Abstract
Acute kidney injury (AKI) is frequently encountered in the older patients. While some of the increased susceptibility to the development of AKI in older patients can be attributed to clinical variables such as underlying comorbid conditions and exposure to multiple potentially nephrotoxic medications and procedures, specific structural, functional, hemodynamic, and cellular changes occur with aging that predispose the kidney to injury when subjected to stress. Recent studies have identified key pathways that could offer potential therapeutic targets to reduce susceptibility to injury as well as to increase the potential for restorative repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Glomerular Filtration Rate
- Acute Kidney Injury
- Renal Vascular Resistance
- Nephrotoxic Medication
- Renal Artery Occlusion
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
FormalPara Key Messages-
1.
The aging kidney undergoes specific structural and functional changes that lead to increased susceptibility to injury when exposed to toxic insults.
-
2.
Cellular and molecular changes also account for increased susceptibility to injury and include reduced regenerative capacity, changes in antioxidant defenses, alterations in growth factors, telomere shortening, mitochondrial changes, and increases in apoptosis.
-
3.
These structural, functional, cellular, and molecular changes also impair repair processes, meaning that the injured kidney may not recover completely and older patients suffering from acute kidney injury may have a greater likelihood of developing chronic kidney disease or end-stage renal disease.
5.1 Introduction
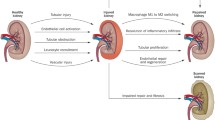
Acute kidney injury (AKI) is frequently encountered in the older patients [1–7]. While some of the increased susceptibility to the development of AKI in older patients can be attributed to clinical variables such as underlying comorbid conditions and exposure to multiple potentially nephrotoxic medications and procedures (many of which can be addressed with careful attention to risk factors and avoidance of toxic insults), specific structural, functional, hemodynamic, and cellular changes occur with aging that predispose the kidney to injury when subjected to stress (summarized in Fig. 5.1). An understanding of these changes in the kidney with aging is critical in allowing for rational design of novel preventative and therapeutic strategies in AKI.
5.2 Structural Alterations in the Pathogenesis of AKI (Box 5.1)
In the absence of a specific disease such as hypertension or diabetes mellitus, the kidney undergoes age-dependent structural changes that ultimately lead to a significant decrease in renal mass and functioning nephron numbers [8, 9]. Wald demonstrated that there is a 19 % decline in male and 9 % decline in female kidney weight in individuals aged 70–79 years as compared with those 20–29 years of age [10]. Importantly, the loss of renal mass is primarily cortical, with relative sparing of the medulla, and the number of functioning glomeruli declines roughly in parallel with the changes in renal weight [11–13]. Thus, the incidence of sclerotic glomeruli rises with advancing age, increasing from less than 5 % of the total at the age of 40, to 10–30 % of the total glomeruli by the eighth decade [14, 15]. On renal histology, glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis often occur together (termed nephrosclerosis when two or more such changes are present) and become more common with aging [16]. The prevalence of nephrosclerosis was 2.7 % for patients aged 18–29 years, 16 % for patients aged 30–39 years, 28 % for patients aged 40–49 years, 44 for patients aged 50–59 years, 58 % for patients aged 60–69 years, and 73 % for patients aged 70–77 years [16]. These microanatomical changes of tubular atrophy and glomerulosclerosis with aging may account for the macroanatomical reduction in kidney size by 10 % per decade of age seen on the computed tomographic scans of adults [17].
There are also important renal vascular changes that occur with aging: intimal thickening, capillary dropout, dysfunctional responses to the autonomic nervous system, and atherosclerosis. These vascular changes may contribute to the process of nephrosclerosis through relative ischemia of the renal parenchyma [18, 19]. One could hypothesize that in the setting of hypoxic and hemodynamic stress such as with sepsis or cardiac failure that these vascular changes may render the renal tubules more susceptible to injury than would be expected in a “younger” kidney with more vascular reserve.
Apoptosis, or programmed cell death, is important in the depletion in the number of cells that occur with aging [20–23]. Not only is there an age-related increase in apoptosis, but under stress conditions (e.g., ischemia/reperfusion injury) the number of apoptotic cells is greater in aged rats as compared to younger animals [22]. This increased basal rate of apoptosis may, in part, also increase the risk for any nephrotoxic insult to result in irreversible cell death.
