Abstract
Cicadas are iconic insects that use conspicuously loud and often complexly structured stereotyped sound signals for mate attraction. Focusing on acoustic communication, we review the current data to address two major questions: How do males generate specific and intense acoustic signals and how is phonotactic orientation achieved? We first explain the structure of the sound producing apparatus, how the sound is produced and modulated and how the song pattern is generated. We then describe the organisation and the sensitivity of the auditory system. We will highlight the capabilities of the hearing system in frequency and time domains, and deal with the directionality of hearing, which provides the basis for phonotactic orientation. Finally, we focus on behavioural studies and what they have taught us about signal recognition.
This work is dedicated to Franz Huber and Axel Michelsen for teaching me so much….
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
About 2,500 species of cicadas live in temperate and tropical regions around the world. Among insects they are notorious for their conspicuous loud and complex sound signals, which are stereotyped and species-specific (e.g. Fonseca 1991; but see Sueur and Aubin 2003, Sueur et al. 2007). Their particular temporal and spectral structure depends on the biomechanics of the sound production apparatus, and on the neural networks underlying song pattern generation. The latter determine the timing and bilateral coordination of timbal muscle contractions, i.e. the song pattern (Fonseca et al. 2008).
Signals are produced by males for mate attraction, courtship induction, as a distress sound or in the context of male–male interactions (Fonseca 1991). Since mate finding in cicadas is usually mediated by acoustic signals, females must be able to recognise the male signal and to orientate towards the singing male(s). Additionally, a female entering a chorus may need to discriminate among different males in an acoustically noisy environment. The complexity of the courtship behaviour allows females to select a mate, possibly upon multimodal assessment of mechanosensory information, conveyed through airborne sound or substrate vibrations, and other sensory channels such as vision (e.g. Cooley and Marshall 2001). The issue of sexual selection is still poorly addressed in these insects.
Female phonotaxis depends on the sensitivity and directionality of the hearing organs and on the extraction of behaviourally relevant information within the nervous system. Information can be imbedded in amplitude modulations of the male signal and/or in its frequency spectrum. Ultimately, sound signalling and sound reception should co-evolve to allow mate finding. In this context, behavioural studies provide an invaluable tool to reveal subtleties of a species communication system.
Here, we shall address two major questions: First, how do males generate specific and intense acoustic signals and second, how do female cicadas achieve phonotactic orientation? We will explain the structure of the sound producing apparatus, and how the sound is produced and modulated to generate the song pattern. We then will describe the organisation and the sensitivity of the hearing organs. We will highlight the processing capabilities of the auditory pathway in the frequency and time domains, and deal with the directionality of hearing, which provides the basis for phonotactic orientation. Finally, we will focus on behavioural studies and what they have taught us about signal recognition.
7.2 How do Males Generate Specific and Intense Acoustic Signals?
7.2.1 The Structure of the Sound Producing Apparatus
Most male cicadas produce their sound signals through a timbal apparatus (Pringle 1954; Moore and Sawyer 1966; Young and Bennet-Clark 1995; Bennet-Clark 1997; Fonseca and Bennet-Clark 1998). It is located dorso-laterally on both sides in the first abdominal segment (Fig. 7.1) and is generally lacking in females. The central feature of the apparatus is a bi- to multistable convex membrane, the timbal (Fig. 7.1c) with variable thickened sclerotised ribs. In some species, a dorsal bar couples a number of ribs. Small sclerotised patches, the small ribs, may be present between the ribs. Posterior to the ribbed area is the timbal plate where the timbal muscle attaches through a tendon-like structure. The timbal is delimited by a strong rim, the timbal frame. The tensor muscle (Fig. 7.1b, d) inserts and pulls at the anterior region of the timbal frame often differentiated as a tensor sclerite. The frame is surrounded anteriorly and ventrally by a folded membrane which allows for movements of the tensor sclerite as well as dorso–ventral and lateral movements of the abdomen. Internally, a large cavity is formed by fused tracheal air sacs (Fig. 7.1b). These project anteriorly forming a smaller thoracic cavity that backs the timbals and the folded membrane. It connects to the exterior when the prominent metathoracic spiracles open. The posterior cavity fills most of the abdomen. The size of the abdominal cavity and the posture of the abdomen can be varied. Ventrally to the sound producing apparatus and facing frontward lie the tympana of the hearing organs (Fig. 7.1a, b, d) which are described below.
General anatomy of a male cicada. a Lateral view with indication of the structures influencing sound production. b Schematic longitudinal section revealing the position and extension of the internal air cavities (view from above). c External view of the left timbal and surrounding structures in Tettigetta josei. d Longitudinal section depicting an internal view of the right timbal apparatus of T. josei
The internal cavities, the structure of the abdominal wall, the tympana, the folded membranes, opercula and timbal covers may all contribute to modify and/or radiate the sound produced by the timbals; their diversity is depicted in Moulds (1990).
7.2.2 Sound Production and Modulation
The primary sound generators are the timbals. When the powerful timbal muscle contracts, the convex timbal is loaded with mechanical energy and eventually collapses inward (Young and Bennet-Clark 1995; Bennet-Clark 1997; Fonseca and Hennig 1996) allowing a fast energy release. The timbal is driven either in one or in successive steps leading to sequential sudden bending of the long ribs. This inward movement is accompanied by one or a group of sound pulses (IN). Due to these mechanisms and the biomechanical properties of the timbal membrane, the timbal acts as a frequency multiplier. This partly explains how timbal muscle contractions, with a rate of 20–550 Hz (Hagiwara 1955; Young 1972a; Young and Josephson 1983a, b; Josephson and Young 1985; Fonseca 1996), can generate peak call components ranging from about 1 kHz (e.g. Cystosoma saundersii, Young 1972a) to ultrasonic frequencies (e.g. Tettigetta josei: Fonseca 1991, Cicadetta iphigenia: Trilar et al. 2006). Upon relaxation of the timbal muscle, the timbal pops out again to its resting position driven by elastic energy stored in the timbal by resilin (Bennet-Clark 1997; Fonseca and Bennet-Clark 1998). This movement may also be accompanied by sound (OUT). In addition, the timbals load and set in motion the air in the internal cavity that can create cavity resonances (Young 1990; Bennet-Clark and Young 1992, 1998) and/or drive other structures such as the abdomen wall (Fonseca and Popov 1994). As the timbal apparatus is bilaterally organised, activation of both timbal muscles may range from simultaneous to alternating. The sound pulses generated by each timbal muscle contraction compose the basic song element, i.e. the syllable. The syllables can be repeated over time in groups to form echemes, which may in turn delineate longer and more complex song sequences, the phrases (Fig. 7.6a; cf. Fonseca 1991 for terminology).
