Abstract
Since the turn of the millennium, PET/CT devices evolved as the first generation of hybrid imaging systems integrating nuclear and radiological imaging from a valuable research tool into a clinically useful and accepted technique. These innovative devices combined morphological and metabolic-functional information in an elegant way and reached widespread distribution. Therefore, it seemed quite obvious to develop a PET/MR system. However, the technical hurdles for this kind of integration were for physic’s reasons much higher – but were finally solved: for more than 2 years, integrated whole-body systems are now available and, in view of the success of PET/CT, the expectations are high that PET/MR provides an actual clinical benefit. MRI’s advantage of an excellent soft tissue contrast and the capability of functional imaging at the molecular level by PET should have the potential to create a unique multimodality imaging. However, PET/MR in general and in cardiac imaging in particular needs to demonstrate its suitability in everyday clinical practice. In this review we give an overview of the requirements and features of this new hybrid imaging system and provide an outlook based on clinical examples, in which areas PET/MR could potentially find a place in the armamentarium of cardiac imaging.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Positron Emission Tomography
- Late Gadolinium Enhancement
- Myocardial Blood Flow
- Positron Emission Tomography Data
- Cardiac Sarcoidosis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
9.1 Introduction
Cardiac imaging is in the fortunate situation that a wide variety of noninvasive imaging modalities is available. This includes echocardiography, computed tomography (CT), magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT), and positron emission tomography (PET). Each of these methods has certain advantages and disadvantages. Through the combination of single modalities into hybrid imaging systems (such as SPECT/CT or PET/CT), the disadvantages of each component can not only be mitigated or eliminated but can yield synergistic value. Thus, the PET/CT has experienced an impressive growth since its introduction in 2000, and several thousand systems were installed worldwide. This is primarily driven by oncological imaging, where the added value of hybrid imaging with PET/CT compared to stand-alone PET was demonstrated [1]. The increased availability of PET/CT systems resulted also in an increased utilization in cardiac imaging. Those devices provided rapid assessment of the data needed for attenuation correction which is the prerequisite of any image quantification up to the delineation of myocardial blood flow. In addition, CT angiography allowed the visualization and quantification of the coronary anatomy, which yielded incremental diagnostic value over perfusion information alone [2]. Thus, the questions arise whether integrated PET/MR systems can repeat the success of PET/CT in general and whether such a hybrid device adds incremental value in cardiac imaging in particular.
This review will provide (a) an overview over the technical challenges and implementations in the context of thoracic imaging, (b) report on our initial experiences, and based on those (c) outline areas of interest and relevance for cardiac PET/ MR.
9.2 PET/MR in Cardiac Imaging: Technical Considerations
As part of a major research initiative of the Deutsche Forschungsgesellschaft (DFG: German Research Foundation), an integrated whole-body PET/MR scanner was installed in our department in late 2010. Since then we gained considerable experience with this novel and complex imaging approach – although no final evaluation or assessment in comparison to the established modalities such as PET/CT and pure MRI systems was possible [3, 4].
From a technical point of view, two fundamental implementations are available today: Philips Healthcare (Best, Netherlands) provides a system with two separated units, which are connected with a common patient table [5]. Siemens Healthcare (Erlangen, Germany) implemented a fully integrated PET/MR system based on earlier prototypes of brain-only PET inserts [6], which is housed directly within the bore of a conventional 3 T magnet.