Loss of glomerular and peritubular capillary in the aging kidney correlated with alterations in vascular endothelial growth factor and with the development of glomerulosclerosis and tubulointerstitial fibrosis [24]. Impaired angiogenesis associated with progressive loss in renal microvasculature and may thus also have a pivotal role in age-related nephropathy. Also, serum levels of epidermal growth factor (EGF) as well as responsiveness of EGF receptors are decreased with aging in various cell types [25, 26]. As functional EGF receptor activity is an essential component of the kidney’s ability to recover from acute injury, there is first evidence of impaired repair and cell survival in the aging kidney linked to renal growth factors.
The link between parenchymal loss and structural nephron changes in the aging kidney and a higher susceptibility to acute damage is somewhat tenuous and not clearly delineated. For example, a substantial reduction in renal mass surprisingly protected against ischemia/reperfusion injury in a 5/6 nephrectomy model [27]. Thus, it may be that other alterations that occur with aging may be more important than simply a loss in nephron numbers. Furthermore, there is little correlation between the amount of nephrosclerosis and actual glomerular filtration rate, suggesting that other factors may be operative [16].
Box 5.1 Anatomical and Functional Changes of the Aging Kidney
1. Structural changes |
1.1. Glomerular changes |
Glomerulosclerosis |
Tubular atrophy/interstitial fibrosis |
Increase in glomerular basement membrane (GBM) permeability |
Progressive folding and thickening of the GBM |
1.2. Vascular changes |
Arteriosclerosis/vascular sclerosis (fibrointimal hyperplasia) |
Intimal thickening |
Medial hypertrophy |
Arteriolar hyalinosis |
Microvasculopathy |
1.3. Tubulointerstitial changes |
Tubular atrophy |
Decreased volume and length of proximal tubules |
Increased number of diverculi of the distal convoluted tubule |
2. Functional changes |
Decreased GFR (not clearly explained by changes in glomerular size, density, or glomerulosclerosis) |
Decrease in afferent arteriolar resistance > rise in glomerular capillary pressure |
Decreasing ultrafiltration coefficient associates with increased glomerular capillary pressure |
Decreased autoregulatory capacity and decreased functional reserve |
5.3 Renal Functional Changes Associated with Aging (see Box 5.1)
With renal senescence, there is a variable decrease in glomerular filtration rate (GFR) [28–31]. Rowe and others demonstrated a reduction in creatinine clearance with age, beginning at age 34 and accelerating after age 65 (an approximate 1 ml/min per 1.73 m2 per year decline in GFR occurring after age 50). Another study from the Mayo Clinic on living donors demonstrated a linear GFR decline of 6.3 ml/min/1.73 m2 per decade of age [16]. Of critical importance is that this age-related decline in GFR is not predictable nor an inevitable consequence of aging. For example, 35 % of elderly subjects had a stable creatinine clearance over 20 years [31]. Why are some elderly individuals able to maintain GFR, while others have variable but inexorable declines in kidney function [32]? Some factors that may account for this variable decline in GFR associated with aging include: (1) racial differences (African-Americans have a faster decline in creatinine clearance as compared to Caucasians) [33]; (2) ill-defined genetic factors that may impact on cellular and molecular pathways involved in aging; (3) underlying comorbid conditions (and their treatment) such as hypertension, heart failure, diabetes mellitus, and vascular disease; and (4) environmental factors such as exposure to nephrotoxins (lead, heavy metals).
It is important to realize that a rise in serum creatinine may not be evident with increasing age despite a decrease in GFR. This is, in part, due to decreases in muscle mass and protein intake with age which directly lower the serum creatinine independently of changes in kidney function [32]. The use of serum creatinine as a surrogate to estimate GFR in the older individuals often overestimates the true creatinine clearance. This is critically important when determining the proper dosing of medications and in the assessment of risk to the aged kidneys from toxic, metabolic, and ischemic events. To some extent this issue is addressed with the use of regression formulas that aim to correct confounding variables on the relationship of serum creatinine to GFR or creatinine clearance. Two of the most common equations in clinical use are the Modification of Diet in Renal Disease (MDRD) equation and Cockcroft-Gault equation [34]. A study investigated the ability of these GFR-estimating equations to predict survival in community-dwelling elderly subjects and demonstrated superiority of the Cockcroft-Gault equation as compared to the MDRD equation [35]. Recently, Schaeffner and colleagues have proposed a new GFR-estimating equation to be specifically utilized in patients aged >70 years [36]. This equation was derived from 610 subjects who were greater than 70 years old and used iohexol clearance as the gold standard. The Berlin Initiative Study (BIS) equation worked particularly well in classifying patients with mild to modest kidney function [36]. More data is needed in determining which estimated GFR equation performs the best in the older patient.
