Abstract
The interaction between mineral structures and living beings is increasingly attracting the interest of research. The formation of skeletons, geomicrobiology, the study of the origin of life, soil biology, benthos biology, human and mammalian diseases generated by the inhalation of dust and biomaterials are some examples of scientific areas where the topic has a relevance. In this chapter we focus on cell reactivity to siliceous rocks and to the various forms of silicon dioxide, in particular. The examples here reported carefully review how such minerals may strongly affect different living beings, from simple ones to humans. The biomineralogy concept is explained, focusing on the effects of rocks on cell growth and development. The toxic action of silicon dioxide in mammalian lungs is the oldest evidence of crystalline silica bioactivity. More recently, we could demonstrate that crystalline silica has a deep impact on cell biology throughout the whole animal kingdom. One of the most illustrative case studies is the marine sponge Chondrosia reniformis, which has the amazing ability to incorporate and etch crystalline silica releasing dissolved silicates in the medium. This specific and selective action is due to the chemical reaction of ascorbic acid with quartz surfaces. One consequence of this is an increased production of collagen. The discovery of this mechanism opened the door to a new understanding of silica toxicity for animal cells and mammalian cells in particular. The presence of silica in sea water and substrates also affects processes like the settlement of larvae and the growth of diatoms. The following sections review all such aspects.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Biomineralogy: Minerals Shaping Organisms, Organism Shaping Minerals
The ways nature shapes the life on Earth are so original that man has just to observe and get inspired. Biomimetics, the science that seeks innovative technologies by emulating nature’s time-tested ideas, dates back to the first man who observed in awe a natural phenomenon and tried imitation. Among the early scholars of such a science, the best known is likely Leonardo da Vinci who, in his Codex of Flight, describes the flight of birds and draws detailed sketches of several flying machines. Nature fascinates scientists and engineers with numerous examples of highly efficient building materials. These often show a complex hierarchical organisation from the nano- to the macroscopic scale. Biomineralogy is the science that studies biomineralisation processes (Bouligand 2004), i.e. the way living organisms can model the mineral world. Thanks to biomimetics and biomineralogy, biomaterials are increasingly considered a promising avenue towards a genuine “chimie douce” for the building of innovative tools for technology and biomedicine. Today, biomimetics and biomineralogy are gaining a strong foothold in the scientific arena, with exponential growth in the number of publications and active research groups in academia and, to a lesser extent yet, in industry. Botanists and zoologists working on the morphofunctional adaptations of living organisms have a “front row seat” in the discipline. They disseminate their observations to fertilise the studies on the mechanisms involved in the construction of these original natural models and on the way society can benefit from them.
The mineral and the living worlds are compatible—since the latter arose from the former—and still coexist, so that the study of mineral metabolism and of skeleton formation could provide ideas for experiments in pure inorganic chemistry. In this perspective, the concept of biomineralogy has been reinterpreted (Cerrano et al. 1999a), considering not only how living organisms can handle mineral elements to build amazing and low-energy frameworks (e.g. diatom shells, siliceous spicules, calcareous sclerites) but also how ambient mineral elements affect living organisms. This has been embedded in clinical studies since the eighteenth century but is still poorly considered by marine biologists and ecologists. If we look at the species that are able to incorporate foreign mineral particles, the relevance of the interactions between living and nonliving realms can be better realised. Several metazoans use foreign bodies to set up frameworks. However, these are usually external protective structures and no incorporation of foreign bodies inside tissues occurs. In demosponges and zoanthids, on the contrary, foreign bodies are actively incorporated in their tissues. Among Porifera, sediment incorporation is a widespread phenomenon yet without an evident phylogenetic pattern, suggesting a polyphyletic origin of this peculiar behaviour (Cerrano et al. 2007a). Among Cnidaria the incorporation of sediment is known only in the order Zoanthidea (Previati et al. 2010), the oldest hexacorals (Brugler and France 2007) probably originating from a mesopsammic lifestyle (Fujii and Reimer 2011).
The present review will describe the evolution of our understanding of such interactions starting from the first relevant evidence of effects on humans, moving to the development of new model organisms in the study of this field with implications for research in the clinical, biological, ecological and biomaterials fields.
6.2 Lung Diseases and Silica
6.2.1 Silica and Mammalian Pathologies
Silica, namely silicon dioxide, is the most abundant mineral on the Earth’s crust and exists in two forms: amorphous and crystalline (also called free silica). This molecular species is formed from silicon and oxygen under conditions of increased pressure and heat; the amorphous form has no crystalline structure and shows relatively low toxicity to the lung (IARC Monographs 1987), while the crystalline form is based on a tetrahedral structure with silicon as a central atom and oxygen at the corners. Free silica has three principal polymorphs, all of them highly toxic to the lung (IARC Monographs 1987), tridymite, cristobalite and quartz, the latter being by far the most common. Quartz is abundant in most rock types, such as marbles, sandstones, flint and slate as well as in sands and soils, while cristobalite and tridymite are found in volcanic rocks. The drilling, grinding, cutting, breaking, crushing and abrasive blasting of all these materials may produce fine silica dust; indeed, because of the wide usage of quartz-containing materials, workers all over the world are exposed to respirable silica in a large variety of industries and occupations (Gamble 2011). Even if ambient dust control measures are applied to prevent lung diseases in most of developed countries, the incidence of lung pathologies continues to occur at an alarming rate also here. As such, exposure to silica and its detrimental effects on human health are a global occupational problem associated with numerous different diseases, not necessarily restricted to the pulmonary compartment, including various forms of silicosis (acute, accelerated, simple and silico-tuberculosis), industrial bronchitis, emphysema, rheumatoid complications, vascular diseases, glomerulonephritis, autoimmune systemic disorders (lupus, scleroderma) and last, but not least, bronchogenic carcinoma (Ding et al. 2002).
Due to the diversity of pathologies associated to silica exposure, it is quite unlikely that a single, common mechanism is responsible for all the possible diseases. It is generally accepted that alveolar macrophages (AMs) are a relevant cell type to study (Davis 1986). Since the main role of AMs in the lung is to clear the alveolar surface of inhaled debris, it is reasonable to assume that macrophages are the first cells of the body that will have crucial contact with silica particles. Indeed, once inhaled, silica particles are captured by AMs and the subsequent cellular and molecular cascades of events establish the conditions for the development of health complications, the severity of which is determined by the extension and intensity of respirable dust exposure. Although the precise sequence of events (from silica inhalation to development of disease) is not completely known, numerous investigators have been analysing these first fundamental steps and the following mechanisms/pathways have been proposed (Maeda et al. 2010):
-
1.
Initial recognition of silica by cell membrane receptors on the macrophages
-
2.
Engulfment of silica by macrophages and entrapment within lysosomes
-
3.
Production of reactive oxygen species (ROS) in the macrophages
-
4.
Induction of cellular and tissue damages due to the production of ROS
-
5.
Production of various chemokines/cytokines such as tumour necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1/2 (MIP1/2), IL-1β and IL-8 causing chronic inflammation and proliferation of fibroblasts
-
6.
Apoptosis of the AM
-
7.
Release of silica particles by the AM and repetition of similar cellular reactions by additional macrophages
If the AM survives the engulfment of silica, it will either migrate out of the lungs (to the proximal lymph nodes or out of the respiratory tract through the mucosal-ciliary escalator) or stay in the lung and move to the interstitial space becoming an activated interstitial macrophage (IM), able to directly contribute to lung disease (Migliaccio et al. 2005). Some evidence suggests that, indeed, IMs could play an important role in the development of silica-induced lung disease (Bowden et al. 1989; Adamson et al. 1991).
The following sections will briefly describe the salient features of the overall-induced silica diseases and the hypotheses currently most accredited to explain how crystalline silica (CS) is toxic to the AM, or in other words, how it all begins.
6.2.2 Silica-Induced Diseases
6.2.2.1 Silicosis
Silicosis is a type of pneumoconiosis caused by the inhalation of CS dust and characterised by chronic inflammation, progressive fibrosis and by the presence of peculiar scars in the form of nodular lesions in the upper lobes of the lungs (Davis 1986). This lung disorder, also known as Potter’s rot, is one of the oldest known occupational diseases. The recognition of respiratory problems from respirable dust dates to ancient Greeks and Romans. Agricola, in the mid-sixteenth century, wrote about lung problems from dust inhalation in miners, while Bernardino Ramazzini, in 1713, noted asthmatic symptoms and sandlike substances in the lungs of stone cutters (Rosen 1943). The term silicosis comes from the Latin silex, or flint, and was originally coined by the Italian pathologist and prosecutor Achille Visconti in 1870 at the Ospedale Maggiore in Milan as a result of his observations during anatomical dissection studies on a flint worker with almost perfectly sclerotic lungs which presented many irregular, opaque corpuscles spread all over the tissue. The chemical analysis revealed a 7 % content of ashes in the lungs of the cadaver (referred to the dry weight of the organ), half of which consisting of silica (Rovida 1871). With industrialisation, as opposed to hand tools, came also an increased production of dust. The pneumatic hammer drill and sandblasting were introduced in the early 1900s, both significantly contributing to the increased prevalence of silicosis (Rosen 1943). Recent reports indicate that, nowadays, over 23 million workers are exposed to CS in China and over ten million in India alone (Chen et al. 2012). In the United States and Europe, the respective figures are 1.7 million and over three million (Chen et al. 2012).
The classification of silicosis is made according to the disease onset, severity and rapidity of progression. The various forms include:
-
1.
Chronic simple silicosis. This is the most common form of silicosis usually resulting from prolonged exposure (10 years or more) to relatively low concentrations of silica dust. It may appear 10–30 years after the first contact of the lung with silica particles and is characterised by the formation of distinct, nodular, hyalinised lesions in the lung, frequently containing silica inclusions (Green and Vallyathan 1996). These nodular lesions are unique pathological entities of silicosis alone. The lesions are mainly present in the upper lobes and nodules can measure from 4 to 10 mm in diameter with a tendency to enlarge and become densely profuse with continued exposure. Cellular infiltrates and dust-engulfed macrophages are usually present at variable amounts in the peripheral zones. A completely developed silicotic nodule is composed of concentric fibrotic laminar layers with calcifications and minimal amounts of dust in the central part. With time and continued exposure to silica, the lesions of simple silicosis, measuring less than 1 cm, enlarge symmetrically and fuse to one another disrupting the surrounding pulmonary architecture and resulting in massive fibrosis, then called complicated or conglomerate silicosis. Because chronic silicosis is very slow to develop, patients may not show symptoms until the overt of the disease, when a decreased lung function, with dyspnea, tachypnea, fatigue, cough, chest pain and fever, becomes obvious.