The convexity and stiffness of the timbal, and thus the sound generated, may be modified by activity of the tensor muscle (Fig. 7.1c, d) (Pringle 1954; Simmons and Young 1978; Hennig et al. 1994; Fonseca and Hennig 1996).
In species where the abdomen appears to act as a Helmholtz resonator, the abdomen cavity and the gap abdomen-opercula can be adjusted to influence the sound quality (Young 1990; Bennet-Clark and Young 1992, 1998; but see Morse and Ingard 1987, for a detailed description of the physics of an Helmholtz resonator and Bennet-Clark 1999, for a general description of resonance models in insects). In species with thick abdomen walls, sound radiation may be primarily via the tympana (Weber et al. 1988; Young 1990); strong amplitude modulations are correlated with vertical movements of the abdomen.
7.2.3 How is the Song Pattern Generated?
Song specificity is determined by the mechanical characteristics of the sound producing structures, and by the coordinated contraction of the muscles affecting the timbals. The large timbal muscle is innervated by a single large timbal motorneuron (Hagiwara and Watanabe 1956; Simmons 1977; Wohlers et al. 1979; Wohlers and Bacon 1980) whereas at least 2–3 motorneurons innervate the tensor muscle (Wohlers et al. 1979; Popov 1981, and functional evidence by Stokes and Josephson 2004). The fast timbal muscle is neurogenic, i.e. each timbal motorneuron action potential causes a twitch contraction. A remarkable exception is Platypleura capitata, in which the timbal muscle appears to be myogenic (Pringle 1954).
Little is known about the organisation of the song pattern generator (SPG) in cicadas. Simmons (1977) found a group of interneurons at the metathoracic-abdominal ganglion complex (MAC) that oscillated at twice the frequency of the timbal motorneuron spikes. These were one quarter of a cycle out of phase. Simmons (1977) concluded that several interneurons were involved in generating the song rhythm and that at least some of these should be non-spiking interneurons. In spite of the indirect evidence that these interneurons might be part of the SPG, current injection rarely changed the waves’ frequency in the interneuron or in the timbal motorneuron, and thus apparently did not strongly influence the rhythm generator.
Each timbal motorneuron received input from several interneurons. The motorneuron initiating a sound sequence often swapped after pauses in singing and they may be activated at different phases (Fonseca 1996). The timbal motorneurons do not appear to be directly coupled as current injection in one timbal motorneuron did not produce any recognisable effect on the other timbal motorneuron (Simmons 1977; R.M. Hennig and P.J. Fonseca unpublished).
Preliminary work using extra- and intracellular recording and staining investigated the activity of descending and local inter- and motorneurons during singing in the cicada Tympanistalna gastrica (R.M. Hennig and P.J. Fonseca unpublished data). A singing pattern similar to the natural calling song was elicited as an after-effect of electrical brain stimulation (for details of this technique introduced by the late A.V. Popov see Fonseca and Popov 1994; for an oscillogram see Fonseca and Bennet-Clark 1998).
As in the flight of orthoperans (Robertson and Pearson 1982, 1984), no evidence was found that the timbal motorneurons are part of the SPG. The activity of the timbal motorneuron (inset in Figs. 7.2a, 7.4c) starts later than most other neurons (Fig. 7.2a). Depolarising current injection, up to 10 nA, applied to the motorneuron never resulted in singing activation. In contrast, in one large local interneuron depolarisation could result in patterned timbal motorneuron activity (inset in Fig. 7.2a, b), i.e. fictive singing. This omega-shaped neuron in MAC, labelled Singing Interneuron 1 (Si-Int-1) never generated spikes, and preceded the activity of the timbal motorneuron by about one cycle (Fig. 7.2a, b). Its arborisations in both hemiganglia overlapped with arborisation of the timbal motorneuron (inset in Fig. 7.2a) which may indicate a connection of Si-Int-1 with the timbal motorneuron. Si-Int-1 established excitatory connections with Si-Int-2, another non-spiking omega-shaped neuron, which closely followed the activity of Si-Int-1. Since Si-Int-1 and Si-Int-2 establish excitatory connections (data not shown), they might constitute an important core of the SPG. Interestingly, the amplitude of Si-Int-1 depolarisation was strongly correlated with the frequency of the timbal motorneuron action potentials (Fig. 7.2c), an observation that deserves further investigation. Differently to Simmons (1977), no evidence was found for continuous oscillation of the SPG activity.
Interneurons active during singing by the cicada Tympanistalna gastrica. a Comparison of the timing of activation of several interneurons relative to the activity of the timbal motorneuron. Inset is a confocal image of simultaneous Luciffer yellow fills of the local interneuron Singing Interneuron 1 (Si-Int-1) and the timbal motorneuron. b Details of the activity of the omega-shaped cell Si-Int-1 in a double intracellular recording with the timbal motorneuron (TiMn1), together with the extracellular recording of the other timbal motorneuron (TiMn2). Singing activity was elicited by depolarising current injection in Si-Int-1. Both timbal motorneurons are activated. c The representation of the frequency of the timbal motorneuron rhythm superimposed to an intracellular recording of Si-Int-1, points to a correlation with the amplitude of depolarisation of Si-int-1 (graph below)
Surprisingly, although both timbal motorneurons are activated almost simultaneously (Fig. 7.2b), EMGs from the timbal muscles reveal a much larger delay (about 2.5 ms, Fonseca 1996), also observed in the sound output. In fact, the IN pulses alternate with a phase ca. 1/6th–1/7th of a period (cf. Fonseca 1996) and singing always initiates with the right timbal (Fonseca and Bennet-Clark 1998). This asymmetry is thus not created at the level of the neuronal oscillators. Instead it must be an attribute of the periphery, probably caused by the asymmetric timbals (Fonseca and Bennet-Clark 1998) that may request different force to buckle inwards.
As in other systems (Hedwig 1996, 2000), it is very likely that singing is elicited by descending activity from command neurons in the brain. Descending neurons whose activation preceded the timbal motorneuron activity by about 2–4 timbal periods could be recorded (Fig. 7.2a).