One of the most critical issues in determining estimated GFR is that it allows risk stratification of patients that may be exposed to nephrotoxic medications or procedures. In all cases, the worse the baseline GFR, the higher the risk of acute kidney injury [37]. The estimated GFR allows for the identification of high-risk individuals and designing strategies to protect older patients from the development of AKI. Reliance simply on serum creatinine may not uncover these high-risk patients.
5.4 Renal Hemodynamics and Decreases in Functional Reserve in Aging
As described, there are decreases in renal mass with aging and under normal conditions; these changes may be functionally compensated for by adaptations in renal hemodynamics that maintain a sufficient GFR. These compensatory changes may be lacking in the aging kidney [38]. A common test of renal hemodynamic reserve is the ability of the kidney vasculature to vasodilate in response to intravenous amino acids or a high-protein meal. Fliser et al. compared renal hemodynamics before and after an amino acid infusion in healthy normotensive young (median age 26 years) and older subjects (median age 70 years) and demonstrated that the increase in renal blood flow in the elderly was markedly impaired with a much higher renal vascular resistance in the elderly group [39]. This finding has been confirmed by others using various other techniques in healthy elderly individuals and has demonstrated that advancing age is associated with a decrease in baseline renal blood flow (RBF) [39–41]. More previous work has demonstrated that RBF is maintained through approximately the fourth decade; thereafter, there is a 10 % decline per decade [40–42]. This decrease in RBF is greater than can be accounted for by simply loss of renal mass [43]. A partial explanation for the increases in renal vascular resistance and fall in RBF with age may be the increased irregularity and tortuosity of the preglomerular vessels that occurs with aging [44]. Functionally, the increase in renal vascular resistance associated with the fall in RBF may indicate that the aged kidney is compensating for underlying glomerulosclerosis to maintain GFR through efferent arteriolar vasoconstriction.
Changes in vascular response to vasoconstrictor and vasodilating substances may be critical in accounting for the fall in renal function with aging [39]. Renal sympathetic-mediated vasoconstriction appears to be exaggerated in the aging kidney, and there is poor response to vasodilatory mediators such as atrial natriuretic peptide (ANP) and prostacyclin (PGI2) [45–47]. Furthermore, most studies support that the aging renal vasculature appears to exhibit exaggerated angiotensin II-mediated vasoconstriction [48, 49]. This sensitivity to angiotensin II can lead to exaggerated vasodilation in response to angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs) and perhaps a higher risk for fall in GFR when these drugs are used, especially in the setting of volume depletion when angiotensin II levels may be high.
It is likely that changes in renal hemodynamics, which maintain GFR in the basal state, may lead to an increased risk for AKI during stress [38]. As an example, during a forced water diuresis, magnetic resonance imaging demonstrated the inability to improve medullary oxygenation in the older subjects as compared to the younger ones [50].
Impairment in nitric oxide (NO) production in the elderly kidney may also lead to an increased risk for AKI [51–54]. Normally, NO has an important role in renal protection from ischemic insults and this protective role may be blunted in the aged kidney [55]. Interestingly, older rats fed l-arginine for 7 days prior to renal artery occlusion have a marked improvement in GFR and renal plasma flow with a decrease in renal vascular resistance as compared to rats receiving placebo when exposed to ischemic insult [56]. A strategy for renal protection of the elderly kidney is suggested by recent experimental evidence suggesting that statin use may increase NO production and mitigate the pronounced decrease in GFR and renal blood flow seen in a model of renal artery occlusion in older experimental animals [57]. Two retrospective studies analyzed the effect of preoperative statin use in more than 100,000 patients undergoing cardiac surgery [58, 59]. Huffmyer and colleagues found an age-dependent decrease in the risk of renal replacement therapy, however with no decreases in the risk of AKI [58]. Across various AKI definitions, statin use was consistently associated with a decreased risk with adjusted odds ratios varying from 0.74 to 0.80 [59].
These findings are not in line with a pilot double-blind randomized controlled trial in 100 cardiac surgical patients (with more than 50 % being older than 70 years) at increased risk of postoperative AKI demonstrating that short-term perioperative statin use was not associated with a reduced incidence of postoperative AKI [60]. Selecting all randomized controlled trials comparing any statin treatment before cardiac surgery to no preoperative statin therapy or placebo, a recent Cochrane review concluded that preoperative statin therapy reduces the risk of postoperative atrial fibrillation and shortens the stay on the intensive care unit and in the hospital; however, statin pretreatment had no influence on perioperative mortality, stroke, myocardial infarction, or AKI [61].
