Abstract
Production of reactive oxygen, chlorine, and nitrogen species is a pivotal and effective mechanism utilized by different immune cells to respond to invading pathogens. During the last decades the molecular pathways involved in the production of reactive species and their intersection with the cellular molecular sensors (nicotinamide adenine dinucleotide phosphate-oxidase, inflammasomes, Toll-like receptor) have been elucidated. At the same time, it has also been recognized that excessive or chronic production of reactive species, as occurred in chronic inflammatory, degenerative, and autoimmune diseases, is detrimental to the immune system. This integrated view of both the physiological and pathological role of reactive species in maintaining the cellular redox balance is coming to light.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reactive Oxygen Species
- Reactive Oxygen Species Production
- NADPH Oxidase
- Reactive Species
- Chronic Granulomatous Disease
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
23.1 Introduction
Reactive oxygen, chlorine, and nitrogen species are generated under physiological conditions by different cellular organelles and molecular pathways. In most cells, reactive species are the by-product of oxidative phosphorylation, the mitochondrial molecular chain which synthesizes adenosine triphosphate (ATP); as such they are readily inactivated in order to prevent cellular oxidative damage. Reactive species are also produced in immune cells by other molecular pathways as an important innate immune mechanism against invading pathogens. Indeed, “damping” phagosome-bound pathogens with reactive species is the most effective and successful way to readily neutralize the pathogen. In this chapter, we will review the different molecular pathways involved in the production of reactive species and how reactive species play a role in innate and adaptive immune responses. Excessive or chronic production of reactive species, as observed in chronic inflammatory and degenerative conditions however, has a negative effect on immune cell function. Herein, we will also review the effects of chronic oxidative stress on dendritic cells, macrophages, granulocytes and T cells, redox homeostasis, and immune cell function.
23.2 Mitochondrial Respiratory Chain
The main function of mitochondria is to convert the energy found in nutrient molecules and to store it in the form of ATP in a process known as oxidative phosphorylation. The energy required to phosphorylate ADP into ATP, by the ATP synthase, is provided by the transfer of electrons through the mitochondria respiratory chain which is coupled with the pumping of protons from the matrix to the inner mitochondrial membrane (Mitchell 1961) (Fig. 23.1). Five protein complexes (I–V) form the respiratory chain. However, two complexes (complex I and III) constitute the site where electron transfer is associated with proton translocation across the mitochondrial membrane (Chance 1961). Complex I (nicotinamide adenine dinucleotide (NADH)-ubiquinone oxidoreductase) and complex III (ubiquinol-cytochrome c oxidoreductase) are the ones that generate the proton gradient required to produce the energy to synthesize ATP (Chance et al. 1979). As by-products of oxidative phosphorylation, the process of proton translocation generates reactive oxygen species (ROS) on both sides of the mitochondrial membrane. A minor source of ROS is also generated by reactions which are part of the Krebs cycle (Starkov et al. 2004).
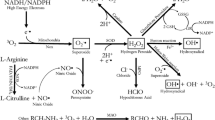
Mitochondrial production of reactive species. The electron transport chain and oxidative phosphorylation are coupled by a proton gradient across the inner mitochondrial membrane. Electrons (e−) from NADH and FADH2 previously generated by glycolysis and citric acid cycle are transported through the five complexes (I–V) of the mitochondrial electron transport chain (MITO-ETC) to generate electrochemical gradient (proton gradient). Q – coenzyme Q (CoQ, Q or ubiquinone); C – cytochrome c; complexes I (NADH dehydrogenase), II ( succinate-CoQ reductase), III (CoQ-cyt c Reductase), IV (Cytochrome Oxidase), V (ATP synthase). The oxygen from a molecule of water receives the electron forming an O2 – superoxide anion and an H+, which accumulate in the intermembrane space. Translocation of H+ across the membrane through the ATP synthase provides the energy to convert ADP and a molecule of inorganic phosphate Pi into ATP. L-arginine and a molecule of oxygen generates nitric oxide radical catalyzed by the enzyme nitric oxide synthase NOS. Additionally, peroxynitrite (ONOO−) formation in mitochondria is due to the constant supply of superoxide radical O2 −• by the electron transport chain plus the facile diffusion of nitric oxide (NO•) to this organelle. To dispose free radicals a molecule of O2 − is converted by the superoxide dismutases (SOD) into oxygen and hydrogen peroxide. Catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen. The glutathione peroxidase (GPx) reduces hydrogen peroxide to water, where GSH represents reduced monomeric glutathione and GS–SG represents glutathione disulfide. Glutathione reductase (GSR) then reduces the oxidized glutathione to complete the cycle
Under physiological conditions cells are equipped with a variety of enzymes to quickly dispose of ROS including superoxide dismutase, glutathione (GSH) peroxidase, and catalase as well as some antioxidant molecules like glutathione and vitamin E (Bai and Cederbaum 2001). However, in pathological conditions and in aging, due to an increased ROS production or decreased activity/synthesis of scavenging enzymes, cells are often unable to deal with the large amount of ROS produced. This will generate a biochemical imbalance, often referred to as “oxidative stress” where ROS molecules will quickly react with any neighboring biomolecules and oxidize them.
