Abstracts
Three recombinant octameric mutants of human normal adult hemoglobin (Hb A), rHb (αN78C), rHb (αN78C/L29F), and rHb (αN78C/L29W), were expressed in our Eschericia coli expression system and purified. They were used as resuscitation fluids in our unique mouse model of traumatic brain injury (TBI) combined with severe hemorrhagic shock (HS). A sulfhydryl group was introduced onto the surface of the α-subunits of rHb A by substituting Asn78 with cysteine. The rHb (αN78C) form octamers by linking the two tetramers with 2 intermolecular disulfide bonds. Nuclear magnetic resonance (NMR) spectroscopic studies indicate that rHb (αN78C) has the same quaternary and tertiary structures as those of Hb A. Furthermore, the oxygen-binding activity (as measured by P 50 ) and the cooperativity of the oxygenation process (as measured by the Hill coefficient) of this mutant have not been altered compared to Hb A. The Leu29 residue on the α-subunits of the octamers was then mutated into either phenylalanine (F) or tryptophan (W) to yield rHb (αN78C/L29F) and rHb (αN78C/L29W), respectively. Compared to Hb A, rHb (αN78C/L29F) has high- while rHb (αN78C/L29W) has low-oxygen affinity. Both mutants are cooperative in their oxygen-binding properties, but lower than that observed for Hb A. They maintain their quaternary structure as those of Hb A, but exhibit perturbation of their tertiary structure at or near the heme pockets as detected by NMR measurements. Although all octameric rHbs would be expected to have reduced nitric oxide (NO) binding based on their size, rHb (αN78C/L29F) and rHb (αN78C/L29W) may have a further reduction in NO binding as a result of introducing a bigger aromatic amino acid residue at the distal heme pocket of the α-chain. These three rHbs were used as resuscitation solutions in mice after TBI combined with HS. TBI was induced by a controlled cortical impact (CCI) to the left parietal cortex. Blood was then withdrawn (2.4 mL/100 g body weight) over 15 min to induce HS. A pressure-controlled model was used with the mean arterial pressure (MAP) maintained at 25–27 mm Hg for an additional 20 min. At the end of the HS Phase (35 min total), i.e., the beginning of Pre-Hosptial Phase, lactated Ringers (LR) or rHb (120 mg/mL) solutions were administered to the mice at 2 mL/100 g. Additional LR or rHb solution was given if needed at 1 mL/100 g to maintain the MAP at > 70 mm Hg for the next 90 min. The shed blood was then returned simulating definitive care with transfusion in a “Hospital Phase”, lasting 15 min. The mice were then recovered, returned to cages and observed for 24 h before sacrificing for neuropathology. A marked difference in the fluid requirements was observed between the LR and the rHb groups. At the end of the Pre-Hospital Phase, the LR group received >4 times more resuscitation fluid (21.5 ± 0.75 mL/100 g) than the rHb groups (5.0 ± 0 mL/100 g; P < 0.05), while the arterial Hb level in rHb groups were ~3 g/dL higher than that of the LR group. More importantly, the LR group exhibited persistent refractory hypotension during the Pre-Hospital Phase. The MAP of rHb groups stayed near baseline level over the entire Pre-Hospital Phase and Hospital Phase. However, an initial elevation in MAP above baseline followed by gradual diminution was observed only for the rHb (αN78C) group (P < 0.05). Brain tissue oxygen (PbtO2) levels in the hippocampus ipsilateral to the site of CCI did not differ significantly between groups, although the LR group showed deterioration in PbtO2 during late Pre-Hospital Phase. Numerically, the normal oxygen affinity rHb (αN78C) group and the high oxygen affinity rHb (αN78C/L29F) groups exhibited the highest and lowest PbtO2, respectively. Surprisingly, the high oxygen affinity rHb conferred a neuroprotective effect in the CA1 region of the selectively vulnerable region of the hippocampus vs. all other groups. No difference in neuronal survival was seen between groups in the CA3 hippocampus. Our present results suggest that these novel octameric rHbs have the potential to develop into a small-volume resuscitation fluid for treatment of TBI combined with HS.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Tremendous effort has gone into developing formulations to substitute red blood cells transfusion. Most of these formulations are hemoglobin-based oxygen-carriers (HBOCs) [See (Natanson et al. 2008) for review]. Acellular hemoglobin (Hb) is cytotoxic by reacting with endothelium-derived NO to form bioinactive nitrate (Minneci et al. 2005), which may lead to vascular thrombosis of the heart and other organs (Rother et al. 2005). Furthermore, Hb without the confine of red blood cells is prone to dissociation into dimers, with subsequent loss in oxygen-binding activity and allostery (Baudin-Creuza et al. 2011). Hence, hemoglobins have been cross-linked (Chatterjee et al. 1986), polymerized (Sehgal et al. 1984) or surface-conjugated (Conover et al. 1996; McCarthy et al. 2001) to create larger and more stable molecules based on the postulation that larger HBOCs could prevent extravasation and have lower toxicities. With the advent of protein engineering, recombinant hemoglobin (Shen et al. 1993, 1997) can be prepared in sufficient quantity for HBOC studies. By substituting protein surface residue(s) with cysteine(s), polymeric (Fronticelli et al. 2001) or octameric (Fablet et al. 2003; Vasseur-Godbillon et al. 2006) hemoglobins can be generated. Furthermore, Hb molecules with unique oxygen-binding and/or autoxidative properties can be obtained by mutating certain amino acid residue(s) on the protein. For instance, replacing the Leu29 residue of the α-subunit (αL29) with phenylalanine inhibits oxidation accompanied by an increase in oxygen affinity (Eich et al. 1996; Olson et al. 1997; Jeong et al. 1999). However, substituting the αL29 residue with tryptophan produces a tetramer with low oxygen affinity (Wiltrout et al. 2005). Nonetheless, octameric hemoglobins possessing these interesting properties have not been tested in vivo with any clinically relevant models.
Traumatic brain injury (TBI) is an important public health problem world-wide. Outcomes from TBI are substantially worsened by secondary insults, such as hemorrhagic shock (HS), which occurs in approximately 30 % of TBI victims (Chesnut et al. 1993). In experimental models, TBI combined with HS can cause neuronal death in the hippocampus beneath the cerebral contusion at mild injury levels where no neuronal death is seen in TBI alone. The situation poses a special challenge to the field of resuscitation medicine. A number of strategies have been explored with limited success (Bhardwaj and Ulatowski 2004; Brasel et al. 2008; Doyle et al. 2001; Forsyth et al. 2008). Blood substitutes, however, may present a promising strategy for resuscitation of patients with TBI plus HS. Recent studies suggest resuscitation with solutions of polymerized hemoglobin provided substantial benefits on intracranial pressure (ICP), brain tissue oxygenation (PbtO2), and neuropathology (Patel et al. 2006; Rosenthal et al. 2008; Stern et al. 2009). Unfortunately, enthusiasm for blood substitutes was blunted considerably when multiple clinical trials concluded that current generation of HBOCs increased incidence of myocardial infarction and mortality (Natanson et al. 2008). Hence, a better Hb-based resuscitation solution is desperately needed for polytrauma casualties suspected of suffering TBI.