The mechanism for decreased NO production in aged kidneys is, in part, due to an age-associated increase in the levels of N(G)-asymmetric dimethylarginine (ADMA), an endogenous NO synthetase inhibitor [62]. There was a direct and independent correlation with age-related increases in ADMA levels and falls in effective renal plasma flow that occur with aging [62].
5.5 Cellular and Molecular Changes Associated with Aging
Aging renal cells may be more vulnerable to damaging insults due to changes in cellular function that decrease their ability to withstand stress.
For instance, telomere length decreases in the aging renal cortex and may be a marker of a limited survival capability for this cell population and impair the ability to regenerate injured cells [63–65]. In an animal experiment using renal ischemia/reperfusion injury as AKI-triggering event, critical telomere shortening in the kidney led to increased senescence and apoptosis, limiting regenerative capacity in response to injury [65].
In addition, there is increased expression of messenger RNA (mRNA) and proteins associated with senescence including the cell-cycle inhibitor p16INK4a, p53, cyclooxygenases 1 and 2, transforming growth factor β-1, and heat shock protein A5 [66].
Microarray analysis from renal tissue harvested from aged animals has been analyzed to assess the response to ischemic injury and investigate the differences in gene expression between young and older animals [67, 68]. The expression of 92 genes was changed by aging (either increased or decreased) including claudin-7, kidney injury molecule-1, zinc-α(2)-glycoprotein (Zag), and matrix metalloproteinase-7. [67, 68]. Zag had been previously implicated in epithelial cell proliferation inhibition. Thus, the increased expression of Zag in the aged kidneys may mechanistically explain some of the increased susceptibility of aged kidneys to nephrotoxic insults [68]. This approach of identifying key injury-response genes that are altered during the aging process yields potential mechanistic targets for therapeutic approaches in the future.
Another change that occurs with aging is increased vulnerability to ischemic damage secondary to decreases in cellular antioxidant defenses [69]. Studies have demonstrated both an increase in free radical generation and a deficiency of antioxidant enzymes in aging renal tissue in response to increased oxidative stress and a concomitant higher propensity for more severe damage [70, 71]. An example of this is a study in aged rats, where there were increased markers of oxidative and lipid peroxidation, isoprostanes, advanced glycosylation end products, and heme oxygenase induction [72]. Interestingly, these markers of oxidative stress decreased with antioxidant treatment (high-dose vitamin E) [72]. Vitamin E has also been shown to be protective in an ischemia/reperfusion model of renal injury [73].
Peroxisome proliferator-activated receptor (PPAR)-γ agonist may ameliorate aging-related progressive renal injury in an animal experiment [74]. The use of PPAR-γ agonist reduced systemic and renal oxidative stress, attenuated mitochondrial injury, reduced proteinuria, and improved GFR. Pioglitazone, a synthetic PPAR-γ agonist, reduced markers of oxidative stress in a renal IR injury model in rats [75] and in an animal model of drug-induced nephrotoxicity [76].
Another interesting observation is that caloric restriction suppresses age-related oxidative stress as well as the susceptibility to ischemic injury [77, 78]. Restriction of calories is more powerful than any other specific dietary manipulation (protein or lipid restriction) in preserving renal function in senescent rodents [79, 80]. Not only does this retard the onset of chronic progressive nephrosclerosis, but it completely prevents the development of renal failure in very old animals [81–83]. Mechanistically, caloric restriction may act through increasing the levels of a group of proteins termed sirtuins [84]. Sirtuins are members of the silent information regulator 2 (Sir2) family, a family of Class III histone/protein deacetylases that are increased in expression after caloric restriction [85, 86]. SIRT1 deacetylates a large number of transcriptional factors and cofactors involved in cell growth, differentiation, stress resistance, reducing oxidative damage, and metabolism [86]. SIRT1 levels decrease in the aged kidneys which is associated with increased mitochondrial oxidative stress and morphological changes in mitochondria [87]. Resveratrol, a plant polyphenol, is a potent activator of SIRT1 activity and has been shown to have renal protective effects in several nephrotoxic and ischemic model systems [88, 89].
Further, both local and systemic and direct and indirect pathways are involved in processes of age-related fibrosis and are impacted by bone marrow-derived cells. Young bone marrow alleviates renal aging, including decreasing deposition of collagen IV in the mesangium and less β-galactosidase staining, an indicator of cell senescence [90].