Mitochondria are the first target of oxidative damage cause by ROS as evidenced by extensive lipid peroxidation, protein oxidation, and mitochondrial DNA mutations (Anders et al. 2006; Lenaz 1998). Extramitochondrial ROS, on the other hand, will oxidize nuclear DNA, as well as cytosolic and organelle proteins, lipids, and carbohydrates (Turrens 2003; Pickrell et al. 2009). Aging-related oxidative stress and functional impairment of mitochondria have been linked to several chronic inflammatory and degenerative diseases as well as cancer (Pawelec et al. 2010; Muster et al. 2010).
23.3 Oxidative Burst
The importance of ROS and reactive nitrogen species (RNS) in innate immunity was first recognized in professional phagocytes undergoing a “respiratory burst” upon activation (Rada and Leto 2008; Netzer et al. 2009). This robust ROS and RNS production, in particular O2 •− and H2O2, is generated by a membrane-bound, superoxide-generating enzyme, the phagocytic nicotinamide adenine dinucleotide phosphate-oxidase (NADPH). The NADPH oxidase enzyme was discovered in 1957 when it was recognized that patients with chronic granulomatous disease (CGD) failed to generate products of the respiratory burst (Holmes et al. 1967). NADPH oxidase contains six cytochrome subunit homologs: NOX1, NOX3, NOX4, NOX5, DUOX1, and DUOX2 (Guzik and Griendling 2009; Katsuyama et al. 2012) (Fig. 23.2). The superoxide anions (O2 –), formed by NADPH oxidase, are reduced to a more stable molecule of hydrogen peroxide (H2O2) and unstable radicals of oxygen (O−) and hydroxyl (OH•) (Bedard and Krause 2007). While not a free radical, hydrogen peroxide is also an oxidant capable of initiating lipid peroxidation chain reaction (Janero et al. 1991). In the cytosol hydrogen peroxide can be reconverted into hydroxyl radical (OH−), one of the most reactive ROS, through the Fenton reaction (Korantzopoulos et al. 2007) in the presence of a catalyst such as transitional metals (manganese, copper, and iron) (Fisher 2009).
Activation of the NADPH oxidative burst. NADPH oxidase generate free radicals (O2 −•) by transferring electrons from NADPH to molecular oxygen. This oxidase is controlled by hormones, growth factors, and other ligands which bind to receptors in the plasma membrane. NADPH oxidase consists of five subunits: gp91phox (glycoprotein-91 kDa-phagocytic-oxidase-NOX1), p22phox, p47phox-NOX4, p67phox, and p40phox and the GTPase, flavocytochrome b588 (Cyt b558), and Rac. MPO is a peroxidase enzyme which produces hypochlorous acid (HOCl) from H2O2. Catalase catalyzes the decomposition of hydrogen peroxide to water and oxygen. To dispose free radicals a molecule of O2 − is converted by the superoxide dismutases (SOD) into oxygen and hydrogen peroxide. GSR reduces glutathione disulfide (GSSG) in presence of NADPH and H+ to the sulfhydryl form GSH and NADP+
The NADPH oxidase enzyme complex serves many cellular functions requiring ROS generation, including oxygen sensing (Leto et al. 2009) redox-based cellular signaling (Fisher 2009) and biosynthetic processes (Hazen et al. 1996). However, in professional phagocytes the most important NADPH-dependent function is generation of ROS and RNS as a defense mechanism towards pathogen invasion (Winterbourn et al. 2000). ROS and RNS induce oxidative inactivation of microbial enzymes, constitutive proteins, and nucleic acids. In cells of the myelomonocytic lineage (including monocytes and polymorphonuclear leukocytes (PMN)), two additional enzymes can generate reactive species, namely, the myeloperoxidase (MPO) and bromoperoxidase (Bouayed and Bohn 2010). H2O2 produced by inflammatory cells oxidizes myeloperoxidase to a higher oxidation state with a redox potential in excess of 1 V. This higher oxidation state (a ferryl-oxo complex) oxidizes Cl– to HOCl, which is capable of oxidizing or chlorinating pathogen-derived macromolecules (Winterbourn et al. 2000). HOCl also reacts with amines to form chloramines or with Cl– to form Cl2 gas; the latter chlorinates the nucleic acid or protein of invading pathogens (Hazen et al. 1996).
23.4 The Effect of Environment and Pollution on Cellular ROS Production
The link between production of reactive oxygen and nitrogen species and environmental factors such as chemicals, ultraviolet (UV) radiation, tobacco, alcohol, toxins, and pollutants has been established by many (Bouayed and Bohn 2010; Bargagli 2000). Exposure to heavy metals, such as cadmium, cobalt, and lead as well as synthetic polymers like polyester terephthalate (Tang et al. 1993) and polystyrene (Liu et al. 2011) has also been shown to increase ROS production (El-Sonbaty and El-Hadedy 2012). This heterogeneous category of molecules and compounds increases reactive species production by interfering either with the mitochondrial respiratory chain, the membrane NADPH, or the metal catalyzed Fenton reaction (Poli et al. 2004).