9.3 Attenuation Correction
As mentioned before, the correction of attenuated and scattered annihilation photons is a cornerstone in PET quantification. In PET/MR, this topic is of particular interest as both system designs lack the conventional (PET-only) approach, namely, rotating germanium rod sources, and also have no access to the technique used in PET/CT systems, i.e., extrapolating the attenuation for the 511 keV annihilation photons from the CT data acquired with 80–140 keV. Without access to such an attenuation map or “μ-map,” PET quantification – which is a fundamental building block of cardiac PET – is impossible. In the special case of cardiac PET/CT, this is of even higher importance as even modest alignment errors between PET and CT decrease the diagnostic accuracy [7, 8], Consequently, the generation of the attenuation map for 511-keV photons poses a new challenge, as there is no physical relationship between MR images and the attenuation coefficients for biological tissues. In order to address this problem, various approaches are being pursued: (a) image segmentation and value mapping, (b) template- or atlas-based, or (c) utilizing the PET data itself. Image segmentation is currently the technique utilized on both available systems and is described briefly: image data is classified into different types of tissue, which then are associated with fixed attenuation coefficients [9] using DIXON image sequences [10]. Water and fat images are generated and each voxel is assigned to be either air, lung tissue, fat, or soft tissue. As cortical bone is basically invisible in these images, it is ignored (which was also the case of the “classic” PET scanner with rotating rod sources). For every bed position, the acquisition time is about 18 s. The major limitations of this technique are the small number of tissue classes with a general (not patient-specific) attenuation coefficient which is of particular relevance in cardiac studies as in some cases significant fluctuations in the lung tissue signal can be seen. The advantages of this approach are, however, that the attenuation map may be computed relatively quickly and in a reproducible way. The Siemens Biograph mMR uses a variant of this approach [9], and we could already show a good correlation of tracer uptake between PET/CT and PET/MR examinations in oncological studies [11, 12]. The Philips PET/MR device uses a similar approach integrating soft and fat tissue into one class, however [13]. Of special relevance is the fact that the transaxial field of the MR unit typically is only 40–45 cm. This results in a potential truncation of the attenuation map in some parts of the body – especially the arms, which are positioned usually next to the body [14]. An extension of the MR’s field-of-view is technically very complex [15], and in the current Biograph mMR, the following approach is implemented to compensate for this effect: in the truncated parts, PET emission data is utilized to estimate the presence of tissue as many PET tracers show nonspecific uptake. This data is also segmented and included in the attenuation map [7, 16]. Finally, it should be mentioned that the MR imaging-related equipment such as patient’s bed (which is more massive than the one found in PET/CT scanners), MRI coils which can be freely placed, and positioning and monitoring devices such as ECG electrodes, headphones, and respiratory belts all contribute to scattering and attenuation of the 511 keV annihilation photons. Since these parts are not visible in conventional MR sequences and thus are not used in the attenuation map, they may lead to image inhomogeneities and adversely affect quantification. Despite these technical problems, the data obtained so far in cardiac PET/MR imaging in general show a relatively good correlation between PET/CT and PET/MR [17, 18], but larger studies are clearly warranted.
9.4 Applications in Cardiac Imaging
It has to be acknowledged that in the past, the probability that a patient underwent MR and PET sequentially was rather low – the only moderate body of literature to be found reflects this. The main reasons were the availability of both techniques in spatial and temporal proximity as well as cost issues. Realistically, if both MR and PET were used in the same patients, the objective was the comparison of methods and the proof of superiority or at least non-inferiority of one approach over the other. However, there are quite some aspects where the combination of both modalities could be advantageous.
9.5 Myocardial Viability and Tissue Characterization
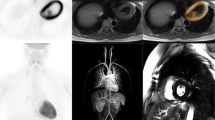
The imaging-based assessment of myocardial viability is a standard approach applied in patients with advanced coronary heart disease or in those who are in early or advanced states of heart failure. The diagnostic goal is to distinguish between under-perfused but vital heart tissue (so-called hibernating myocardium) and poorly perfused but nonvital tissue (scar). Patients in the first group would benefit from invasive revascularization, whereas patients in the latter group have no advantage of this intervention. Of the various imaging methods, PET using 18 F-fluorodeoxyglucose (FDG) is widely considered as the clinical gold standard. In such an examination, both myocardial perfusion (e.g., with the 13 N-ammonia as tracer) and the glucose metabolism using FDG are investigated ideally applying the euglycemic-hyperinsulinemic clamp technique [19]. From a conceptual point of view, hypoxia or ischemia leads to a change in myocardial metabolism from the utilization of free fatty acids toward glucose metabolism. It could also be shown that the extent of the ischemically compromised myocardium is associated with a poorer long-term outcome [20]. Furthermore, this approach allows the classification of the myocardial tissue into fully viable, partially vital, and nonvital tissue – however, always within the limits of PET’s modest spatial resolution. MR imaging could provide here a valuable addition to PET. Late gadolinium enhancement (LGE) which is acquired using T1-weighted inversion recovery sequences 5–20 min after the administration of gadolinium-DTPA directly represents myocardial scar tissue [21–23]. Normal myocardium can be “nulled” and infarcted tissue shows a hyperintense signal due to different washout constants in normal versus abnormal tissue. MRI’s high spatial resolution allows not only the distinction between transmural and non-transmural myocardial infarction (MI), it can also detect even small subendocardial infarcts, which is common in patients with suspected coronary artery disease without previous MI and shows prognostic significance [24]. Whereas the specificity of PET and MRI is comparable (63 %), PET shows a higher sensitivity (92 % vs. 84 %) [25]. This stems from the fundamentally different processes in the imaging process: while the LGE signal is based solely on an increased interstitial volume of distribution (gadolinium chelates are too large to enter a cell), the PET signal is truly metabolic. Furthermore, the PET also allows the identification of myocardium at a metabolical risk – thus, the PET provides an indication of therapeutic relevance in addition to the known relationship of infarct size and prognosis [26]. In summary, for the assessment of myocardial viability, both methods provide similar information [18]. However, a potentially relevant opportunity of hybrid imaging could arise from the observation that myocardial tissues that were classified as vital prior to the revascularization show no contractile improvement [27]. Here, the PET/MR imaging could offer an interesting approach. By integrating the information from morphology and left ventricular wall motion from the MR and the functional information from PET, a better understanding of the underlying mechanisms as well as an improved prediction of myocardial recovery could arise. Figure 9.1 shows an example of multimodality imaging with PET/MRI of a patient after acute MI.