Angiopoietin 2, an autocrine activator of endothelial cells, seems to be increased in older mice priming the endothelial cells for an exaggerated response to a second hit, e.g., an inflammatory stimulus [91].
5.6 Is Renal Repair Impaired in Older Individuals?
In general, one of the hallmarks of aging is impairment in the ability to repair and regenerate injured cells. In the aging kidney, a decline of renal progenitor cells, a reduction of the peritubular capillary – the area that supplies renal tubules with oxygen – and a decrease in the degree of DNA synthesis in renal tubules after injury were observed.
This impairment in repair processes can be reflected in several ways: (1) minor insults may lead to cumulative damage that normally would have been repaired, (2) AKI may be more prolonged due to impaired healing, and (3) AKI may never recover and lead to end-stage renal disease.
Several clinical observations support the importance of defects in renal repair in clinical important outcomes, for example, (1) kidneys that are harvested from donors above age 65 years suffer from a rate of delayed graft function (essentially ischemic acute tubular necrosis) that is twice as high as that seen in younger donors [92], (2) older patients suffering an episode of AKI have a 13-fold higher relative risk of developing end-stage renal disease (this number rises to 41.2-fold if patients have baseline chronic kidney disease) [93], and (3) a recent meta-analysis of recovery rates of kidney function after AKI in the older patients has demonstrated that recovery after AKI is approximately 28 % less likely to occur when the patient is older than 65 years [94].
This loss of proliferative potential will likely lead to significant impairment in the repair processes and increased susceptibility to cumulative stresses.
5.7 Summary and Implications for Therapy
Older patients are at higher risk for the development of AKI and the likelihood of complete renal recovery is impaired. This is due to age-related changes in kidney structure, cellular and molecular function, and hemodynamic reserve. Recent studies have identified key pathways that could offer potential therapeutic targets to reduce susceptibility to injury as well as to increase the potential for restorative repair. For instance, therapeutic activation of the sirtuin pathways or antioxidant defenses may hold promise but require much experimental work. Currently, therapy rests on prevention of ischemic and nephrotoxic insults, and where these insults are unavoidable or unanticipated, we must focus on limiting exposure and restoring normal hemodynamics. Given the anticipated growth in the elderly population, devising strategies for nephroprotection and therapy for AKI will be critical in improving outcomes.
References
Feest TJ, Round A, Hamad S (1993) Incidence of severe acute renal failure in adults: results of a community-based study. BMJ 306:481–489
Turney JH, Marshall DH, Brownjohn AM, Ellis CM, Parsons FM (1990) The evolution of acute renal failure. QJM 74:83–89
Groeneveld ABJ, Tran DD, Van der Meulen J, Nauta JJ, Thijs LG (1991) Acute renal failure in the intensive care unit: predisposing, complicating factors affecting outcome. Nephron 59:602–607
Pascual J, Orofino L, Liano F, Marcén R, Orte L, Ortuño J (1990) Incidence and prognosis of acute renal failure in older patients. J Am Geriatr Soc 38:25–32
Ali T, Khan I, Simpson W et al (2007) Incidence and outcome in acute kidney injury: a comprehensive population-based study. J Am Soc Nephol 18:1292–1298
Macias-Nunez JF, Lopez-Novoa JM, Martinez-Maldonado M (1996) Acute renal failure in the aged. Semin Nephrol 16:330–342
Jerkic M, Vojvodic S, Lopez-Novoa JM (2001) The mechanism of increased renal susceptibility to toxic substances in the elderly. Int Urol Nephrol 32:539–547
Choudhury DRD, Levi M (2004) Effect of aging on renal function ad disease. In: Brenner B (ed) Brenner and Rector’s the kidney, 7th edn. Saunders, Philadelphia, p 2305
Esposito C, Plati A, Mazzullo T et al (2007) Renal function and functional reserve in healthy elderly individuals. J Nephrol 20:617–623
Wald H (1937) The weight of normal adult human kidneys and its variability. Arch Pathol Lab Med 23:493–500
Tauchi H, Tsuboi K, Okutomi J (1971) Age changes in the human kidney of the different races. Gerontologia (Basel) 17:87–97