-
2.
Acute silicosis. A form of silicosis that develops rapidly from a few weeks to 5 years after exposure to high concentrations of silica dust. Alveoli are usually filled with lipid-rich pulmonary oedema, also called silicolipoproteinosis, and interstitial inflammation is widespread (Green and Vallyathan 1996). The disease progresses rapidly with severe loss of pulmonary function which can be fatal.
-
3.
Accelerated silicosis. It’s a silicosis that develops between 5 and 10 years after exposure to concentrations of respirable silica dust higher than those leading to acute silicosis. It is morphologically very similar to the latter with alveoli showing silicoproteinosis and chronic inflammation and the rapid development of silicotic lesions (Green and Vallyathan 1996). Similarly to acute silicosis, accelerated silicosis is mostly common in occupations where silica is mechanically crushed or fractured and intensely inhaled.
-
4.
Silico-tuberculosis. Patients with silicosis are at an increased risk of tuberculosis infection despite that the prevalence of this disease is now reduced in the overall population (Hnizdo and Murray 1988; Cowie 1994). It is well known that the inhalation of silica can lead to impaired cell-mediated immunity with alterations of the number of lymphocyte subsets (lower T cells and higher B cells) and of serum immunoglobulins (Green and Vallyathan 1996).
6.2.2.2 Lung Cancer
In 1987 the International Agency for Research on Cancer (IARC) decided that CS was a probable carcinogen to humans, belonging to Group 2A agents. This was based on the evaluation that there was sufficient evidence for carcinogenicity in experimental animals but limited evidence in humans (IARC Monographs 1987). Ten years later, when a second IARC working group met, CS was classified as a Group I carcinogen based on the reviewing of more scientific papers, as well as nine cohorts and mortality studies published since the earlier meeting (IARC Monographs 1997), stating that CS exposure gave sufficient evidence for carcinogenicity in humans. Actually, there was no unanimous agreement on this classification among the working group (anyway not an unusual situation in an IARC working group). Doubts were based on several issues including (1) the consistency of a strong association between silicosis and lung cancer and (2) the negative outcome of coal mine dust as a lung carcinogen established in the same meeting. Nevertheless, recognising a clear heterogeneity of the quartz hazard in the epidemiological and toxicological studies revised by the working group, the final evaluation included the statement “…the Working Group noted that carcinogenicity in humans was not detected in all industrial circumstances studied. Carcinogenicity may be dependent on inherent characteristics of the crystalline silica or on external factors affecting its biological activity or distribution of its polymorphs…” (IARC Monographs 1997). Since then, and probably in response to the IARC statement, many papers have been published focusing on the differential hazards of different forms of natural or commercially available CS. These studies provide a potential explanation for the dramatic differences in the exposure response curves observed in many epidemiological studies (Donaldson and Borm 1998). Extensive research has also addressed the genotoxic mechanisms underlying the occurrence of lung cancer mainly questioning whether the tumour is a result of a direct genotoxicity of CS or of indirect genotoxicity acting through inflammation (Borm et al. 2011). Direct primary genotoxicity refers to particles that cause genetic damage in the absence of inflammation. This is by a mechanism involving a direct physical interaction or an oxidative attack by reactive oxygen species (ROS) at the particle surface on the genomic DNA or its associated components (Schins and Knaapen 2007). Indirect primary genotoxicity arises from the enhanced production of ROS and/or depletion of antioxidant defences by particle-stimulated or particle-engulfed cells. On the other hand, indirect genotoxicity is driven by inflammatory cells such as macrophages and granulocytes permanently recruited to the sites of particle deposition. This leads to chronic inflammation, persistent oxidative stress and DNA damage in a pro-survival and proliferation signal-rich environment. The genetic defects that can be accumulated in this situation facilitate the transformation of cells into malignant forms (Azad et al. 2008). An apparent agreement on the mechanism of quartz genotoxicity comes from a third IARC meeting, held in May 2009 (Straif et al. 2009). In that occasion the working group met to reassess the carcinogenicity of ten separate agents (including CS), previously classified as Group I carcinogens, and to identify additional tumour sites and mechanisms of carcinogenesis. The working group reaffirmed the carcinogenicity of “silica dust, crystalline in the form of quartz or cristobalite”, indicating the lung as the sole tumour site. Moreover, in the “established mechanism events”, the working group indicated an “impaired particle clearance leading to macrophage activation and persistent inflammation” without mentioning any direct genotoxicity mechanism, as such supporting the idea that lung cancer caused by quartz in vivo is a secondary, inflammation-driven event.
6.2.2.3 Dysregulation of the Immune System
There is evidence that silica exposure is also associated to the development of autoimmune diseases such as scleroderma (systemic sclerosis), rheumatoid arthritis, chronic renal disease and systemic lupus erythematosus (Cooper et al. 2002). Indeed, it is well known that silicotic patients often suffer complications from the above-mentioned autoimmune diseases (Hess 2002). More in general, silica is considered one of the most important environmental substances, like vinyl chloride and epoxy resins, that give rise to autoimmune diseases in exposed hosts (Hess 2002; Cooper et al. 2009). An increase of serum polyclonal immunoglobulins associated with high levels of circulating rheumatoid factor, anti-nuclear antibodies and immune complexes is often linked to some of these autoimmune conditions (Craighead et al. 1988). CS, as well as amorphous silica, shares such common pathogenic responses, but little insight into the underlying mechanism has been provided. It is generally believed that the effect of silica on the human immune system results, in part, from the potential adjuvant activity of silica and, additionally, from the increasing extracellular presence of various autoantigens such as RNA, DNA and organelles released by AMs undergoing apoptosis after silica engulfment. Accordingly, it would derive from the increased opportunity for reactive T-lymphocytes to meet circulating autoantigens favouring the development of a detrimental, self-reactive environment in the organism (Hamilton et al. 2008; Thakur et al. 2008).
6.2.2.4 Toxicity of Crystalline Silica in Alveolar Macrophages
Research is currently strongly focused on CS toxicity to the AM. Indeed, most researchers agree that the initial toxicity of silica upon binding and phagocytosis by resident macrophages is a crucial step towards the development of further diseases.
The literature can be divided into three main threads that support as many hypotheses developed to explain how CS particles are toxic to the macrophage cell (1) the free radical hypothesis, (2) the receptor-mediated hypothesis and (3) the lysosomal permeability hypothesis (Hamilton et al. 2008).
-
1.
The free radical hypothesis. There are many possible sources of free radicals resulting from silica internalisation, i.e. particle-derived ROS, cell-derived ROS and reactive nitrogen species (RNS), as well as ROS arising from the interaction of cell-derived and particle-derived free radicals such as peroxynitrite and superoxide anion (Fubini and Hubbard 2003). Upon silica phagocytosis, AMs respond with a respiratory burst that produces superoxide anions, hydrogen peroxide and hydroxyl radicals, all potentially toxic to the AM and the surrounding lung tissue being able to damage all types of cellular macromolecules (Valko et al. 2007). Silanol groups, on silica surface, are also thoroughly studied for their ability to generate silicon-based free radicals from freshly fractured silica (Vallyathan et al. 1988) and because they can form hydrogen bonds with oxygen and nitrogen groups on biological membranes, affecting their structure and integrity (Fubini and Hubbard 2003). Furthermore, it is well documented that water reacts with freshly fractured silica producing all types of ROS also thanks to the presence of traces of iron catalysing the Fenton reaction (Fubini and Hubbard 2003). Researchers sustaining the free radical hypothesis (Fig. 6.1, panel a) maintain that ROS may be mediators of silica-induced responses from macrophages and lung epithelial cells such as acute cell injury and proliferation, inflammation and fibrosis, and also DNA modifications (Ding et al. 2002), although some admit that ROS are not completely responsible for silica toxicity (Fubini and Hubbard 2003).
Fig. 6.1 Schematic representation of the three main hypotheses to explain the toxicity of CS to alveolar macrophages. Panel (a) The free radical hypothesis: overview of the reported effects of silica phagocytosis, the following respiratory burst, inflammatory pathway activation and consequential cell and tissue damage. Panel (b) The receptor-mediated hypothesis: schematic representation of class A scavenger receptors involved in the binding of CS. The extracellular domains proposed for silica binding are indicated for SR-AI, SR-AII and MARCO. Panel (c) The lysosomal permeability hypothesis: diagram of the events leading to apoptosis (following lysosomal leakage of cathepsin D in CS-engulfed alveolar macrophages), production of ceramide, mitochondrial depolarisation and cell death
-
2.
The receptor-mediated hypothesis. Some researchers believe that the specific receptors involved in the first critical step of particle recognition and internalisation (upon physical contact with the particle surface) are the ultimate mediators of silica toxicity by inducing an intracellular signalling pathway leading to apoptosis of the AM (Palecanda and Kobzik 2001). It has been described that these silica-binding receptors are also involved in normal immune function and inflammation regulation, the disruption of which can occur by excessive exposure to silica (Gough and Gordon 2000). The most accredited silica-binding receptors belong to the scavenger receptor (SR) family (Murphy et al. 2005), which are known to bind a wide variety of ligands. The SR typically associated with silica binding in the AM are the SR-AI, -AII and MARCO, where the collagen-like domain (present in all mentioned protein structures) has been implicated as the putative binding site for CS (Fig. 6.1, panel b). There are a few reports that address the potential signalling pathway elicited by SR-A and MARCO upon CS binding, some authors describing heat shock proteins and GAPDH recruitment at the level of the intracellular SR domain (Nakamura et al. 2002), while others pointing at tyrosine phosphorylation of the intracellular SR domain followed by activation of PKC (Hsu et al. 1988). Although it is not clear how these receptors can initiate different signalling pathways discriminating between various ligands, there is evidence that such phenomenon can actually occur (Hsu et al. 2001), triggering different responses that lead to the activation of macrophages or, alternatively, to apoptosis.
-
3.