Short-axis images of myocardial perfusion and glucose metabolism (top). Both reduced 13N-ammonia and 18F-FDG uptake are clearly depicted in the basal areas of the anterior, anterolateral, and lateral wall. Four-chamber and two-chamber views show large areas of transmural LGE in the anterior and lateral wall (middle and bottom rows). While clearly reduced perfusion and glucose metabolism is observed in the basal lateral wall, the majority of the anterior wall still shows significant amount of 18F-FDG and 13N-ammonia uptake
In addition to the conventional approach of viability imaging, recently, interesting insights were gained into the inflammatory process after acute MI. The compromised ischemic myocardium undergoes a complex healing process, which includes inflammation, neoangiogenesis, fibroblast proliferation, and collagen deposition. Studies indicate that an excessive inflammatory response could increase myocardial remodeling after acute MI and thus directly affect the prognosis [28]. This inflammatory response is primarily maintained by different subpopulations of monocytes. In a recent study in mice, it has been shown that the (sequentially measured) PET and MR scans can be used to describe and monitor this inflammatory response [29]. It was shown that FDG is taken up mainly by monocytes in the acutely infarcted myocardium. However, to reliably determine this particular FDG uptake, it is necessary to suppress its uptake in the healthy (remote) myocardium. This can be achieved by a special low-carbohydrate diet (so-called Atkins diet) in combination with “glucose fasting.” [30] PET/MRI could play an important role in elucidating the underlying pathophysiology of the inflammatory response after acute MI in humans as well as in facilitating such a complex protocol as the FDG signal can be only moderate and an improved morphological co-localization is crucial. In addition, patient compliance is improved if only one examination is performed shortly after myocardial infarction.
The same fasted protocol is applicable in hybrid PET/MR imaging for myocardial tissue characterization. These applications target pathological entities such as sarcoidosis or Takotsubo cardiomyopathy. Those applications are clearly not for everyday clinical use, but they can point into a direction where a complex imaging system such as the PET/MRI could show its potential.
Cardiac sarcoidosis represents an inflammatory cardiomyopathy where, in the case of cardiac involvement, the myocardium is replaced by fibrotic, fibrogranulomatous tissue. MR using LGE can image those fibrotic changes of the heart [31]. In sarcoidosis, LGE is patchy and appears primarily in subepicardial regions but only rarely in the subendocardium. In PET, different patterns of glucose metabolism under fasting conditions and myocardial perfusion are known [32] (Fig. 9.2). Vivid glucose metabolism and normal perfusion indicate active inflammation, whereas reduced perfusion and high glucose metabolism represent an advanced stage of the disease. Absent or reduced perfusion and lack of glucose uptake indicate end-stage disease. Thus, hybrid PET/MR not only allows the quantification of the amount of affected myocardium but also helps to assess the disease stage and might be suited to guide therapy.
Illustration of short-axis images of myocardial perfusion (top row) and inflammation (bottom row). Both reduced 13N-ammonia and upregulated 18F-FDG uptake are clearly depicted in the anterolateral and lateral wall as a sign of active cardiac sarcoidosis in these regions (top). Four-chamber view shows an area of transmural LGE in the lateral wall (left). Here, clearly reduced perfusion (middle) and upregulated glucose metabolism (right) are observed as a sign of active inflammation. Note increased 18F-FDG uptake in bilateral hilar lymph nodes (bottom)
Takotsubo cardiomyopathy is an increasingly recognized syndrome with symptoms similar to acute MI, including chest pain and electrocardiographic ST segment elevation, but in the absence of relevant stenosis. It occurs in the majority of cases in postmenopausal women of an advanced age and is characterized by transient left ventricular apical wall motion abnormalities associated with emotional or physical stress. Its pathophysiology is not well understood and the available literature focuses primarily on case studies – however, hybrid imaging offers attractive opportunities [33, 34] and PET/MRI using the fasted FDG protocol as described above shows promising results (Fig. 9.3).