McLachlan MSF (1978) The aging kidney. Lancet 2:143–145
Goyal VK (1982) Changes with age in the human kidney. Exp Gerontol 17:321–331
Kaplan C, Pasternack B, Shah H, Gallo G (1975) Age-related incidence of sclerotic glomeruli in human kidneys. Am J Pathol 80:227–234
Kappel B, Olsen S (1980) Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney related to age and sex. Virchows Arch 387:271–277
Rule AD, Amer H, Cornell LD et al (2010) The association between age and nephrosclerosis on renal biopsy among healthy adults. Ann Intern Med 152:561–567
Gourtsoyiannis N, Prassopoulos P, Cavouras D, Pantelidis N (1990) The thickness of the renal parenchyma decreases with age: a CT study of 360 patients. AJR Am J Roentgenol 155:541–544
Musso CG, Oreopoulos DG (2011) Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol 119(suppl 1):1–5
Thomas SE, Anderson S, Gordon KI, Oyama TT, Shankland SJ, Johnson RJ (1998) Tubulointerstitial disease in aging: evidence for underlying peritubular capillary damage, a potential role for renal ischemia. J Am Soc Nephrol 9:231–242
Ma LJ, Nakamura S, Whitsitt JS et al (2000) Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 58:2425–2436
Qiao X, Chen X, Wu D et al (2005) Mitochondrial pathway is responsible for aging-related increase of tubular cell apoptosis in renal ischemia/perfusion injury. J Gerontol A Biol Sci Med Sci 60:830–839
Lee JH, Jung KJ, Kim JW et al (2004) Suppression of apoptosis by calorie restriction in aged kidney. Exp Gerontol 39:1361–1368
Zhang JH, Zhang Y, Herman B (2003) Caspases, apoptosis and aging. Ageing Res Rev 1:357–366
Kang DH, Anderson S, Kim YG et al (2001) Impaired angiogenesis in the aging kidney: vascular endothelial growth factor and thrombospondin-1 in renal disease. Am J Kidney Dis 37:601–611
Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE (2007) Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine 39:123–129
Tran KT, Rusu SD, Satish L, Wells A (2003) Aging-related attenuation of EGF receptor signaling is mediated in part by increased protein tyrosine phosphatase activity. Exp Cell Res 289:359–367
Vercauteren SR, Ysebaert DK, De Greef KE, Eyskens EJ, De Broe ME (1999) Chronic reduction in renal mass in the rat attenuates ischemia/reperfusion injury and does not impair tubular regeneration. J Am Soc Nephrol 10:2551–2559
Epstein M (1996) Aging and the kidney. J Am Soc Nephrol 7:1106–1122
Andres R (1969) Physiological factors of aging significant to the clinician. J Am Geriatr Soc 17:274–277
Rowe JW, Andres R, Tobin JD, Norris AH, Shock NW (1976) The effect of age on creatinine clearance in man: a cross-sectional and longitudinal study. J Gerontol 31:155–163
Lindeman RD, Tobin JD, Shock NW (1985) Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33:278–285
Lew SQ, Bosch JP (1991) Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J Am Soc Nephrol 2:856–865
Luft FC, Fineberg NS, Miller JZ, Rankin LI, Grim CE, Weinberger MH (1980) The effects of age, race and heredity on glomerular filtration rate following volume expansion and contraction in normal man. Am J Med Sci 279:15–25
Levey AS, Coresh J, Balk E et al (2003) National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification and stratification. Ann Intern Med 139:137–146
Pizzarelli F, Lauretani F, Bandinelli S et al (2009) Predictivity of survival according to different equations for estimating renal function in community-dwelling elderly subjects. Nephrol Dial Transplant 24:1197–1205
Schaeffner ES, Ebert N, Delanaye P et al (2012) Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 157:471–481
Thakar CV, Arrigain S, Whorley S, Yared YP, Paganini EP (2005) A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16:162–168
Lameire N, Hoste E, Van Loo A, Dhondt A, Bernaert P, Vanholder R (1996) Pathophysiology, causes and prognosis of acute renal failure in the elderly. Ren Fail 18:333–340
Fliser D, Zeler M, Nowack R, Ritz E (1993) Renal functional reserve in healthy elderly subjects. J Am Soc Nephrol 3:1371–1377
Davies DF, Shock NW (1950) Age changes in glomerular filtration rate, effective renal plasma flow and tubular excretory capacity in adult males. J Clin Invest 29:496–506
Miller JH, McDonald RK, Shock NW (1951) The renal extraction of p-aminohippurate in the aged individual. J Gerontol 6:213–216