23.5 Dendritic Cells
Dendritic cells (DCs) are sparsely but widely distributed migratory cells of bone marrow origin (Steinman 1991). They provide a critical link between the innate and adaptive immune response and they are specialized in the uptake, processing, and presentation of pathogen-derived antigens to T cells. They are the only antigen-presenting cell (APC) that can activate naive T cells (Banchereau and Steinman 1998). DCs can sense pathogen-derived products (pathogen-associated molecular patterns or PAMPs) through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), present at their plasma membrane and in endosomal compartments (Mogensen 2009), as well as through several cytosolic sensors of the inflammasome family (Krishnaswamy et al. 2013).
Observations that ROS play an important role in innate and adaptive immune responses were first made in the 1970s when it was demonstrated that insulin along with insulin-like growth factor-II (IGF-II) receptors (Kadota et al. 1987) and insulin-like growth factor-binding protein-6 (IGFBP-6) (Xie et al. 2005) stimulated cellular H2O2 production (Czech et al. 1974). In a study by Rutault and co-workers, exposure of DCs to H2O2 was found to increase surface expression of human leukocyte antigen (HLA) class I and II (DQ and DR), as well as the co-stimulatory molecules CD40 and CD86 (Rutault et al. 1999). At the same time, H2O2 downregulated molecules involved in antigen capture such as CD32. The experiments also determined that H2O2-treated DCs had an enhanced ability to promote T cell proliferation as compared with untreated cells. This study was among the first to demonstrate that oxygen species are a “danger” signal produced by the inflammatory microenvironment, leading to DCs activation and increased immune responses. Several additional studies confirmed the role played by ROS in activating human and rodent DCs during inflammation (Sheng et al. 2010; Zhong et al. 2013). Using UV-mediated ROS production, a strong upregulation of CD1a, HLA-DR, B7-1, and B7-2 on Langerhans cells has also been reported (Laihia and Jansen 1997).
Accumulating evidence indicates that the ability of ROS to stimulate DCs is in part due to their targeting of signaling molecules and transcription factors (Bianco et al. 2002). Major targets of ROS include protein tyrosine phosphatases (PTP), protein tyrosine kinases (PTK), mitogen-activated protein (MAP) kinases, ion channels, and phospholipases (Droge 2002). The prototype of a redox-sensitive transcription factor is nuclear factor κB (NFκB) which is sequestered in the cytoplasm in a complex with its inhibitor IκB. ROS promote IκB degradation, initiating NFκB nuclear translocation (Mishra et al. 2008). Other redox-sensitive transcription factors include AP-1, c-Jun, and c-Fos.
An additional major pathway which generates inflammation in response to ROS occurs through the NALP3 inflammasome activation (Fig. 23.3). Inflammasomes are NOD-like receptor (NLR) and caspase-1-recruting cytoplasmic multiprotein complexes formed by a NALP protein, the adapter protein apoptosis-associated speck-like protein containing a CARD (ASC), which contains a caspase recruitment domain (CARD) and procaspase-1. Upon assembly they process and activate the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 (Zhou et al. 2010). The NALP3 inflammasome complex is activated by a plethora of PAMPs, as well as danger-associated molecular patterns (DAMP). The NACHT, LRR, and PYD domains-containing protein 3 (NALP3) inflammasome can be activated by elevated concentrations of ROS (Chen and Nunez 2010) through activation of plasma membrane-associated nicotinamide adenine dinucleotide phosphate-oxidase (NADPH) and cytosolic thioredoxin peroxidase (TXP) and elevated concentrations of uric acid and oxidized protein aggregates.
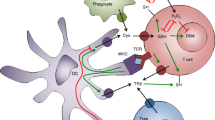
Damage-associated molecular patterns (DAMPs). The arrays of molecular DAMPs recognized by endosomal and plasma membrane TLRs are reported for each receptor. Recognition of each ligand triggers a multitude of signaling cascades, mostly, NFκB and AP-1 mediated, leading to the secretion of pro-inflammatory cytokines including IL-1 β, IL-6, IL-8, and TNFα. Additional DAMPs (iEDAP – g-D-glutamyl-meso-diaminopimelic acid and MDP – muramyl dipeptide) trigger the Nalp3 inflammasome complex (ASC and procaspase-1) via the NOD1 and NOD2, respectively. Reactive oxygen species, uric acid crystals, and protein aggregates induce inflammasome via NADPH activation or thioredoxin reductases (TRX). IL-1 and IL-18 are released following Nalp3 inflammasome activation
The connection between antigen phagocytosis, phagosome formation, antigen processing, and ROS production has been explored by several laboratories (Kotsias et al. 2013). Phagosomal NADPH oxidase (NOX2) was originally shown to be recruited to the DC phagosomes to induce ROS-mediated alkalinization and to decrease antigen processing, favoring cross-presentation. It was later reported that in macrophages, NOX2 mediates the inhibition of phagosomal proteolysis through a pH-independent mechanism, by reversible oxidative inactivation of local cysteine cathepsins (Rybicka et al. 2010) (Fig. 23.4). On the other hand, increased proteolytic activity has been reported in M2 skewed MΦ through inhibition of endosomal/lysosomal NOX2 which increases cathepsin activity (Balce et al. 2011).