Given the failures of HBOCs in clinical trials (Natanson et al. 2008), it seems appropriate to consider a paradigm shift in the approach to the development of blood substitutes. We believe that this paradigm shift should incorporate two strategies. First, it should develop novel and targeted HBOCs that are molecularly and/or chemically designed not only to minimize toxicities, but also such that HBOC performance is optimized for specific indications. For example, in the setting of shock, benefit might be maximized using an HBOC that not only features reduced NO binding but also exhibits specific oxygen affinity characteristics and/or other modifications critical to the disease process being targeted. Recombinant Hbs represent perfect candidates in this regard. Using this methodology, one has the opportunity to design a Hb molecule with designed oxygen affinity, reduced rate of autoxidation, NO binding affinity, etc. Olson and colleagues have written an excellent review on the design of recombinant Hbs for HBOCs with emphasis on autoxidation and NO binding affinity (Varnado et al. 2012). Second, we posit that pre-clinical studies should be carried out in experimental models that more closely mimic the complex clinical scenarios that have the greatest need for HBOC therapies. For example, when polytrauma and HS include TBI, hypotensive resuscitation, which prevents “blood washout” in uncontrolled hemorrhage shock, may be inadequate for the injured brain—which has high metabolic demands early after the injury (Bauman et al. 2009). TBI guidelines (Bratton et al. 2007) recommend avoiding hypotension in this setting which is quite different from HS alone. And in TBI plus HS, resuscitation with large volumes of crystalloid can exacerbate brain edema and intracranial hypertension. Thus, TBI plus HS represents a specific form of polytrauma that could greatly benefit from an HBOC. TBI plus HS may also represent a special opportunity for HBOC therapy because it generally occurs in the young, and is of importance in combat casualty care from blast TBI in service persons who are, once again, young and free of cardiovascular diseases. These trauma victims may develop the fewest side effects from HBOC therapy and thus may be best for initial demonstration of efficacy (Elmer et al. 2012).
In this communication, we report on the administration of three octameric hemoglobins as resuscitation solutions in mice after experimental TBI plus HS. The systemic hemodynamics, resuscitation volume requirements, survival, PbtO2, and neuropathology of the mice were assessed after treatment. The recombinant octameric hemoglobin derives from substituting a surface asparagine at the 78 position of the α-subunit with cysteine (αN78C). The rHb (αN78C) remains as octamers in the presence of human plasma (Brillet et al. 2012). Its large molecular size offers the possibility of diminished NO binding. In addition, the leucine residue locates in the distal heme pocket (B10 helix) of the α-subunit was mutated to either phenylalanine or tryptophan to generate mutants with high- [rHb (αN78C/L29F)] and low-oxygen [rHb (αN78C/L29W)] affinities, respectively, when compared to that of Hb A. Our results demonstrate that these octameric rHbs can serve as small volume resuscitation solutions in mice suffering TBI with HS, compared to conventional lactated Ringer’s (LR) solution. Acute hemodynamic effects of these rHbs may be predicted based on theoretical effects on NO binding. Increasing Hb oxygen affinity also produces a trend towards lower PbtO2 and surprisingly, reduced neuronal death in the CA1 region of the hippocampus, a brain region that is highly sensitive to ischemic insults.
2 Materials and Methods
2.1 Materials
Hb A was isolated from human normal blood samples obtained from local blood bank with a published protocol from our laboratory (Russu et al. 1984). Restriction enzymes were purchased from New England BioLabs. QuikChange site-directed mutagenesis kit was a product of Stratagene. All other chemicals are of reagent grade and obtained from Sigma unless specified.
2.2 Expression and Purification of rHbs
Construction of the plasmid pHE2073 that encodes rHb (αN78C) for co-expression with methionine aminopeptidase has been reported (Baudin-Creuza et al. 2011). With standard molecular biology techniques, the pHE2073 plasmid was used as template in polymerase chain reactions with appropriate mutation primers to replace the Leu29 residue of the α-subunit (αL29) with either phenylalanine or tryptophan. The resulting pHE2053 and pH2054 plasmids encode rHb (αN78C/L29F) and rHb (αN78C/L29W), respectively.
These plasmids were transformed separately into E. coli JM109 for protein expression. Transformed cells were cultured in a 20 L fermentor (B. Braun Biotech International, model Biostat C) in DM-4 medium (Looker et al. 1994) at 32 °C. Glucose in the medium was maintained at 0.8–1 % throughout the culturing period. Recombinant protein expression was induced with 0.1 mM isopropyl β-thiogalactopyranoside (IPTG) for 6 h when the culture reached an optical density of 10 at 600 nm. Hemin (25 mg/L) was added to the culture at 0 and 3 h after IPTG induction. At the end of the induction period, cells normally reached an optical density of 30 or higher at 600 nm. Cells were then collected by centrifugation and the wet paste was stored at −80 °C.
The recombinant proteins were purified as published (Shen et al. 1997; Wiltrout et al. 2005) with minor modifications at 4 °C under CO environment. Briefly, cells were suspended in Buffer A (40 mM Tris–HCl, pH 8.6, and 1 mM benzamidine) at 3 g/mL. Cell lysis was carried out in a high-pressure homogenizer (Avestin, EmulsiFlex-C3). Cell debris and large DNA fragments were removed by centrifugation at 22,000 x g for 2.5 h. Polyethyleneimine was then added to a concentration of 0.5 % and the precipitated nucleic acids were removed by centrifugation at 15,000 x g for 30 min. The sample was concentrated with a Vivaflow 200 system (Sartorius Stedim Biotech GmbH), then dialyzed overnight against Buffer B (20 mM Tris–HCl, pH 8.6, 0.5 mM EDTA). The recombinant proteins were loaded onto a Q-Sepharose Fast-Flow (GE Healthcare Life Sciences) column equilibrated and washed with Buffer B. Proteins were eluted from the column with Buffer C (20 mM Tris–HCl, pH 6.5, 0.5 mM EDTA) then oxidized for 1 h with four-fold molar excess of K3Fe(CN)6. Excess chemicals were removed by gel permeation through a Sephadex G-25 column with Buffer D (50 mM sodium phosphate, pH 6.8) as carrier. The sample was incubated overnight at room temperature then reduced with four-fold molar excess of sodium dithionite dissolved in Buffer D under nitrogen. The reduced protein sample was loaded immediately onto a Sephadex G-25 column that has been equilibrated with Buffer E (10 mM sodium phosphate, pH 6.8, 0.5 mM EDTA) freshly equilibrated with CO and eluted with the same buffer. Final purification was carried out on a Mono S 16/10 column (GE Healthcare Life Sciences) equilibrated in Buffer E and eluted with Buffer F (20 mM sodium phosphate, pH 8.3, 0.5 mM EDTA) at an increment of 2 % Buffer F per column volume. The octamers eluted off the column at approximately 22–25 % Buffer F. The molecular weights of the rHb subunits were affirmed with mass spectrometry and the amount of N-terminal methionine cleavage was estimated with Edman degradation (Shen et al. 1993, 1997). All rHbs used in this study had the correct molecular weights and less than 5 % N-terminal methionine.
For rHbs used as resuscitation solutions, they were converted to oxy-Hb then passed through an EndoBind-R column (BioDtech, Inc) for endotoxin removal. Samples were collected in pyrogen free test tubes (Lonza Walkersville, Inc.) and further concentrated to 120 mg/mL with ultrafiltration columns (Vivaspin, Satorius Stedim Biotech GmbH) for studies.