Faulstick D, Yiengst MJ, Oussler DA, Schock NW (1962) Glomerular permeability in young and old subjects. J Gerontol 17:40–44
Hollenberg NK, Adams DF, Solomon HS, Rashid A, Abrams HL, Merrill JP (1974) Senescence and the renal vasculature in normal man. Circ Res 34:309–316
Davidson AJ, Talner LB, Downs WM (1969) A study of the angiographic appearance of the kidney in an aging normotensive population. Radiology 92:975–983
Lakatta EG (1993) Cardiovascular regulatory mechanisms in advanced age. Physiol Rev 73:413–420
Moritoki H, Yoshikawa T, Hisayama T, Takeuchi S (1992) Possible mechanisms of age-associated reduction of vascular relaxation caused by atrial natriuretic peptide. Eur J Pharmacol 210:61–67
Sato I, Kaji K, Morita I, Nagao M, Murota S (1993) Augmentation of endothelin-1, prostacyclin and thromboxane A2 secretion associated with in vitro ageing in cultured human umbilical vein endothelial cells. Mech Ageing Dev 71:73–80
Zhang XZ, Qui C, Baylis C (1997) Sensitivity of the segmental renal arterioles to angiotensin II in the aging rat. Mech Ageing Dev 97:183–188
Baylis C (1993) Renal responses to acute angiotensin II inhibition and administered angiotensin II in the ageing, conscious, chronically catheterized rat. Am J Kidney Dis 22:842–849
Prasad PV, Epstein FH (1999) Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int 55:294–303
Reckelhoff JF, Manning RD (1993) Role of endothelium-derived nitric oxide in control of renal microvasculature in aging male rats. Am J Physiol 265(suppl):R1123–R1128
Moncada S, Palmer RM, Higgs EA (1991) Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol Rev 43:109–114
Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC (1994) Changes in nitric oxide precursor, L-arginine and metabolites, nitrate and nitrite with aging. Life Sci 55:1895–1901
Tan D, Cernadas MR, Aragoncillo P et al (1998) Role of nitric oxide-related mechanisms in renal function in ageing rats. Nephrol Dial Transplant 13:594–600
Rivas-Cabanero L, Rodriguez-Barbero A, Arevalo M, López-Novoa JM (1995) Effect of Ng-nitro-L-arginine methyl ester on gentamicin-induced nephrotoxicity in rats. Nephron 71:203–208
Sabbatini M, Sansone G, Uccello F et al (1994) Functional versus structural changes in the pathophysiology of acute ischemic renal failure in aging rats. Kidney Int 45:1355–1362
Sabbatini M, Pisani A, Uccello F et al (2004) Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 15:901–909
Huffmyer JL, Mauermann WJ, Thiele RH, Ma JZ, Nemergut EC (2009) Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 23:468–473
Brunelli SM, Waikar SS, Bateman BT et al (2012) Preoperative statin use and postoperative acute kidney injury. Am J Med 125:1195–1204
Prowle JR, Calzavacca P, Licardi E et al (2012) Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology 17:215–224
Liakopoulos OJ, Kuhn EW, Slottosch I, Wassmer G, Wahlers T (2012) Preoperative stain therapy for patients undergoing cardiac surgery. Cochrane Database Syst Rev (4):CD008493. doi:10.1002/14651858.CD008493.pub2
Kielstein JT, Bode-Boger SM, Frolich JC, Ritz E, Haller H, Fliser D (2003) Asymmetric dimethylarginine, blood pressure, and renal perfusion in elderly subjects. Circulation 107:1891–1899
Melk A, Ramassar V, Helms LM et al (2000) Telomere shortening in kidneys with age. J Am Soc Nephol 11:44–51
Melk A, Kittikowit W, Sandhu I et al (2003) Cell senescence in rat kidneys in vivo increases with growth and age despite lack of telomere shortening. Kidney Int 63:2134–2141
Westhoff JH, Schildhorn C, Jacobi C et al (2010) Telomere shortening reduces regenerative capacity after acute kidney injury. J Am Soc Nephrol 21:327–336
Melk A, Schmidt BM, Takeuchi O, Sawitzki B, Rayner DC, Halloran PF (2004) Expression of p16INK4a and other cell cycle regulator and senescence associated genes in aging human kidney. Kidney Int 65:510–519
Chen G, Bridenbaugh EA, Akintola AD (2007) Increased susceptibility of aging kidney to ischemic injury: identification of candidate genes changed during aging, but corrected by caloric restriction. Am J Physiol Renal Physiol 293:F1272–F1281
Schmitt R, Marlier A, Cantley LG (2008) Zag expression during aging suppresses proliferation after kidney injury. J Am Soc Nephrol 19:2375–2383