The role of the NADPH oxidase in antigen processing. The phagosomal NADPH oxidase (NOX2) mediates the sustained production of low levels of reactive oxygen species. Low ROS levels induce reversible oxidative inactivation of local cysteine cathepsins and decreased ability to reduce disulfides. As a result, the proteolysis, cathepsins activity, and redox potential are reduced
Taken together these reports solidified the notion that ROS production by DCs is an important signaling mechanism that forms part of a rapid induction of innate immune responses. At the same time, however, other reports indicated that a sustained and prolonged ROS production could compromise innate immune responses (Chan et al. 2006). Indeed, chronic ROS production in DCs has been shown to negatively regulate NF-κB phosphorylation in response to lipopolysaccharide (LPS) and zymosan, although it did not compromise activation of p38 MAPK by LPS (Brown and Goldstein 1983). An additional anti-inflammatory effect by ROS production in DCs was recently reported by Jendrysik et al. (2011) who found that NOX2-dependent ROS production in DCs negatively regulated pro-inflammatory IL-12 expression by inhibiting p38-MAPK activity. It has also been proposed that perturbation of DCs function by oxidative stress induces the activation of the Nrf2-mediated pathway, which exerts negative regulatory effects on NFκB signaling, the major pathway involved in IL-12 production, co-stimulatory receptor expression, and DCs maturation. The induction of the Nrf2 pathway, in ROS-stimulated DCs, has also been linked to the induction of a Th1 toTh2 skewing and overall inhibition of Th1 immunity (Kidd 2003).
Altogether, it appears that a dichotomy between the beneficial and detrimental effects of ROS has been observed according to the length of DCs exposure to the reactive species. Indeed, in chronic inflammation, following infection or degenerative conditions, the sustained ROS production appears to be detrimental to DCs functions leading to progressive decline of their biological activities and irreversible damage.
23.6 Macrophages
Macrophages are important effectors of innate immune responses and their primary function is to phagocytose pathogens for degradation and initiate innate immune responses (VanderVen et al. 2010). Macrophages like DCs express the phagocyte NADPH oxidase, a member of NOX family, which generates reactive species to kill microbes.
As reported for other immune cells, prolonged macrophage ROS production, as observed in chronic inflammatory diseases, can be damaging to the macrophages as well as the surrounding tissue. Indeed, macrophage production of reactive carbonyls alters the extracellular matrix (ECM) network and induces tissue damage and cellular apoptosis (Cathcart 2004). Carbonyl modifications of collagens also cause increased macrophage adhesion and activation through receptors that are involved in phagocytosis (Fig. 23.5) including opsonic phagocytosis, mediated by the Fc receptor family (FcγRI, FcγRIIA, and FcγRIIA), complement receptors (CR1, CR3, and CR4), and α5β1 integrin and non-opsonic phagocytosis mediated by Dectin 1, macrophage receptor MARCO, scavenger receptor A, and αVβ5 integrin (Underhill and Goodridge 2012).
Macrophage scavenger receptors bind oxidized lipoproteins. Highly oxidized aggregated LDLs are generated by ROS and different enzymes including SMase, lipases, and MPO. The oxidized aggregated LDLs (acLDL acetylated LDL, oxLDL oxidized LDL, mlDL maleylated LDL) are recognized by macrophage scavenger receptors such as SR-A (class A), CD36 (class B), and CD68 (class C). Scavenger receptor expression is upregulated by cytokines such as TNF-α and IFN-γ
TLRs are a recognition system for PAMPS. However, several oxidatively damaged biomolecules activate the TLR system such as DAMPS including heat shock protein (HSP)60, 70, 72, 22, Gp96, high mobility group box 1 (HMGB1), HMGB1-nucleosome complexes, β-defensin 3, D, eosinophil-derived neurotoxin, antiphospholipid antibodies, serum amyloid A, cardiac myosin, pancreatic adenocarcinoma upregulated factor (PAUF), carboxyethylpyrrole (CEP), monosodium urate crystals, biglycan, versican, hyaluronic acid fragments, surfactant proteins A and D, β-defensin 2, S100A8, S100A9, neutrophil elastase, antiphospholipid antibodies, lactoferrin, oxidized low-density lipoprotein (LDL), saturated fatty acids, resistin, fibronectin EDA, fibrinogen, and tenascin-C heparin sulfate fragments.
Additionally, macrophage scavenger receptors (SR), categorized into class A (SR-A1-SR-A5), class B (SR-B1-SR-B3), and class C (CD68, mucin, and lectin-like oxidized LDL receptor-1), normally function in the recognition and internalization of pathogens and apoptotic cells (Matsumoto et al. 1990) (Fig. 23.5). However, scavenger receptors also recognize altered molecular patterns present on oxidized low-density lipoprotein. For instance, SCARA1 or MSR1 multifunctional, multiligand pattern recognition receptors can bind an extraordinarily wide range of ligands, including bacterial pathogens (Dunne et al. 1994); however, it is also implicated in the pathological deposition of cholesterol during atherogenesis as a result of receptor-mediated uptake of oxidatively modified low-density lipoproteins (mLDL) (Krieger and Herz 1994; Brown and Goldstein 1983).
As a result of the action of ROS and different enzymes including sphingomyelinase (SMase), secretory phospholipase 2 (sPLA2), lipases, and MPO, the highly oxidized aggregated LDL is formed in different cells. The oxidized aggregated LDL is recognized by macrophage scavenger receptors such as SR-A, CD36, and CD68 (Brown and Goldstein 1983).