2.3 Oxygen-Binding Measurements
Data were acquired on a Hemox Analyzer (TCS Medical Products) at 29 °C as a function of pH in 0.1 M sodium phosphate (Shen et al. 1993, 1997). The solution contained 0.1 mM hemoglobin (in terms of heme) and a methemoglobin (met-Hb) reductase system to slow down the formation of met-Hb (Hayashi et al. 1973). Results from each equilibrium binding curve were fit to the Adair equation and oxygen affinity was determined from the P 50 value (in millimeters of Hg) which was taken at 50 % O2 saturation. The cooperativity of the hemoglobin samples was estimated with the Hill coefficient (n 50 ), which was calculated from the slope of the Hill plot at 50 % saturation and had an accuracy of ±10 %.
2.4 Structural Study with 1H NMR Spectroscopy
1H NMR spectra were collected on Bruker Avance DRX-300 or DRX-600 spectrometer. Samples were 5 % (3.1 mM in terms of heme) hemoglobin in 0.1 M sodium phosphate buffer at pH 7.0 containing 95 % H2O and 5 % deuterium oxide (D2O). Experiments were performed at 29 °C. A jump-and-return pulse sequence was used to suppress the water signal (Plateau and Gueron 1982). Proton chemical shifts are referenced to the methyl proton resonance of 2, 2-dimethyl-2-silapentene-5-sulfonate (DSS) indirectly by using the water signal at 4.76 ppm downfield of DSS at 29 °C.
2.5 Mouse Model of Traumatic Brain Injury Combined with Hemorrhagic Shock
C57BL6 mice (Jackson Laboratories) 12–15 weeks of age and weighing of 27.7 ± 0.5 g were housed in controlled environmental condition. Food and water were availed ad libitum until the start of the study. Induction of TBI by controlled cortical impact (CCI) with subsequent hemorrhage was carried out according to (Hemerka et al. 2012), with modifications. Mice were anesthetized with 4 % isofluorane in 2:1 N2O/O2 via nose cone. Femoral venous and arterial catheters were inserted via an inguinal cut-down and the animals were placed in a stereotactic frame. A 5 mm craniotomy was then performed and the bone flap removed. The brain temperature was controlled with a heat lamp at 37.0–37.5 °C and monitored with a probe (Physitemp) inserted into the right parietal cortex via a burr hole. The body temperature of the animal was checked with a rectal probe.
CCI was performed with a pneumatic impactor (Bimba) using a flat 3 mm tip at a velocity of 5 m/s to a depth of 1 mm. TBI at this level without HS produced a mild to moderate level of injury with no appreciable loss of CA1 hippocampal neurons and no mortality (Dennis et al. 2009). A tissue PO2 probe was then inserted via the CCI craniotomy with its tip stayed beneath the CCI injury and approximate to the core of the hippocampus. The initial reading 5 min after insertion was taken as “baseline” and subsequent readings were converted to percent of baseline. Anesthesia was then changed to 1.5 % isofluorane in room air for 10 min, after which baseline hemodynamic and PbtO2 readings were recorded.
Blood was withdrawn from the mice via the femoral arterial catheter over 15 min to a total volume of 2.4 mL/100 g body weight. Over the next 20 min, the mean arterial blood pressure (MAP) of the mice was maintained between 25–27 mm Hg to mimic HS, via further withdrawal or reinfusion of blood as needed. The HS phase was 35 min in length.
2.6 Experimental Protocol for In Vivo Testing
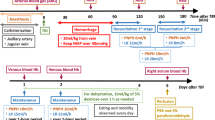
The experimental protocol is detailed in Fig. 13.1. The time course includes a 35 min HS Phase, a 90 min “Pre-Hospital” resuscitation Phase, a 15 min “Hospital” resuscitation Phase, and a 24 h Observation Phase. The Pre-Hospital Phase was initiated by administering a bolus of LR or rHbs (12 % solution in terms of heme) at 2 mL/100 g body weight. LR was then infused continuously at 2 mL/100 g/hr as a maintenance fluid. Additional LR was given at 1 mL/100 g/5 min if MAP of the animal decreased below 70 mm Hg. After 90 min of “Pre-Hospital” resuscitation, the Hospital Phase was started with 100 % oxygen and return of all shed blood to mimic the definitive care in an emergency room. At the end of this phase, all catheters and probes were removed and the animals were sutured. Mice were returned to cage and observed for 24 h, then sacrificed for neuropathology by perfusion fixation. Blood pressure and PbtO2 were assessed continuously in the hippocampus ipsilateral to the CCI. Arterial blood gases were determined after CCI (baseline), and at the end of each phase. Total blood Hb levels were determined using Hemocue 201 (Hemocue Inc.) which measured Hb in both erythrocytes and plasma. The number of animals used in our study with resuscitation fluids was: LR (n = 15); rHb (αN78C) (n = 7); rHb (αN78C/αL29F) (n = 9); and rHb (αN78C/αL29W) (n = 7). Differences in sample size related to differences in availability of the various rHbs.
Experimental protocol for studies of combined traumatic brain injury (TBI) and hemorrhagic shock (HS) designed to mimic the clinical scenario of a polytrauma victim, with TBI and a secondary insult from severe hemorrhagic hypotension. TBI, was induced using the controlled cortical impact (CCI) model in C57BL6 mice at a mild-moderate injury level. This was followed by severe pressure controlled HS (mean arterial blood pressure [MAP] at 25–27 mm Hg) induced by blood withdrawal for a period of 35 min. To mimic pre-hospital resuscitation, a 90-min period followed with administration of either lactated Ringers (LR) solution or recombinant hemoglobin (rHb). A 15-min phase mimicking hospital emergency department care followed during which time the shed blood was re-infused and pure oxygen was administered. Anesthesia in the model was provided using isoflurane (Iso). This model thus presents a highly clinically relevant scenario for testing novel resuscitation solutions such as rHbs
2.7 Histology
Mice were anesthetized with 4 % isofluorane and by transcardial perfusion with ice-cold saline followed by 10 % buffered formalin. Brain tissues were fixed with 10 % buffered formalin and embedded in paraffin. Multiple 5 mm sections, 200 mm apart from bregma −1.86 to −2.26, were prepared from each brain. Sections were stained with hematoxylin and eosin (Thermo Scientific). The number of surviving neurons in the CA1 and CA3 regions of the hippocampus were quantified by a blinded observer in the hematoxylin and eosin stained sections (ImageJ software, NIH). Neuron counts were quantified as densities per 100 μm segments of the hippocampal subfields.
2.8 Statistical Analysis
Data are presented as mean ± standard error unless it was stated otherwise. Analysis of variance was used to compare continuous physiologic variables between groups. Appropriate post hoc analysis was performed using the Student–Newman–Keuls (for all group-wise comparisons) or Dunnetts test (for comparison to LR resuscitation control). Kruskal–Wallis tests were used to determine the significance of non-parametric data.
3 Results
3.1 rHb Characterization
3.1.1 Oxygen-Binding Properties
The oxygen-binding properties of the rHb mutants were determined and compared to that of Hb A in 0.1 M phosphate buffer as a function of pH at 29 °C. The properties of mouse Hb were also determined and plotted together for direct comparison. The rHb (αN78C) mutant has similar P 50 values as that of Hb A between pH 5.7 to pH 8.5 (Fig. 13.2a). The results agree closely to that of Brillet et al. (2012) and Baudin-Creuza et al. (2011) and show clearly that the substitution of a surface residue, αAsn78, with Cys does not change the function of the macromolecule. However, additional replacement of the B10 residue (αLeu29) alters the oxygen-binding affinity of the protein. The rHb (αN78C/L29F) and the rHb (αN78C/L29W) mutants have low and high P 50 values, respectively. Data indicate rHb (αN78C/L29F) is a mutant with high oxygen affinity while rHb(αN78C/L29W) is a low oxygen affinity mutant. The mouse Hb has P 50 values higher than those of Hb A, but substantially lower than those of the rHb (αN78C/L29W) mutant (Fig. 13.2a).