The lysosomal permeability hypothesis. Immediately after encountering silica, the AM initiates a yet partially undeciphered engulfment process. Silica particles are then entrapped in the lysosomal compartment where the acidic pH activates strong digestive enzymes, eventually unable to disrupt silica particles. Failure of silica destruction can lead to the loss of lysosomal membrane integrity, and some investigators believe that the failed attempt at silica digestion in the AM leads to the activation of caspases and apoptosis (Thibodeau et al. 2003, 2004). This specific apoptotic signalling pathway caused by silica would involve lysosomal leakage of cathepsin D with activation of an acidic sphingomyelinase and would result in mitochondrial depolarisation and in the activation of caspase 3 and 9 (Fig. 6.1, panel c).
6.2.2.5 Conclusion
The wide range of diseases caused by the inhalation of CS testifies that CS toxicity is indeed a very complex process. Several biological rationales, summarised in Fig. 6.1, have been presented trying to describe silica binding and toxicity to the AM. It is important to underline that none of the hypotheses presented is mutually exclusive, as there may be more than one mechanism of silica-induced cell death, or the different toxicity mechanisms described may work in tandem to produce their detrimental effects on the exposed subjects. This heterogeneity of mechanisms might help explain why there is a diversity of pathologies linked to silica exposure and inhalation. Furthermore, not all silica-exposed individuals develop a disease. Therefore, there must also be some interaction with genetic and epigenetic factors and possibly polymorphisms that predispose groups of individuals and not others (Qu et al. 2007). Some diseases such as silicosis take years to develop, while others occur relatively rapidly after silica exposure as in the case of scleroderma and other silica-induced autoimmune disorders (Castranova et al. 1996; Cooper et al. 2002). Understanding how exactly mammalian AMs interact with inhaled silica remains a fundamental milestone to be achieved to unravel the silica-induced disease process.
6.2.3 Mammalian Macrophages, Crystalline Silica, and Ascorbic Acid
6.2.3.1 Cytotoxic Potential of Ascorbic Acid-Pretreated Quartz
Several physico-chemical features have been suggested as the cause of silica toxicity, among which are particles’ shape, the distribution of silanols at the surface of the crystal—and consequently its hydrophilic nature—and the generation of free radicals and ROS (Ding et al. 2002; Castranova 2004). The cellular response to crystalline silica dust is a complex matter regulated by multifactorial events (Hamilton et al. 2008). Consequently, various experimental models have been proposed to explain the molecular mechanisms of quartz toxicity in detail.
Almost two decades ago, Bavestrello et al. (1995) reported that ascorbic acid (AA) is able to partially dissolve the surface of quartz, highly increasing the concentration of soluble silica in the surrounding medium. Later, Fenoglio et al. (2000) demonstrated that during this peculiar chemical reaction, while AA progressively disappears, important modifications of the quartz surface occur, leading to an increased production of free hydroxyl radicals and hydrogen peroxide. These findings are relevant to mammalian quartz toxicity: by reacting with AA, quartz could deprive the alveolar epithelium of one of its most effective antioxidant defences, while the surface modifications induced by AA increase the concentration of particle-derived ROS in the alveolar space—one of the mechanisms proposed for quartz fibrogenicity and carcinogenicity (Ding et al. 2002). Furthermore, AA-driven quartz dissolution is specific for crystalline silica, as the amorphous silica particulate is not modified at its surface by ascorbate treatment and does not produce hydroxyl radicals, possibly explaining the dramatic difference of toxicity between amorphous and crystalline silica in the lung (Fenoglio et al. 2000).
This prompted Giovine and collaborators to further investigate the cytotoxicity of AA-treated quartz (QA) particles on the murine macrophage cell line RAW 264.7, a cell model widely used for molecular studies on cell-particle interaction. They showed that QA determines a significantly higher cytotoxicity than untreated quartz (Q) (Giovine et al. 2003), suggesting an active role of AA as a cofactor involved in the early stages of quartz-induced pathology. These results became the basis for further research on the effect of AA on the quartz-induced inflammatory response in the same cell model.
6.2.3.2 Inflammatory Potential of Ascorbic Acid-Pretreated Quartz
As cytotoxicity is one of the many aspects of macrophage reactivity to quartz, Scarfì and collaborators investigated the inflammatory response of RAW 264.7 murine macrophages in the presence of QA compared to Q. In particular they focused on the inducible enzyme cyclo-oxygenase-2 (COX-2), one of the key molecules involved in the immediate cellular inflammatory response. The protein was studied in terms of transcription, expression and enzymatic activity (PGE2 production) on the RAW cell line and on bronchoalveolar lavage (BAL) macrophages, of the transcription factors involved and of the possible role of ROS in triggering the COX-2 synthesis (Scarfì et al. 2007).
They found that in RAW 264.7 cells and in BAL macrophages, only QA particles, and not AA-treated amorphous silica, induced a higher transcription and biosynthesis of COX-2, as well as a greater production of PGE2 compared to Q. This overall COX-2 increase stimulated by QA seemed to be driven by an enhanced production of hydroxyl and hydrogen peroxide radicals (Fig. 6.2), which in turn stimulated the nuclear translocation and transcriptional activity of NFκB, pCREB and AP-1 transcription factors. These results could be particularly relevant because the over-expression of COX-2 seems strictly related not only to inflammation development but also to cancer progression (Hinz and Brune 2002; Brown and DuBois 2004). Interestingly, the enhancing effect of QA over Q on the synthesis of COX-2 was even more evident in the presence of IFN-γ, a cytokine produced by activated lymphocytes, which are believed to be recruited by macrophages at a later stage of the lung inflammatory process. The result of those experiments suggested that the quartz surface modifications operated by AA could be relevant not only in the first steps of the lung inflammatory response but also subsequently. The fact that macrophages are unable to dissolve the internalised quartz particles indeed prolongs the activation through multiple ingestion-reingestion cycles and exposes the same cells to cytokines produced by activated lymphocytes (Kreyling 1992). The long-lasting presence of quartz in the lung may expose the particles to AA present in the bronchoalveolar fluid, inducing the chemical modifications demonstrated by previous in vitro experiments (Fenoglio et al. 2000; Giovine et al. 2003). The resulting in vivo AA-modified quartz could eventually favour an escalation of the inflammatory response by macrophages, also stimulated by cytokines released by other cell types during the ongoing inflammation.
Schematic representation of the mechanisms underlying the toxicity of ascorbic acid-modified quartz (QA) to macrophages [as proposed by Scarfì et al. (2009)], and consequent to QA endocytosis, free radicals production, TNF-α and PGE2 release. Q quartz, AA ascorbic acid, QA ascorbic acid-modified quartz, DXS dextran sulphate, a scavenger receptor inhibitor; cytochalasin B; phagocytosis inhibitor; catalase and mannitol, inhibitors of hydrogen peroxide and hydroxyl radicals, respectively
6.2.3.3 Fibrogenic Potential of Ascorbic Acid-Pretreated Quartz
In a further study, taking advantage of the enhanced macrophage response to AA-treated quartz (QA) compared to untreated quartz (Q), Scarfì and collaborators investigated the first steps of cell activation and the contribution of early signals directly generated by the plasma membrane to the production of TNF-α, a cytokine that is crucial to develop silicosis by activating both inflammatory and fibrogenic pathways (Scarfì et al. 2009). Results demonstrated that the secretion of TNF-α and the synthesis of mRNA were significantly increased in RAW 264.7 macrophages challenged with QA than with Q and that, as in the case of COX-2 production, the higher pro-inflammatory effect of QA over Q was even more evident in the presence of IFN-γ. To assess the direct role of the plasma membrane in the production of silica-induced TNF-α, the research group investigated the very first step of macrophage quartz phagocytosis, which is known to be mediated by scavenger receptors (Kobzik 1995). They demonstrated that membrane lipid peroxidation was significantly higher in QA- than Q-treated cells, and the use of phagocytosis inhibitors (i.e. cytochalasin D and dextran sulphate) further increased lipid peroxidation in both cell samples. These results indicated that the contact of quartz particles with the plasma membrane, which is prolonged in the absence of quartz internalisation, is responsible for lipid peroxidation (Fig. 6.2). Furthermore, the production of TNF-α increased significantly over controls after exposure to Q (and even more after exposure to QA) in cells where phagocytosis was inhibited, indicating that membrane lipid peroxides generated independently of phagocytosis could induce the production of TNF-α.
This was the first demonstration that the contact with quartz by the plasma membrane, in the absence of phagocytosis, is sufficient to trigger membrane lipid peroxidation, TNF-α release and cell death. This appeared to be more detrimental to macrophage survival than particle phagocytosis itself and to increase the fibrogenic potential of crystalline silica by means of an enhanced release of its principal mediator.
As such, an impairment of macrophage phagocytosis could exacerbate lung disease in silica-exposed individuals. This might be the case of alcoholic subjects, where pulmonary macrophage function is compromised resulting in defective phagocytosis (Brown et al. 2007). Additionally, it would imply that alcohol abuse or other pathological conditions impairing macrophage phagocytosis could be a risk factor for silica-induced lung disease and a detrimental condition facilitating complications by means of the prolonged macrophage contact with unphagocytised particles.
6.2.3.4 Conclusion
Taken altogether these studies (summarised in Fig. 6.2) demonstrate that reaction of quartz with AA in the lung is indeed a possible event. Contrary to common belief, this reaction does not neutralise the hazard of quartz as it would be expected given the well-known antioxidant properties of the vitamin. Rather, it increases the toxicity and inflammatory/fibrotic potential of crystalline silica in the lung. The subsequent events, then, lead to higher macrophage cell death and enhanced release of important mediators such as PGE2 and TNF-α, the pro-inflammatory cytokines currently believed to be mainly responsible of lung fibrosis, and eventually cancer, after exposure to crystalline silica (Piguet et al. 1990; Brown and DuBois 2004).
6.3 The Effects of Minerals on Animal Growth and Development
Minerals in the aquatic environment may affect the growth and development of benthic animals, as suggested by the following examples (1) the development of thicker primmorphs of Petrosia ficiformis on quartz rock than on marble suggests that different minerals (here calcium carbonate vs. silica) elicit different responses in sponges (Pozzolini et al. 2010), (2) evidence regarding the positive effects of quartz on collagen production suggests that the thickness of the ectosome in Chondrosia reniformis may be enhanced by quartz dissolution (see Sect. 6.4.2.5) and (3) a long-term field experiment on the gamma (massive) stage of the boring sponge Cliona viridis (=nigricans) maintained on gravels with different mineralogy evidenced a negative effect of quartz on sponge growth compared to a calcareous substrate (Cerrano et al. 2007b).