7.3 How is the Auditory System Organised?
7.3.1 Structure of the Hearing Organ
Cicadas have a highly specialised auditory system (Fig. 7.3) with a basic structure similar across species (Vogel 1923; Myers 1928; Pringle 1954; Michel 1975; Young and Hill 1977; Doolan and Young 1981; Fonseca 1993, 1994; Fonseca and Popov 1997). The ears are situated latero-ventrally in the first abdominal segment (Fig. 7.3a, b). The delicate tympana, which are much larger in males than in females, may in parts be under 1 μm thick. They are backed and acoustically coupled by tracheal air cavity(ies) (Fig. 7.3a) that open through the metathoracic spiracles (Figs. 7.1d, 7.3a). The folded membranes integrate the anterior cavity wall which, in males, backs the timbals (Fig. 7.1b, d). The male abdomen can be very thin and translucent. Thus, sound may reach the internal surfaces of the tympana via the contralateral tympanum, the metathoracic spiracles and the folded membranes and, in males, also through the timbals and the thin abdominal wall (Fig. 7.1d). The tension of the tympanum can be varied by the action of detensor tympani muscles (Pringle 1954), which can modulate hearing sensitivity (Hennig et al. 1994). The tympanic ridge, a sclerite that sits on the thin tympanic membrane, connects the tympanum to the auditory organ, which is protected within the auditory capsule, through a lever, the tympanic apodeme (Fig. 7.3b). About 500–2,200 auditory receptors (Fig. 7.4b) attach to the apodeme tip (Fig. 7.4a) and form the onion-shaped auditory organ (Figs. 7.3b, 7.4a) (Wohlers et al. 1979; Doolan and Young 1981; Fonseca 1994). The receptors’ axons coalesce in an auditory nerve that joins the MAC (Figs. 7.3b, 7.4a) and project into a complex auditory neuropile (Fig. 7.4c, d) (Wohlers et al. 1979; Fonseca 1994). The auditory nerve may also contain other sensory fibres, and the axon of the timbal motorneuron (Fig. 7.4c).
Anatomy of the cicada hearing structures. a Diagrammatic lateral view revealing the components of the hearing system and their position relative to the male timbal apparatus. b Transverse cut at the level of the auditory organ in a female and a male of the cicada Tettigetta josei. AC auditory capsule; AN auditory nerve; AO auditory organ; AS air sac; CV chitinous V; MAC metathoracic-abdominal ganglion complex; MS metathoracic spiracle; Op operculum; TA tympanic apodeme; Ti timbal; TiM timbal muscle; TR tympanic ridge; Ty tympanum
Details of elements in the auditory pathway of the cicada Tettigetta josei. a Two-photon confocal image showing the distribution of auditory receptor cell bundles and their connection to the complex-shaped crescent-like tip of the auditory apodeme. The axons of the receptors coalesce in several branches that integrate the auditory nerve. The wide arrow indicates the direction of the tympanic apodeme towards the tympanum. b Electron microscope view of a transverse cut of the auditory nerve just exiting the auditory organ. Each profile corresponds to one axon of one auditory receptor cell. Many axons exhibit sub-micrometre profiles. c Two-photon confocal image of the auditory neuropile revealed by backfilling of both auditory nerves with Lucifer Yellow fluorescent dye. The auditory receptors project into a complex auditory neuropile that spans several segmental areas in the fused metathoracic-abdominal ganglion complex. The two large timbal motorneurons, whose axons run in this, and in many other cicada species, in the auditory nerve, cross at the mid line. d In other species, such as in Cicada barbara, the timbal motorneuron runs in an independent nerve. Here the auditory neuropile is revealed by backfilling one auditory nerve with nickel chloride (dark blue) and the other with cobalt chloride (dark orange). Some axons appear not to end at the neuropile (see arrows)
7.3.2 The Sensitivity of the Auditory System
Only anecdotal data are available from single auditory afferent recordings (e.g. Münch 1999) as axon diameters are in the range of 1 micron (Fig. 7.4b), Sensitivity of the hearing organ has been analysed with recordings of the whole auditory nerve or auditory interneurons. Nerve recordings in different species (Popov 1981; Popov et al. 1985; Huber et al. 1990; Fonseca 1994; Daws and Hennig 1996; Fonseca et al. 2000; Fonseca and Cooley unpublished) revealed threshold curves with sensitivity of 25–40 dB SPL (i.e. re. 20 μPa) to frequencies ranging 2–6 kHz (but see Young and Hill 1977). The best frequency range is the same across many species, even in cases where the songs’ spectra are almost devoid of energy at this range. This puzzling mismatch (Popov 1990; Fonseca 1994) is an artefact at least in some species. Intracellular recordings in Tettigetta josei (Fonseca et al. 2000) (Fig. 7.5) and Cicada barbara (Fonseca 1994), revealed auditory interneurons sensitive to frequencies higher than expected from auditory nerve recordings. This unsuspected interneuron sensitivity may be attributed to auditory afferents with very thin axons (Fig. 7.4b) (Fonseca et al. 2000), which are not properly represented in whole nerve recordings. The ubiquitous strong auditory sensitivity within the low frequency range suggests the activation of a large number of afferents, and may be related to selection pressure to detect predators rather than conspecifics (e.g. Popov 1990; Mason 1991).
7.3.3 Processing in the Frequency Domain
Songs vary widely across cicada species and include wide band and pure-tone spectra, ranging from few hundred Hz to ultrasound (e.g. Young 1972b; Popov 1975; Popov et al. 1985; Fonseca 1991, 1994; Gogala 1995; Sueur and Aubin 2003; Sueur et al. 2004; Moulds 2005; Trilar et al. 2006). Songs can show pronounced frequency modulations, especially in tropical species (e.g. Gogala 1995). Based on the very large number of auditory afferents and at least 15 auditory ascending interneurons (Huber et al. 1980; Fonseca et al. 2000; Fonseca and Correia 2007) cicadas may be able to process details in their songs, both in frequency and in time domains.
Tympanic membrane vibrations (Fonseca 1993; Fonseca and Popov 1997; Sueur et al. 2006, 2008, 2010) are transmitted to the onion-shaped auditory organ by the flattened rod-like tympanic apodeme in a way that may contribute to frequency analysis in the auditory pathway. Some underlying mechanisms revealed in T. josei point to (1) the complex vibrations observed at the tympanic ridge, (2) the shape of the apodeme tip and (3) the distribution and orientation of the afferent neurons’ attachment to the apodeme (Fig. 7.4a) (Michelsen and Fonseca, in preparation). The apodeme originates from, and forms an angle with, the tympanic ridge which vibrates in a complex mode. Depending on frequency it may rock, move up and down or back and forth, movements that are communicated to the apodeme. At the edge of the tympanum, the apodeme barely moves at most frequencies and appears like a lever anchored at an intermediate point, i.e. it inverts and transmits the movement to the crescent-like apodeme tip, which has the freedom to move in three dimensions.
