L. Plants. In: Aftab, T., Ferreira, J., Khan, M., Naeem, M. (eds) Artemisia annua - Pharmacology and Biotechnology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-41027-7_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-41027-7_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-41026-0
Online ISBN: 978-3-642-41027-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)