Similarly, if the interaction with different mineral particles can cause a response in a single species discriminating calcium carbonate from silica, which are the effects of mineral substrates on benthic assemblages? Can the mineralogical features of substrates affect larval settlement and survival?
These considerations are not new to environmental scientists but, as reported in a recent review on the topic (Davis 2009), it is difficult to disentangle the properties of natural mineral substrata that may affect the structure of benthic communities, both on soft and hard bottoms. Owing to the rapidity of biofilm formation, it has always been assumed that larvae interact with surface biofilms, rather than with natural rock surfaces (Wahl 1989; Hadfield 2011). Therefore, until recently authors have not considered the crystallographic structure of sand or rock as a relevant abiotic factor in the structuring of benthic assemblages. In addition, the texture and the crystallographic structure of sand grains, and the roughness in case of rock surfaces, can have confounding effects. This is enhanced by the effects of the different biofilms that may be specific to soft (Manini and Luna 2003; Van Colen et al. 2009) and hard substrates (Doiron et al. 2012; McDougald et al. 2012), whether they are biogenic or not (e.g. Gil-Turnes et al. 1989), and may alter the biological response to minerals (Faimali et al. 2004; Totti et al. 2007). Field experiments indicate that microflora or bioorganic films can play an important role in modifying the patterns of settlement of sessile invertebrates (Todd and Keough 1994; Keough and Raimondi 1995, 1996).
While unravelling the specific role of the mineralogical composition of rocks in benthic ecology is far from easy, scientific evidence increasingly indicates that it may therefore affect the settlement of larvae (Cerrano et al. 1999a) and algal propagules, which may occur by direct as well as indirect mechanisms and be facilitating, inhibiting or neutral to the various organisms. Understanding how larvae respond to such cues across a variety of spatial and temporal scales is a real challenge for marine ecologists. Different rock types can have both physical (roughness) and chemical effects on settling organisms. Calcareous rocks can be eroded by bioborers while quartz rocks (e.g. granite) and those rich in silicates cannot. Generally, pitted substrates are easier to colonise as they offer a wide range of microhabitats, thus enhancing biodiversity. Siliceous rocks cannot be bioeroded and only their surfaces are colonised. However, the production of basal collagen or of a calcareous sheet during larval settlement provides for a physical barrier that enables benthic organisms to compete for space by avoiding several effects derived from substrate features.
Regarding the chemical effects, the ability of a particular rock type to sequester/partition solutes and the scale of its physico-chemical heterogeneity (Holmes et al. 1997) must be considered. Bavestrello and co-authors (2000) provided evidence that quartz-rich rocks and sands negatively affect species richness and epibenthic assemblages. Laboratory experiments revealed that the planulae of a hydroid tended to avoid quartz sands and preferentially settled on marble ones; however, no difference in metamorphosis was recorded (Cerrano et al. 1999a).
The formation of silicon-based and OH radicals in the surrounding water may affect community structure at the fish, algal and sessile invertebrate levels (Guidetti et al. 2004). Cattaneo-Vietti et al. (2002) also studied non-granitic shores detecting statistically significant differences between epibenthic assemblages developed on different substrates, with higher total abundance for sandstone and basalt than serpentine rocks. While leaving many questions open, these works highlight that the effects of rock type on the structure of benthic communities still deserve experimental attention.
6.4 Reactivity of Marine Sponges to Silica Particles
6.4.1 Historical Overview
The incorporation of foreign materials has been described in sponges, many of which appeared to be rich in spongin fibres or collagen. This peculiar behaviour was originally observed 140 years ago by Haeckel (1872). In the following years other authors remarked this behaviour, describing different examples of inorganic particles embedded into spongin skeletons. Specifically, the regular presence of inorganic particles inside the skeletal fibres was used as a taxonomic tool to classify some horny sponges (Von Lendenfeld 1889; de Laubenfels 1950). However, whether the sponges actively select such material is controversial (Haeckel 1872; Schulze 1879; Von Lendenfeld 1889; Sollas 1908; Shaw 1927; Teragawa 1986a, b). Haeckel (1872) suggested a selective uptake. Schulze (1879), on the contrary, considered it passive, since the type of foreign matter incorporated depends on the features of the sponge surface, such as the ectosome texture or the presence of a thin mucous layer. The active selection hypothesis was also supported by Von Lendenfeld (1889), who observed that the incorporation is species specific and stable for the same species from different locations. In his “Monograph of Horny Sponges”, a widely documented description of the selective incorporation of sand grains in some species or of foreign siliceous spicules in others is provided, and their peculiar embedment within sponging fibres is detailed. At pages 768–769 of the volume, the author underlined the structural role of the selected inorganic material incorporated, which is organised differently in the different species (see an example in Fig. 6.3). In all cases, Lendenfeld remarked that inorganic materials are firmly associated with spongin fibres and evidenced a different organisation of spongin in the same species grown in the presence of different amounts of suitable sand grains or foreign spicules (Von Lendenfeld 1889). These observations anticipated some ideas resumed and expanded more than 100 years later (Bavestrello et al. 2003).
Details of skeleton organisation of Dysidea villosa (Lendenfeld 1886), where sand grains are embedded within spongin fibres [partially reproduced by Von Lendenfeld (1889), Table 10, Figs. 1 and 2]
The occurrence of sand grains or foreign spicules in horny sponges has been a matter of scientific interest also at the beginning of twentieth century. Minchin (1900) thought that sand grains adhering to the surface of sponges were surrounded by the growing points of spongin fibres and were therefore incorporated as the fibres grew upwards. Sollas (1908) studied the incorporation process in Liosina paradoxa (=Migas porphyrion), suggesting another way by which the inclusion of sand may take place: pseudopodia-like extensions of surface cells surround sand grains, and the inclusion of sand and foreign bodies is due to the action of amoebocytes, also responsible for their conveyance to and orientation in the spongin fibres. In the same report it is shown that some species include only sand in their fibres while closely related ones incorporate only foreign spicules, underlining the highly selective action of sponge cells towards foreign materials. In the technical description of the procedures used to obtain good histological preparations, Sollas advised to pretreat sponge materials with hydrofluoridric acid before cutting slices. This determined the almost complete removal of sand grains (Sollas 1908). This again remarked for the first time a selective incorporation of siliceous sands through amoeboid movements of cells: a similar behaviour is reported for mammalian lung macrophages after the inhalation of silica dust (see Sect. 6.2.1).
In 1927 Shaw continued this research line. In her “Note on the Inclusion of Sand in Sponges”, she confirmed the prevalence of the phenomenon in species characterised by almost total absence of endogenous spicules, counterbalanced by a high production of spongin fibres. In the same report the author evidenced the absence of detectable amounts of sand granules inside sponges of the genera Chondrosia and Chondrilla, cited as a peculiarity. Now we know that also Chondrosia species, when living in environments rich in siliceous sand, incorporate and select large amounts of foreign material as described below (see Sect. 6.4.2.2). Chondrilla species produce their own spicules. In the conclusive remarks, and on the basis of histological observations of fixed materials, the author supported the idea that sponges actively select sand grains. She agreed on the hypothesis previously suggested by Sollas to explain how sand is incorporated and underlined the inverse correlation between the abundance of foreign material and the amount of endogenous spicules inside sponges (Shaw 1927). To our best knowledge, apart from some additional (taxonomical) remarks by De Laubenfels (1950), no further research has been carried out on the topic until the late 1980s (Teragawa 1986a, b; Cerrano et al. 2007a). Teragawa worked on the keratose demosponge Dysidea etheria de Laubenfels, a species building a spongin skeleton that can be filled with a wide variety of foreign particles. In a first work based on histological techniques, Teragawa showed a remarkable interaction of dermal cells with particles, suggesting a direct role of the same cells in moving, internalising and orienting sand grains (Teragawa 1986a). In a second contribution, she used D. etheria to perform relevant experiments directly demonstrating particle transport and incorporation during the formation of sponge skeleton. The loose construction and the relative transparency of the sponge species she used allowed for the direct observation of the interactions and movements of foreign particles between the outer surface and the underlying distal skeletal components in the living animals, using a time lapse cinemicrography technique. The results clearly demonstrated an active role of the sponge in the selection and incorporation process. To perform her experiments, the author used sand grains of different typology and dimension, revealing different reactions of the sponge surface towards particulates, depending on size and abundance. As a whole, the smallest particles are inhaled by the aquiferous system; the others are retained by the surface but only ca. 20 % are definitively incorporated into fibres (Teragawa 1986b). To better evaluate the contribution of sand to skeleton formation, two different types of sand grains were used: siliceous sand, as a reference, and carbonatic sand, as test grains. The results showed how the incorporation of sand grains positively contributes to build up spongin fibres (Teragawa 1986b), but the experimental design applied did not allow the unequivocal discrimination between the specific contributions of siliceous and calcareous grains. The latter consideration is pivotal in the interpretation of current research that considers sponges, especially collagen-rich demosponges, as an evolutionary cornerstone for the production of the proteins of the extracellular matrix in the animal kingdom. Recently, Cerrano and co-authors reviewed how sponges incorporate foreign matter by comparing the strategies of species living in soft and hard bottoms. They showed that in both habitats, sponges are able to incorporate sand grains or foreign spicules, often discriminating carbonate materials from silica (according to the species). However, if the animal or parts of it get detached from the substrate, they invariably lose the ability to select particles until they find a new settlement place (Cerrano et al. 2007a).