Apodeme geometry may be simpler in other species. Bundles of receptor cells oriented in all three space dimensions attach just before the tip and along the crescent-like structure (Fig. 7.4a). Such special arrangement, also found in the bladder cicada Cystosoma saundersii (Doolan and Young 1981), allows the sensory neurons to be maximally activated according to their directions of attachment. Since the orientation of the apodeme movement is frequency dependent, frequency discrimination could be due to the activation of differently oriented receptors. In addition, intrinsic cellular mechanisms were proposed (Fonseca and Correia 2007) and demonstrated in other insects (Göpfert and Robert 2001; Göpfert et al. 2005; Kernan 2007) and in vertebrates (Fettiplace 1987; Dallos 1992; Kennedy et al. 2005). If present in cicadas, such mechanisms could enhance frequency discrimination.
Interneurons seem to represent mechanosensory information from very low frequency substrate-born vibrations to high frequency air-born signals (cf. Fig. 7.5). In Tettigetta josei, a set of ascending interneurons with high Q10dB values is tuned to different frequencies in the range 1–25 kHz (Fig. 7.5) (Fonseca et al. 2000). In addition to air-born sounds, cicadas also detect substrate vibrations mostly via subgenual organs since afferent activity is mainly found in the leg’s nerve. Interneurons tuned to different frequencies within 20–1,000 Hz with sensitivities ranging from 0.02 to 0.3 ms−2 (Fig. 7.5) have been described (Fonseca and Santos 2001) some of which were activated by vibrations induced in a plant during a cicada landing or takeoff. In small-sized cicadas, like T. josei, males move around with short flights intercalated by short calling sequences (“sing-fly” behaviour) and females wait deeply within the vegetation until a male lands and sings close by and, only then signal e.g. by wing flicking (e.g. Gwynne1987; Lane 1995; Marshall and Cooley 2000, 2001; Cooley 2001). Therefore substrate vibrations may also be important in intraspecific communication (Fonseca and Santos 2001; Stölting et al. 2002) and may be invaluable to detect approaching predators (Kühne 1982; Kühne et al. 1984; Hill 2001).
7.3.4 Processing in the Time Domain
Cicadas’ acoustical signals show great variability in temporal patterns among species (e.g. Popov 1975; Popov et al. 1985; Fonseca 1991; Moulds 1990, 2005; Gogala 1995; Sueur et al. 2004; Gogala and Trilar 2004; Gogala et al. 2005; Trilar et al. 2006). Mate finding is usually mediated by the calling song but final acceptance of a male by the female depends on subtleties of the courtship behaviour (Cooley 1999; Cooley and Marshall 2001), including courtship song. This makes sound an important pre-mating mechanism for species isolation and raises the question to what degree cicadas can process the time pattern of conspecific songs.
Different methods have been used to estimate auditory temporal resolution in animals (Michelsen 1985; Tougaard 1998), but only a few studies have approached the question in cicadas (Huber et al. 1990; Fonseca 1994).
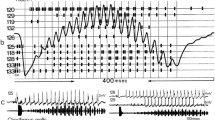
Using the calling song and natural sounds evidence for time resolution in the auditory pathway was obtained in T. josei (Alves and Fonseca, unpublished). This species produces calling song phrases with two distinctive parts (Fig. 7.6a); a succession of echemes (part 1) which ends with a more continuous buzz (part 2) (details in Fonseca 1991). This pattern creates sound pulse periods of 3–5 ms within the echemes, echeme periods around 30 ms and periods of 10–15 ms between consecutive loud IN pulses during the buzz (cf. Fig. 7.6a). The representation of these calling song features was investigated at the level of summed auditory nerve activity and auditory interneuron responses. Auditory nerve activity represented any sound pulses down to at least periods of 1 ms provided the first pulse was quieter than the second (Fig. 7.6b, OUT1-IN2), the normal condition in echemes. If the first pulse was considerably louder, as in the last part of an echeme, a weak nerve response occurred for pulse periods of about 4 ms, augmenting with increasing intervals (Fig. 7.6b, IN2-OUT2). Within auditory interneurons, the best response occurred in ascending neurons very sensitive to the calling song (Fig. 7.7a). At least one neuron represented the loud IN pulses of the buzz down to a period of 6 ms (note that the IN pulses in buzzes are always loud). Manipulation of the silent interval between the quiet (OUT1) and the loud (IN2) pulses characteristic of the echemes revealed that the same cell represented the onset of the two pulses down to at least 4 ms (Fig. 7.7b). Similar temporal resolution down to 1–4 ms occurs in other insects (e.g. von Helversen 1979; Ronacher and Stumpner 1988; Tougaard 1996; Prinz and Ronacher 2002; Franz and Ronacher 2002). In contrast, if the quiet pulse followed the loud one, the neuron only responded with some irregular activity if periods were longer than 7–9 ms (Fig. 7.7c). Confirmation that the responses to the first (quieter OUT1) and the second (loud IN2) pulses were actually caused by the onset of the sounds, was further obtained by suppressing the quieter first (OUT1), the quieter last (OUT2) or both of them keeping only the loud (IN2) pulse (Fig. 7.7d). This unequivocally ruled out that responses to the first two pulses in an echeme (i.e. OUT1–IN2) might be artefacts created by sustained spiking activity due to suprathreshold stimulation. It demonstrated that the first two pulses in an echeme, OUT1–IN2, but not the last OUT2 were represented in the neuron’s spiking activity. A conceivable physiological reason for the representation of two pulses with the second being louder than the first was advanced by Münch (1999).