Oxygen binding properties of Hb A, octameric rHbs, and mouse Hb in 0.1 M sodium phosphate buffer as a function of pH at 29 °C: a Oxygen binding as a function of pH and b Hill coefficient as a function of pH. (dot) Hb A, (X) mouse Hb, (triangle) rHb (αN78C), (inverted triangle) rHb (αN78C/L29F), and (diamond) rHb (αN78C/L29W)
The Hill coefficients (n 50 ), a measure of the cooperative oxygenation process, of Hb A, mouse Hb, and the rHbs are plotted in Fig 13.2b. The experimental values obtained for the rHb (αN78C) are lower than those of Hb A, but similar to the results observed for mouse Hb. Mutation in the distal heme pocket further decreases the n 50 values. Nonetheless, all the mutants show cooperativity in oxygen binding, even for those containing distal heme pocket substitution.
3.1.2 NMR Spectra of Hbs in the CO Form
1H NMR spectroscopy is an excellent tool to probe tertiary and quaternary structural changes in hemoglobin (Ho 1992). The exchangeable protons in the inter-subunit interfaces give resonance signals between 9 and 14 ppm. The resonances at 12.9 and 12.1 ppm from DSS have been assigned to the NH1 of αHis122 and αHis103, respectively (Russu et al. 1987; Simplaceanu et al. 2000). These two residues locate in the α1β1 interface. The resonance at 10.6 ppm has been assigned to the NHε1 of βTrp37 (Simplaceanu et al. 2000; Fang et al. 2000), which is located in the α1β2 interface. Figure 13.3a shows clearly that no noticeable shift has been detected in these resonances among the spectra generated for Hb A and the three mutants. Hence, the quaternary structure at the α1β1 and the α1β2 interfaces has been preserved.
Spectra representing the non-exchangeable ring-current shifted proton resonances from 0 to −3 ppm from DSS are presented in Fig. 13.3b. The resonances at −1.75 and −1.82 ppm from DSS have been assigned to the γ2-CH3 group of αVal62 and βVal67, respectively (Lindstrom et al. 1972; Dalvit and Ho 1985). These two residues located on the E helix and the E11Val methyl resonances provide information about the tertiary structure of the heme pockets. As expected, the NMR spectrum representing the rHb (αN78C) mutant is similar to that of Hb A and the resonance at −1.82 ppm has not been shifted for all three mutants. However, the resonance at −1.75 ppm shifted noticeable in the rHb (αN78C/L29F) and the rHb (αN78C/L29W) mutants, but not the rHB (αN78C) mutant. These results demonstrate clearly the αAsn78 mutation does not change the structure of the heme pockets while the αLeu29 substitutions affect only the tertiary structure of the α-subunit heme pocket.
3.1.3 NMR Spectra of Hbs in the Deoxy Form
The hyperfine-shifted proton resonances spectra representing Hb A and the three mutants covering the region 55–80 ppm from DSS are shown in Fig. 13.4a. For deoxy-Hb, the NδH exchangeable proton of αHis87 and βHis92 yield signals at 63 and 76 ppm, respectively (Takahashi et al. 1980). These two histidine residues locate at the proximal heme pockets and any shift in resonance signal indicates a change in the tertiary structure at the heme pocket. There is no shift in these two resonances in rHb (αN78C) compared to Hb A. However, we have detected a downfield shift of 4–5 ppm for the resonance at 63 ppm while the resonance at 76 ppm remains unaffected for the rHb (αN78C/L29F) and the rHb (αN78C/L29W) mutants. Therefore, the tertiary structure of the proximal heme pocket has been disturbed in the α-subunits of the Hb molecule carrying αLeu29 mutations as expected. The tertiary structure of their β-subunit heme pocket remains intact.
The spectra for exchangeable protons in the inter-subunit interfaces are presented in Fig. 13.4b. The resonances at 13.0 and 12.2 ppm from DSS are generated by the NHε1 of αHis122 and αHis103, respectively (Ho 1992). We cannot detect any significant shift in these resonances from the mutants. Hence, the quaternary structure of these three octamers in the deoxy form at the α1β1 interface has not been perturbed.
βTrp37 is located in the α1β2 interface and forms an H-bond to αAsp94 (Dickerson and Geis 1983). Its exchangeable indole proton gives a resonance signal at 11.2 ppm from DSS. A slight shift of 0.3 ppm was detected for this signal in the rHb (αN78C/L29F) mutant. Another important T-structure marker is the resonance at 14.1 ppm, which is contributed by the H-bond between αTyr42 and βAsp99 in the α1β2 interface of deoxy-Hb A (Fung and Ho 1975). We have observed a slight 0.3–0.5 ppm shift for this resonance in both rHb (αN78C/L29F) and rHb (αN78C/L29W) mutants. Combining these results, we conclude that the rHb (αN78C/L29F) and rHb (αN78C/L29W) mutants have slight but noticeable changes in the α1β2 interface.
3.2 rHb as a Resuscitation Solution
3.2.1 Endotoxin Level in Octameric rHbs
The purified octameric rHbs were further isolated from an EndoBind-R column for endotoxin removal. Parts of the final products were sent to Lonza Walkersville, Inc for endotoxin analysis. The samples have on the average 131 and 2.43 EU/mL of endotoxin before and after passing through the column, respectively.
3.2.2 Survival
TBI combined with HS is a severe insult. Nevertheless, mice in all groups can generally survive through the Pre-Hospital Phase (Table 13.1). Statistically, no difference in survival rate was detected among groups considering either survival to the Hospital Phase or to 24 h post CCI (P = 0.435, Chi Square test).
3.2.3 Hemodynamics
MAP was normalized in all rHb groups 5 min after administration of the rHb solution. MAP in these groups stayed near baseline level over the entire Pre-Hospital Phase (90 min, Fig. 13.5). Furthermore, the rHb groups did not need additional boluses after the initial dosage of 2 mL/100 g body weight of a 12 % rHb solution. In contrast, the LR group had persistent refractory hypotension (p < 0.01 vs rHb groups) and additional boluses were needed to maintain the MAP > 70 mm Hg which is consistent with prior reports of this model (Dennis et al. 2009). Consequently, the LR group received 4 times more resuscitation fluid during the Pre-Hospital Phase than that of the rHb groups (Table 13.1). It is of interest to note that an increase in MAP was noted in the LR group during the Hospital Phase after returning all shed blood to the animals. This increment of MAP was not observed in the rHb-treated groups. Among the rHb-treated groups, the initial elevation in MAP was significantly higher in the group receiving rHb (αN78C) (Fig 13.5, p < 0.05 vs other 2 rHb groups).
3.2.4 Arterial Blood Gas Analysis and Hemoglobin Levels
Arterial blood gas values do not differ among the 4 treatment groups at baseline. At the end of the Pre-Hospital Phase, Hb levels in the rHb groups were approximately 3 g/dL higher than that of the LR group (p < 0.05, Table 13.2). This significant increase in Hb for all of the rHb-treated groups versus the LR-treated group also remained at the end of the Hospital phase—i.e., after the shed blood had been re-infused (Table 13.2). All groups had almost normalized arterial blood gas and lactate levels and no difference is detected among groups at any time point (including baseline, end of HS phase, end of Pre-Hospital Phase, and end of Hospital Phase) (Table 13.2).