6.4.2 Present Time Chondrosia reniformis Nardo 1847: A Case Study
6.4.2.1 The Sponge
Chondrosia reniformis (Nardo 1847) is a marine sponge that lacks calcite or siliceous spicules. Its body consists of a collagenous mesohyl, which is located between the external epithelium and the internal epithelia that line the inhalant and exhalant canals (Wilkie et al. 2006). Although the mesohyl confers the whole animal a cartilaginous texture, it is also plastic in terms of shape and mechanical properties. For example, within 40–60 h after excision, fragments of C. reniformis (fragmorphs) undergo rounding off of cut surfaces and marked bending (Nickel and Brümmer 2003). Whole sponges or tissue explants can lose rigidity under continuous compression (Garrone et al. 1975; Garrone 1978). The animal, in its natural environment, may lose its adhesion substrate and slowly elongate under gravity (Fig. 6.4) until its eventual separation from the still-attached portion, a process that may be regarded as a form of opportunistic asexual reproduction (Sarà and Vacelet 1973; Bonasoro et al. 2001; Zanetti 2002). This plasticity is mainly due to the extraordinary attributes of C. reniformis collagen, which has a behaviour similar to the contractile collagens described in echinoderms (Wilkie et al. 2006). The structure and features of the collagen from C. reniformis (Heinemann et al. 2007), the sponge’s amazing ability to incorporate and select foreign inorganic materials (Bavestrello et al. 1995) and the relationship between the incorporation of particles and the biosynthesis of collagen (Pozzolini et al. 2012) make this species a unique model to study the evolution of the extracellular matrix.
6.4.2.2 Incorporation of Foreign Material
In horny sponges, foreign inorganic particles are embedded in the spongin fibres and in the ectosome, while in C. reniformis, they form a layer underneath the sponge surface (Bavestrello et al. 1996). The cells of the exopinacoderm are responsible for their subsequent incorporation. In C. reniformis, particles that settle on the mucous ectosome (Fig. 6.5) are translocated, at varying rates, to special areas where they are quickly engulfed (Bavestrello et al. 1996). In this sponge the incorporation of foreign materials is a complex phenomenon involving the passive settling of suspended sediment on the sticky sponge surface (Fig. 6.5), the active selection of particles by pinacocytes and their eventual engulfment (Bavestrello et al. 1996, 1998a). The availability of particles depends on environmental conditions. As shown comparing two locations in the Ligurian Sea, the incorporation of foreign material by C. reniformis depends on the rate of sedimentation (Cerrano et al. 1999b). The rate of incorporation also depends on the amount of sediment suspended in the water column, directly related to the intensity of water movement. The size of the particles incorporated depends on environmental conditions, too. When the sea is rough, only fine particles (about 10 μm in diameter) can adhere to the mucous surface of C. reniformis, while coarser particles (about 30 μm) may be collected only during calm sea conditions (Cerrano et al. 1999b).
6.4.2.3 Selection
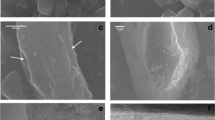
Field observations and laboratory experiments indicate that C. reniformis selects and incorporates only siliceous materials, in particular quartz particles (crystalline silica) and sponge spicules (amorphous biogenic silica) from the sediment, even though carbonate particles are often dominant in the same sediments. Laboratory experiments demonstrated that the cells of the ectosome are the main actors in this process. Here siliceous particles are incorporated while carbonate ones are agglutinated and drop off the sponge surface (Bavestrello et al. 1998b). Nevertheless, a striking finding was the remarkable ability of C. reniformis to discriminate between crystalline (quartz) and amorphous biogenic silica (foreign spicules) (Bavestrello et al. 1998a). When crystalline quartz particles settle on the sponge, ectosome cells contract uniformly, creating a ruffled surface that remains throughout the process of quartz incorporation. Indeed, when exopinacocytes interact with crystalline quartz, they contract and become globular, creating a slightly raised layer that contrasts with the flattened epithelium of undisturbed sponges. Quartz particles are gradually engulfed by pinacocytes that stretch to form a rim around them (Bavestrello et al. 2003). Despite this reaction, a gap is maintained between the exopinacocytes and the foreign grains. As the grains sink into the collagenous cortical matrix of the sponge, the cellular ring shrinks until the grain is completely incorporated. Eventually, the exopinacoderm returns to its original state (Bavestrello et al. 2003). In contrast, siliceous spicules elicit a motile response in exopinacocytes. SEM analysis showed that these cells, without losing their epithelial configuration, release an organic matrix that covers the spicules. This process creates a coat towards which other exopinacocytes protrude long and thin pseudopodia. As time goes by, other exopinacocytes line up and cover the spicules that become completely incorporated (Bavestrello et al. 2003).
The selection and incorporation of foreign inorganic matter in C. reniformis relies on the ability of exopinacocytes to contract and to move (Bavestrello et al. 1998a). The peculiar ability of this sponge to recognise and select mineralogical species (calcite, quartz, opal, chalcedony, etc.) also depends on the life cycle phase and the anatomical region concerned. Usually, only the upper surface recognises and eliminates calcium carbonate, while the lower surface, which settles on every kind of substratum, does not. During fragmentation, the exclusive affinity for silica is lost by the sponge propagules, allowing the incorporation of calcareous particles as well (Bavestrello et al. 1998b). This behaviour is shared by other sponge species (see Sect. 6.4.1) and is probably related to the anchoring strategy of sponge fragments (Cerrano et al. 2007a).
6.4.2.4 The Destiny of Incorporated Particles: The Dissolution of Quartz
What happens after particles have been incorporated by the sponge ectosome is remarkably different, depending on the cellular reactivity towards crystalline or amorphous structure and on the final destiny of the particles incorporated: whereas foreign spicules remain unaltered within the sponge tissue, quartz particles are quickly etched and show typical corrosion patterns at the SEM analysis (Bavestrello et al. 1995). The experimental administration of quartz sand to several specimens of C. reniformis showed that their size became uniform (about 30 μm) after a week, irrespective of the initial size. The etching of quartz particles by C. reniformis is the first report of such an activity in the animal kingdom (Bavestrello et al. 1995). It is remarkable that only crystalline quartz is dissolved. Not only opal biominerals like sponge spicules, but also inorganic amorphous minerals remain unaltered. This is particularly evident for benthic specimens in the volcanic Aeolian Archipelago (Tyrrhenian Sea), where the only available siliceous material is volcanic glass. In this area, C. reniformis incorporates several fragments of such amorphous material, which is not etched inside sponge tissues, so grain size is unusually large (about 60 μm) (Bavestrello 1996). The ability to etch quartz sand is ascribed to a specific chemical reaction. Previous evidence that several polyvalent organic acids are able to dissolve quartz (Bennet 1991) suggested the involvement of ascorbic acid in the quartz etching process seen in sponges (Bavestrello et al. 1995). Ascorbic acid is the reducing agent in proline hydroxylation during collagen synthesis; its concentration in C. reniformis ranges from 1–10 μg/g of wet weight (Cerrano et al. 1999c), the same order of magnitude observed in plants (Loewus 1980). Ascorbic acid is continuously released from the ectosome, especially when the sponge is disturbed (Bavestrello et al. 1995). Its extrusion may also be deduced by the presence of calcium oxalate crystals on the sponge surface (Cerrano et al. 1999c). In slightly alkaline environments, like sea water (pH 7.8–8.0), ascorbic acid is indeed spontaneously oxidised to oxalic acid (Cerrano et al. 1999c). Fenoglio et al. (2000) observed that ascorbic acid dissolves quartz more rapidly than it does with amorphous silica. The author suggested that ascorbic acid interacts via hydrogen bonding with the surface of silica, followed by the attack on the silicon atom and the removal of the silicate from the crystal. This peculiar reaction is favoured by the specific distance between surface silanols (Si-OH) in crystalline quartz but not in amorphous silica (Fenoglio et al. 2000). The dissolved silica seems to form a complex with an unidentified ascorbate derivative, which could be similar to the hexacoordinate organosilicon complexes that are normally generated by catechol reaction with quartz (Iler 1979). Ascorbic acid, like catechol, exhibits a planar 1.2 diols moiety. Moreover, evidence of organosilicon complexes in the presence of diols in neutral solutions has been reported (Kinrade et al. 2001).
In both fresh and sea water, the rate of silica dissolution as Si(OH)4 is greatly affected by crystallinity, and it is generally accepted that the most soluble forms of silica are amorphous, while crystalline forms are characterised by a very low solubility (Kamatani 1971). Inside C. reniformis the dynamic of dissolution is remarkably different: amorphous hydrated silica is engulfed and “protected” against the slow dissolution operated by sea water, while the almost insoluble quartz grain is incorporated in a sort of “chamber” inside the ectosome (Bavestrello et al. 2003). Here, it is dissolved by ascorbic acid, which selectively reacts with crystalline silica rather than with the amorphous biogenic structure (Fenoglio et al. 2000).
The chemical study of this process evidenced an unexpected but extremely interesting contribution to the modification of quartz surface features generated by ascorbic acid. Indeed, once pretreated with vitamin C, quartz grains show relevant alterations in the silanol population, specifically a reduced number of free silanols (Fenoglio et al. 2000). The same scientific study also evidenced a remarkable increase of •OH in the presence of hydrogen peroxide in an aqueous suspension of quartz pretreated with ascorbic acid, while radical production was not detected with amorphous silica (Fenoglio et al. 2000). This may explain why the sponge interacts in a different way with quartz sand or opal spicules, suggesting a new perspective in the earlier molecular events of lung injuries generated by the inhalation of quartz dust in human being, as reviewed above (see Sect. 6.2.3).
6.4.2.5 Biological Meaning of Quartz Etching
Although the process of selection, incorporation and modification of foreign materials has been clearly documented for different sponges, its adaptive function has not been fully understood yet. Sponges seem to incorporate foreign materials to strengthen their structure. Therefore, in a high-sedimentation environment, sponges select the settled material at the epithelial level, thus avoiding the incorporation of high amounts of calcite (often the most abundant fraction in the sediment). In the specific case of C. reniformis, while opal remains unaltered, quartz is rapidly dissolved and expelled as pellets. One consequence of quartz dissolution is an increased concentration of dissolved silica in the intercellular medium and in the nearby water environment (Bavestrello et al. 1995). Recent studies on this peculiar sponge definitively demonstrated a positive role of dissolved silica in the expression the collagen gene (Pozzolini et al. 2012). The authors identified one of the most represented collagen genes in C. reniformis (COLch), describing it for the first time at the molecular level. Its full length cDNA codes for a nonfibrillar collagen type, characterised by two noncollagenic and three collagenic domains (Fig. 6.6). On these grounds, COLch proves to belong to the short-chain spongin-like collagen family, with a high structural similarity to type IV collagen, even if phylogenetic analysis shows it evolved independently from a common ancestor (Pozzolini et al. 2012). This suggests a specific role by COLch in mediating the attachment to proper substrata by sponges (Exposito et al. 2002) as well as in reinforcing the ectosome structure. Using the in situ hybridisation technique, Pozzolini and co-authors confirmed this hypothesis. The results demonstrated that the mRNA encoding for COLch is mainly expressed in the peripheral areas of the sponge, both in the upper and in the basal region. In the same work, the authors unequivocally demonstrated the role of soluble silicon compounds in the up-regulation of the expression of this spongin-related gene. They used two different compounds: Na2SiO3, which generates the same anion normally found in sea water, and Na-hexafluorosilicate (Krasko et al. 2000). Both of them activated the transcription of COLch mRNA in fragmorphs model (Pozzolini et al. 2012). These findings are relevant to the molecular biology research about the proteins of the extracellular matrix. Indeed, it is widely known that silicon compounds are pivotal in the production of collagen in many living beings, from sponges (Krasko et al. 2000) to vertebrates (Carlisle 1972, 1980; Maehira et al. 2008). However, to our knowledge, there is no strong evidence yet to support a direct role of these compounds in the regulation of gene expression. The etching process has a physiological role in C. reniformis. The incorporation of crystalline silica causes an increase of dissolved silicon concentration and consequently of the up-regulation of collagen gene expression (Fig. 6.7).