Two-chamber views during diastole (top left) and systole (top right) show midventricular hypokinesia with marked ballooning during systole. Left ventricular ejection fraction was reduced (46 %). LGE shows no enhancement of the myocardium (bottom left). 18F-FDG PET demonstrates an upregulated glucose metabolism in the hypokinetic midventricular segments while glucose consumption is suppressed in remote myocardium (bottom right)
9.6 Myocardial Ischemia
The most widespread application in nuclear cardiology is the detection (or exclusion) of a hemodynamically significant coronary artery disease (CAD). Although the majority of patients are examined with SPECT, PET offers certain advantages. It shows a sensitivity and specificity of about 90 % for this indication [35, 36] and has proven suitable to make quantitative statements for the prognostic assessment of patients and guidance for treatment [37, 38]. MRI researchers introduced “first-pass” MRI almost two decades ago where significant coronary stenoses are detected visually after the injection of a fast bolus of a contrast agent such as gadolinium-DTPA using the different wash-in rates at rest and during exercise. The sensitivity and specificity of this method vary substantially in the literature but can reach values of about 91 and 81 % [39–41]. Most SPECT and PET studies are also analyzed in a visual manner; however, PET’s major advantage is the possibility to quantify myocardial blood flow in absolute terms. This allows the determination of blood flow at rest and during exercise and consequently the calculation of the myocardial flow reserve. This is particularly of interest in patients with 3-vessel disease or myocardial dysfunction, since a reduced perfusion under stress might be missed if only a visual or semiquantitative assessment is utilized due to the phenomenon of “balanced ischemia.” [42] While the absolute quantification of blood flow is increasingly used in many hospitals (especially in the United States) with PET due to the availability of FDA-approved software, dynamic perfusion MR is still in clinical trials and exclusively for research purposes. Consequentially, the body of literature is rather sparse although a good correlation with 13N- ammonia PET was shown [41, 43].
Indeed, both methods have limitations which could point to a mutual benefit, if combined: MRI’s weak part is the reliable definition of the arterial input function (as a prerequisite for absolute flow delineation), the relatively low “volume of distribution” for gadolinium-DTPA, the very rapid diffusion of gadolinium-DTPA into the extracellular space, and the limited spatial coverage of the left ventricle during acquisition (usually 3–5 slices and not the entire ventricle). The biggest disadvantage of PET is that no morphological information is obtained, but only data about the perfused myocardium. Therefore, PET cannot distinguish whether a reduced blood flow is a result due to an epicardial stenosis or a microvascular dysfunction (e.g., diabetes). Furthermore, PET cannot clearly determine whether such a perfusion defect is caused by thin scar tissue, which often poses a problem in dilated cardiomyopathy [44].
Thus, parallel PET/MR imaging allows for the first time a direct comparison of the delineation of myocardial blood flow using PET and MR under identical physiological (resting or stress) conditions. In addition to validation studies, this permits the combination of MRI-derived information such as morphology (e.g., coronary anatomy (MR angiography)), wall thickness, or scar (LGE) with the functional PET data so that an improved tissue characterization becomes possible.
9.7 Considerations for the Clinical Workflow
It is important to remember that hybrid scanners always require close collaboration between nuclear medicine physicians, radiologists, cardiologists, and technical personnel. This holds especially true for MR imaging, as the methodological complexity (different contrasts, image geometry, artifacts, etc.) and the patient-specific adjustments are higher and more time consuming than those found in stand-alone PET and/or CT systems.
But also technical workflow aspects need to be addressed: any reading of hybrid data requires PET and MR images to be superimposed as accurately as possible. In thoracic imaging in general and in cardiac imaging in particular, this represents a challenge as was learned already from cardiac PET/CT. Both cardiac motion due to contraction of the heart as well as thorax movement due to respiration have to be taken into account [7]. As mentioned before, any misalignment between emission and “transmission” negatively affects the accuracy of attenuation correction: but in contrast to PET/CT, MRI does not require ionizing radiation and thus the attenuation map can be generated as often as needed. In general, we found a good agreement between the PET data, which is acquired over many respiratory cycles, and the MR attenuation map, which is acquired in 18 s at end-expiration. It is relevant to keep in mind that alignment issues are present both for fully integrated as well as for integrated PET/MR systems. Even in the latter design, PET and MR data will be acquired often not simultaneously but rather in parallel. MR imaging is usually done sequentially, i.e., the image data represents a volume usually acquired sequentially slice by slice with slice acquisition times ranging from about 50 m s for perfusion scans up to several seconds for high-resolution images. This differs in PET where truly volumetric acquisitions with typical frame times – depending on the protocol – from 5 s to 20 min are acquired. Basically, this translates into the fact that fully simultaneous acquisitions using PET/MR are not trivial and thus continue to pose a major problem with respect to patient movement. However, integrated PET/MR scanners could offer real-time motion correction and partial volume correction in the future.