3.2.5 Brain Tissue Oxygen Concentration
At the end of the HS Phase, PbtO2 consistently decreased to approximately 20 % of baseline. Resuscitation with LR or rHb solutions can slowly increase PbtO2, but none of the groups has normalized PbtO2 (Fig. 13.6). Numerically, the group receiving the high oxygen affinity rHb (αN78C/L29F) has the lowest PbtO2 throughout the Pre-Hospital Phase (P > 0.05). During the Hospital Phase, when 100 % oxygen was administered and all shed blood re-infused, as anticipated PbtO2 increased to values markedly greater than baseline (results not shown) and again no difference was detected among groups.
Brain tissue oxygen concentration (PbtO2) during HS and Pre-Hosptital Phases on the ipsilateral side of controlled cortical impact induced traumatic brain injury in mice resuscitated with lactated Ringer solution, normal oxygen affinity rHb (αN78C), high oxygen affinity rHb (αN78C/L29F), or low oxygen affinity rHb (αN78C/L29W). Although there is a trend toward the lower PbtO2 levels during resuscitation in the rHb (αN78C/L29F) group, this does not reach significance
3.2.6 Neuropathology
As previously reported (Dennis et al. 2009), TBI combined with HS causes significant neuronal death in both CA1 and CA3 regions on the hippocampus ipsilateral to the side of CCI in all groups. Surprisingly, neuronal survival is greatest in CA1 hippocampus in the high oxygen affinity rHb (αN78C/L29F) treated group. However, neuronal counts in CA3 hippocampus do not differ significantly among treatment groups (Fig. 13.7). No neuronal loss was seen in the hippocampus contralateral to the impact (data not shown).
Surviving neurons in the CA1 and CA3 sectors of the hippocampus at 24 h after traumatic brain injury plus hemorrhagic shock in mice resuscitated with lactated Ringer solution, normal oxygen affinity rHb (αN78C), high oxygen affinity rHb (αN78C/L29F), or low oxygen affinity rHb (αN78C/L29W). In the CA1 region of the hippocampus, resuscitation with the rHb (αN78C/L29F) significantly increased neuronal survival (*P < 0.05 vs all other groups; ANOVA and Dunnett’s test). In contrast, in the CA3 region of the hippocampus, there was no difference between groups on neuronal survival. Neuron densities in CA1 and CA3 regions were quantified in coronal brain sections taken through the dorsal hippocampus beneath the impact site and counts assessed per 100 μm length segments
4 Discussion
The overall goal of this study is to develop high molecular weight hemoglobins with unique oxygen binding properties for use as resuscitation fluids. Development of an oxygen carrier of larger size can theoretically prolong the circulation time (Fronticelli et al. 2001; Chauvierre et al. 2010). To achieve this end, we have substituted αAsn78 on the surface of the tetrameric hemoglobin with cysteine to form an octameric structure that is stable in the presence of fresh human plasma (Brillet et al. 2012). The rHb (αN78C) is functionally similar to Hb A (Brillet et al. 2012) and our NMR studies here (Figs. 13.3 and 13.4) demonstrated that the heme pockets, the α1β1 and the α1β2 inter-subunit interfaces are also structurally similar to Hb A.
NO scavenging by extracellular HBOCs has been suggested to be the cause of hypertension and this effect can possibly be negated by employing Hbs with lower rates of NO-induced oxidation (Doherty et al. 1998). The leucine residue at B10 is part of the distal heme pocket of myoglobin and hemoglobin (Dickerson and Geis 1983). Previous studies have shown that mutation of Leu29 (the B10 residue) of myoglobin and the α-subunit of hemoglobin can reduce the rate of autoxidation and NO-induced oxidation (Wiltrout et al. 2005; Carver et al. 1992). Hence, we replaced αLeu29 on rHb (αN78C) with phenylalanine or tryptophan to generate hemoglobin octamers with B10 mutations.
rHb (αL29F) and rHb (αL29W) have been studied by our group (Wiltrout et al. 2005). rHb (αL29F) has high while rHb (αL29W) has low oxygen binding affinity (P 50 ). The octamers behave similarly, rHb (αN78C/L29F) mutant has high while the rHb (αN78C/L29W) has P 50 value (Fig. 13.2). Accompanying the change in oxygen binding affinity is a perturbation in the tertiary structure of the α-subunit heme pocket. The heme pocket of the β-subunit has not been disturbed (Figs. 13.3 and 13.4). Furthermore, these B10 mutations have not changed the subunit interfaces of the ligated hemoglobin (Fig 13.3). However, slight tertiary structural changes occur at the α1β2 interface in the unligated (deoxy) B10 mutants (Fig. 13.4). Whether these structural changes in the mutants are related to the diminished n 50 values remain to be investigated.
These three octamers have been used as resuscitation solutions in our mouse model. This is the first application of octameric rHb in any clinically relevant in vivo model. We have chosen to study TBI combined with HS because this combined insult has emerged as a major combat casualty scenario in the United States Army due to improvised explosive devices (Bauman et al. 2009). Secondary insults also are common in civilian TBI and contribute to increases in morbidity and mortality (Chesnut et al. 1993). We have demonstrated that CCI (at the injury level used) or HS alone produces little damage to the CA1 and CA3 hippocampal regions, but injury and resultant neuronal death are significantly exacerbated when CCI and HS are combined (Dennis et al. 2009). In addition, this combined insult represents a setting where a cell-free Hb solution could have special potential, given the need to resuscitate with a small volume solution to limit brain edema, while attempting to optimize oxygen delivery to the highly vulnerable traumatically injured brain where metabolic demands are great (Hovda et al. 1995).
Our study with these novel rHbs demonstrated several interesting findings. First, rHbs, regardless of their oxygen affinity, restored systemic hemodynamics after TBI combined with HS and normalized markers of global tissue metabolism as suggested by systemic lactate and pH. This is in contrast to the failing resuscitation efforts that results from use of the standard LR. Even at 4 times the resuscitation volume as that of rHbs, LR does not improve MAP into the target range during the Pre-Hospital Phase. It is well known that TBI increases markedly the sensitivity to secondary hemorrhage and contributes to a refractory hypotension during conventional resuscitation (Yuan and Wade 1992). And our prior reports in murine models of TBI plus HS confirm that observation (Hemerka et al. 2012; Dennis et al. 2009).
A second interesting effect of octameric rHbs in this study is that the MAP profiles produced during resuscitation were predicted by the molecular structure of the rHbs. rHb (αN78C/L29F) and rHb (αN78C/L29W) have an amino acid substitute at the distal heme pocket (B10). In vitro experiments have concluded that rHbs carrying a single phenyalanine or tryptophan substitute at αLeu29 have reduced NO-induced oxidation rates (Eich et al. 1996; Olson et al. 1997; Wiltrout et al. 2005). The results imply that these mutants have diminished interaction with NO. We observed a supra-baseline hypertensive response after resuscitation only for rHb (αN78C) that has surface residue replacement. The other two octamers tested carry additional αLeu29 substitutions and they do not exhibit this hypertensive response (Fig. 13.5). Indeed, based on testing of numerous crystalloid, colloid, and Hb-based resuscitation fluids in our mouse model (Dennis et al. 2009; Exo et al. 2009; Shellington et al. 2011), rHb (αN78C) is the only agent that we have tested that has produced a hypertensive response. We recognize, however, that the effect of these octameric rHbs on ‘vasoactivity’ in vivo is likely to be complex and could involve mechanisms other than a simple change in the NO interacting site, such as differences in Hb-endothelial interaction or pre-mature arteriolar oxygen release, among other possibilities (Rohlfs et al. 1998). Nevertheless, given the general similarities of these octameric Hbs, we believe that the differences in molecular structure at αLeu29 are most likely the cause of this finding. This interesting finding suggests that specific rHb modifications can potentially translate to in vivo effects. Future studies should specifically address this interesting mechanistic question.