Deduced organisation of COLch amino acid sequence domains. The non-collagenous regions at the N terminus and C terminus of the protein (NC0 and NC1, respectively) are marked in dark grey. In NC0, the 25-amino acid signal peptide (SP) is in red, and the cleavage site is between glycine25 and threonine26. In NC1, the four amino acid residues involved in the two putative C-proteinase cleavage sites, Ala578–Asp579 and in Arg630–Asp631, are indicated in green. The collagen-like domain (C1) is indicated as a blue block while the three conventional collagen domains (C2, C3 and C4) are indicated as yellow blocks. The GXG triplet imperfection is shown inside each of them; additionally, the RGD motif is shown in C3. The three linker peptides (LP1, LP2 and LP3) are indicated as pink lines. The potential glycosylation sites on lysine residues are marked in blue, the potential glycosylation sites on threonine and serine are marked in yellow and the potential glycosylation sites on asparagine are marked in white
Biological meaning of quartz etching. Quartz sand is incorporated inside the sponge ectosome and etched (Bavestrello et al. 2003). The release of dissolved silicon compounds stimulates the expression of COLch gene, with the consequent production of new fibres
Figure 6.8 shows how the interaction with different silica dusts in C. reniformis results in a clear modification of the body shape. Fragmorphs of C. reniformis (Pozzolini et al. 2012) were treated for 10 days with MinUSil (a well characterised quartz dust from US Silica Company), with Aerosil (amorphous silica dust from Degussa) or with P. ficiformis minced spicules. At the end of the treatment, the external epithelium was partially restored under each condition, but the morphology of the respective fragmorphs was significantly different. The samples treated with quartz dust were larger than those treated either with amorphous silica dust or minced spicules that were much more rounded. The difference may be due to the latter models trying to minimise their surface/volume ratio hence exposing the least surface to the powder. Yet, the influence of different silica dust on the organisation of collagen fibres in C. reniformis is still to be elucidated at the ultrastructural and molecular levels.
6.5 Different Silica Affect Diatoms Growth
Diatoms are the dominant species in many ecosystems and account for up to 35 % of total primary production in oligotrophic oceans and up to 75 % in coastal waters (Tréguer et al. 1995). Jin et al. (2006) estimated their global contribution to net primary production at 15 % and to carbon export at 40 %. Moreover, diatoms are the most important group of organisms affecting silicon balance in the marine ecosystem as they form polymerised frustules (Jézéquel et al. 2000; Ragueneau et al. 2000). Silicon constitutes the diatom cell wall and its availability is a key factor in regulating diatom growth (Shipe and Brzezinski 1999). Silicon enters the oceans by river run-off (either dissolved, in monomeric or oligomeric form, or as particulate solids of varying size and crystallinity) and by the dissolution of particulate silica present in the lithogenic and biogenic marine environment. Not much is known on Si biomineralisation processes (Finkel and Kotrc 2010), while the biochemical pathways involved in silicification and their regulation are still being investigated (Hildebrand 2008; Ramanathan et al. 2011). Moreover, little information is available about the possible influence of different silica sources on the growth of marine diatoms. This has an ecological relevance for Si-biogeochemical patterns in coastal sea water, where dissolved silica may be depleted during diatom blooms and under grazing pressure, while siliceous lithogenic material and biogenic silica from settled diatoms are abundant (Schultes et al. 2010).
The marine silica cycle is dominated by the production and subsequent dissolution of biogenic silica in the water column. The complex chemical balance of different silica compounds in aqueous solutions with respect to the source type (i.e. biogenic silica or quartz) and the physico-chemical features of the aqueous environment are known (Dove and Rimstidt 1994; Dove et al. 2008). In sea water, dissolved silica occurs in different chemical forms SiO x (OH) x2 and concentrations depending on the polymorphs from which it originates. At the natural pH of sea water (pH = 8), undissociated silicic acid Si(OH)4 is the dominant form (97 % of total dSi), the remainder largely being SiO(OH)3 −. However, little is known on the actual bioavailability to diatoms of different silicon compounds (Demarest et al. 2009).
The group of Penna focused on the role of silica sand in regulating the growth of marine diatoms. They demonstrated that cell culture media containing different lithogenic and biogenic/amorphous silica sands (such as crystalline quartz—natural quartz sand—diatomaceous earth or sponge spicules) significantly affect the growth rate of marine diatoms (Penna et al. 2003). In particular, quartz sand induced higher growth than amorphous biogenic silica, likely due to surface-driven uptake mechanisms. The original hypothesis to explain these results was that diatoms excrete organic compounds to dissolve silica and make it bioavailable (Penna et al. 2003). Present-day experiments demonstrated that the differences are due to different bioavailability of different soluble silicon compounds generated by spontaneous dissolution of the various substrates (Penna, personal communication). More specifically, the analysis of parameters typical for diatoms (such as cell growth, maximum cell density, biovolume and silicon uptake) suggested that the soluble silicon compounds generated by crystalline sources in sea water are highly bioavailable compared to those generated by biogenic and amorphous materials (Data not shown, Penna, personal communication). These findings are of considerable ecological importance and may contribute to clarify anomalous spatial and temporal distributions of siliceous organisms with respect to the presence of lithogenic or biogenic silica sources in marine environments.
6.6 Conclusive Remarks and Open Questions
In this chapter we have given an overview of the complex interactions between rocks and living cells, focusing on case studies that range from human pathology to the aquatic environment. The apparent heterogeneity of these examples is actually solved by several shared points (1) in aqueous solutions, crystalline silica behaves differently from amorphous phases; (2) one of the molecular keys at the basis of this difference is the specific reactivity of ascorbic acid with crystalline silica; (3) dissolved silica stimulates collagen production in marine sponges; and (4) different silica substrates affect the growth of diatoms in different ways. These points remark the deep interaction between living beings and silica. However, little is still known about the molecular mechanism at the basis of such processes. The main open question is related to the chemistry of silica in aqueous solution. The generally accepted idea is that in aqueous solution, silica generates orthosilicic acid although with different kinetics and yields depending on several factors. Yet, the specific reactivity of ascorbic acid with crystalline silica and the findings related to diatoms are not consistent with this assumption. “Silicon bioavailability” is the correct perspective hereby introduced and “bioavailable silicon” is likely the result of peculiar chemical species not yet identified.