Calling song and temporal resolution of the auditory pathway of the cicada Tettigetta josei exhibited by the summed activity of the auditory receptors. a The calling song consists in a sequence of temporally complex phrases with two distinct parts. Echemes in part 1 exhibit a characteristic amplitude modulation with the IN sound pulse increasing strongly from the first syllable, where it can be barely noticeable, to the second syllable, where the IN is the loudest pulse. The pattern is different in the second part, where the IN pulses are always loud. This amplitude modulation pattern generates different temporal cues. b Nerve recordings represent any timing between two sound pulses provided the first is quitter, a characteristic of the first syllable in part 1 echemes. In the second syllable, when the second sound pulse has a lower amplitude, it appears in the averaged activity of the auditory nerve (average of 16 echemes, t = 28 °C) only for periods above 3–4 ms
Representation of the temporal characteristics of the song by an auditory ascending interneuron type sensitive to the calling song in the cicada Tettigetta josei (averages of 16 stimuli). a Song and interneuron activity at 25 °C. The timing of the echemes is well represented in the two parts of a phrase. b Manipulation of the period in the first audible two pulses of a part 1 echeme, i.e. Out1-In2, shows that this period is faithfully represented in the spiking activity at least down to 3.5–4 ms (graph inset when x ≈ y), In contrast, the timing at the end of an echeme is only represented for much longer periods (>7–8 ms) (c). d Selectively removing the softer (OUT) pulses of a part 1 echeme unequivocally shows that this pulse is correctly represented in the first syllable of the echeme, but not in the second syllable
These results were obtained with recordings at 25–28 °C and the performance reduced with lower temperatures. The temporal resolution measured in the experiments, however, corresponds to temporal characteristics of songs produced at higher temperatures (Fonseca and Allen-Revez 2002a), indicating that all time periods within the calling song of T. josei are represented by ascending neurons and forwarded to the brain. However, more electrophysiological and behavioural work is needed to reveal the capabilities of ascending interneurons and especially of auditory brain neurons to deal with temporal features of the songs. The latter question may also be approachable by behavioural experiments.
7.3.5 Directional Hearing
Localisation of a sound source depends primarily on the ability of the peripheral auditory organs to be differently activated according to sound direction, and/or on the ability to process the time difference of the sound arrival at both hearing organs (reviews by Michelsen 1998 and Michelsen and Larsen 2008).
In cicadas, directional hearing was studied in few species by measuring tympanic membrane vibrations (Fonseca 1993; Fonseca and Popov 1997; Fonseca and Hennig 2004) or auditory nerve activity (Young and Hill 1977; Fonseca 1994; Daws and Hennig 1996; Fonseca and Hennig 2004). Significant directionality occurred both at low frequencies and around the peak of the calling song spectrum, with the exception of C. saundersii males, where no directionality was found (Young and Hill 1977). Experiments with selective and reversible blocking of putative sound inputs to the auditory system (Fonseca 1993; Fonseca and Popov 1997) indicated that in males the sound generating timbal acted as an important input responsible for the directionality at the spectral peak of the song, a frequency that corresponds to the natural resonance of the timbal (e.g. Fonseca 1993; Fonseca and Hennig 2004). The input through the contralateral tympanum caused high directionality at middle range frequencies (3–8 kHz) in males of T. gastrica and T. josei but not in Cicada barbara males. Instead, in C. barbara males, the hollow and thin abdomen was responsible for a directionality response at low frequencies (1–2 kHz) (Fonseca and Popov 1997). By contrast, the most important acoustic inputs conditioning hearing directionality in females were, in addition to the contralateral tympanum, the metathoracic spiracles. Usually, the auditory directionality of females encompasses a large frequency range starting at low frequencies and includes the loud frequency components of the male song. Interestingly, the contralateral tympanum not only acts as an important sound input, but also determines the correct phase lag between the bilateral sound inputs to create pronounced directionality (Figs. 7.6, 7.7 in Fonseca 1993; see Löhe and Kleindienst 1994; Michelsen and Löhe 1995 for a similar observation involving the central membrane in crickets).
In two cicada species, T. josei and T. gastrica, the directional differences of tympanic vibrations are encoded by the activity of the auditory afferents. Directionality with frontal sound stimulation at ±30º could differ by more than 5 dB (Fonseca and Hennig 2004). In addition, ascending interneurons in T, josei showed threshold differences up to 15 dB with frontal stimulation at ±45º, both at lower frequency ranges (3–6 kHz) and around the calling song spectral peak of 16 kHz (Fonseca, unpublished).
7.3.6 What Behavioural Studies have Taught us About Signal Recognition in Cicadas?
The ultimate answer to questions regarding the capacity of an animal to recognise and orient towards sound signals comes from behavioural experiments. Successful experiments rely on subjects receptive and prone to react to the stimulus, what may depend on their physiological state, e.g. circadian influences (Daws et al. 1997) or the receptivity of the female (Cooley and Marshall 2001). This imposes considerable difficulties if the insects cannot be bred in the laboratory and for cicada probably is a cause for the scarcity of behavioural data, compared to the extensive work involving other acoustically communicating insects. For example, receptive female cicadas, or isolated males, fly towards a singing male or a chorus. However, flight phonotaxis experiments have been difficult to conduct in captivity.
In some cicada species, a stereotyped and well-timed short wing flicking signal is produced by receptive females upon listening to the male song (e.g. Cooley 1999; Cooley and Marshall 2001). Such duets, with males producing loud sounds and females responding with wing flicks, should allow to evaluate the relevant parameters for species-specific song recognition. For instance, in many New Zealand cicadas, the songs were found to possess an introductory section and a cueing section, which is responsible for releasing the wing flick response (Marshall, Hill and Cooley personal communication; cf. Fig. 7.1 in Marshall et al. 2008). Therefore, taxa like the genus Kikihia and other Cicadettini may be interesting groups to analyse auditory signal and song recognition. Based on the female wing flick response, Marshall and Cooley (2000) demonstrated that females of the 13-year periodical species Magicicada tredecim and M. neotredecim responded selectively to the dominant frequency of the species-specific male calling songs; both species have nearly pure-tone calls that lack temporal patterns. The dominant frequency of the male song, but not the temporal pattern, was also found to be key to elicit flight phonotaxis in female C. saundersii (Doolan and Young 1989); Daws et al. (1997) argued that this frequency-dependent phonotaxis did not necessarily result from fine frequency selectivity of the females, but rather appeared to be based on the overall level of excitation of the auditory system. In fact, an increase in the amplitude of a stimulus outside the best hearing range compensated the reduction in overall auditory excitation and re-established the level of female phonotaxis. Female courtship responses, however, were only elicited if the temporal parameters of the natural song were present in the stimuli, even when synthesised with different carrier frequencies. Thus, the carrier frequency of calling songs appears to be more important in long range communication, to attract flying females, while details of the temporal structure may be essential for short range courtship interactions (Doolan and Young 1989). In this way, the cicada communication system would circumvent the constraint of random amplitude modulation that inevitably affects distant sound propagation, especially within vegetation (e.g. Richards and Wiley 1980). Behavioural data from Tibicina haematodes, which aggregate to form choruses, are in line with these results. Males responded to conspecific as well as to allospecific calling songs with overlapping song spectra but distinct temporal pattern. However, they did not react to playbacks of heterospecific songs with disjunct frequency spectra (Sueur and Aubin 2002). In contrast, an apparent absence of frequency selectivity was found in Cyclochila australasiae (Daws et al. 1997).