A representative receptor of class B, the plasma membrane glycoprotein receptor CD163, is a cysteine-rich receptor highly expressed on resident tissue of macrophages which acts as an innate immune sensor and inducer of local inflammation (Fabriek et al. 2009). The CD68 scavenger receptor, whose expression is restricted to mononuclear phagocytes, is a unique SR member, owing to its lysosome-associated membrane protein (LAMP)-like domain and predominant endosomal distribution (Song et al. 2011). Its function was reported to either negatively regulate antigen uptake, loading, or major histocompatibility complex class II (MHC-II) trafficking. Class C receptors recognize lipoprotein (LDL) (acLDL, acetylated LDL; and oxLDL, oxidized LDL), while CD68 recognizes primarily oxLDL.
At a more direct level, both oxidative and carbonyl stress inhibit activity of the transcriptional corepressor HDAC-2 (histone deacetylase 2), which under normoxic conditions helps to suppress pro-inflammatory gene expression (Kirkham 2007). Consequently, macrophages activated under conditions of oxidative or carbonyl stress can lead to a more enhanced inflammatory response via increased production of tumor necrosis factor alpha (TNF-α) and IL-8. Coupled with an impairment of the phagocytic response, this can lead to ineffective clearance of apoptotic cells and secondary necrosis, with the result being failure to resolve the inflammatory response and the establishment of a chronic inflammatory state (Song et al. 2011).
In conclusion, macrophages are phagocytic cells that produce and release ROS in response to phagocytosis or stimulation with various agents. The enzyme responsible for the production of superoxide and hydrogen peroxide is a multicomponent NADPH oxidase that requires assembly at the plasma membrane to function as an oxidase. In addition to participating in bacterial killing, ROS have been implicated in inflammation, in tissue injury, and in modulating cellular function.
23.7 Granulocytes
Granulocytes, discovered by Paul Ehrlich in 1879, are leukocyte subpopulations characterized by the presence of “granules,” which store enzymes and other molecules important in antimicrobial functions and as mediators of inflammatory responses. Granulocytes are divided in subpopulations which include neutrophils, eosinophils, and basophils. Neutrophils, also known as PMN, are the most abundant type of granulocytes.
Neutrophils along with macrophages and dendritic cells play a key role in host defenses against invading microorganisms and have a crucial role in inflammatory processes. Neutrophils infiltrate inflamed tissues, degranulate their secretory vesicles, and release large amounts of bioactive compounds. As early as within the first minutes of stimulation, neutrophilic NADPH oxidase is activated, and cells release large quantities of superoxide anion (O2 −) as part of the so-called respiratory burst (Ciz et al. 2012). Together with NADPH oxidase activation, several oxygen-independent events take place, such as the release of proteolytic enzymes, defensins, myeloperoxidase, and bactericidal peptides from stored intracellular granules. Neutrophils (PMNs) can exist in three different states, namely, resting, primed, and activated (Dang et al. 1999). The priming process has been demonstrated in vitro by pretreating PMNs with a sub-stimulatory concentration of pharmacological agents, which subsequently enhances the PMN response to a second stimulus. Studies show that pro-inflammatory cytokines such as granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-1b, interferon-γ (IFN-g), and IL-8 modulate NADPH oxidase activity through a “priming” phenomenon (Gougerot-Pocidalo et al. 2002). Others reported “priming” by inducing a very weak oxidative response by neutrophils while strongly enhancing neutrophil release of ROS on exposure to a secondary applied stimulus such as bacterial N-formyl peptides (Elbim et al. 1994).
Altogether, the neutrophil oxidative burst is pivotal to pathogen defenses; however, in several chronic inflammatory conditions, it can contribute to tissue oxidative damage. Oxidative burst in rheumatoid arthritis (RA) joints is a result of the activation of innate immune system cells (Newkirk et al. 2003). Activated phagocytic cells such as neutrophils and macrophages produce free radicals in the joint area. Activated phagocytes produce reactive oxidants through the NADPH oxidase and the nitric oxide synthase (NOS). Mechanisms of free radical production differs between these cell groups. While macrophages are stimulated by the NADPH oxidase system to produce free radicals, the presence of NOS accompanied by the NADPH oxidase is necessary for neutrophils to secrete free radicals. RA neutrophils also generate enhanced amount of ONOO− by NOS (El Benna et al. 2002). A similar chronic activation of NADPH oxidase which has been linked to tissue oxidative stress has been shown in diabetes (Omori et al. 2008). It was suggested that hyperglycemia and increased advanced glycation end products prime neutrophils and increase oxidative stress inducing the translocation of p47phox to the cell membrane and preassembly with p22phox by stimulating a RAGE-ERK1/2 pathway (Erlemann et al. 2004).
Eosinophils, another subpopulation of granulocytes, are under homeostatic conditions, mostly found within the mucosal immune system (Straumann and Safroneeva 2012). They are released from hematopoietic stem cells (HSCs) into the peripheral blood in a phenotypically mature state, where they spend a short amount of time before being recruited to mucosal tissue in the lungs, gastrointestinal tract, and urogenital tract (Blanchard and Rothenberg 2009). Under inflammatory conditions, for instance, in chronic asthma or pulmonary eosinophilia, the production of type 2-associated cytokines and chemokines, particularly IL-4, IL-5, IL-13, and eotaxin-1, is increased. Eotaxin, a chemokine that selectively recruits eosinophils by inducing their chemotaxis, was found to prime the production of ROS and play a role in inducing chronic oxidative stress (Honda and Chihara 1999).