A major advantage of parallel imaging, however, is the patient management, because sequential imaging of PET and MR is not only uncomfortable for the patient but also for the operating personnel. Consequently, cardiac PET/MR imaging could lead to both increased patient compliance as well as to better device utilization, and thus better cost-effectiveness.
9.8 Clinical Cases
9.8.1 Acute Infarction
Clinical History: A 31-year-old male patient presented to his general practitioner (GP) because of chest pain. While talking to the GP, the patient suddenly collapsed. The GP immediately started cardiopulmonary resuscitation and called the ambulance which transported the patient to our emergency department where myocardial infarction was suspected. Cardiac catheterization confirmed left main occlusion, and subsequently PCI was performed. 13 days later the patient underwent PET/MR viability imaging (Fig. 9.1).
Findings: MR images indicate mostly transmural myocardial infarction of the anterior, lateral, and anteroseptal wall. LV ejection fraction was significantly reduced. PET images show both reduced glucose metabolism and perfusion in the basal regions indicating transmural myocardial infarction. However, PET confirms still a large amount of viable myocardium.
Advantages of PET/MR: The integrated information of PET and MR may allow more accurate prediction of the left ventricular remodeling and outcome after acute myocardial infarction.
9.8.2 Cardiac Sarcoidosis
Clinical History: A 30-year-old male patient was referred to our institution because of suspected cardiac involvement of a newly diagnosed sarcoidosis. Coronary angiography excluded coronary artery disease and showed a severely reduced left ventricular function. A CT of the chest demonstrated bihilar lymphadenopathy. Subsequently, the patient was transferred to our institution to confirm cardiac involvement of the sarcoidosis by PET/MR (Fig. 9.2).
Findings: Bihilar lymphadenopathy with increased FDG uptake in the lymph nodes confirms the diagnosis of sarcoidosis. Areas in the left ventricle with increased FDG uptake and reduced perfusion indicate active inflammation and reveal cardiac involvement of the sarcoidosis. Also, a severely reduced left ventricular ejection fraction with severe hypokinesia of the lateral wall demonstrates dilated cardiomyopathy (DCM) as a consequence of cardiac sarcoidosis. Furthermore, areas demonstrating late gadolinium enhancement (LGE) indicate myocardial infiltration and scarring.
Advantages of PET/MR: The integrated information of PET and MR may thus not only allow assessing the amount of affected myocardium by LGE but also exact assessment of the stage and inflammatory state of the disease, and consequently therapy monitoring is feasible.
9.8.3 Takotsubo Cardiomyopathy
Clinical History: A 70-year-old woman presented with acute chest pain to our institution. She reported of progressive dyspnea while experiencing personal stress during this time. Angiographically inconspicuous epicardial coronary arteries were found during cardiac catheterization. The ventriculography, however, showed midventricular dilatation and a hypercontractile apex and base. Because of these findings a variant of Takotsubo cardiomyopathy (TTC) was suspected and the patient was referred for PET/MR (Fig. 9.3).
Findings: Left midventricular hypokinesia was found on cine MR images; however, no late gadolinium enhancement (LGE) could be depicted in the entire left ventricular myocardium. FDG PET showed a markedly increased glucose metabolism in the dysfunctional midventricular myocardium while FDG uptake was suppressed in the remote area.
Advantages of PET/MR: Takotsubo cardiomyopathy is a rare but increasingly recognized, reversible disease of which the underlying pathophysiology is unclear. However, stress-induced catecholamine excess to the myocardium seems to play an important role in pathophysiology. PET/MR allows the integrated imaging of hypokinetic areas of the myocardium, which demonstrate a metabolic shift from fatty acid to glucose consumption in the fasted state while no myocardial enhancement (LGE) after MR contrast application is seen.
9.8.4 Outlook: Advanced Molecular Imaging and Neoangiogenesis
In addition to those more clinically motivated imaging approaches, previous work with sequential PET and MR imaging identified potential applications. Although those come mostly from preclinical work, their translation would be greatly facilitated by the use of a hybrid imaging system.
9.8.4.1 Stem Cell Therapy
After an acute MI many of the affected cardiomyocytes die and the ischemically compromised myocardium loses its function and thus contractility – which may eventually lead to heart failure. A promising therapeutic approach is the use of stem cells. Here, the transplanted cells (e.g., embryonic stem cells or bone marrow stem cells) will replace the lost cardiomyocytes and thus contribute to the regeneration of the myocardium. A frequently observed problem is that only a fraction of the transplanted cells remains actually in the myocardium [45]. PET/MR could assist for a better understanding of “stem cell engraftment” and the underlying cellular and molecular processes. Our group demonstrated earlier the advantage of a (sequential) hybrid imaging approach [46]. In this rodent study, human endothelial precursor cells were labeled with iron particles. In addition, the cells were modified using a viral vector in such a way that they expressed the human sodium iodide symporter (NIS) and then were transplanted into immunodeficient rats. The MR was well suited to represent the left ventricular morphology and to identify the location of transplanted cells. The vitality of the cells was shown by PET, which delineated uptake of radionuclide 124I only in living cells. Basically, the iron particles were taken up by macrophages after the death of the transplanted cells and thus were still visible on the MR. The PET signal (I-124), however, was only visible if the transplanted cells were still vital, which involves a sustained expression of the NIS.