Evidence for frequency analysis and evaluation of temporal pattern was obtained in males of C. barbara, which also aggregate in choruses (Fonseca and Allen-Revez 2002b). The readiness of males to sing once another male initiated singing can be used in behavioural experiments. Males responded to the conspecific song as well as to a continuous pure-tone of 6 kHz, the song’s spectral peak component. However, in spite of the species’ broadband calling song spectrum, and in spite of the maximal peripheral excitation at 3–4 kHz, the males’ stereotyped response decreased significantly when tested with pure-tone stimuli at 3 or 4 kHz. This revealed at least some ability for frequency discrimination among 6, 4 and 3 kHz, compatible with the frequency selectivity found at the level of auditory interneurons in T. josei (Fonseca et al. 2000). At 90 dB SPL playback intensities, no significant differences in the response occurred between 6 and 9 kHz. This is in line with the finding that this cicada readily reacts to loud songs of another sympatric species (Tibicina garricola), also with a continuous song but a spectral maximum at about 9 kHz, a frequency well represented in the C. barbara song. The response at 9 kHz deteriorated if the playback amplitude was lowered by 20 dB. This might be attributed to a considerable lower peripheral excitation at 9 kHz when compared to the excitation at 6 kHz (Fonseca and Allen-Revez 2002b), as argued for C. saundersii by Daws et al. (1997); this should also effectively prevent a response to singing males of T. garricola singing at a distance in natural conditions.
When pauses were introduced in the song of C. barbara, the males’ responsiveness was maintained if the pauses were shorter than 30 ms, irrespective of the sound duration. The response decreased steeply when pauses exceeded 30 ms (cf. Fonseca and Allen-Revez 2002b). Only this temporal discrimination prevents a response to the calling song of a sympatric and synchronic sister species (Cicada orni), which has a largely overlapping frequency spectrum but is composed by a succession of echemes separated by silent intervals longer than 40 ms (Fonseca 1991). Remarkably, C. orni males stop responding to song models with silent intervals shorter than 40 ms (Simões and Quartau 2006), suggesting that the duration of the silent intervals is paramount for species discrimination. Studying the responses of females of these two species to the same playback signals should confirm the importance of the pause length for segregation of these species, but so far has not been possible.
Evidence from the few behavioural studies suggests that cicada species may exhibit different capabilities to extract information from the songs both in time and frequency domains. During evolution, these abilities may have been shaped by male–male competition, by the need to detect acoustic cues of individuals within crowded and noisy choruses (Cooley and Marshall 2001) and by the requirement to recognise species-specific song features in noisy habitats with many sympatric species.
References
Bennet-Clark HC (1997) Tymbal mechanics and the control of song frequency in the cicada Cyclochila australasiae. J Exp Biol 200:1681–1694
Bennet-Clark HC (1999) Resonators in insect sound production : how insects produce loud pure-tone songs. J Exp Biol 202:3347–3357
Bennet-Clark HC, Young D (1992) A model of the mechanism of sound production in cicadas. J Exp Biol 173:123–153
Bennet-Clark HC, Young D (1998) Sound radiation by the bladder cicada Cystosoma saundersii. J Exp Biol 201:701–715
Cooley JR (1999) Sexual behavior in North American cicadas of the genera Magicicada and Okanagana. PhD dissertation, University of Michigan, Ann Arbor
Cooley JR (2001) Long-range acoustical signals, phonotaxis, and risk in the sexual pair-forming behaviors of Okanagana canadensis and O. rimosa (Hemiptera: Cicadidae). Ann Ent Soc Am 94:755–760
Cooley JR, Marshall DC (2001) Sexual signaling in periodical cicadas, Magicicada spp. Behaviour 138:827–855
Dallos P (1992) The active cochlea. J Neurosci 12:4575–4585
Daws AG, Hennig RM (1995/1996). Tuning of the peripheral auditory system of the cicada, Cyclochila australasiae. Zoology 99:175–188
Daws AG, Hennig RM, Young D (1997) Phonotaxis in the cicadas Cystosoma saundersii and Cyclochila australasiae. Bioacoustics 7:173–188
Doolan JM, Young D (1981) The organization of the auditory organ of the bladder cicada Cystosoma saundersii. Phil Trans R soc Lond 291(1055):525–540
Doolan JM, Young D (1989) Relative importance of song parameters during flight phonotaxis and courtship in the bladder cicada Cystosoma saundersii. J Exp Biol 141:113–131
Fettiplace R (1987) Electrical tuning of hair cells in the inner ear. Trends Neurosci 10:421–425
Fonseca PJ (1991) Characteristics of the acoustic signals in nine species of cicadas (Homoptera, Cicadidae). Bioacoustics 3:173–182
Fonseca PJ (1993) Directional hearing of a cicada: biophysical aspects. J Comp Physiol A 172:767–774
Fonseca PJ (1994) Acoustic communication in cicadas (Homoptera, Cicadoidea): sound production and sound reception. PhD dissertation, Universidade de Lisboa, Lisboa
Fonseca PJ (1996) Sound production in cicadas: timbal muscle activity during calling song and protest song. Bioacoustics 7:13–31
Fonseca PJ, Allen Revez M (2002a) Temperature dependence of cicada songs (Homoptera, Cicadoidea). J Comp Physiol A 187:971–976
Fonseca PJ, Allen Revez M (2002b) Song discrimination by male cicadas Cicada barbara lusitanica (Homoptera, Cicadidae). J Exp Biol 205:1285–1292
Fonseca PJ, Bennet-Clark HC (1998) Sound radiation in a cicada: the role of tymbal asymmetry in the production of complex songs. J Exp Biol 201:717–730
Fonseca PF, Correia T (2007) Effects of temperature on tuning of the auditory pathway in the cicada Tettigetta josei (Hemiptera, Tibicinidae). J Exp Biol 210:1834–1845
Fonseca PJ, Hennig RM (1996) Phasic action of the tensor muscle modulates the calling song in cicadas. J Exp Biol 199:1535–1544