While initially similar responses of neutrophils and eosinophils to oxidative burst were reported (Petreccia et al. 1987) by Petreccia et al, a few distinctive features were found indicating substantial differences in the oxidative burst of eosinophils with respect to activation, function, and regulation (Shult et al. 1985). For example, eotaxin specifically induces significantly higher amounts of ROS in eosinophils and induces expression of the well-known eosinophil activator C5a. Eotaxin is also a GM-CSF (Peled et al. 1998).
The synthesis of a potent eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid (5-oxo-ETE) can be enhanced by exposure to H2O2 which induces dramatic increases in the levels of both GSSG and NADP+ (Grant et al. 2011). Its effect on neutrophils, basophils, and monocytes is similar, promoting their infiltration into inflammatory sites (Erlemann et al. 2004). In addition, 5-oxo-ETE was able to induce infiltration of eosinophils into the skin after intradermal injection in humans (Grant et al. 2009) and to promote the survival of tumor cells by blocking the induction of apoptosis by 5-LO (Sundaram and Ghosh 2006). Because of 5-oxo-ETE chemoattractant effects on neutrophils and monocytes, this knowledge could be applied in the future in treatment of atherosclerosis, asthma, ischemia-reperfusion injury, as well as a variety of other inflammatory diseases.
23.8 Conclusions
During physiological conditions, a homeostatic balance between biosynthesis and disposal of oxygen, nitrogen, and chlorine species by immune cells is maintained. As such immune cells can take advantage of the powerful activity of ROS and RSN to kill invading pathogens or to control endosomal antigen processing and quickly dispose them to minimize unwanted oxidative reaction. However, this balance is lost in conditions associated with prolonged cellular stress, including chronic, septic, or aseptic, inflammation and degenerative conditions (Sies and Cadenas 1985); under these circumstances, the increased production/half-life of ROS and RSN will induce oxidative damage to cellular structure and contribute to decreased functionality or even cellular apoptosis (Fariss et al. 2005; Le et al. 2007).
Thus, reactive species can have beneficial effects on immune cells as well as damaging ones according to the experimental system analyzed. This can explain contradictory findings in the same cells analyzed under different experimental conditions or when experiments are performed in young or senescent cells or cells under different stressors. It can also explain why antioxidant therapy can be beneficial or detrimental under different experimental settings. Altogether, the extensive literature on reactive species in immune cells indicates that a more balanced approach is required towards the interpretation of the effects of reactive species on immune functions.
In conclusion, during the last decade several important findings and detailed molecular analysis have been reported on how reactive species are formed and their interaction with different cellular pathways. An integrative view on how the cellular redox system behaves under physiological and pathological conditions and how the same molecular players can have sometimes even opposite function under different stressors is coming to light.
References
Anders MW, Robotham JL, Sheu SS (2006) Mitochondria: new drug targets for oxidative stress-induced diseases. Expert Opin Drug Metab Toxicol 2(1):71–79
Bai J, Cederbaum AI (2001) Mitochondrial catalase and oxidative injury. Biol Signals Recept 10(3–4):189–199
Balce DR, Li B, Allan ER et al (2011) Alternative activation of macrophages by IL-4 enhances the proteolytic capacity of their phagosomes through synergistic mechanisms. Blood 118(15):4199–4208
Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392(6673):245–252
Bargagli R (2000) Trace metals in Antarctica related to climate change and increasing human impact. Rev Environ Contam Toxicol 166:129–173
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313
Bianco AC, Salvatore D, Gereben B et al (2002) Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23(1):38–89
Blanchard C, Rothenberg ME (2009) Biology of the eosinophil. Adv Immunol 101:81–121
Bouayed J, Bohn T (2010) Exogenous antioxidants – double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3(4):228–237
Brown MS, Goldstein JL (1983) Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem 52:223–261
Cathcart MK (2004) Regulation of superoxide anion production by NADPH oxidase in monocytes/macrophages: contributions to atherosclerosis. Arterioscler Thromb Vasc Biol 24(1):23–28
Chan RC, Wang M, Li N et al (2006) Pro-oxidative diesel exhaust particle chemicals inhibit LPS-induced dendritic cell responses involved in T-helper differentiation. J Allergy Clin Immunol 118(2):455–465
Chance B (1961) The interaction of energy and electron transfer reactions in mitochondria. V. The energy transfer pathway. J Biol Chem 236:1569–1576
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59(3):527–605