9.8.4.2 Imaging of Neoangiogenesis
This seems to play a key role after myocardial infarction and accordingly is an attractive target for molecular imaging. Integrins are of importance in cell migration, the regulation of cell proliferation, cell survival, as well as in cell differentiation. 18F-labeled galacto-RGD was initially developed for oncological PET imaging [47] but was investigated also for imaging of ανβ3 integrin expression after acute MI, both in animals and humans [48, 49]. In these studies the co-visualization of this “hot spot” PET imaging using MRI for anatomical localization and functional correlation was helpful. Furthermore, a recent study revealed that significant F-18 galacto-RGD uptake was a predictor for the absence of left ventricular remodeling after MI in rats [50]. Thus, whole-body PET/MR has the potential to facilitate further research, especially since it allows the delineation of scar extent, left ventricular function, and the integrin expression within a single examination.
9.9 Conclusion
Both PET and MRI as stand-alone modalities are accepted imaging methods for the assessment of myocardial perfusion and vitality. Hybrid PET/MR combines these two methods in one system, but it remains to be seen whether this integration provides synergistic, diagnostic value. Currently, PET/MR is mostly used for research purposes, where it provides an excellent tool for cross-validation of new imaging methods under identical experimental conditions. However, it seems not unlikely that PET/MR could become an indispensable method for the development of radiopharmaceuticals and contrast agents. However, it requires complex workflows and excellent interdisciplinary cooperation. The latter factor in addition to the proof of cost-effectiveness will determine a wider distribution.
References
Von Schulthess GK, Hany TF. Imaging and pet-pet/ct imaging. J Radiol. 2008;89:438–47; quiz 448.
Knuuti J, Saraste A. Combined functional and anatomical imaging for the detection and guiding the therapy of coronary artery disease. Eur Heart J. 2013;34:1954–7.
Nekolla SG, Martinez-Moeller A, Saraste A. Pet and mri in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging. 2009;36 Suppl 1:S121–30.
Rischpler C, Nekolla SG, Dregely I, Schwaiger M. Hybrid pet/mr imaging of the heart: potential, initial experiences, and future prospects. J Nucl Med. 2013;54:402–15.
Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, Ratib O, Izquierdo-Garcia D, Fayad ZA, Shao L. Design and performance evaluation of a whole-body ingenuity tf pet-mri system. Phys Med Biol. 2011;56:3091–106.
Schlemmer HP, Pichler BJ, Schmand M, Burbar Z, Michel C, Ladebeck R, Jattke K, Townsend D, Nahmias C, Jacob PK, Heiss WD, Claussen CD. Simultaneous mr/pet imaging of the human brain: feasibility study. Radiology. 2008;248:1028–35.
Martinez-Moller A, Souvatzoglou M, Navab N, Schwaiger M, Nekolla SG. Artifacts from misaligned ct in cardiac perfusion pet/ct studies: frequency, effects, and potential solutions. J Nucl Med. 2007;48:188–93.
Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac pet/ct due to misregistration of ct attenuation and emission pet images: a definitive analysis of causes, consequences, and corrections. J Nucl Med. 2007;48:1112–21.
Martinez-Moller A, Souvatzoglou M, Delso G, Bundschuh RA, Chefd’hotel C, Ziegler SI, Navab N, Schwaiger M, Nekolla SG. Tissue classification as a potential approach for attenuation correction in whole-body pet/mri: evaluation with pet/ct data. J Nucl Med. 2009;50:520–6.
Coombs BD, Szumowski J, Coshow W. Two-point dixon technique for water-fat signal decomposition with b0 inhomogeneity correction. Magn Reson Med. 1997;38:884–9.
Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Moller A, Nekolla SG, Ziegler S, Ganter C, Rummeny EJ, Schwaiger M. First clinical experience with integrated whole-body pet/mr: comparison to pet/ct in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–55.
Souvatzoglou M, Eiber M, Takei T, Furst S, Maurer T, Gaertner F, Geinitz H, Drzezga A, Ziegler S, Nekolla SG, Rummeny EJ, Schwaiger M, Beer AJ. Comparison of integrated whole-body [c]choline pet/mr with pet/ct in patients with prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40(10):1486-99.