Fonseca PJ, Hennig RM (2004) Directional characteristics of the auditory system of cicadas: is the sound producing tymbal an integral part of directional hearing? Physiol Entomol 29:1–9
Fonseca PJ, Popov AV (1994) Sound radiation in a cicada: the role of different structures. J Comp Physiol A 175:349–361
Fonseca PJ, Popov AV (1997) Directionality of the tympanal vibrations in a cicada: a biophysical analysis. J Comp Physiol 180:417–427
Fonseca PJ, Santos T (2001) Sensitivity of substrate vibrations in the cicada Tettigetta josei (Homoptera, Cicadoidea). In: Elsner N, Kreutzberg GW (eds) Proceedings 28th gottingen neurobiology conference, vol 2. G Thieme Verlag, Stuttgart, p 374
Fonseca PJ, Münch D, Hennig RM (2000) How cicadas interpret acoustic signals. Nature 405:297–298
Fonseca PJ, Serrão EA, Pina-Martins F, Silva P, Mira S, Quartau JA, Paulo OS, Cancela L (2008) The evolution of cicada songs contrasted with the relationships inferred from mitochondrial DNA (Insecta, Hemiptera). Bioacoustics 18:17–34
Franz A, Ronacher B (2002) Temperature dependence of temporal resolution in an insect nervous system. J Comp Physiol A 188:261–271
Gogala M (1995) Songs of four cicada species from Thailand. Bioacoustics 6:101–116
Gogala M, Trilar T (2004) Biodiversity of cicadas in Malaysia—bioacoustic approach. Serangga (Bangi) 9(1/2):63–81
Gogala M, Trilar T, Krpač V (2005) Fauna of singing cicadas (Auchenorrhyncha: Cicadoidea) of Macedonia: a bioacoustic survey. Acta Entomol Slov 13(2):103–126
Göpfert MC, Robert D (2001) Active auditory mechanics in mosquitoes. Proc R Soc Lond B 268:333–339
Göpfert MC, Humphris ADL, Albert JT, Robert D, Hendrich O (2005) Power gain exhibited by motile mechanosensory neurons in Drosophila ears. PNAS 102:325–330
Gwynne DT (1987) Sex-biased predation and the risky mate-locating behavior of male tick-tock cicadas (Homoptera: Cicadidae). Anim Behav 35:571–576
Hagiwara S (1955) Neuro-muscular mechanism of sound production in the cicada. Physiol Comp Oecol 4:142–153
Hagiwara S, Watanabe A (1956) Discharges in motoneurons of cicada. J Cell Comp Physiol 47:415–428
Hedwig B (1996) A descending brain neuron elicits stridulation in the cricket Gryllus bimaculatus (de Geer). Naturwissenschaften 83:428–429
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on behavioural state. J Neurophysiol 83:712–722
Hennig RM, Weber T, Huber F, Kleindienst H-U, Moore TE, Popov AV (1994) Auditory threshold change in singing cicadas. J Exp Biol 187:45–55
Hill PSM (2001) Vibration and animal communication: a review. Amer Zool 41:1135–1142
Huber F, Wohlers D, Moore TE (1980) Auditory nerve and interneurone responses to natural sounds in several species of cicadas. Physiol Entomol 5:25–45
Huber F, Kleindienst H-U, Moore TE, Schildberger K, Weber T (1990) Acoustic communication in periodical cicadas: neuronal responses to songs of sympatric species. In: Gribakin FG, Wiese K, Popov AV (eds) Sensory systems and communication in arthropods. advances in life sciences, Verlag, Berlin, pp 217–228
Josephson RK, Young D (1985) A synchronous insect muscle with an operating frequency greater than 500 Hz. J Exp Biol 118:185–208
Kennedy HJ, Crawford AC, Fettiplace R (2005) Force generation by mammalian hair bundles supports a role in cochlear amplification. Nature 433:880–883
Kernan MJ (2007) Mechanotransduction and auditory transduction in Drosophila. Pflügers Arch 454:703–720
Kühne R (1982) Neurophysiology of the vibration sense in locusts and bushcrickets: the responses of ventral-cord neurons. J Insect Physiol 28(7):615–623
Kühne R, Silver S, Lewis B (1984) Processing of vibratory and acoustic signals by ventral cord neurones in the cricket Gryllus campestris. J Insect Physiol 30(7):575–585
Lane DH (1995) The recognition concept of species applied in an analysis of putative hybridization in New Zealand cicadas of the genus Kikihia (Insecta: Homoptera: Tibicinidae). In: Lambert DM, Spencer HG (eds) Speciation and the recognition concept: theory and application. John Hopkins University Press, Baltimore, pp 367–421
Löhe G, Kleindienst H-U (1994) The role of the medial septum in the acoustic trachea of the cricket Gryllus bimaculatus. II. Influence on directionality of the auditory system. J Comp Physiol A 174:601–606
Marshall DC, Cooley JR (2000) Reproductive character displacement and speciation in periodical cicadas, with description of a new species, 13-year Magicicada neotredecim. Evolution 54(4):1313–1325
Marshall DC, Slon K, Cooley JR, Hill KBR, Simon C (2008) Steady plio-pleistocene diversification and a 2-million-year sympatry threshold in a New Zealand cicada radiation. Mol Phylog Evol 48:1054–1066
Mason AC (1991) Hearing in a primitive ensiferan: the auditory system of Cyphoderris monstrosa (Orthoptera: Haglidae). J Comp Physio A 168:351–363
Michel K (1975) Das tympanalorgan von Cicada orni L. (Cicadina, Homoptera). Eine Licht- und Elektronenmikroskopische Untersuchung. Zoomorphologie 82:63–78
Michelsen A (1985) Time resolution in auditory systems. Springer, New York
Michelsen A (1998) Biophysics of sound localization in insects. In: Hoy RR, Popper AN, Fay RR (eds) Comparative hearing: insects. Springer, New York, pp 18–62
Michelsen A, Larsen ON (2008) Pressure difference receiving ears. Bioinsp Biomim 3(011001):18
Michelsen A, Löhe G (1995) Tuned directionality in cricket ears. Nature 375:639
Moore TE, Sawyer RT (1966) The mechanism of cicada timbal action (Insecta: Homoptera: Cicadidae). Am Zool 5:509
Morse PM, Ingard KU (1987) Theoretical acoustics. Princeton University Press, New Jersey 949p
Moulds MS (1990) Australian Cicadas. New South Wales University Press, New South Wales
Moulds MS (2005) Song Analyses of Cicadas of the genera aleeta moulds and tryella moulds (Hemiptera: Cicadidae). Proc Linn Soc New South Wales 126:133–142
Münch D (1999) Frequenz- und zeitverarbeitung durch thorakale auditorische interneurone bei zikaden (tettigetta josei). Diploma dissertation, Humboldt-University, Berlin
Myers JG (1928) The morphology of the Cicadidae. Proc Zool Soc Lond 25:365–472