References
Adamson IY, Letourneau HL, Bowden DH (1991) Comparison of alveolar and interstitial macrophages in fibroblast stimulation after silica and long or short asbestos. Lab Invest 64:339–344
Azad N, Rojanasakul Y, Vallyathan V (2008) Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev 11:1–15
Bavestrello G (1996) Particolarità nella formazione dello scheletro osservato in alcune specie di poriferi delle isole Eolie. In: Faranda FM, Povero P (eds) Caratterizzazione ambientale marina del sistema Eolie e dei bacini limitrofi di Cefalù e Gioia (EOCUMM95). G. Lang Artigrafiche, Genova, pp 317–322
Bavestrello G, Benatti U, Cattaneo-Vietti R, Cerrano C, Giovine M (1995) Quartz dissolution by the sponge Chondrosia reniformis (Porifera, Demospongiae). Nature 378:374–376
Bavestrello G, Cerrano C, Cattaneo-Vietti R, Sarà M, Calabria F, Cortesogno L (1996) Selective incorporation of foreign material in Chondrosia reniformis (Porifera, Demospongiae). Ital J Zool 63:215–220
Bavestrello G, Arillo A, Calcinai B, Cerrano C, Lanza S, Sarà M, Cattaneo-Vietti R, Gaino E (1998a) Siliceous particles incorporation in Chondrosia reniformis (Porifera, Demospongiae). Ital J Zool 65:343–348
Bavestrello G, Benatti U, Calcinai B, Cattaneo-Vietti R, Cerrano C, Favre A, Giovine M, Lanza S, Pronzato R, Sarà M (1998b) Body polarity and mineral selectivity in the demosponge Chondrosia reniformis. Biol Bull 195:120–125
Bavestrello G, Bianchi CN, Calcinai B, Cattaneo-Vietti R, Cerrano C, Morri C, Puce S, Sarà M (2000) Bio-mineralogy as a structuring factor for rocky bottom communities. Mar Ecol Prog Ser 193:241–249
Bavestrello G, Benatti U, Cattaneo-Vietti R, Cerrano C, Giovine M (2003) Sponge cell reactivity to various forms of silica. Microsc Res Tech 62:327–335
Bennet PC (1991) Quartz dissolution in organic-rich aqueous systems. Geochim Cosmochim Acta 55:1781–1797
Bonasoro F, Wilkie IC, Bavestrello G, Cerrano C, Candia Carnevali MD (2001) Dynamic structure of the mesohyl in the sponge Chondrosia reniformis (Porifera, Demospongiae). Zoomorphology 121:109–121
Borm PJ, Tran L, Donaldson K (2011) The carcinogenic action of crystalline silica: a review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism. Crit Rev Toxicol 41(9):756–770
Bouligand Y (2004) The renewal of ideas about biomineralisations. CR Palevol 3:617–628
Bowden DH, Hedgecock C, Adamson IY (1989) Silica-induced pulmonary fibrosis involves the reaction of particles with interstitial rather than alveolar macrophages. J Pathol 158:73–80
Brown JR, DuBois RN (2004) Cyclooxygenase as a target in lung cancer. Clin Cancer Res 10:4266s–4269s
Brown LA, Ping XD, Harris FL, Gauthier TW (2007) Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol 292:L824–L832
Brugler MR, France SC (2007) The complete mitochondrial genome of the black coral Chrysopathes formosa (Cnidaria: Anthozoa: Antipatharia) supports classification of antipatharians within the subclass Hexacorallia. Mol Phylogenet Evol 42:776–788
Carlisle EM (1972) Silicon: an essential element for the chick. Science 178:619–621
Carlisle EM (1980) Biochemical and morphological changes associated with long bone abnormalities in silicon deficiency. J Nutr 110:1046–1056
Castranova V (2004) Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/nitrogen species. Free Radic Biol Med 37(7):916–925
Castranova V, Vallyathan V, Wallace WE (1996) Silica and silica-induced lung diseases. CRC, Boca Raton, FL
Cattaneo-Vietti R, Albertelli G, Bavestrello G, Bianchi CN, Cerrano C, Chiantore M, Gaggero L, Morri C, Schiaparelli S (2002) Can rock composition affect sublittoral epibenthic communities? Mar Ecol 23:65–77
Cerrano C, Arillo A, Bavestrello G, Benatti U, Calcinai B, Cattaneo-Vietti R, Cortesogno L, Gaggero L, Giovine M, Puce S, Sarà M (1999a) Organism-quartz interactions in structuring benthic communities: towards a marine bio-mineralogy? Ecol Lett 2:1–3
Cerrano C, Bavestrello G, Cattaneo-Vietti R, Giovine M, Benatti U, Sarà M (1999b) Incorporation of inorganic matter in Chondrosia reniformis (Porifera, Demospongiae): the role of water turbulence. Mem Queensl Mus 44:85–90
Cerrano C, Arillo A, Bavestrello G, Benatti U, Bonpadre S, Cattaneo-Vietti R, Gaggero L, Giovine M, Leone L, Lucchetti G, Sarà M (1999c) Calcium oxalate production in the marine sponge Chondrosia reniformis. Mar Ecol Prog Ser 179:297–300
Cerrano C, Calcinai B, Di Camillo CG, Valisano L, Bavestrello G (2007a) How and why do sponges incorporate foreign material? Strategies in Porifera. In: Custódio MR, Lôbo-Hajdu G, Hajdu E, Muricy G (eds) Porifera research: biodiversity, innovation and sustainability. Museu Nacional, Série Livros 28, Rio de Janeiro
Cerrano C, Sambolino P, Calcinai B, Azzini F, Bavestrello G (2007b) Growth of the massive morph of Cliona nigricans (Schmidt, 1862) (Porifera, Clionaidae). Ital J Zool 74:13–19
Chen W, Liu Y, Wang H (2012) Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Med 9(4):e1001206
Cooper GS, Miller FW, Germolec DR (2002) Occupational exposures and autoimmune diseases. Int Immunopharmacol 2:303–313
Cooper GS, Makris SL, Nietert PJ, Jinot J (2009) Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Perspect 117:696–702
Cowie RL (1994) The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med 150:1460–1462
Craighead JE, Kleinerman J, Abraham JL, Gibbs AR, Green FHY, Harley RA, Ruettner JR, Vallyathan V, Juliano EB (1988) Diseases associated with exposure to silica and non-fibrous silicate minerals. Arch Pathol Lab Med 112:673–720
Davis GS (1986) The pathogenesis of silicosis: state of the art. Chest 89:166S–169S
Davis AR (2009) The role of mineral, living and artificial substrata in the development of subtidal assemblages. In: Wahl M (ed) Marine hard bottom communities. Springer, New York, NY
De Laubenfels MW (1950) The Porifera of Bermuda Archipelago. Trans Zool Soc Lond 27:1–154
Demarest MS, Brzezinski MA, Beucher C (2009) Fractionation of silicon isotopes during biogenic silica dissolution. Geochim Cosmochim Acta 73:5572–5583
Ding M, Chen F, Shi X, Yucesoy B, Mossman B, Vallyathan V (2002) Diseases caused by silica: mechanisms of injury and disease development. Int Immunopharmacol 2:173–182
Doiron K, Linossier I, Fay F, Yong J, Wahid EA, Hadjiev D, Bourgougnon N (2012) Dynamic approaches of mixed species biofilm formation using modern technologies. Mar Environ Res 78:40–47, http://dx.doi.org/10.1016/j.marenvres.2012.04.00
Donaldson K, Borm PJ (1998) The quartz hazard: a variable entity. Ann Occup Hyg 42:287–294
Dove PM, Rimstidt JD (1994) Silica-water interactions. In: Heaney P, Prewitt C, Gibbs G (eds) The silica polymorphs, vol 29, Reviews in mineralogy. Mineralogical Society of America, Washington, DC, pp 259–308
Dove PM, Han N, Wallace AF, De Yoreo JJ (2008) Kinetics of amorphous silica dissolution and the paradox of the silica polymorphs. Proc Natl Acad Sci USA 105:9903–9908
Exposito J, Cluzel C, Garrone R, Lethias C (2002) Evolution of collagen. Anat Rec 268:302–316
Faimali M, Garaventa F, Terlizzi A, Chiantore M, Cattaneo-Vietti R (2004) The interplay of substrate nature and biofilm formation in regulating Balanus amphitrite Darwin, 1854 larval settlement. J Exp Mar Biol Ecol 306:37–50
Fenoglio I, Martra G, Coluccia S, Fubini B (2000) Possible role of ascorbic acid in the oxidative damage induced by inhaled crystalline silica particles. Chem Res Toxicol 13:971–975
Finkel ZV, Kotrc B (2010) Silica use through time: macroevolutionary change in the morphology of the diatom frustule. Geomicrobiol J 27:596–608
Fubini B, Hubbard A (2003) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34:1507–1516
Fujii T, Reimer JD (2011) Phylogeny of the highly divergent zoanthid family Microzoanthidae (Anthozoa, Hexacorallia) from the Pacific. Zool Scr 40:418–431
Gamble JF (2011) Crystalline silica and lung cancer: a critical review of the occupational epidemiology literature of exposure response studies testing this hypothesis. Crit Rev Toxicol 41(5):404–465
Garrone R (1978) Phylogenesis of connective tissue: morphological aspects and biosynthesis of sponge intercellular matrix, vol 5, Frontiers of matrix biology. Karger, Basel
Garrone R, Huc A, Junqua S (1975) Fine structure and physicochemical studies on the collagen of the marine sponge Chondrosia reniformis Nardo. J Ultrastruct Res 52:261–275
Gil-Turnes MS, Hay ME, Fenical W (1989) Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 246:116–118
Giovine M, Pozzolini M, Fenoglio I, Scarfi S, Ghiazza M, Benatti U, Fubini B (2003) Crystalline silica incubated in ascorbic acid acquires a higher cytotoxic potential. Toxicol Ind Health 18:249–255
Gough PJ, Gordon S (2000) The role of scavenger receptors in the innate immune system. Microbes Infect 2:305–311
Green FHY, Vallyathan V (1996) Pathologic responses to inhaled silica. In: Castranova V, Vallyathan V, Wallace WE (eds) Silica and silica-induced lung diseases. CRC, Boca Raton, FL, pp 39–59
Guidetti P, Bianchi CN, Chiantore MC, Schiaparelli S, Morri C, Cattaneo-Vietti R (2004) Living on the rocks: substrate mineralogy and the structure of subtidal rocky substrate communities in the Mediterranean Sea. Mar Ecol Prog Ser 274:57–68
Hadfield MG (2011) Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann Rev Mar Sci 3:453–470
Haeckel E (1872) Die Kalkschwämme. G. Reiser, Berlin
Hamilton RE Jr, Thakur SA, Holian A (2008) Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med 44:1246–1258
Heinemann S, Ehrlich H, Douglas T, Heinemann C, Worch H, Schatton W, Hanke T (2007) Ultrastructural studies on the collagen of the marine sponge Chondrosia reniformis Nardo. Biomacromolecules 8:3452–3457
Hess EV (2002) Environmental chemicals and autoimmune disease: cause and effect. Toxicology 181–182:65–70
Hildebrand M (2008) Diatoms, biomineralization processes, and genomics. Chem Rev 108:4855–4874
Hinz B, Brune K (2002) Cyclooxygenase-2 10 years later. J Pharmacol Exp Ther 300:367–375
Hnizdo E, Murray J (1988) Risk of pulmonary tuberculosis relative to silicosis and exposure to silica dust in South Africa gold miners. Occup Environ Med 55:496–502
Holmes SP, Sturgess CJ, Davies MS (1997) The effect of rock-type on the settlement of Balanus balanoides (L) cyprids. Biofouling 11:137–147
Hsu H, Hijjar DP, Khan KM, Falcone DJ (1988) Ligand binding to scavenger receptor-A induces urokinase-type plasminogen activator expression by a protein kinase C delta-dependent. J Biol Chem 273:1240–1246
Hsu H, Chiu SL, Wen MH, Chen KY, Hua KF (2001) Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signalling pathways. J Biol Chem 276:28719–28730