Schulz V, Torres-Espallardo I, Renisch S, Hu Z, Ojha N, Bornert P, Perkuhn M, Niendorf T, Schafer WM, Brockmann H, Krohn T, Buhl A, Gunther RW, Mottaghy FM, Krombach GA. Automatic, three-segment, mr-based attenuation correction for whole-body pet/mr data. Eur J Nucl Med Mol Imaging. 2011;38:138–52.
Delso G, Martinez-Moller A, Bundschuh RA, Nekolla SG, Ziegler SI. The effect of limited mr field of view in mr/pet attenuation correction. Med Phys. 2010;37:2804–12.
Blumhagen JO, Ladebeck R, Fenchel M, Scheffler K. Mr-based field-of-view extension in mr/pet: B(0) homogenization using gradient enhancement (huge). Magn Reson Med. 2012. doi:10.1002/mrm.24555.
Nuyts J, Bal G, Kehren F, Fenchel M, Michel C, Watson C. Completion of a truncated attenuation image from the attenuated pet emission data. IEEE Trans Med Imaging. 2013;32:237–46.
Lau JM, Sharma S, Laforest R, McConathy J, Barnwell J, Priatna A, Becker LM, Foster GJ, Gropler RJ, Woodard PK. Feasibility of mri attenuation correction in cardiac fdg-pet. J Cardiovasc Magn Reson. 2013;15 Suppl 1:O61.
Nensa F, Poeppel TD, Beiderwellen K, Schelhorn J, Mahabadi AA, Erbel R, Heusch P, Nassenstein K, Bockisch A, Forsting M, Schlosser T. Hybrid pet/mr imaging of the heart: feasibility and initial results. Radiology. 2013;268(2):366–73.
Knuuti MJ, Nuutila P, Ruotsalainen U, Saraste M, Harkonen R, Ahonen A, Teras M, Haaparanta M, Wegelius U, Haapanen A, et al. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med. 1992;33:1255–62.
Beanlands RSB, Hendry PJ, Masters RG, de Kemp RA, Woodend K, Ruddy TD. Delay in revascularization is associated with increased mortality rate in patients with severe left ventricular dysfunction and viable myocardium on fluorine 18-fuorodeoxyglucose positron emission tomography imaging. Circulation. 1998;98:Ii51–6.
Klein C, Nekolla SG, Balbach T, Schnackenburg B, Nagel E, Fleck E, Schwaiger M. The influence of myocardial blood flow and volume of distribution on late gd-dtpa kinetics in ischemic heart failure. J Magn Reson Imaging. 2004;20:588–93.
Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, Schnackenburg B, Delius W, Mudra H, Wolfram D, Schwaiger M. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation. 2002;105:162–7.
Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2007;9:653–8.
Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733–43.
Schinkel AFL, Poldermans D, Elhendy A, Bax JJ. Assessment of myocardial viability in patients with heart failure. J Nucl Med. 2007;48:1135–46.
Ibrahim T, Hackl T, Nekolla SG, Breuer M, Feldmair M, Schomig A, Schwaiger M. Acute myocardial infarction: serial cardiac mr imaging shows a decrease in delayed enhancement of the myocardium during the 1st week after reperfusion. Radiology. 2010;254:88–97.
Wu YW, Tadamura E, Yamamuro M, Kanao S, Marui A, Tanabara K, Komeda M, Togashi K. Comparison of contrast-enhanced mri with (18)f-fdg pet/201tl spect in dysfunctional myocardium: relation to early functional outcome after surgical revascularization in chronic ischemic heart disease. J Nucl Med. 2007;48:1096–103.
van der Laan AM, Nahrendorf M, Piek JJ. Healing and adverse remodelling after acute myocardial infarction: Role of the cellular immune response. Heart. 2012;98:1384–90.
Lee WW, Marinelli B, van der Laan AM, Sena BF, Gorbatov R, Leuschner F, Dutta P, Iwamoto Y, Ueno T, Begieneman MPV, Niessen HWM, Piek JJ, Vinegoni C, Pittet MJ, Swirski FK, Tawakol A, Di Carli M, Weissleder R, Nahrendorf M. Pet/mri of inflammation in myocardial infarction. J Am Coll Cardiol. 2012;59:153–63.
Williams G, Kolodny GM. Suppression of myocardial 18f-fdg uptake by preparing patients with a high-fat, low-carbohydrate diet. AJR Am J Roentgenol. 2008;190:W151–6.
Watanabe E, Kimura F, Nakajima T, Hiroe M, Kasai Y, Nagata M, Kawana M, Hagiwara N. Late gadolinium enhancement in cardiac sarcoidosis: characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging. 2013;28:60–6.