Chen GY, Nunez G (2010) Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 10(12):826–837
Ciz M, Denev P, Kratchanova M et al (2012) Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev 2012:181295
Czech MP, Lawrence JC Jr, Lynn WS (1974) Evidence for the involvement of sulfhydryl oxidation in the regulation of fat cell hexose transport by insulin. Proc Natl Acad Sci U S A 71(10):4173–4177
Dang PM, Dewas C, Gaudry M et al (1999) Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox). J Biol Chem 274(29):20704–20708
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82(1):47–95
Dunne DW, Resnick D, Greenberg J et al (1994) The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci U S A 91(5):1863–1867
El Benna J, Hayem G, Dang PM et al (2002) NADPH oxidase priming and p47phox phosphorylation in neutrophils from synovial fluid of patients with rheumatoid arthritis and spondylarthropathy. Inflammation 26(6):273–278
Elbim C, Bailly S, Chollet-Martin S et al (1994) Differential priming effects of proinflammatory cytokines on human neutrophil oxidative burst in response to bacterial N-formyl peptides. Infect Immun 62(6):2195–2201
El-Sonbaty SM, El-Hadedy DE (2012) Combined effect of cadmium, lead, and UV rays on Bacillus cereus using comet assay and oxidative stress parameters. Environ Sci Pollut Res Int. Epub
Erlemann KR, Rokach J, Powell WS (2004) Oxidative stress stimulates the synthesis of the eosinophil chemoattractant 5-oxo-6,8,11,14-eicosatetraenoic acid by inflammatory cells. J Biol Chem 279(39):40376–40384
Fabriek BO, van Bruggen R, Deng DM et al (2009) The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood 113(4):887–892
Fariss MW, Chan CB, Patel M et al (2005) Role of mitochondria in toxic oxidative stress. Mol Interv 5(2):94–111
Fisher AB (2009) Redox signaling across cell membranes. Antioxid Redox Signal 11(6):1349–1356
Gougerot-Pocidalo MA, el Benna J, Elbim C et al (2002) Regulation of human neutrophil oxidative burst by pro- and anti-inflammatory cytokines. J Soc Biol 196(1):37–46
Grant GE, Rokach J, Powell WS (2009) 5-Oxo-ETE and the OXE receptor. Prostaglandins Other Lipid Mediat 89(3–4):98–104
Grant GE, Rubino S, Gravel S et al (2011) Enhanced formation of 5-oxo-6,8,11,14-eicosatetraenoic acid by cancer cells in response to oxidative stress, docosahexaenoic acid and neutrophil-derived 5-hydroxy-6,8,11,14-eicosatetraenoic acid. Carcinogenesis 32(6):822–828
Guzik TJ, Griendling KK (2009) NADPH oxidases: molecular understanding finally reaching the clinical level? Antioxid Redox Signal 11(10):2365–2370
Hazen SL, Hsu FF, Duffin K et al (1996) Molecular chlorine generated by the myeloperoxidase-hydrogen peroxide-chloride system of phagocytes converts low density lipoprotein cholesterol into a family of chlorinated sterols. J Biol Chem 271(38):23080–23088
Holmes B, Page AR, Good RA (1967) Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest 46(9):1422–1432
Honda K, Chihara J (1999) Eosinophil activation by eotaxin–eotaxin primes the production of reactive oxygen species from eosinophils. Allergy 54(12):1262–1269
Janero DR, Hreniuk D, Sharif HM (1991) Hydrogen peroxide-induced oxidative stress to the mammalian heart-muscle cell (cardiomyocyte): lethal peroxidative membrane injury. J Cell Physiol 149(3):347–364
Jendrysik MA, Vasilevsky S, Yi L et al (2011) NADPH oxidase-2 derived ROS dictates murine DC cytokine-mediated cell fate decisions during CD4 T helper-cell commitment. PLoS One 6(12):e28198
Kadota S, Fantus IG, Deragon G et al (1987) Stimulation of insulin-like growth factor II receptor binding and insulin receptor kinase activity in rat adipocytes. Effects of vanadate and H2O2. J Biol Chem 262(17):8252–8256
Katsuyama M, Matsuno K, Yabe-Nishimura C (2012) Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr 50(1):9–22
Kidd P (2003) Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 8(3):223–246
Kirkham P (2007) Oxidative stress and macrophage function: a failure to resolve the inflammatory response. Biochem Soc Trans 35(Pt 2):284–287
Korantzopoulos P, Kolettis TM, Galaris D et al (2007) The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol 115(2):135–143
Kotsias F, Hoffmann E, Amigorena S et al (2013) Reactive oxygen species production in the phagosome: impact on antigen presentation in dendritic cells. Antioxid Redox Signal 18(6):714–729
Krieger M, Herz J (1994) Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP). Annu Rev Biochem 63:601–637
Krishnaswamy JK, Chu T, Eisenbarth SC (2013) Beyond pattern recognition: NOD-like receptors in dendritic cells. Trends Immunol 34:224–233
Laihia JK, Jansen CT (1997) Up-regulation of human epidermal Langerhans’ cell B7-1 and B7-2 co-stimulatory molecules in vivo by solar-simulating irradiation. Eur J Immunol 27(4):984–989
Le SB, Hailer MK, Buhrow S et al (2007) Inhibition of mitochondrial respiration as a source of adaphostin-induced reactive oxygen species and cytotoxicity. J Biol Chem 282(12):8860–8872
Lenaz G (1998) Role of mitochondria in oxidative stress and ageing. Biochim Biophys Acta 1366(1–2):53–67