IARC (1987) Silica, some silicates, vol 42, IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. IARC, Lyon
IARC (1997) Silica, some silicates, coal dust and para-aramid fibrils, vol 68, IARC monographs on the evaluation of carcinogenic risk of chemicals to humans. IARC, Lyon
Iler RK (1979) The chemistry of silica. Plenum, New York, NY
Jézéquel MV, Hildebrand M, Brzezinski MA (2000) Silicification in diatoms. Implication for growth. J Phycol 36:821–840
Jin X, Gruber N, Dunne JP, Sarmiento JL, Armstrong RA (2006) Diagnosing the contribution of phytoplankton functional groups to the production and export of particulate organic carbon, CaCO3, and opal from global nutrient and alkalinity distributions. Global Biogeochem Cy. doi:10.1029/2005GB002532
Kamatani A (1971) Physical and chemical characteristics of biogenous silica. Mar Biol 68:91–96
Keough MJ, Raimondi PT (1995) Responses of settling invertebrate larvae to bioorganic films: effects of different types of films. J Exp Mar Biol Ecol 185:235–253
Keough MJ, Raimondi PT (1996) Responses of settling invertebrate larvae to bioorganic films: effects of large-scale variation in films. J Exp Mar Biol Ecol 207:59–78
Kinrade SD, Schach AS, Hamilton RJ, Knight CT (2001) NMR evidence of pentaoxo organosilicon complexes in dilute neutral aqueous silicate solutions. Chem Commun (Cambr) 7:1564–1565
Kobzik L (1995) Lung macrophage uptake of unopsonized environmental particulates. Role for scavenger-type receptors. J Immunol 155:367–376
Krasko A, Lorenz B, Batel R, Schröder HC, Müller IM, Müller WEG (2000) Expression of silicatein and collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur J Biochem 267:4878–4887
Kreyling WG (1992) Intracellular particle dissolution in alveolar macrophages. Environ Health Perspect 97:121–126
Loewus FA (1980) L-ascorbic acid: metabolism, biosynthesis, function. In: Press J (ed) The biochemistry of plants, vol 3. Academic, New York, NY
Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, Katoh M, Miyahara N, Yamamoto S, Hirastuka J, Otsuki T (2010) Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol 7(4):268–278
Maehira F, Iinuma Y, Eguchi Y, Miyagi I, Teruya S (2008) Effect of soluble silicon compound and deep sea water on biochemical and mechanical properties of bone and the related gene expression in mice. J Bone Miner Metab 26:446–455
Manini E, Luna GM (2003) Influence of the mineralogical composition on microbial activities in marine sediments: an experimental approach. Chem Ecol 19:399–410
McDougald D, Rice SA, Barraud N, Steinberg PD, Kjelleberg S (2012) Should we stay or should we go: mechanisms and ecological consequences for biofilm dispersal. Nat Rev Microbiol 10(1):39–50
Migliaccio CT, Hamilton RF Jr, Holian A (2005) Increase in a distinct pulmonary macrophage subset possessing an antigen-presenting cell phenotype and in vitro APC activity following silica exposure. Toxicol Appl Pharmacol 205:168–176
Minchin EA (1900) In: Ray Lancaster E (ed) A treatise on zoology, p 42
Murphy JE, Tedbury PR, Homer-Vanniasinkam S, Walker JH, Ponnambalam S (2005) Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 182:1–15
Nakamura T, Hinagata J, Tanaka T, Imanishi T, Wada J, Kodama T, Doi T (2002) HSP90, HSP70 and GAPDH directly interact with the cytoplasmic domain of macrophage scavenger receptors. Biochem Biophys Res Commun 290:858–864
Nickel M, Brümmer F (2003) In vitro sponge fragment culture of Chondrosia reniformis (Nardo, 1847). J Biotechnol 100:147–159
Palecanda A, Kobzik L (2001) Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med 1:589–595
Penna A, Magnani M, Fenoglio I, Fubini B, Cerrano C, Giovine M, Bavestrello G (2003) Marine diatom growth on the different forms of particulate silica: evidence of cell/particle interaction. Aquat Microb Ecol 32:299–306
Piguet PF, Collart MA, Grau GE, Sappino AP, Vassalli P (1990) Requirement of tumor necrosis factor for development of silica-induced pulmonary fibrosis. Nature 344:245–247
Pozzolini M, Valisano L, Cerrano C, Menta M, Schiaparelli S, Bavestrello G, Benatti U, Giovine M (2010) Influence of rocky substrata on three-dimensional sponge cells model development. In Vitro Cell Dev Biol Anim 46(2):140–147
Pozzolini M, Buzzone F, Berilli V, Mussino F, Cerrano C, Benatti U, Giovine M (2012) Molecular characterization of a nonfibrillar collagen from the marine sponge Chondrosia reniformis Nardo 1847 and positive effects of soluble silicates on its expression. Mar Biotechnol 14(3):281–293
Previati M, Palma M, Bavestrello G, Falugi C, Cerrano C (2010) Reproductive biology of Parazoanthus axinellae (Schmidt 1862) and Savalia savaglia (Bertoloni 1819) (Cnidaria, Zoanthidea) from the NW Mediterranean coast. Mar Ecol Evol Perspect 31:555–565
Qu Y, Tang Y, Cao D, Wu F, Liu J, Lu G, Zhang Z, Xia Z (2007) Genetic polymorphisms in alveolar macrophage response-related genes, and risk of silicosis and pulmonary tuberculosis in chinese iron miners. Int J Hyg Environ Health 210:679–689
Ragueneau O, Tréguer P, Leynaert A, Anderson RF, Brzezinski MA, DeMaster DJ, Dugdale RC, Dymond J, Fischer G, François R, Heinze C, Maier-Reimer E, Jézéquel MV, Nelson DM, Quéguiner B (2000) A review of the Si cycle in the modern ocean: recent progress and missing gaps in the application of biogenic opal as paleoproductivity proxy. Global Planet Change 26:317–365
Ramanathan R, Campbell JL, Soni SK, Bhargava SK, Bansal V (2011) Cationic amino acids specific biomimetic silicification in ionic liquid: a quest to understand the formation of 3-D structures in diatoms. PLoS One 6:e17707
Rosen G (1943) The history of miners’ diseases: a medical and social interpretation. Schuman’s, New York, NY, pp 459–476
Rovida CL (1871) Un caso di silicosi del polmone con analisi chimica. Ann di Chim Appl alla Med 13:102
Sarà M, Vacelet J (1973) Écologie des démosponges. In: Grassé PP (ed) Traité de Zoologie, Tome III: spongiaires. Masson et Cie, Paris
Scarfì S, Benatti U, Pozzolini M, Clavarino E, Ferraris C, Magnone M, Valisano L, Giovine M (2007) Ascorbic acid pre-treated quartz enhances cyclooxygenase-2 expression in raw 264.7 murine macrophages. FEBS J 274:60–73
Scarfì S, Magnone M, Ferraris C, Pozzolini M, Benvenuto F, Benatti U, Giovine M (2009) Ascorbic acid pre-treated quartz stimulates TNF-α release in RAW 264.7 murine macrophages through ROS production and membrane lipid peroxidation. Respir Res 10:25. doi:10.1186/1465-9921-10-25
Schins RP, Knaapen AM (2007) Genotoxicity of poorly soluble particles. Inhal Toxicol 19(S1):189–198
Schultes S, Lambert C, Pondaven P, Corvaisier R, Jansen S, Ragueneau O (2010) Recycling and uptake of Si(OH)4 when protozoan grazers feed on diatoms. Protist 161:288–303
Schulze FE (1879) Untersuchungenüber den Bau und die Entwicklung der Spongien. VI. Die Gattung Spongelia. Z Wiss Zool 32:117–157
Shaw ME (1927) Note on the inclusion of sand in sponges. Ann Mag Nat Hist 19:601–609
Shipe RF, Brzezinski MA (1999) A study of Si deposition study in Rhizosolenia (Bacillariophyceae) mats using novel 32Si autoradiographic method. J Phycol 35:995–1004
Sollas IBJ (1908) The inclusion of foreign bodies by sponges, with a description of a new genus and species of Monaxonida. Ann Mag Nat His 1:395–401
Straif K, Benbrahim-Tallaa L, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens—part C: metals, arsenic, dusts and fibers. Lancet Oncol 10:453–454
Teragawa CK (1986a) Sponge dermal membrane morphology: histology of cell mediated particle transport during skeletal growth. J Morphol 190:335–348
Teragawa CK (1986b) Particle transport and incorporation during skeleton formation in a keratose sponge Dysidea etheria. Biol Bull 170:321–334
Thakur SA, Hamilton RE Jr, Holian A (2008) Role of scavenger receptor: a family in lung inflammation from exposure to environmental particles. J Immunotoxicol 5:151–157
Thibodeau M, Giardina C, Hubbard AK (2003) Silica-induced caspase activation in mouse alveolar macrophages is dependent upon mitochondrial integrity and aspartic proteolisis. Toxicol Sci 76:91–101
Thibodeau M, Giardina C, Knecht DA, Helble J, Hubbard AK (2004) Silica-induced apoptosis in mouse alveolar macrophages is initiated by lysosomal enzyme activity. Toxicol Sci 80:34–48
Todd CD, Keough MJ (1994) Larval settlement in hard substratum epifaunal assemblages: a manipulative field study of the effects of substratum filming and the presence of incumbents. J Exp Mar Biol Ecol 181:159–187
Totti C, Cucchiari E, De Stefano M, Pennesi C, Romagnoli T, Bavestrello G (2007) Seasonal variations of epilithic diatoms on different hard substrates, in the northern Adriatic Sea. J Mar Biol Assoc UK 87:649–658
Tréguer P, Nelson DM, Van Bennekom AJ, DeMaster DJ, Leynaert A (1995) The silica balance in the world ocean: a re-estimate. Science 268:375–379
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
Vallyathan V, Shi XL, Dalal NS, Irr W, Castranova V (1988) Generation of free radicals from freshly fractured silica dust: potential role in acute silica-induced lung injury. Am Rev Respir Dis 138:1213–1219
Van Colen C, Lenoir J, De Backer A, Vanelslander B, Vincx M, Degraer S, Ysebaert T (2009) Settlement of Macoma balthica larvae in response to benthic diatom films. Mar Biol 156:2161–2171
Von Lendenfeld R (1889) A monograph on the horny sponges. Trübner, London
Wahl M (1989) Marine epibiosis. I. Fouling and antifouling: some basic aspects. Mar Ecol Prog Ser 58:75–89
Wilkie IC, Parma L, Bonasoro F, Bavestrello G, Cerrano C, Carnevali MD (2006) Mechanical adaptability of a sponge extracellular matrix: evidence for cellular control of mesohyl stiffness in Chondrosia reniformis Nardo. J Exp Biol 209(22):4436–4443
Zanetti G (2002) Dinamismo strutturale nella spugna Chondrosia reniformis (Porifera, Demospongiae): osservazioni in habitat. Dissertation, University of Milan
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. KBBE-2010-266033 to MG and from University of Genova Funding (PRA 2012) to SS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Giovine, M., Scarfì, S., Pozzolini, M., Penna, A., Cerrano, C. (2013). Cell Reactivity to Different Silica. In: Müller, W., Wang, X., Schröder, H. (eds) Biomedical Inorganic Polymers. Progress in Molecular and Subcellular Biology, vol 54. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-41004-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-41004-8_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-41003-1
Online ISBN: 978-3-642-41004-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)