Mc Ardle BA, Leung E, Ohira H, Cocker MS, de Kemp RA, DaSilva J, Birnie D, Beanlands RS, Nery PB. The role of f(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:297–306.
Kurisu S, Kihara Y. Tako-tsubo cardiomyopathy: clinical presentation and underlying mechanism. J Cardiol. 2012;60:429–37.
Hasbak P, Kjaer A, Skovgaard D, Bang LE, Grande P, Holmvang L. Preserved myocardial blood flow in the apical region involved in takotsubo cardiomyopathy by quantitative cardiac pet assessment. J Nucl Cardiol. 2012;19:169–71.
Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O’Gara PT, Carabello BA, Russell RO, Cerqueira MD, Sutton MGS, DeMaria AN, Udelson JE, Kennedy JW, Verani MS, Williams KA, Antman EM, Smith SC, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO, Heart ACCA. Acc/aha/asnc uidelines for the clinical use of cardiac radionuclide imaging - executive summary - a report of the american college of cardiology/american heart association task force on practice guidelines (acc/aha/asnc committee to revise the 1995 guidelines for the clinical use of cardiac radionuclide imaging). J Am Coll Cardiol. 2003;42:1318–33.
Parker MW, Iskandar A, Limone B, Perugini A, Kim H, Jones C, Calamari B, Coleman CI, Heller GV. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5:700–7.
Yoshinaga K, Chow BJW, Williams K, Chen L, Dekemp RA, Garrard L, Szeto ALT, Aung M, Davies RA, Ruddy TD, Beanlands RSB. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48:1029–39.
Merhige ME, Breen WJ, Shelton V, Houston T, D’Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with pet and rb-82 on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med. 2007;48:1069–76.
Manning WJ, Atkinson DJ, Grossman W, Paulin S, Edelman RR. First-pass nuclear magnetic resonance imaging studies using gadolinium-dtpa in patients with coronary artery disease. J Am Coll Cardiol. 1991;18:959–65.
Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2007;50:1343–53.
Morton G, Chiribiri A, Ishida M, Hussain ST, Schuster A, Indermuehle A, Perera D, Knuuti J, Baker S, Hedstrom E, Schleyer P, O’Doherty M, Barrington S, Nagel E. Quantification of absolute myocardial perfusion in patients with coronary artery disease: comparison between cardiovascular magnetic resonance and positron emission tomography. J Am Coll Cardiol. 2012;60:1546–55.
Bengel FM. Leaving relativity behind: quantitative clinical perfusion imaging. J Am Coll Cardiol. 2011;58:749–51.
Schwitter J, Nanz D, Kneifel S, Bertschinger K, Buchi M, Knusel PR, Marincek B, Luscher TF, von Schulthess GK. Assessment of myocardial perfusion in coronary artery disease by magnetic resonance: a comparison with positron emission tomography and coronary angiography. Circulation. 2001;103:2230–5.
O’Neill JO, McCarthy PM, Brunken RC, Buda T, Hoercher K, Young JB, Starling RC. Pet abnormalities in patients with nonischemic cardiomyopathy. J Card Fail. 2004;10:244–9.
Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu JH, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–26.
Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, Welling A, Hofmann F, Oostendorp RAJ, Gansbacher B, Nekolla SG, Bengel FM, Botnar RM, Schwaiger M. Combined reporter gene pet and iron oxide mri for monitoring survival and localization of transplanted cells in the rat heart. J Nucl Med. 2009;50:1088–94.
Beer AJ, Schwaiger M. Imaging of integrin alphavbeta3 expression. Cancer Metastasis Rev. 2008;27:631–44.
Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, Reder S, Nekolla SG, Wester HJ, Schwaiger M. Assessment of alpha(v)beta(3) integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res. 2008;78:395–403.
Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting alpha(v)beta(3) integrin expression, in a patient after acute myocardial infarction. Eur Heart J. 2008;29:2201.
Sherif HM, Saraste A, Nekolla SG, Weidl E, Reder S, Tapfer A, Rudelius M, Higuchi T, Botnar RM, Wester HJ, Schwaiger M. Molecular imaging of early alpha(v)beta(3) integrin expression predicts long-term left-ventricle remodeling after myocardial infarction in rats. J Nucl Med. 2012;53:318–23.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Nekolla, S.G., Rischpler, C. (2014). MR-PET in Cardiology: An Overview and Selected Cases. In: Carrio, I., Ros, P. (eds) PET/MRI. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40692-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-40692-8_9
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40691-1
Online ISBN: 978-3-642-40692-8
eBook Packages: MedicineMedicine (R0)