Although statistically significant differences in PbtO2 ipsilateral to the injury have not been seen between groups resuscitated with octameric rHbs of varying oxygen affinities, there are trends that suggest that the differences in oxygen affinity might have played some role. Specifically, the high oxygen affinity rHb (αN78C/L29F) exhibited a trend toward the lowest PbtO2 during the Pre-Hospital Phase—with values lower than the LR group. Our study represented an initial exploration of this concept, which certainly warrants additional studies. TBI is well recognized to produce heterogeneous injury and the local PbtO2 can vary greatly. A much larger sample size and additional experiments would be needed to appropriately test the potential efficacy of rHbs in this regard. Indeed, the “ideal” design of an rHb in the treatment of TBI combined with HS or other conditions is an intriguing concept that certainly merits further investigation. For blood substitutes (but not necessarily resuscitation agents), the advantages and disadvantages of Hbs with higher or lower oxygen affinity have been extensively explored. Kunert et al. (1996) reported that the administration of allosteric effector that reduces Hb affinity for oxygen can increase tissue PO2, but decrease arteriolar diameters and blood flow. Because of the vasoactivity associated with low oxygen affinity Hb, some researchers believe that Hbs with high oxygen affinity are more desirable. Rohlfs et al. (1998) found that Hb vasoactivity was closely linked to decreased oxygen affinity, but not to NO consumption rates. They hypothesized that Hbs with lower oxygen affinity may cause premature O2 release in arterioles and triggering autoregulatory vasoconstriction. In support of this hypothesis, a polymerized low oxygen affinity bovine Hb (P 50 of 54 mm Hg) reduced tissue oxygenation in a hamster cheek-pouch model (Cabrales et al. 2008; Tsai et al. 2006). Consistent with that hypothesis, the high oxygen affinity rHb was the only one that exhibited a neuroprotective effect–enhancing neuronal survival in CA1 hippocampus (Fig. 13.7). It should be noted, that the CA1 hippocampus is highly vulnerable to both ischemia and oxidative stress after TBI, and is a highly vulnerable target in the setting of TBI plus HS (Dennis et al. 2009). In contrast, many investigators have suggested that a low oxygen affinity Hb would be desirable to facilitate O2 delivery in resuscitation. Surprisingly, there are few data to support this intuitive hypothesis. Watanabe et al. (2008) transplanted transgenic bone marrow cells that express an extremely low oxygen affinity Hb (P 50 of 99 mm Hg vs P 50 of 48 mm Hg for normal mouse) into mice with chronic heart failure and reported increased O2 supply to skeletal muscles and improved treadmill performance. Similary, Huang et al. (2005) reported that transfusion of low oxygen affinity Hb (Presbeterian Hb) to septic mice can improve survival. As for our high and low oxygen affinity octamers, we have shown previously that substitution of αLeu29 with either phenyalanine or tryptophan would lower the NO-induced oxidation of the rHbs (Wiltrout et al. 2005). Hence, further experiments are needed before we can delineate the oxygen affinity from NO interacting effect in animal models.
The debate regarding optimal O2 affinity for blood substitutes will continue. Unfortunately, our data do not yield a definitive answer with regard to resuscitation of injured brain. We have found that the high oxygen affinity rHb [rHb (αN78C/L29F)] has the lowest numerical PbtO2 (Fig. 13.6) and the paradoxically best acute neuronal survivial. TBI combined with HS is a complex model and additional studies should be pursued in other models.
Accompanying the potential benefits of rHbs related to hemodynamics, O2 delivery and their small resuscitation volume requirements are their potential neurotoxicity. This may be an important issue when Hb solutions are used in patients with TBI, where blood–brain barrier injury is well recognized. It is known that the amount of native Hb that leaks into brain parenchyma after TBI may be sufficient to cause direct neuronal injury (Hellal et al. 2004; Wang et al. 2002). We have seen improved neuropathology only in the high affinity group in spite of greatly improved hemodynamics in all of the rHb groups versus LR. One possibility is that direct neurotoxicity may play a role. A covalently modified bovine polynitroxylated pegylated Hb has shown neuroprotection in this TBI with HS model (Shellington et al. 2011). This modified Hb also exhibits surprising neuroprotection in primary neuronal culture rather than the neurotoxicity seen with the parent unmodified bovine Hb (Shellington et al. 2011). Direct neurotoxicity, thus, may contribute to our ability to show neuroprotection only in the high oxygen affinity rHb group. The potential of an optimized rHb or covalently modified rHb to show neuroprotection in TBI combined with HS also deserves further investigation. Other markers of neuroprotection, such as brain edema, intracranial pressure, and long-term histological and cognitive outcomes, should also be studied.
There are several concerns to this initial exploratory study with novel octameric rHbs. First, it would be of interest if native human Hb and mouse Hb solutions were also tested. However, our primary focus was to compare these three rHbs with different oxygen affinities. Secondly, there are concerns that endotoxin in the rHb solutions could be problematic (Okajima et al. 2005). The approach taken by our group in purifying these rHbs appears to have addressed this concern and endotoxin levels are acceptable and hypotension has not been seen with our rHb administration. Nevertheless, we cannot rule out unrecognized effects of endotoxin, cell free Hb, or differences in oxygen affinity on either mortality or neuropathology in our model. Our study focused on comparing oxygen affinity and was not powered to assess mortality. Although not significantly different, mortality was numerically greater at 24 h in the low affinity rHb (αN78C/L29W) group. Nonetheless, no appreciable Pre-Hospital mortality was seen with any of the rHbs. Third, the pharmacokinetics of octameric rHbs has not been studied previously. There is no assay available to determine readily the blood levels of the octamers. Our hemodynamic data suggest that MAP is maintained for at least ~2 h. Fourth, although we believe the most critical period of resuscitation was the Pre-Hospital Phase, administration of 100 % O2 and shed blood in the Hospital Phase in all groups may have limited differences between groups. Finally, the study was not conducted in a randomized fashion.
In summary, octameric rHbs normalized MAP and blood lactate with much smaller than LR in mice suffered TBI combined with HS. In principle, the difference in NO reactivity rather than oxygen affinity of Hbs appears to account for their difference in hemodynamic effects. The high oxygen affinity rHb (αN78C/L29F) shows the lowest PbtO2 during resuscitation and is the only rHb to confer acute neuroprotection assessed at 24 h after injury. The impact of these interesting new rHbs on resuscitation of TBI, HS, and their combination deserve further explorations.