Miura K, Goldstein RS, Morgan DG, Pasino DA, Hewitt WR, Hook JB (1987) Age-related differences in susceptibility to renal ischemia in rats. Toxicol Appl Pharmacol 87:284–292
Papa S, Skulachev VP (1997) Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem 174:305–311
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Reckelhoff JF, Kanji V, Racusen LC et al (1998) Vitamin E ameliorates enhanced renal lipid peroxidation and accumulation of F2-isoprostanes in aging kidneys. Am J Physiol 274:R767–R774
Özkaya D, Naziroğlu M, Armağan A et al (2011) Dietary vitamin C and E modulates oxidative stress induced-kidney and lens injury in diabetic aged male rats through modulating glucose homeostasis and antioxidant systems. Cell Biochem Funct 29:287–293
Yang HC, Deleuze S, Zuo Y, Potthoff SA, Ma LJ, Fogo AB (2009) The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 20:2380–2388
Reel B, Guzeloglu M, Bagriyanik A et al (2013) The effects of PPAR-γ agonist pioglitazone on renal ischemia/reperfusion injury in rats. J Surg Res 182(1):176–184. doi:10.1016/j.jss.2012.08.020, pii: S0022-4804(12)00745-7
Jesse CR, Bortolatto CF, Wilhelm EA, Roman SS, Prigol M, Nogueira CW (2012) The peroxisome proliferator-activated receptor-γ agonist pioglitazone protects against cisplatin-induced renal damage in mice. J Appl Toxicol. doi:10.1002/jat.2818 [Epub ahead of print]
Yu BP (1996) Aging and oxidative stress: modulation by dietary restriction. Free Radic Biol Med 21:651–668
Baylis C, Corman B (1998) The aging kidney: insights from experimental studies. J Am Soc Nephrol 9:699–709
Masoro E (1988) Food restriction in rodents, an evaluation in its role in the study of aging. J Gerontol 43:59–64
Kennedy G (1957) Effect of old age and over-nutrition on the kidney. Br Med Bull 13:67–81
Wyndham J, Everitt A, Everitt S (1983) Effect of isolation and food restriction begun at 50 days on the development of age-associated renal disease in the male Wistar rat. Arch Gerontol Geriatr 2:317–332
Tucker S, Mason R, Beauchene R (1976) Influence of diet and feed restriction on kidney function of aging male rats. J Gerontol 31:264–270
Bras G, Ross H (1964) Kidney disease and nutrition in the rat. Toxicol Appl Pharmacol 6:247–252
Allard JS, Perez E, Zou S, de Cabo R (2009) Dietary activators of Sirt1. Mol Cell Endocrinol 299:58–63
Cohen HY, Miller C, Bitterman KJ et al (2004) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305:390–392
Dali-Youcef N, Lagouge M, Froelich S, Koehl C, Schoonjans K, Auwerx J (2007) Sirtuins: the “magnificent seven”, function, metabolism and longevity. Ann Med 39:335–345
Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D (2013) Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci (Lond) 124:153–164
Do Amaral CL, Francescato HD, Coimbra TM et al (2008) Resveratrol attenuated cisplatin-induced nephrotoxicity in rats. Arch Toxicol 82:363–370
Sebai H, Ben-Attia M, Sani M, Aouani E, Ghanem-Boughanmi N (2008) Protective effect of resveratrol on acute endotoxemia-induced nephrotoxicity in rat through nitric oxide independent mechanism. Free Radic Res 42:913–920
Yang HC, Rossini M, Ma L-J, Zuo Y, Ma J, Fogo AB (2011) Cells derived from young bone marrow alleviate renal aging. J Am Soc Nephrol 21:2028–2036
Wulfert FM, van Meurs M, Kurniati NF et al (2012) Age-dependent role of microvascular endothelial and polymorphonuclear cells in lipopolysaccharide-induced acute kidney injury. Anesthesiology 117:126–136
United States Renal Data System (USRDS) (2007) The 2007 USRDS Annual Data Report (ADR) reference tables. http://www.usrds.org/reference.htm
Ishani A, Xue JL, Himmelfarb J et al (2009) Acute kidney injury increases risk of ESRD among the elderly. J Am Soc Nephrol 20:223–228
Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR (2008) Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am J Kidney Dis 52:262–271
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Rosner, M.H., Cruz, D.N., Ronco, C. (2014). Pathogenesis and Susceptibility to Injury. In: Haase, M., Haase-Fielitz, A. (eds) Managing Renal Injury in the Elderly Patient. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39947-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-642-39947-3_5
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39946-6
Online ISBN: 978-3-642-39947-3
eBook Packages: MedicineMedicine (R0)