Popov AV (1975) The structure of the tymbals and the characteristics of the sound signals in singing cicadas (Homoptera, Cicadidae) in the southers regions of the USSR. Entomol Obozr 54:258–291
Popov AV (1981) Sound production and hearing in the cicada Cicadetta sinuatipennis Osh. (Homoptera: Cicadidae). J Comp Physiol 142:271–280
Popov AV (1990) Co-evolution of sound-production and hearing in insects. In: Gribakin FG, Wiese K, Popov AV (eds) Sensory systems and communication in arthropods, advances in life sciences. Birkhäuser, Berlin, pp 301–304
Popov AV, Aronov IB, Sergeeva MV (1985) Calling songs and hearing in cicadas from Soviet Central Ásia. Zh Evol Biokh Fiziol 21:288–298
Pringle JWS (1954) A physiological analysis of cicada song. J Exp Biol 31:525–560
Prinz P, Ronacher B (2002) Temporal modulation transfer functions in auditory receptor fibres of the locust (Locusta migratoria L.). J Comp Physiol A 188:577–587
Richards DG, Wiley RH (1980) Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am Nat 115(3):381–399
Robertson RM, Pearson KG (1982) A preparation for the intracellular analysis of neuronal activity during flight in the locust. J Comp Physiol 146:311–320
Robertson RM, Pearson KG (1984) Interneuronal organization in the flight system of the locust. J Insect Physiol 30(1):95–101
Ronacher B, Stumpner A (1988) Filtering of behaviourally relevant temporal parameters of a grasshopper’s song by an auditory interneuron. J Comp Physiol A 163:517–523
Simmons PJ (1977) Neuronal generation of singing in a cicada. Nature 270:243–245
Simmons PJ, Young D (1978) The tymbal mechanism and song patterns of the bladder cicada Cystosoma saundersii. J Exp Biol 76:27–45
Simões PC, Quartau JA (2006) Selective responsiveness in males of Cicada orni to conspecific and allospecific calling songs (Hemiptera: Cicadidae). Entomol Gener 29(1):47–60
Stokes DR, Josephson RK (2004) Power and control muscles of cicada song: structural and contractile heterogeneity. J Comp Physiol A 190:279–290
Stölting H, Moore TE, Lakes-Harlan R (2002) Substrate vibrations during acoustic signaling in the cicada Okanagana rimosa. J Insect Science 2(2):1–7
Sueur J, Aubin T (2002) Acoustic communication in the palaearctic red cicada, Tibicina haematodes: chorus organization, calling-song structure, and signal recognition. Can J Zool 80:126–136
Sueur J, Aubin T (2003) Specificity of cicada calling songs in the genus Tibicina (Hemiptera: Cicadidae). Syst Entomol 28:481–492
Sueur J, Puissant S, Simões PC, Seabra S, Boulard M, Quartau JA (2004) Cicadas from Portugal: revised list of species with eco-ethological data (Hemiptera: Cicadidae). Insect Syst Evol 35:177–187
Sueur J, Windmill JFC, Robert D (2006) Tuning the drum: the mechanical basis for frequency discrimination in a mediterranean cicada. J Exp Biol 209:4115–4128
Sueur J, Vanderpool D, Simon C, Ouvrard D, Bourgoin T (2007) Molecular phylogeny of the genus Tibicina (Hemiptera: Cicadicae): rapid radiation and acoustic behaviour. Biol J Linn Soc 91:611–626
Sueur J, Windmill JFC, Robert D (2008) Sexual dimorphism in auditory mechanics: tympanal vibrations of Cicada orni. J Exp Biol 211:2379–2387
Sueur J, Windmill JFC, Robert D (2010) Sound emission and reception tuning in three cicada species sharing the same habitat. J Acoust Soc Am 127(3):1681–1688
Tougaard J (1996) Energy detection and temporal integration in the noctuid A1 auditory receptor. J Comp Physiol A 178:669–677
Tougaard J (1998) Detection of short pure-tone stimuli in the noctuid ear: what are temporal integration and integration time all about? J Comp Physiol A 183:563–572
Trilar T, Gogala M, Popa V (2006) Contribution to the knowledge of the singing cicadas (Auchenorrhyncha: Cicadoidea) of Romania. Acta Entomol Slovenica 14(2):175–182
Vogel R (1923) Ube rein tympanales sinnesorgan, das mutmassliche hororgan der singzikaden. Z Anat Entwickl-Gesh 67:190–231
von Helversen O (1979) Angeborenes erkennen akustischer schlüsselreize. Verh Dtsch Zool Ges 1979:42–59
Weber T, Moore TE, Huber F, Klein U (1988) Sound production in periodical cicadas (Homoptera: Cicadidae: Magicicada septendecim, M. cassini). In: Proceedings 6th auchen meeting, Turin, 7–11 Sept 1987, pp 329–336
Wohlers D, Bacon J (1980) Sexual dimorphism of motoneurons: timbal muscle innervation in male periodical cicadas and homologous structures in females. Cell Tissue Res 209:371–382
Wohlers D, Williams JDL, Huber F, Moore TE (1979) Central projections of fibers in the auditory and tensor nerves of cicadas (Homoptera: Cicadidae). Cell Tissue Res 203:35–51
Young D (1972a) Neuromuscular Mechanism of Sound Production in Australian Cicadas. J Comp Physiol 79:343–362
Young D (1972b) Analysis of songs of some Australian cicadas (Homoptera: Cicadidae). J Aust Ent Soc 11:237–243
Young D (1990) Do cicadas radiate sound through their ear-drums? J Exp Biol 151:41–56
Young D, Bennet-Clark HC (1995) The role of the tymbal in cicada sound production. J Exp Biol 198:1001–1019
Young D, Hill KG (1977) Structure and function of the auditory system of the cicada, Cystosoma saundersii. J Comp Physiol 117:23–45
Young D, Josephson RK (1983a) Mechanisms of sound-production and muscle contraction kinetics in cicadas. J Comp Physiol 152:183–195
Young D, Josephson RK (1983b) Pure-tone songs in cicadas with special reference to the genus Magicicada. J Comp Physiol 152:197–207
Acknowledgments
I thank Berthold Hedwig, John Cooley, Sofia Seabra, Maria Clara Amorim and Axel Michelsen for their comments that improved the manuscript, and José Feijó for the confocal microscope images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Fonseca, P.J. (2014). Cicada Acoustic Communication. In: Hedwig, B. (eds) Insect Hearing and Acoustic Communication. Animal Signals and Communication, vol 1. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-40462-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-642-40462-7_7
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-40461-0
Online ISBN: 978-3-642-40462-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)