Abbreviations
- HBOC:
-
Hemoglobin-based oxygen-carrier
- Hb:
-
Hemoglobin
- rHb:
-
Recombinant hemoglobin
- TBI:
-
Traumatic brain injury
- CCI:
-
Controlled cortical impact
- HS:
-
Hemorrhagic shock
- ICP:
-
Intracranial pressure
- PbtO2 :
-
Brain tissue oxygen concentration
- MAP:
-
Mean arterial blood pressure
- LR:
-
Lactated Ranger’s solution
- DSS:
-
2, 2-dimethyl-2-silapentene-5-sulfonate
- rHb (αN78C):
-
Recombinant hemoglobin with Asn78 to Cys substitution on the α-subunit
- rHb (αN78C/L29F):
-
Recombinant hemoglobin with Asn78 to Cys and Leu29 to Phe substitutions on the α-subunit
- rHb (αN78C/L29W):
-
Recombinant hemoglobin with Asn78 to Cys and Leu29 to Trp substitutions on the α-subunit
- Iso:
-
Isofluorane
References
Baudin-Creuza V, Ho C, Marden MC (2011) Hb octamers by introduction of surface cysteins. In: Maozzarelli A, Bettati S (eds) Chemistry and biochemistry of oxygen therapeutics: from transfusion to artificial blood. John Wiley and Sons, LTd, pp 372–380
Bauman R, Ling G, Tong L, Januszkiewicz A, Agoston D, Delanerolle N, Kim Y, Ritzel D, Bell R, Ecklund J, Armonda R, Bandak F, Parks S (2009) An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma 26:841–860
Bhardwaj A, Ulatowski JA (2004) Hypertonic saline solutions in brain injury. Curr Opin Crit Care 10:126–131
Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, Manley GT, Nemecek A, Newell DW, Rosenthal G, Schouten J, Shutter L, Timmons SD, Ullman JS, Videtta W, Wilberger JE, and Wright DW (2007) Guidelines for the management of severe traumatic brain injury. I. blood pressure and oxygenation, J Neurotraum 24(1):S7–13
Brasel KJ, Bulger E, Cook AJ, Morrison LJ, Newgard CD, Tisherman SA, Kerby JD, Coimbra R, Hata JS, Hoyt DB (2008) Hypertonic resuscitation: design and implementation of a prehospital intervention trial. J Am Coll Surg 206:220–232
Brillet T, Baudin-Creuza V, Kettering R, Yeh JI, Shen TJ, Ho NT, Vasseur C, Domingues E, Ho C, Marden MC (2012) Interaction of haptoglobin with hemoglobin octamers based on the mutation alphaAsn78Cys or betaGly83Cys”. Am J Mol Biol 2:1–10
Cabrales P, Tsai AG, Intaglietta M (2008) Isovolemic exchange transfusion with increasing concentrations of low oxygen affinity hemoglobin solution limits oxygen delivery due to vasoconstriction. Am J Physiol Heart Circ Physiol 295:H2212–H2218
Carver TE, Brantley RE Jr, Singleton EW, Arduini RM, Quillin ML, Phillips GN Jr, Olson JS (1992) A novel site-directed mutant of myoglobin with an unusually high O2 affinity and low autooxidation rate. J Biol Chem 267:14443–14450
Chatterjee R, Welty EV, Walder RY, Pruitt SL, Rogers PH, Arnone A, Walder JA (1986) Isolation and characterization of a new hemoglobin derivative cross-linked between the alpha-chains (lysine 99-alpha-1-lysine 99-alpha-2). J Biol Chem 261:9929–9937
Chauvierre C, Manchanda R, Labarre D, Vauthier C, Marden MC, Leclerc L (2010) Artificial oxygen carrier based on polysaccharides-poly(alkylcyanoacrylates) nanoparticle templates. Biomaterials 31:6069–6074
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34:216–222
Conover CD, Malatesta P, Lejeune L, Chang CL, Shorr RG (1996) The effects of hemodilution with polyethylene glycol bovine hemoglobin (PEG-Hb) in a conscious porcine model. J Investig Med 44:238–246
Dalvit C, Ho C (1985) Proton nuclear Overhauser effect investigation of the heme pockets in ligated hemoglobin—conformational differences between oxy and carbonmonoxy forms. Biochemistry 24:3398–3407
Dennis AM, Haselkorn ML, Vagni VA, Garman RH, Janesko-Feldman K, Bayir H, Clark RSB, Jenkins LW, Dixon CE, Kochanek PM (2009) Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotraum 26:889–899
Dickerson RE, Geis I (1983) Hemoglobin: structure, function, evolution, and pathology. Benjamin/Cummings, Menlo Park, CA
Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD (1998) Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat Biotechnol 16:672–676
Doyle JA, Davis DP, Hoyt DB (2001) The use of hypertonic saline in the treatment of traumatic brain injury. J Trauma 50:367–383
Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN Jr, Olson JS (1996) Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35:6976–6983
Elmer J, Alam HB, Wilcox SR (2012) Hemoglobin-based oxygen carriers for hemorrhagic shock. Resuscitation 83:285–292
Exo JL, Shellington DK, Bayir H, Vagni VA, Janesco-Feldman K, Ma L, Hsia CJ, Clark RS, Jenkins LW, Dixon CE, Kochanek PM (2009) Resuscitation of traumatic brain injury and hemorrhagic shock with polynitroxylated albumin, hextend, hypertonic saline, and lactated Ringer’s: effects on acute hemodynamics, survival, and neuronal death in mice. J Neurotraum 26:2403–2408
Fablet C, Marden MC, Green BN, Ho C, Pagnier J, Baudin-Creuza V (2003) Stable octameric structure of recombinant hemoglobin α2β283 Gly - > Cys, Protein Sci 12:690–695
Fang TY, Simplaceanu V, Tsai CH, Ho NT, Ho C (2000) An additional H-bond in the α1β2 interface as the structural basis for the low oxygen affinity and high cooperativity of a novel recombinant hemoglobin (βL105W). Biochemistry 39:13708–13718
Forsyth LL, Liu-DeRyke X, Parker D Jr, Rhoney DH (2008) Role of hypertonic saline for the management of intracranial hypertension after stroke and traumatic brain injury. Pharmacotherapy 28:469–484
Fronticelli C, Arosio D, Bobofchak KM, Vasquez GB (2001) Molecular engineering of a polymer of tetrameric hemoglobins. Proteins 44:212–222
Fung LWM, Ho C (1975) Proton nuclear magnetic-resonance study of quaternary structure of human hemoglobins in water. Biochemistry 14:2526–2535
Hayashi A, Suzuki T, Shin M (1973) An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim Biophys Acta 310:309–316
Hellal F, Bonnefont-Rousselot D, Croci N, Palmier B, Plotkine M, Marchand-Verrecchia C (2004) Pattern of cerebral edema and hemorrhage in a mice model of diffuse brain injury. Neurosci Lett 357:21–24
Hemerka J, Wu X, Dixon CE, Garman RH, Exo J, Shellington DK, Blasiole B, Vagni VA, Janesko KL, Xu M, Wisniewski SR, Bayir H, Jenkins LW, Clark R, Tisherman S, Kochanek PM (2012) Severe brief pressure controlled hemorrhagic shock after traumatic brain injury exacerbates functional deficits and long-term neuropathological damage in mice. J Neurotraum 29:2192–2208
Ho C (1992) Proton nuclear-magnetic-resonance studies on hemoglobin—Cooperative interactions and partially ligated intermediates. Adv Protein Chem 43:153–312
Hovda DA, Lee SM, Smith ML, Von Stuck S, Bergsneider M, Kelly D, Shalmon E, Martin N, Caron M, Mazziotta J, Phelps M, Becker DP (1995) The neurochemical and metabolic cascade following brain injury: moving from animal models to man. J Neurotraum 12:903–906
Huang F, Nojiri H, Shimizu T, Shirasawa T (2005) Beneficial effect of transfusion with low-affinity red blood cells in endotoxemia. Transfusion 45:1785–1790