Leto TL, Morand S, Hurt D et al (2009) Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal 11(10):2607–2619
Liu WF, Ma M, Bratlie KM et al (2011) Real-time in vivo detection of biomaterial-induced reactive oxygen species. Biomaterials 32(7):1796–1801
Matsumoto A, Naito M, Itakura H et al (1990) Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A 87(23):9133–9137
Mishra D, Mehta A, Flora SJ (2008) Reversal of arsenic-induced hepatic apoptosis with combined administration of DMSA and its analogues in guinea pigs: role of glutathione and linked enzymes. Chem Res Toxicol 21(2):400–407
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148
Mogensen TH (2009) Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev 22(2):240–273
Muster B, Kohl W, Wittig I et al (2010) Respiratory chain complexes in dynamic mitochondria display a patchy distribution in life cells. PLoS One 5(7):e11910
Netzer N, Goodenbour JM, David A et al (2009) Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462(7272):522–526
Newkirk MM, Goldbach-Mansky R, Lee J et al (2003) Advanced glycation end-product (AGE)-damaged IgG and IgM autoantibodies to IgG-AGE in patients with early synovitis. Arthritis Res Ther 5(2):R82–R90
Omori K, Ohira T, Uchida Y et al (2008) Priming of neutrophil oxidative burst in diabetes requires preassembly of the NADPH oxidase. J Leukoc Biol 84(1):292–301
Pawelec G, Derhovanessian E, Larbi A (2010) Immunosenescence and cancer. Crit Rev Oncol Hematol 75(2):165–172
Peled A, Gonzalo JA, Lloyd C et al (1998) The chemotactic cytokine eotaxin acts as a granulocyte-macrophage colony-stimulating factor during lung inflammation. Blood 91(6):1909–1916
Petreccia DC, Nauseef WM, Clark RA (1987) Respiratory burst of normal human eosinophils. J Leukoc Biol 41(4):283–288
Pickrell AM, Fukui H, Moraes CT (2009) The role of cytochrome c oxidase deficiency in ROS and amyloid plaque formation. J Bioenerg Biomembr 41(5):453–456
Poli G, Leonarduzzi G, Biasi F et al (2004) Oxidative stress and cell signalling. Curr Med Chem 11(9):1163–1182
Rada B, Leto TL (2008) Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol 15:164–187
Rutault K, Alderman C, Chain BM et al (1999) Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med 26(1–2):232–238
Rybicka JM, Balce DR, Khan MF et al (2010) NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc Natl Acad Sci U S A 107(23):10496–10501
Sheng KC, Pietersz GA, Tang CK et al (2010) Reactive oxygen species level defines two functionally distinctive stages of inflammatory dendritic cell development from mouse bone marrow. J Immunol 184(6):2863–2872
Shult PA, Graziano FM, Wallow IH et al (1985) Comparison of superoxide generation and luminol-dependent chemiluminescence with eosinophils and neutrophils from normal individuals. J Lab Clin Med 106(6):638–645
Sies H, Cadenas E (1985) Oxidative stress: damage to intact cells and organs. Philos Trans R Soc Lond B Biol Sci 311(1152):617–631
Song L, Lee C, Schindler C (2011) Deletion of the murine scavenger receptor CD68. J Lipid Res 52(8):1542–1550
Starkov AA, Fiskum G, Chinopoulos C et al (2004) Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 24(36):7779–7788
Steinman RM (1991) The dendritic cell system and its role in immunogenicity. Annu Rev Immunol 9:271–296
Straumann A, Safroneeva E (2012) Eosinophils in the gastrointestinal tract: friends or foes? Acta Gastroenterol Belg 75(3):310–315
Sundaram S, Ghosh J (2006) Expression of 5-oxoETE receptor in prostate cancer cells: critical role in survival. Biochem Biophys Res Commun 339(1):93–98
Tang L, Lucas AH, Eaton JW (1993) Inflammatory responses to implanted polymeric biomaterials: role of surface-adsorbed immunoglobulin G. J Lab Clin Med 122(3):292–300
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552(Pt 2):335–344
Underhill DM, Goodridge HS (2012) Information processing during phagocytosis. Nat Rev Immunol 12(7):492–502
VanderVen BC, Hermetter A, Huang A et al (2010) Development of a novel, cell-based chemical screen to identify inhibitors of intraphagosomal lipolysis in macrophages. Cytometry A 77(8):751–760
Winterbourn CC, Vissers MC, Kettle AJ (2000) Myeloperoxidase. Curr Opin Hematol 7(1):53–58
Xie L, Tsaprailis G, Chen QM (2005) Proteomic identification of insulin-like growth factor-binding protein-6 induced by sublethal H2O2 stress from human diploid fibroblasts. Mol Cell Proteomics 4(9):1273–1283
Zhong J, Rao X, Deiuliis J et al (2013) A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 62(1):149–157
Zhou R, Tardivel A, Thorens B et al (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11(2):136–140
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Urbanska, A.M., Zolla, V., Verzani, P., Santambrogio, L. (2014). Physiological and Pathological Role of Reactive Oxygen Species in the Immune Cells. In: Massoud, A., Rezaei, N. (eds) Immunology of Aging. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-39495-9_23
Download citation
DOI: https://doi.org/10.1007/978-3-642-39495-9_23
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-39494-2
Online ISBN: 978-3-642-39495-9
eBook Packages: MedicineMedicine (R0)