Jeong ST, Ho NT, Hendrich MP, Ho C (1999) Recombinant hemoglobin(α29Leucine - > Phenylalanine, α96valine - > tryptophan, β108Asparagine - > Lysine) exhibits low oxygen affinity and high cooperativity combined with resistance to autoxidation. Biochemistry 38:13433–13442
Kunert MP, Liard JF, Abraham DJ, Lombard JH (1996) Low-affinity hemoglobin increases tissue PO2 and decreases arteriolar diameter and flow in the rat cremaster muscle. Microvasc Res 52:58–68
Lindstrom TR, Lehmann H, Charache S, Noren IBE, Ho C (1972) Nuclear magnetic-resonance studies of hemoglobins. 7. Tertiary structure around ligand binding-site in carbonmonoxyhemoglobin. Biochemistry 11:1677–1681
Looker D, Mathews AJ, Neway JO, Stetler GL (1994) Expression of recombinant human hemoglobin in escherichia coli. Methods Enzymol 231:364–374
McCarthy MR, Vandegriff KD, Winslow RM (2001) The role of facilitated diffusion in oxygen transport by cell-free hemoglobins: implications for the design of hemoglobin-based oxygen carriers. Biophys Chem 92:103–117
Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB (2005) Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115:3409–3417
Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM (2008) Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299:2304–2312
Okajima K, Isobe H, Uchiba M, Harada N (2005) Role of sensory neuron in reduction of endotoxin-induced hypotension in rats. Crit Care Med 33:847–854
Olson JS, Eich RF, Smith LP, Warren JJ, Knowles BC (1997) Protein engineering strategies for designing more stable hemoglobin-based blood substitutes. Artif Cells Blood Substit Immobil Biotechnol 25:227–241
Patel MB, Feinstein AJ, Saenz AD, Majetschak M, Proctor KG (2006) Prehospital HBOC-201 after traumatic brain injury and hemorrhagic shock in swine. J Trauma 61:46–56
Plateau P, Gueron M (1982) Exchangeable proton Nmr without base-line distortion, using new strong-pulse sequences. J Am Chem Soc 104:7310–7311
Rohlfs RJ, Bruner E, Chiu A, Gonzales A, Gonzales ML, Magde D, Magde MD Jr, Vandegriff KD, Winslow RM (1998) Arterial blood pressure responses to cell-free hemoglobin solutions and the reaction with nitric oxide. J Biol Chem 273:12128–12134
Rosenthal G, Morabito D, Cohen M, Roeytenberg A, Derugin N, Panter SS, Knudson MM, Manley G (2008) Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J Neurosurg 108:575–587
Rother RP, Bell L, Hillmen P, Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobinA novel mechanism of human disease. JAMA 293:1653–1662
Russu IM, Ho NT, Ho C (1987) A proton nuclear overhauser effect investigation of the subunit interfaces in human normal adult hemoglobin. Biochim Biophys Acta 914:40–48
Russu IM, Lin A, Ferro-Dosch S, Ho C (1984) A proton nuclear magnetic-resonance investigation of human hemoglobin-A2—implications on the intermolecular contacts in sickle hemoglobin fibers and on the Bohr effect of human normal adult hemoglobin. Biochim Biophys Acta 785:123–131
Sehgal LR, Gould SA, Rosen AL, Sehgal HL, Moss GS (1984) Polymerized pyridoxylated hemoglobin: a red cell substitute with normal oxygen capacity. Surgery 95:433–438
Shellington DK, Du L, Wu X, Exo J, Vagni V, Ma L, Janesko-Feldman K, Clark RS, Bayir H, Dixon CE, Jenkins LW, Hsia CJ, Kochanek PM (2011) Polynitroxylated pegylated hemoglobin: a novel neuroprotective hemoglobin for acute volume-limited fluid resuscitation after combined traumatic brain injury and hemorrhagic hypotension in mice. Crit Care Med 39:494–505
Shen TJ, Ho NT, Simplaceanu V, Zou M, Green BN, Tam MF, Ho C (1993) Production of unmodified human adult hemoglobin in scherichia coli. 8108–8112
Shen TJ, Ho NT, Zou M, Sun DP, Cottam PF, Simplaceanu V, Tam MF, Bell DA, Ho C (1997) Production of human normal adult and fetal hemoglobins in scherichia coli. Protein Eng 10:1085–1097
Simplaceanu V, Lukin JA, Fang TY, Zou M, Ho NT, Ho C (2000) Chain-selective isotopic labeling for NMR studies of large multimeric proteins: application to hemoglobin. Biophys J 79:1146–1154
Stern S, Rice J, Philbin N, McGwin G, Arnaud F, Johnson T, Flournoy WS, Ahlers S, Pearce LB, McCarron R, Freilich D (2009) Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201, in a swine model of severe uncontrolled hemorrhage and traumatic brain injury. Shock 31:64–79
Takahashi S, Lin A, Ho C (1980) Proton nuclear magnetic-resonance studies of hemoglobin-M-Boston (α58e7 His-->Tyr) and hemoglobin-M-Milwaukee (β67e11 Val --> Glu)—spectral assignments of hyperfine-shifted proton resonances and of proximal histidine (E7) NH resonances to the alpha-chains and beta-chains of normal human adult hemoglobin. Biochemistry 19:5196–5202
Tsai AG, Cabrales P, Manjula BN, Acharya SA, Winslow RM, Intaglietta M (2006) Dissociation of local nitric oxide concentration and vasoconstriction in the presence of cell-free hemoglobin oxygen carriers. Blood 108:3603–3610
Varnado CL, Mollan TL, Birukou I, Smith BJZ, Henderson DP, Olson JS (2012) Development of recombinant hemoglobin-based oxygen carriers, Antioxidants & Redox Signaling.
Vasseur-Godbillon C, Sahu SC, Domingues E, Fablet C, Giovannelli JL, Tam TC, Ho NT, Ho C, Marden MC, Baudin-Creuza V (2006) Recombinant hemoglobin beta G83C-F41Y, Febs J 273:230–241
Wang X, Mori T, Sumii T, Lo EH (2002) Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke 33:1882–1888
Watanabe T, Takeda T, Omiya S, Hikoso S, Yamaguchi O, Nakano Y, Higuchi Y, Nakai A, Abe Y, Aki-Jin Y, Taniike M, Mizote I, Matsumura Y, Shimizu T, Nishida K, Imai K, Hori M, Shirasawa T, Otsu K (2008) Reduction in hemoglobin-oxygen affinity results in the improvement of exercise capacity in mice with chronic heart failure. J Am Coll Cardiol 52:779–786
Wiltrout ME, Giovannelli JL, Simplaceanu V, Lukin JA, Ho NT, Ho C (2005) A biophysical investigation of recombinant hemoglobins with aromatic B10 mutations in the distal heme pockets. Biochemistry 44:7207–7217
Yuan XQ, Wade CE (1992) Traumatic brain injury attenuates the effectiveness of lactated Ringer’s solution resuscitation of hemorrhagic shock in rats. Surg Gynecol Obstet 174:305–312
Acknowledgements
This work is supported by research grants from the National Institutes of Health (R01GM084614 to CH, and P01NS30318 to PMK).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Wu, X. et al. (2013). Recombinant Octameric Hemoglobins as Resuscitation Fluids in a Murine Model of Traumatic Brain Injury Plus Hemorrhagic Shock. In: Kim, H., Greenburg, A. (eds) Hemoglobin-Based Oxygen Carriers as Red Cell Substitutes and Oxygen Therapeutics. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40717-8_13
Download citation
DOI: https://doi.org/10.1007/978-3-642-40717-8_13
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40716-1
Online ISBN: 978-3-642-40717-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)